Abstract
Ligand activation of the epidermal growth factor receptor (EGFR) leads to its rapid internalization and eventual delivery to lysosomes. This process is thought to be a mechanism to attenuate signaling, but signals could potentially be generated after endocytosis. To directly evaluate EGFR signaling during receptor trafficking, we developed a technique to rapidly and selectively isolate internalized EGFR and associated molecules with the use of reversibly biotinylated anti-EGFR antibodies. In addition, we developed antibodies specific to tyrosine-phosphorylated EGFR. With the use of a combination of fluorescence imaging and affinity precipitation approaches, we evaluated the state of EGFR activation and substrate association during trafficking in epithelial cells. We found that after internalization, EGFR remained active in the early endosomes. However, receptors were inactivated before degradation, apparently due to ligand removal from endosomes. Adapter molecules, such as Shc, were associated with EGFR both at the cell surface and within endosomes. Some molecules, such as Grb2, were primarily found associated with surface EGFR, whereas others, such as Eps8, were found only with intracellular receptors. During the inactivation phase, c-Cbl became EGFR associated, consistent with its postulated role in receptor attenuation. We conclude that the association of the EGFR with different proteins is compartment specific. In addition, ligand loss is the proximal cause of EGFR inactivation. Thus, regulated trafficking could potentially influence the pattern as well as the duration of signal transduction.
INTRODUCTION
The structure and function of the epidermal growth factor receptor (EGFR) is evolutionarily conserved from Caenorhabditis elegans to Homo sapiens (Aroian et al., 1990) and its activity regulates the proliferation, motility, and differentiation of many different cell types (Sibilia and Wagner, 1995; Threadgill et al., 1995). Binding of any one of at least five ligands activates the intrinsic tyrosine kinase domain of the EGFR (van der Geer et al., 1994), which phosphorylates itself and activates other members of the EGFR family, such as HER2 (Stern and Kamps, 1988; van der Geer et al., 1994). Receptor phosphotyrosine residues act as nucleation sites for additional proteins such as Shc, Grb2, mSOS, ras-GAP, phospholipase C-γ, Eps8, and c-Cbl (Rozakis-Adcock et al., 1992; Fazioli et al., 1993; van der Geer et al., 1994; Levkowitz et al., 1998). These receptor signaling partners are activated by allosteric effects or by tyrosine phosphorylation, leading to recruitment of additional signaling molecules (van der Geer et al., 1994). Downstream kinase cascades and specific protein-protein assemblages can, in turn, determine cell type-specific responses (Tan and Kim, 1999).
Activated EGFR are rapidly internalized by coated pits, sorted through early endosomes, and ultimately degraded in lysosomes by a process generally known as receptor down-regulation (Wiley et al., 1991; Sorkin and Waters, 1993). G-protein coupled receptors, as well as other receptor tyrosine kinases, are also down-regulated after ligand activation (Sorkin and Waters, 1993; Kallal et al., 1998). Although degradation is the ultimate fate of internalized receptors, the rate of receptor degradation is much slower than their rate of internalization. Thus, substantial intracellular pools of receptors and ligands can accumulate (Wiley et al., 1985). It is clear that receptors are initially activated at the plasma membrane, but it is much less certain whether internalized receptors remain active until they are degraded. It is also unknown whether signals from internalized receptors are qualitatively different from those generated at the cell surface.
For more than a decade, investigators have debated the existence of “signaling endosomes.” Experiments with rat liver have demonstrated that, after the administration of a bolus of EGF, intracellular EGFR are associated with Shc, Grb2, and mSOS (Di Guglielmo et al., 1994). These signaling cofactors are thought to be responsible for initiating signals at the cell surface (van der Geer et al., 1994). Additionally, other receptor substrates, such as c-src and rho-B, are enriched in endosomes (Adamson et al., 1992; Kaplan et al., 1992). The strongest evidence supporting the signaling endosome hypothesis comes from recent genetic and biochemical experiments with the EGFR and the β-adrenergic receptor. Schmid and colleagues used a conditional dynamin mutant to block EGFR endocytosis, resulting in specific signal transduction pathways being up-regulated and others being attenuated (Vieira et al., 1996). In similar experiments with the β-adrenergic receptor, endocytosis was inhibited with the use of both the nonspecific conditional dynamin mutation and a specific mutation in β-arrestin. This resulted in inhibition of mitogen-activated protein kinase activation (Daaka et al., 1998; Ahn et al., 1999). Together, these data suggest that specific signals can arise from the endosomal compartment.
