Abstract
In the immune response against a typical T cell-dependent protein antigen, the affinity maturation process is fast and is associated with the early class switch from IgM to IgG. As such, a comprehension of the molecular basis of affinity maturation could be of great importance in biomedical and biotechnological applications. Affinity maturation of anti-protein antibodies has been reported to be the result of small structural changes, mostly confined to the periphery of the antigen-combining site. However, little is understood about how these small structural changes account for the increase in the affinity toward the antigen. Herein, we present the three-dimensional structure of the Fab fragment from BALB/c mouse mAb F10.6.6 in complex with the antigen lysozyme. This antibody was obtained from a long-term exposure to the antigen. mAb F10.6.6, and the previously described antibody D44.1, are the result of identical or nearly identical somatic recombination events. However, different mutations in the framework and variable regions result in an ≈103 higher affinity for the F10.6.6 antibody. The comparison of the three-dimensional structures of these Fab-lysozyme complexes reveals that the affinity maturation produces a fine tuning of the complementarity of the antigen-combining site toward the epitope, explaining at the molecular level how the immune system is able to increase the affinity of an anti-protein antibody to subnanomolar levels.
During the antigen-specific activation of B cells, point mutations generally accumulate in the variable regions of antibodies. This process has been called affinity maturation, because it is believed that the role of these mutations is to affect an increased binding to antigen (1). Studies with hapten antigens have shown a pattern of somatic hypermutations in VH and VL regions, which correlate with observed increases in kinetic association rates and affinity (2, 3). As such, affinity maturation is understood as a process of accumulation of mutations (repertoire drift), favored by long-term exposure to antigen, producing antibodies of higher affinity. During prolonged immunizations, high-affinity antibodies also appear as the result of the recruitment of new clones expressing different antibody genes (repertoire shift) (4). In the immune response against a typical T cell-dependent protein antigen, the affinity maturation process is fast and is associated with the early class switch from IgM to IgG. Moreover, somatic mutations during the switch process help to improve the complementarity of the antibody/antigen-combining site (5, 6). Affinity maturation, therefore, may compensate for the loss in avidity given the decrease in the valence from IgM to IgG.
Little is known, however, about the effects of the somatic mutations responsible for affinity maturation, in terms of the structural changes in the antigen-binding site that result in an increased affinity toward the antigen. To establish the structural basis for affinity maturation against protein antigens, hen egg-white lysozyme (HEL) is an excellent antigen, because much is known about its structure as a free monomer and in complexes with several specific mAbs (7, 8). Structural studies, as well as epitope mapping (9-11), have contributed a wealth of information regarding the structural aspects of the anti-lysozyme response. The three-dimensional structures of eight complexes between HEL and the Fab or Fv fragments of murine anti-HEL antibodies have been reported, identifying several important features of antibody/antigen interactions (12-21). The specificity of binding is determined almost exclusively by the structure of the complementarity-determining regions (CDRs) of the VH and VL domains. VH CDR3, encoded primarily by the D (diversity) gene segment, contributes a significant percentage of the noncovalent bonds stabilizing the antibody/antigen complex. The six antibody CDRs form a contiguous surface (paratope) that affords shape and noncovalent bond complementarity to the antigenic determinant or epitope. These surfaces areas of interaction are ≈600-900 Å2, with the shape and chemical complementarity between antibody and antigen in some cases increased by the burying of solvent water molecules. In addition, large proportions of CDR aromatic residues, excluded from solvent interactions by the antibody/antigen complex, are implicated as hot spots, dominating the free energies of the interaction (22).
In contrast to hapten-specific responses, a study of the mouse immune response to HEL found no correlation between the time of exposure to the antigen and the equilibrium and kinetic association constants (23). Antibodies elicited during short-term (early and late secondary) responses showed an average affinity constant of 5.7 × 108 M-1, whereas antibodies elicited after long-term exposure to the antigen (120 days) showed an average affinity of 1.6 × 109 M-1 (23). Affinity maturation of the anti-lysozyme response has, therefore, been attributed to small structural changes, mostly confined to the periphery of the antigen-combining site (7, 23).
Herein, we present the three-dimensional structure and analysis of the Fab from BALB/c mouse mAbF10.6.6 (IgG1κ) in complex with HEL. This mAb was obtained after long-term exposure to the antigen (23) and belongs to a group of antibodies, including mAb D44.1, which recognize the β-pleated sheet domain of HEL. mAbs D44.1 and F10.6.6 are the result of identical or nearly identical somatic recombination events (VH plus D plus JH and VL plus JL) (23). However, different mutations in recombined germ-line genes result in an ≈103 higher affinity for the F10.6.6 anti-HEL antibody than for D44.1. An understanding of the structural basis of this increase in affinity could be of great interest in biomedical and biotechnological applications, such as optimizing the affinity of neutralizing antibodies to subnanomolar levels.
Materials and Methods
Preparation of Fabs from IgG1 Anti-HEL mAbs. mAbs were purified from ascites, and their Fab fragments were prepared by papain hydrolysis and were purified as reported (23). To ensure that only univalent Fabs were present, the Fabs were purified by gel filtration on a Superdex 75 column (Amersham Pharmacia Biosciences, Uppsala) and the peaks corresponding to 50 kDa were collected and were used for biosensor analysis and crystallization.
Measurements of Kinetic Constants by Biosensor. Affinity sensor analysis experiments were conducted on an IAsys Plus apparatus (Affinity Sensors, Saxon Hill, Cambridge, U.K.). Lysozyme was covalently coupled to carboxymethyl dextran sensor chips (Affinity Sensors) giving 5.1 ng of protein per cuvette. Binding reactions were carried out in PBS, 0.05% Tween 20 at 25°C, with constant stirring set up at 90%. Data were collected at intervals of 0.3 sec. Ligate binding to immobilized ligand was monitored at multiple ligate concentrations, ranging 10-fold below to at least 10-fold above preliminary estimates of equilibrium dissociation constants (Kd) for each reaction. Kinetic analysis was performed by using the fastfit software (Affinity Sensors).
Crystallization, Data Collection, Solution, and Refinement of the F10.6.6-HEL Structure. The Fab F10.6.6/HEL complex was crystallized by using the hanging-drop vapor diffusion method. The Fab F10.6.6/HEL complex at 10 mg/ml in 5 mM Tris, pH 7.5, was crystallized with 28% polyethylene glycol 1000/0.2M CaCl2/pH 4.6. Crystals appeared within 24 h and grew to maximum size of 0.4 × 0.05 × 0.05 mm in 4 days.
Data to 2.0 Å were collected at 100 K by using a Raxis IV (Rigaku/MSC, The Woodlands, TX) area detector with a Rigaku microfocus x-ray source and Osmic confocal focusing system. A total of 111,920 measured intensities were reduced to 60,488 unique reflections. The space group is P1 with a = 44.66, b = 73.75, c = 83.78, α = 66.59, β = 74.74, γ = 85.44. Data were integrated and scaled with the crystalclear (Rigaku/MSC) version of d*trek (24) and are 95% complete to 2.0-Å resolution. The Rmerge for all observed data are 0.013 with an Rmerge of the outer shell data (2.0-2.07 Å) of 0.036.
The structure was solved with molecular replacement procedures by using the program cns (25) and the previously determined D44.1/HEL complex. Two Fab/HEL complexes were located in the unit cell and a rigid body refinement resulted in an R value of 0.37 (8-4 Å, F > 2σ F, residual target).
The model was refined by alternating cycles of simulated annealing with maximum likelihood target (all data to 2.0 Å, F > 2σ) and visual model building with the program turbo (26). During later cycles, peaks in 2Fo - Fc and Fo - Fc Fourier maps, corresponding to appropriate solvent locations, were used to model solvent positions. After 10 cycles of refinement, the overall R value dropped to 0.204 (Rfree = 0.242) for all data to 2.0 Å (58,850 reflections F > 2σ). The rms deviations in the final model are 0.006 Å for bond lengths and 1.395° for bond angles.
Buried surface areas of interaction (ΔASA) between antibody and antigen were calculated with the ms suite of programs (27) by using a probe radius of 1.7 Å, a density of 20 points per Å2, and the buried surface option. Intermolecular nonpolar interactions were assigned to any pair of atoms at <4-Å distance that do not form hydrogen or ionic bonds. Antibody/antigen interface complementarity was analyzed with the program fade (28).
Results
Kinetics and Thermodynamics of the Antibody/Antigen Interaction. Table 1 presents the kinetics and thermodynamics for mAbs D44.1 and F10.6.6 reactions with lysozyme. mAb F10.6.6 has an ≈700 times greater affinity toward HEL with ≈170 times faster association constant (Ka). The F10.6.6/HEL reaction is enthalpically driven with an unfavorable entropic effect. In contrast, the lower entalphy of the D44.1/HEL reaction is partially compensated by a neutral entropic factor (29).
Table 1. Kinetics and thermodynamics of mAbs D44.1 and F10.6.6 reactions with lysozyme at 25°C.
| mAb | Ka*, M−1·s−1 | Kd*, s−1 | Ka, M−1 | ΔG, KJ/mol | ΔH†, KJ/mol | −TΔS, KJ/mol |
|---|---|---|---|---|---|---|
| D44.1 | 4.21 × 104 | 2.92 × 10−3 | 1.44 × 107 | −40.5† | −43.3 | 2.8† |
| F10.6.6 | 7.19 × 106 | 7.00 × 10−4 | 1.02 × 1010 | −56.6‡ | −76.4 | 19.8‡ |
Determined by IAsys biosensor (this work) using Fab fragments.
Taken from Schwarz et al. (29).
Calculated from the equations ΔG = −RT InKa and ΔG = ΔH − TΔS
Sequence Analysis of mAb F10.6.6-Variable Regions. The sequences of the VH and VL domains from mAbs D44.1 and F10.6.6 are very close, with 90% and 96% of homology, respectively (Fig. 1), and are apparently derived from the same germ-line genes.
Fig. 1.