Despite the positive evidence, it has been argued that EGFR signal transduction is primarily restricted to the cell surface (Fiore and Gill, 1999). To a large extent, this idea is based on the correlation between low rates of EGFR internalization and cell transformation (Wells et al., 1990; Huang et al., 1997). Supporting this argument is the observation that v-Cbl transforms cells at least in part by shunting EGFR back to the cell surface (Levkowitz et al., 1998). These data, however, do not directly rule out the possibility that signal transduction can arise from endosomes; nor do they separate the effects of inhibiting receptor endocytosis from the effects of inhibiting ligand or receptor degradation. Endosomes could still make up an important signaling compartment.
A major difficulty in evaluating the role of endosomal signaling is the low sensitivity of current techniques. In general, one must isolate endosomal compartments at different times after ligand stimulation and evaluate their composition (Wada et al., 1992). Because of the low yield and time-consuming nature of this approach, previous studies have been restricted to abundant tissues, such as rat liver, or transformed cells that overexpress receptors or specific signaling components (Levkowitz et al., 1998; Xue and Lucocq, 1998). Although these studies have been informative, they have necessary limitations. Rat liver is not a physiologically important target of EGFR action and overexpression of receptors or signaling molecules can lead to altered trafficking or function. These technical issues have made it difficult to determine whether endosomal signaling is a normal consequence of EGFR activation or is restricted to specific experimental systems.
To investigate the role of EGFR trafficking in its biological actions, we have used responsive human mammary epithelial cells (HMEC). Genetic and biochemical studies in mice have shown that normal EGFR function is critical for the development of the mammary epithelium (Fowler et al., 1995; Xie et al., 1997). In vitro, blocking the EGFR in HMEC leads to cell cycle arrest as well as inhibition of cell migration and organization (Stampfer et al., 1993; Wiley et al., 1998; Dong et al., 1999). Importantly, HMEC normally express high levels of EGFR, facilitating biochemical studies (Bates et al., 1990; Burke and Wiley, 1999). To investigate EGFR trafficking, we developed a new biochemical technique to isolate activated EGFR within endosomes with the use of a reversibly biotinylated nonantagonistic anti-EGFR antibody. In addition, we developed antibodies specific to tyrosine-phosphorylated EGFR to follow activated EGFR by immunofluorescence techniques. With the use of these approaches, we observed that the pattern of EGFR association with substrates and adaptor proteins changed as the EGFR moved from the cell surface through the endosomal compartment. In addition, we found that internalized EGFR lost both phosphotyrosine and associated ligand before degradation. Our results suggest that endosomes make up a major site of regulated EGFR signaling in responsive cells and that ligand loss is the proximal cause of attenuated receptor signaling.