Sequence alignment of variable region of mAbs D44.1 and F10.6.6. Amino acid sequences of VH D44.1, VH F10.6.6, VL D44.1, and VL F10.6.6 (GenBank accession no. AY277254). VL F10.6.6 was cloned and sequenced as described (23). Assignment of antibody CDRs follows the AbM definition, based on sequence variability (Kabat definition, refs. 41 and 42) and the location of the structural loop regions (Chothia definition, ref. 43). CDR residues are underlined in both sequences.
The heavy chains of both antibodies probably originate from the VHJ558.17 and JH4 genes. mAb D44.1 accumulates 10 mutations in the VH region (nine in framework regions and one in CDR1H), whereas mAb F10.6.6 accumulates 16 mutations in VH (13 in framework regions, two in CDR1H, and one in CDR2H). The FR4H in both antibodies present the same three mutations. Despite the lack of information about the origin of V(D)J rearrangement at the CDR3H juncture (putative DH genes are DSP2.7, DSP2.8, and DSP2.9 for both antibodies), mAbs D44.1 and F10.6.6 share a similar and same length CDR3H.
The light chains of both antibodies originate from the VLκ 23.43 and Jk1 germ-line genes. As with the heavy chain, the light chain of mAb F10.6.6 accumulates more mutations (seven mutations; three in framework regions, two in CDR2L, and two in CDR3L) than mAb D44.1 (four mutations: one in framework regions, two in CDR2L, and one in CDR3L). Thus, the increased number of somatic mutations in mAb F10.6.6 (23 mutations compared with 14 mutations of mAb D44.1) correlates with the increase in affinity toward the antigen lysozyme.
Structural Comparison of FabF10.6.6/HEL and FabD44.1/HEL Complexes. The D44.1-HEL (16) and F10.6.6-HEL crystal structures are not isomorphous but each contains two Fab/HEL complexes in the asymmetric unit, allowing duplicate comparisons of antibody/antigen contacts. All amino acids are situated in good electron density, except for residues 162-165 of the CH1 domain, frequently noted as disordered in other crystal structures of Fab fragments.
To identify any structural origin of the differences in HEL binding to F10.6.6 and D44.1, the four complexes were superimposed by using a least-squares procedure and all Cαs of the VL and VH domains (Fig. 2). In the superpositions, the 2σ outliers are in loop regions localized on VL 14, VL 57, VL 82, VH 41, and VH 62, regions not involved in contacts to HEL. Despite the good superposition, the Fvs of the F10.6.6/HEL complexes superimpose slightly better with each other (rms deviation of 0.20 Å) than with the Fvs of D44.1/HEL (rms deviation of 0.42 Å). This result is indicative of the minor conformational differences between F10.6.6 and D44.1 that may play a role in the complementarities of the antibody/antigen complexes.
Fig. 2.
Superposition of F10.6.6-HEL (yellow) and D44.1-HEL (light blue). For clarity, the FV-HEL of only one F10.6.6-HEL and one D44.1-HEL is shown. The superposition identifies no remarkable conformational differences between F10.6.6 and D44.1; however, the HEL in the D44.1 complex shows a displacement away from the antibody, as compared with the F10.6.6-HEL structure.
All seven hydrogen bonds or ion pairs, as well as three of the four buried solvent water molecules mediating antibody/antigen contacts at the F10.6.6/HEL interface, are found in the D44.1-HEL structures. The D44.1/HEL interaction includes two additional hydrogen bonds, detailed below, not found in F10.6.6-HEL. Of the common hydrogen bonds, the average distance in the F10.6.6/HEL complexes is 2.85 Å, whereas the hydrogen bond distance in the D44.1/HEL complexes averages 2.98 Å. Likewise, the salt bridge formed by VH Glu-50 Oε2 and HEL Arg-68 Nh2 is 0.2 Å shorter in the F10.6.6/HEL complexes. Both F10.6.6-HEL structures include a fourth buried solvent water molecule not found in the D44.1-HEL structures (Table 2).
Table 2. Hydrogen bonds, ion pairs, and buried water interactions present in F10.6.6/HEL and D44.1/HEL interfaces.
| Antibody atoms | F10.6.6-HEL(1) | F10.6.6-HEL(2) | D44.1-HEL(1) | D44.1-HEL(2) | |
|---|---|---|---|---|---|
| HEL atoms | |||||
| 47 O | VL 92 Nδ2 | 2.79 | 3.04 | ||
| 49 N | VL 92 O | 2.88 | 2.9 | 2.83 | 3.36 |
| 81Oγ | VH 30 O | 3.02 | 2.74 | 3.42 | 2.96 |
| 53 OH | VH 33 Nε1 | 2.83 | 2.77 | 2.87 | 2.91 |
| 68 Nh2* | VH 35 Oε2 | 3.09* | 2.83* | 2.93* | 3.27* |
| 45 Nε | VH 50 Oε1 | 2.75 | 2.86 | 2.74 | 2.75 |
| 45 Nh2* | VH 50 Oε1 | 2.98* | 2.86* | 3.03* | 2.43* |
| 68 Nh2* | VH Oε2 | 2.4* | 2.42* | 2.51* | 2.71* |
| 41 Nε2 | VH 55 O | 3.01 | 2.74 | ||
| Water molecule | |||||
| 1 | VH 33 N | 2.86 | 2.88 | 2.6 | 2.73 |
| 1 | VH 99 O | 2.79 | 2.92 | 2.75 | 2.95 |
| 1 | HEL 66 O | 2.62 | 2.52 | 2.88 | 2.41 |
| 2 | VL 96 Nh2 | 2.50 | 2.95 | 2.98 | 2.67 |
| 2 | HEL 68 O | 3.03 | 2.63 | 2.78 | 2.57 |
| 2 | HEL 49 O | 2.91 | 2.66 | 2.99 | 3.17 |
| 2 | Water 3 | 2.95 | 2.99 | 2.92 | 2.88 |
| 3 | VL 50 OH | 2.87 | 2.93 | 2.73 | 3.69 |
| 3 | VL 91 Oγ | 2.52 | 2.51 | 2.57 | 2.48 |
| 4 | VH 31 O | 2.84 | 3.02 | ||
| 4 | HEL 65 O | 2.75 | 2.80 | ||
| 4 | HEL 80 N | 3.11 | 2.87 | ||
| 4 | HEL 81 N | 3.18 | 2.84 |
Distances are given in Å. The hydrogen bonds listed above correspond to HEL/antibody direct interactions and water interactions between antibodies and HEL. Numbers in parentheses denote the two complexes found in the asymmetric unit.