MATERIALS AND METHODS
General
Human EGF was obtained from PeproTech (Rocky Hill, NY) and transforming growth factor (TGF)-α was obtained from R&D Systems (Minneapolis, MN). Monoclonal antibody (mAb) 225 against the EGFR (Gill et al., 1984) was purified from hybridomas obtained from American Type Culture Collection. mAb 13A9 against the human EGFR (Winkler et al., 1989) was a generous gift from Genentech (San Francisco, CA). Anti-EGFR antibody C-13 and anti-EEA1 antibody 14 were from Transduction Laboratories (Lexington, KY). Anti-HER2 antibody C18 and anti-EGFR antibody 1005 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-LAMP-2 antibody H4B4, developed by J.T. August and J.E.K. Hildreth, was obtained from the Developmental Studies Hybridoma Bank maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA. Antibodies used in Western blotting were purchased from Transduction Laboratories and used according to the manufacturer's instructions. Horseradish peroxidase-conjugated anti-mouse and anti-rabbit secondary antibodies were purchased from Pierce (Rockford, IL). Western blot detection was done with enhanced chemiluminescence (NEN-Renaissance, Boston, MA) and detected on film. Densitometry was done with the use of Molecular Analyst 2.2 software Bio-Rad (Hercules, CA). Monoclonal 13A9 and antibodies against EEA1 and LAMP-2 were directly labeled with Alexa dye 488 according to the manufacturer's instructions (Molecular Probes, Eugene, OR). Alexa-594 streptavidin and EGF complexed to Texas Red streptavidin were purchased from Molecular Probes.
The HMEC cell line 184A1 was provided by Dr. Martha Stampfer and was cultured in DFCI-1 medium supplemented with 12.5 ng/ml EGF (Stampfer, 1985; Band and Sager, 1989). HB2 cells were obtained from Dr. Joyce Taylor-Papadimitriou and were cultured as described (Bartek et al., 1991). Cell lines 184A1 and HB2 express 3.0 × 105 and 7.0 × 105 EGFR, respectively (Burke and Wiley, 1999). Eighteen hours before experiments, cells were transferred to a 37°C tabletop incubator in either DFCI-1 without bicarbonate but with 1% serum for 184A1 or serum-free and bicarbonate-free DMEM for HB2.
Antibody against EGFR Phosphorylated at Tyr-1173
A phosphopeptide corresponding to the major tyrosine phosphorylation site of the EGFR (ENAE[pY]LRVAPC) was custom synthesized, conjugated to KLH, and used to raise antisera in sheep (QCB, Hopkinton, MA). Sera from immunized sheep were precipitated with 50% ammonium sulfate, resuspended in 10 ml of 50 mM Tris, pH 7.2, 150 mM NaCl, and dialyzed overnight against the same buffer. Antibodies were then isolated by affinity chromatography.
Affinity matrices were made by coupling 1 mg of phosphorylated (Ac-ENAE[pY]LRVAPC-NH) or nonphosphorylated peptide (Ac-ENAE[Y]LRVAPC-NH) to 1 ml of iodoacetyl-agarose (QCB) through the carboxy-terminal cysteine according to the manufacturer's instructions. The matrices were rinsed once with 50 mM Tris, 5 mM EDTA, pH. 8.5, once with 50 mM cysteine in the same buffer, and twice with 50 mM NaH2PO4, 0.5 M NaCl, pH 6.5. Phosphotyramine was synthesized as previously described (Rothberg et al., 1978), purified by precipitation and recrystallization (Ross et al., 1981), confirmed by thin-layer chromatography and mass spectroscopy, and then conjugated to CNBr-activated Sepharose (Amersham Pharmacia Biotech, Piscataway, NJ) according to the manufacturer's instructions.
To obtain monospecific antibodies, the crude antibodies were run through four columns in series: Sepharose CL6B, Y1173 agarose, phosphotyramine Sepharose, and pY1173 agarose. Columns were first equilibrated with phosphate buffer (50 mM NaH2PO4, pH 6.5). The dialyzed ammonium sulfate cut was loaded onto the column series with phosphate buffer in a closed circuit loop overnight at room temperature. The columns were rinsed with a high salt buffer (50 mM NaH2PO4, 0.5 M NaCl, pH 6.5) and then CL6B and Y1173 columns were removed. The temperature of the phosphotyramine column was increased to 45°C to elute weakly binding material, and after reaching baseline, the phosphotyramine column was removed. Antibodies were then eluted from the pY1173 column with 100 mM glycine, pH 2.5, and 4-ml fractions were collected continuously into 200 μl of Tris buffer (1 M Tris, pH 9.5) for neutralization. Peak fractions were pooled and dialyzed (10 mM Tris, 20 mM NaCl, pH 7.0) at 4°C for 2 d with changes. The antibodies obtained with this protocol could bind only to phosphotyrosine in the correct peptide context.