Ion pairs.
Table 3 shows the analysis of differences in ΔASA, the number of noncovalent contacts, and surface complementarity (SC) of the antibody/antigen complexes. The F10.6.6/HEL complex shows an average increase of 9.5% (58.6 Å2) in ΔASA due to a 16.5% increase in nonpolar surface (51.0 Å2), whereas the polar surface area increases only 2.3%. Nonpolar atoms account for 50.3% and 53.6% of the interaction surface areas in D44.1 and F10.6.6, respectively. The number of noncovalent bonds (van der Waals, hydrogen bonds and ion pairs) also increases significantly from 93 in D44.1 to 129 in F10.6.6. In agreement with these results, F10.6.6-HEL shows an improvement of SC, indicating that the F10.6.6/HEL interaction surfaces are closer than the surfaces in the D44.1/HEL complex. The improvement in SC and increase in nonpolar ΔASA and number of noncovalent bonds is distributed along the entire antigen-combining site. In all complexes, VH has a larger interacting surface with HEL than VL (63.7% and 61.2% of the total area in D44.1-HEL and in F10.6.6-HEL, respectively). However, the increase in nonpolar ΔASA is more pronounced in VL of F10.6.6-HEL. Of the 51.5 Å2 increase in nonpolar ΔASA for the F10.6.6 complex, 34.6 Å2 are associated with VL and 16.9 Å2 with the VH domain.
Table 3. Analysis of contacts, surface area, and SC differences between D44.1-HEL and F10.6.6-HEL.
| ΔASA total
|
ΔASA polar
|
ΔASA no polar
|
No. of contacts
|
SC
|
Average contact distances
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fragment | D44 | F10 | D44 | F10 | D44 | F10 | D44 | F10 | D44 | F10 | D44 | F10 |
| HEL | 607.4 | 633.9 | 303.4 | 321.4 | 304.0 | 312.5 | ||||||
| Fv | 617.4 | 676.0 | 306.3 | 313.4 | 311.1 | 362.6 | 93 | 129 | −143.67 | −162.08 | ||
| VH | 393.6 | 413.8 | 175.2 | 178.5 | 218.4 | 235.3 | 57 | 78 | −67.18 | −69.01 | ||
| H1 | 183.9 | 189.6 | 78.0 | 83.5 | 105.9 | 106.1 | 30 | 36 | 3.56 | 3.44 | ||
| H2 | 128.4 | 136.2 | 65.1 | 64.0 | 63.3 | 72.2 | 11 | 18 | 3.46 | 3.49 | ||
| FR3 | 28.8 | 28.9 | 0.9 | 1.9 | 27.9 | 27.0 | 8 | 11 | 3.65 | 3.70 | ||
| H3 | 52.4 | 59.1 | 31.2 | 29.1 | 21.3 | 30.0 | 8 | 13 | 3.68 | 3.46 | ||
| VL | 223.8 | 262.2 | 131.1 | 134.9 | 92.7 | 127.3 | 36 | 51 | −61.57 | −73.57 | ||
| L1 | 33.5 | 64.1 | 31.9 | 48.7 | 1.6 | 15.4 | 1 | 1 | 3.44 | 3.76 | ||
| L2 | 27.3 | 31.2 | 14.4 | 15.6 | 12.9 | 15.6 | 1 | 6 | 3.63 | 3.74 | ||
| L3 | 163.0 | 166.9 | 84.8 | 70.6 | 78.2 | 96.3 | 34 | 44 | 3.66 | 3.39 | ||
Average contact distances were calculated for both crystallographic complexes of each antibody by using a cutoff of 4.0 Å.
Comparison of the CDRs. CDR1H has the same sequence and structural conformation in both complexes [canonical class 1 (30)] except for residue 30 (Thr in F10.6.6, Ser in D44.1). This loop shows no significant differences in ΔASA; however, there is a slight increase in the number of contacts and shorter average contact distances in the F10.6.6/HEL complex (Table 3). Many of these contacts are involved in a nonpolar stacking interaction between Trp 33 and HEL Arg 68. The F10.6.6 and D44.1 complexes include a buried water molecule forming hydrogen bonds bridging antibody and antigen (Wat 1, Table 2). However, the F10.6.6/HEL complex include a buried water not found in D44.1-HEL (Wat 4, Table 2). This solvent water molecule bridges the carbonyl oxygen of H 31 with HEL main-chain atoms 65 O, 50 N, and 81 N.
CDR2H differs at position 56 (Asp in F10.6.6 and Gly in D44.1). F10.6.6 CDR2H is involved with more nonpolar contacts to HEL (18), compared with the loop in D44.1 (11), resulting in an increase in nonpolar ΔASA of 8.9 Å2. The average distance of the hydrogen bonds generated by CDR2H are equivalent in both complexes (2.71 Å in F10.6.6-HEL and 2.70 Å in D44.1-HEL); however, the salt bridge VH50 Oε2-HEL 68 Nh2 distance is shorter in the F10.6.6 structures (2.41 Å) compared with the D44.1 structures (2.61 Å).
In CDR3H, there are two amino acid differences located at positions 102 and 104. However, no conformational differences can be observed in this loop and neither of these residues contribute noncovalent bonds to the antibody/antigen interaction. Again, a few more nonpolar contacts are found in F10.6.6-HEL than in D44.1-HEL (13 versus eight, respectively). An increase in nonpolar ΔASA is noted (8.9 Å2) and shorter contact distances are observed in F10.6.6/HEL complexes.
CDR1L has the same sequence and structural conformation (canonical class 2A), makes no polar bonds, and only one nonpolar contact to HEL in the antibody/antigen complexes. An increase in both polar and nonpolar ΔASA is observed in F10.6.6-HEL; however, no gain in the number of noncovalent bonds is observed. There are two differences in the sequence of CDR2L at positions 51 and 55, neither of which contribute noncovalent bonds to the antibody/antigen complexes. CDR2L has a small contribution to the binding in both complexes and no significant changes in ΔASA are observed; however, the number of contacts is greater in the F10.6.6/HEL complex.
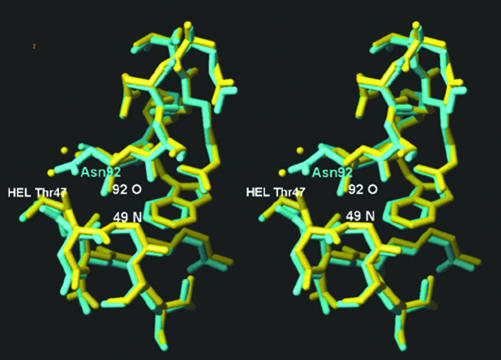
CDR3L (canonical class 1) features a significant difference at position 92, (Gly in F10.6.6 and Asn in D44.1). This loop accounts for 66% of ΔASA of the total contribution of the light chain in both complexes and forms most of the VL contacts (34 of 36, and 44 of 51 in D44.1-HEL and F10.6.6-HEL, respectively). For this loop, no significant changes in ΔASA are observed; however, there is a clear change in the character of the contacting surface. A decrease of 14.2 Å2 in the polar ΔASA is compensated by an increase of 18.1 Å2 in nonpolar ΔASA (Table 3) in the F10.6.6/HEL complex. Moreover, the average distance of the contacting atoms is 0.27 Å closer in F10.6.6-HEL than in D44.1-HEL. In D44.1, Asp 92 makes a hydrogen bond with the carbonyl oxygen of HEL Thr 47 (Table 2). This contact dislocates the carbonyl oxygen of HEL Thr 47 with respect to F10.6.6-HEL (Fig. 3). The carbonyl is rotated 35° in the D44.1-HEL structure, compared with the F10.6.6/HEL complex, strongly suggesting that the conformation of HEL in complex with D44.1 may be strained and energetically less favorable. Possibly as a result of this strain, the numbers of nonpolar contacts between CDR3L and HEL in the antibody/antigen complexes are different (44 in F10.6.6-HEL and 34 in D44.1-HEL) and contact distances are closer in the F10.6.6/HEL complex. The Asn to Gly mutation in F10.6.6 results in the loss of one solvent-accessible hydrogen bond, VL Asn 92 Ne2 to HEL Thr 47 O (Table 2). The loss of this bond, however, is compensated in the F10.6.6/HEL interaction by two water molecules.
Fig. 3.
Stereoview of the CDR L3/HEL interaction in F10.6.6/HEL (yellow) and D44.1/HEL (light blue) complexes. The dislocation of the HEL carbonyl by D44.1 CDRL3 Asn is evident. The average displacement of the HEL Cαs in the D44.1-HEL structures is 0.45 Å.