Biotinylation and Purification of mAb 13A9
Two milligrams of mAb 13A9 (1.25 ml) were exhaustively dialyzed against 50 mM NaHCO3 at 4°C and biotinylated by adding 80 μl of a freshly prepared 1 mg/ml aqueous stock of sulfo-NHS-SS-biotin and incubating for 2 h on ice. After dialysis against 50 mM NaHCO3, biotinylated 13A9 (Btn-13A9) was purified with the use of a 2-cm monomeric avidin column (Pierce) and elution with biotin. The final product (75% recovery) was dialyzed against phosphate-buffered saline (PBS) and stored at −20°C at a concentration of 0.25 mg/ml in PBS, 50% glycerol, 1% bovine serum albumin, 0.02% sodium azide.
To isolate the Btn-13A9 from cells, we used streptavidin agarose. To isolate the nonbiotinylated 13A9, we used precoupled rabbit, anti-mouse protein A Sepharose. This was made by incubating 1.5 ml of 50% protein A Sepharose slurry in the presence of 30 μg of rabbit, anti-mouse IgG antibody (Sigma, St. Louis, MO) overnight at 4°C. The coupled protein A Sepharose was then washed twice with 10% glycerol, 1% Triton X-100, 20 mM HEPES, pH 7.0, 2 mM EDTA, 0.02% azide, 0.1 mM orthovanadate and stored at 4°C in the same buffer.
Separation of Internal from Surface EGFR
Cells were grown to near confluence in 100-mm tissue culture plates. Eighteen hours before the experiment, cells were changed to bicarbonate-free medium and transferred to an air incubator. Cells were changed to media containing 500 ng/ml Btn-13A9 for 2 h at 37°C to achieve steady-state labeling of the receptor pool. The cells were then treated with 50 nM EGF in the absence of Btn-13A9 with the use of a staggered time schedule so that the cells could be processed at the same time. To control for the varying treatment times with Btn-13A9, cell samples treated with and without Btn-13A9 for the entire incubation period were also collected.
All plates of cells were rapidly chilled to 0°C with the use of cold PBS on ice and treated three times for 8 min each with 3.5 ml of a glutathione-stripping solution (50 mM glutathione, 75 mM NaCl, 1 mM EDTA, 1% bovine serum albumin, 0.75% [vol/vol] 10 N NaOH). Bovine serum albumin and NaOH were added just before use. During each 8-min incubation, plates were rocked gently two to three times. Cells were then rinsed three times with 5 ml of PBS at 4°C and incubated for 10 min at 0°C with 1 ml of extraction buffer (10% glycerol, 1% Triton X-100, 20 mM HEPES, pH 7.0, 2 mM EDTA, 0.02% azide, 0.1 mM orthovanadate, 2 mM sodium pyrophosphate, and 1 μg/ml each of pepstatin, chymostatin, leupeptin, and aprotinin). Extracts were collected by scraping and centrifuging at 14,000 × g for 10 min at 4°C. Supernatants (950 μl) were transferred to a new tube containing 50 μl of a streptavidin agarose slurry (Pierce). A 40-μl sample of supernatant was evaluated for protein concentration with the use of a BCA kit (Pierce). After incubation on a rocking platform for 2.5–3.0 h at 4°C, the streptavidin agarose was collected by centrifugation for 1 min at 1000 × g in a swinging bucket rotor. The supernatants were transferred to fresh tubes containing 50 μl of a 50% slurry of precoupled rabbit, anti-mouse protein A Sepharose and incubated with rocking for 2.5 h at 4°C. All samples were washed twice in 1 ml of wash buffer (10% glycerol, 1% Triton X-100, 20 mM HEPES, pH 7.0, 2 mM EDTA, 0.02% azide, 0.1 mM orthovanadate, and 2 mM sodium pyrophosphate) at 4°C. Samples were solubilized by boiling in 1% SDS, 1% β-mercaptoethanol, 5% glycerol, 10 mM Tris-HCl, pH 6.8, for 5 min, snap frozen, and stored at −20°C. Samples were analyzed by Western blot after separation on 7.5% denaturing polyacrylamide gels. To maximize sample utility, blots were probed sequentially for different antigens. For example, one blot was probed first with anti-EGFR antibody (C-13, a mouse antibody) and then with anti-Shc (a rabbit antibody). A similar strategy was used for detecting Grb2 and Eps8.