Discussion
Affinity maturation of the immune response is an unique and sophisticated mechanism of the immune system. A functional immune system relies on the production of an extremely wide range of Ig molecules from a limited genomic repertoire. Ig variable region genes are diversified after gene rearrangement by hypermutation, a fast process driven by selection for high-affinity binding to the antigen. Secondary response IgG antibodies directed to protein antigens have affinities of 107 to 1010 M-1 (23). Whereas lower affinities may not be sufficient to stimulate clones of antibody-producing cells, affinities >1010 M-1 apparently do not confer any selective advantage in terms of cell proliferation (31).
mAbs D44.1 and F10.6.6 recognize the same epitope, are very close in sequence, and typify the lower and upper limits of affinities for anti-protein responses. mAb D44.1 derives from a mouse immunized twice over a period of 15 days and mAb F10.6.6 is the product of six immunizations over a period of 3 months. Therefore, the pair of antibodies studied here are an excellent model to study the structural changes that occur in the antigen-combining site during the affinity maturation process.
Fig. 4 contrasts the contacting surface of both antibodies with HEL. The blue-colored atoms represent the D44.1 atoms interacting with HEL. These atoms on the D44.1 paratope surface (except Asn 92L) are also present in the F10.6.6 paratope. The cyan-colored atoms are those corresponding to contacts formed only in the F10.6.6/HEL interaction. As indicated, more contacting atoms are present in the F10.6.6 paratope producing the increased ΔASA of the F10.6.6/HEL interaction. The increase in contacts is evident across the entire paratope, not just in the peripheral zone, as has been described for antibodies that recognize another epitope of HEL (32). The F10.6.6-interacting surface differing from D44.1 features CDR1H atoms (Thr 31, Cγ2, O), CDR2H (Leu 52, Cδ2), and the peripheral atoms from FR3H (Tyr 59, all atoms), CDR3H (Asp 100, Cα, Cβ, O), CDR2L (Tyr 50, Cε2, Cζ), and CDR3L (Gly 92, Cα, C). These results demonstrate that both VH and VL F10.6.6 establish more contacts than do D44.1, producing an improved accommodation and complementarity to the HEL surface.
Fig. 4.
Atoms contacting HEL in the D44.1 and F10.6.6 antigen-combining sites. The F10.6.6 paratope topography is illustrated. Atoms in blue are those contacting HEL in both antibodies. Atoms in cyan correspond to additional contacts with the antigen in mAb F10.6.6.
Unlike several studies of site-directed antibody mutants (7, 22), the structural comparison of the F10.6.6 and D44.1 interactions with HEL does not identify any mutated residue that presents new bonds or contacts that could account for the differences in the kinetics and thermodynamics of the antibody/antigen association. On the other hand, several subtle differences explain the higher affinity of F10.6.6. Primarily, F10.6.6 presents more contacts with closer distance to HEL than does D44.1.
Furthermore, F10.6.6 forms more direct hydrogen bonds with HEL than D44.1, primarily as the result of the extra water (Wat 4) bridging F10.6.6 and HEL. The absence of this solvent water in the D44.1/HEL complex is most likely due to the increase in the antibody/antigen contact distances, allowing the water to exchange with solvent in the crystal structure. Finally, by providing an improvement in SC to HEL, mAb F10.6.6 presents an increase in nonpolar ΔASA, in comparison with mAb D44.1.
Like most antibodies against HEL (33, 34), the interacting surface of antibodies D44.1 and F10.6.6 have important polar and nonpolar components. A comparative study of four mAbs (H10-H63 system), which recognize an epitope on HEL different from that recognized by D44.1 and F10.6.6, shows that the interacting surfaces are predominantly hydrophilic, with no correlation between the number of contacts (electrostatic and van der Waals interactions) and the increase of affinity (32). In contrast, for antibodies D44.1 and F10.6.6, the increase in affinity arises from the combination of several factors, including improved shape complementarity and the resulting additional interfacial hydrogen bonds, van der Waals contacts, and increased buried surface area. These findings suggest that the D44.1-F10.6.6 system produces similar affinity maturation mechanism to anti-hapten responses (35-37), but with the changes affecting a larger planar surface. In the H10-H63 and D44.1-F10.6.6 systems, SC correlates with affinity, signifying that improved fit contributes to affinity maturation.
As described in a study of site-directed antibody mutations, there is a strong correlation between the loss of buried nonpolar accessible surface area and the increase in the free energy of binding (ΔGb) (22). Assuming a contribution of 21 cal per mol per Å2 to the free energy of reaction, the 51.5 Å2 difference in the nonpolar surface interaction areas of the F10.6.6/HEL and D44.1/HEL complexes could account for 1.08 kcal/mol of the 3.86 kcal/mol differences in free energy of reaction (ΔΔGb) (Table 1). The improved complementarity of the F10.6.6/HEL interaction also allows for the burying of a water molecule that contributes bridging hydrogen bonds between antibody and antigen. With four hydrogen bonds, each contributing 0.6-1.8 kcal/mol (38), it can be envisioned that this buried water molecule along with the increased surface area of interaction and the shorter contact distances, can explain the enthalpy of reaction between F10.6.6 and HEL, as compared with D44.1-HEL. In comparable protein/protein interactions, mutations that increase enthalpies of reaction may also contribute to unfavorable entropies (enthalpy-entropy compensation). The negative change in entropy after F10.6.6/HEL complex formation, as compared with the neutral change in entropy for the D44.1/HEL reaction, can be explained in part by the extra water molecule buried at the interface (39). Other possible causes for this effect include desolvation of a larger surface of interaction and differences in the conformation of uncomplexed and complexed antibody and antigen. We now plan to study the effect of solvent stress on the F10.6.6-HEL reaction to compare it with the D44.1/HEL interaction (40). This study may explain the enthalpy-entropy compensation in the F10.6.6/HEL and D44.1/HEL reactions. We have recently crystallized the uncomplexed Fab F10.6.6. The analysis of this structure, and comparison with complexed F10.6.6 and free and complexed D44.1, should provide information as to whether conformational changes occur after complex formation, which may affect the structural, thermodynamic, and kinetic analysis of affinity maturation we have presented.
In summary, the detailed comparison of F10.6.6 and D44.1 complexed with HEL clearly demonstrates how subtle structural changes in the combining site can produce a physiologically significant higher affinity.
Acknowledgments
We thank the Fundación Antorchas for support, Dr. Roberto Poljak for critical reading of the manuscript, and the reviewers for their thoughtful comments and suggestions about the presentation of the manuscript. This work was supported by a Fogarty International Research Collaboration Award grant from the Fogarty International Center, National Institutes of Health (to B.C.B and F.A.G.), and by a grant from the Agencia Nacional de Promoción Científica y Tecnológica República Argentina.
Abbreviations: HEL, hen egg-white lysozyme; CDR, complementarity-determining region; ΔASA, buried surface area of interaction; SC, surface complementarity.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY277254). The atomic coordinates and structure factors for the F.10.66-HEL structure have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 1P2C).
References
- 1.Eisen, H. N. & Siskind, G. W. (1964) Biochemistry 3, 996-1008. [DOI] [PubMed] [Google Scholar]
- 2.Foote, J. & Milstein, C. (1991) Nature 352, 530-532. [DOI] [PubMed] [Google Scholar]
- 3.Sagawa, T., Oda, M., Ishimura, M., Furukawa, K. & Azuma, T. (2003) Mol. Immunol. 39, 801-808. [DOI] [PubMed] [Google Scholar]
- 4.Berek, C., Jarvis, J. M. & Milstein, C. (1987) Eur. J. Immunol. 1121-1129. [DOI] [PubMed]
- 5.England, P., Nageotte, R., Renard, M., Page, A. L. & Bedouelle, H. (1999) J. Immunol. 162, 2129-2136. [PubMed] [Google Scholar]
- 6.Roost, H. P., Bachman, M. F., Haag, A., Kalinke, U., Pliska, V., Hengartner, H. & Zinkemagel, R. M. (1995) Proc. Natl. Acad. Sci. USA 92, 1257-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braden, B. C. & Poljak, R. J. (1995) FASEB J. 9, 9-16. [DOI] [PubMed] [Google Scholar]
- 8.Davies, D. H. & Cohen, G. H. (1996) Proc. Natl. Acad. Sci. USA 93, 7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman, M. A., Mainhart, C. R., Mallett, C. P., Lavoie, T. B. & Smith-Gill, S. J. (1992) J. Immunol. 149, 3260-3272. [PubMed] [Google Scholar]
- 10.Harper, M., Lema, F., Boulot, G. & Poljak, R. J. (1987) Mol. Immunol. 24, 97-108. [DOI] [PubMed] [Google Scholar]
- 11.Smith-Gill, S. J., Wilson, A. C., Potter, M., Prager, E. M., Feldmann, R. J. & Mainhart, C. R. (1982) J. Immunol. 128, 314-322. [PubMed] [Google Scholar]
- 12.Fischmann, T. O., Bentley, G. A., Bhat, T. N., Boulot, G., Mariuzza, R. A., Phillips, S. E. V., Tello, D. & Poljak, R. J. (1991) J. Biol. Chem. 266, 12915-12920. [PubMed] [Google Scholar]
- 13.Bhat, T. N., Bentley, G. A., Boulot, G., Greene, M. I., Tello, D., Dall'Acqua, W., Souchon, H., Schwarz, F. P., Mariuzza, R. A. & Poljak, R. J. (1994) Proc. Natl. Acad. Sci. USA 91, 1089-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padlan, E. A., Silverton, E. W., Sheriff, S., Cohen, G. H., Smith-Gill, S. J. & Davies, D. R. (1989) Proc. Natl. Acad. Sci. USA 86, 5938-5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kondo, H., Shiroishi, M., Matsushima, M., Tsumoto, K. & Kumagai, I. (1999) J. Biol. Chem. 274, 27623-27631. [DOI] [PubMed] [Google Scholar]
- 16.Braden, B. C., Souchon, H., Eisele, J-L., Bentley, G. A., Bhat, T. N., Navaza, J. & Poljak, R. J. (1994) J. Mol. Biol. 243, 767-781. [DOI] [PubMed] [Google Scholar]
- 17.Li, Y., Li, H., Smith-Gill, S. J. & Mariuzza, R. A. (2000) Biochemistry 39, 6296-6309. [DOI] [PubMed] [Google Scholar]
- 18.Chitarra, V., Alzari, P. M., Bentley, G. A., Bhat, T. N., Eisele, J. L., Houdusse, A., Lescar, J., Souchon, H. & Poljak, R. J. (1993) Proc. Natl. Acad. Sci. USA 90, 7711-7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lescar, J., Pellegrini, M., Souchon, H., Tello, D., Poljak, R. J., Peterson, N., Greene, M. & Alzari, P. M. (1995) J. Biol. Chem. 270, 18067-18076. [DOI] [PubMed] [Google Scholar]
- 20.Ay, J., Keitel, T., Kuttner, G., Wessner, H., Scholz, C., Hahn, M. & Hohne, W. (2000) J. Mol. Biol. 301, 239-246. [DOI] [PubMed] [Google Scholar]
- 21.Cohen, G. H., Sheriff, S. & Davies, D. R. (1996) Acta Crystallogr. D 52, 315-326. [DOI] [PubMed] [Google Scholar]
- 22.Sundberg, E. J., Urrutia, M., Braden, B. C., Isern, J., Tsuchiya, D., Fields, B. A., Malchiodi, E. L., Tormo, J., Schwarz, F. P. & Mariuzza, R. A. (2000) Biochemistry 39, 15375-15387. [DOI] [PubMed] [Google Scholar]
- 23.Goldbaum, F. A., Cauerhff, A., Velikovsky, C. A., Llera, A. S., Riottot, M. M. & Poljak, R. J. (1999) J. Immunol. 162, 6040-6045. [PubMed] [Google Scholar]
- 24.Pflugrath, J. W. (1999) Acta Crystallogr. D 55, 1718-1747. [DOI] [PubMed] [Google Scholar]
- 25.Brünger, A. T., Adams, P. D., Clore, G. M., Delano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N. S., et al. (1998) Acta Crystallogr. D 54, 905-921. [DOI] [PubMed] [Google Scholar]
- 26.Roussel, A. & Inisan, A. G. (1992) TURBO-FRODO (Technopole de Chateau-Gombert, Europarc Bat. C. Marseille, France).
- 27.Connolly, M. L. (1983) J. Appl. Crystallogr. 16, 548-558. [Google Scholar]
- 28.Mitchell, J. C., Kerr, R. & Ten Eyck, L. F. (2001) J. Mol. Graphics 19, 324-329. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz, F. P., Tello, D., Goldbaum, F. A., Mariuzza, R. A. & Poljak, R. J. (1995) Eur. J. Biochem. 228, 388-394. [PubMed] [Google Scholar]
- 30.Al-Lazikani, B., Lesk, A. M. & Chothia, C. (1997) J. Mol. Biol. 273, 927-948. [DOI] [PubMed] [Google Scholar]
- 31.Foote, J. & Eisen, H. N. (1995) Proc. Natl. Acad. Sci. USA 92, 1254-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, Y., Li, H., Yang, F., Smith-Gill, S. J. & Mariuzza, R. A. (2003) Nat. Struct. Biol. 10, 482-488. [DOI] [PubMed] [Google Scholar]
- 33.Mac Callum, R. M., Martin, A. C. R. & Thornton, M. (1996) J. Mol. Biol. 262, 732-745. [DOI] [PubMed] [Google Scholar]
- 34.Sundberg, E. J. & Mariuzza R. A. (2002) Adv. Protein Chem. 61, 119-160. [DOI] [PubMed] [Google Scholar]
- 35.Alzari, P. M., Spinelli, S., Mariuzza, R. A., Boulot, G., Poljak, R. J., Jarvis, J. M. & Milstein, C. (1990) EMBO J. 9, 3807-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wedemayer, G. J., Pattern, P. A., Wang, L. H., Schultz, P. G. & Stevens, R. C. (1997) Science 276, 1665-1669. [DOI] [PubMed] [Google Scholar]
- 37.Yang, P. L. & Schultz, P. G. (1999) J. Mol. Biol. 294, 1191-1201. [DOI] [PubMed] [Google Scholar]
- 38.Fersht, A. R. (1987) Trends Biochem. Sci. 12, 301-304. [Google Scholar]
- 39.Holdgate, G. A., Tunnicliffe, A., Ward, W. H. J., Weston, S. A., Rosembrock, G., Barth, P. T., Taylor, I. W. F., Pauptit, R. A. & Timms, D. (1997) Biochemistry 36, 9663-9673. [DOI] [PubMed] [Google Scholar]
- 40.Goldbaum, F. A., Schwarz, F. P., Eisenstein, E., Cauerhff, A., Mariuzza, R. A. & Poljak, R. J. (1996) J. Mol. Recognit. 9, 6-12. [DOI] [PubMed] [Google Scholar]
- 41.Kabat, E. A., Wu, T. T. & Bilofsky, H. (1997) J. Biol. Chem. 252, 6609-6617. [PubMed] [Google Scholar]
- 42.Kabat, E. A. (1978) Adv. Protein Chem. 32, 1-20. [PubMed] [Google Scholar]
- 43.Chothia, C., Lesk, A. M., Tramontano, A., Levitt, M., Smith-Gill, S. J., Air, G., Sheriff, S., Padlan, E. A., Davies, D., Tulip, W. R., et al. (1989) Nature 342, 877-883. [DOI] [PubMed] [Google Scholar]