Fluorescence Microscopy
Cells were plated on fibronectin-coated coverslips and changed to medium lacking EGF 24 h before the experiment. Cells were treated at 37°C with 200 ng/ml biotinylated EGF complexed with Texas Red streptavidin. At appropriate intervals, coverslips were rinsed in ice-cold saline and fixed with 3.6% paraformaldehyde, 0.024% saponin, freshly prepared in PBS as previously described (Wiley et al., 1998). Cells were incubated with 3.5 μg/ml affinity-purified sheep anti-1173-P for 1 h followed by staining with 1 μg/ml Alexa-488-labeled mAb 13A9, 5 μg/ml Cy5-labeled affinity-purified donkey anti-sheep IgG (Chemicon International, Temecula, CA), and 15 nM 4′6-diamidino-2-phenylindole for 45 min. After rinsing, the coverslips were mounted in 40 μl of Prolong mounting medium (Molecular Probes). Slides were viewed with an inverted microscope (Nikon, Melville, NY) with the use of a 60× objective and an XF57 multiband filter set (Omega Optical, Brattleboro, VT). Images (657 × 517) were captured separately at four wavelengths (460 nm; 4′6-diamidino-2-phenylindole, 520 nm; Alexa-488, 610 nm; Texas Red and 710 nm; Cy5) with the use of a MicroMax cooled CCD camera (Princeton Instruments, Trenton, NJ) attached to a Macintosh workstation running Openlab software (Improvision, Coventry, UK). Images at each wavelength were acquired with the use of a constant exposure time. Composite images were assembled in Adobe Photoshop (Adobe Systems, Mountain View, CA) with no alterations in relative gray scale levels. The lack of cross-reactivity of the Cy5-labeled affinity-purified donkey anti-sheep IgG with mouse monoclonal antibodies was verified experimentally.
To determine colocalization with organelle markers, cells were plated on coverslips, as described above, and incubated in the presence of 500 ng/ml Btn-13A9 for 2–3 h. The medium was aspirated, the coverslips were rinsed, and fresh media lacking Btn-13A9 but containing 50 nM EGF were added. At the appropriate time intervals, the cells were rinsed, fixed, and permeabilized in saponin as described above and then incubated with 1 μg/ml Alexa-594-streptavidin and 1 μg/ml mAbs against either EEA1 or LAMP-2 directly labeled with Alexa-488 for 1 h. The antibodies were dissolved in PBS containing 10 mg/ml bovine serum albumin and 0.012% saponin. Coverslips were mounted in Prolong antifade medium, and images were collected on a Bio-Rad MRC-1024 confocal microscope attached to a microscope (Zeiss, Oberkochen, Germany) with the use of a 40× objective. Alexa-594 and Alexa-488 image pairs were acquired sequentially and imported into the Openlab software package as Tiff files. They were converted to binary images by setting the threshold to remove the bottom 25% of the full scale intensity values (0–64). A logical “AND” between the image pairs was then used to determine the colocalization between the tagged EGFR and the appropriate subcellular marker. The percentage of colocalization was calculated by dividing the number of pixels remaining after the AND operation by the number of pixels in the corresponding EGFR binary image. Composite images were assembled in Adobe Photoshop.
RESULTS
Isolation of Surface and Internal EGFR
To investigate the ability of the EGFR to associate with specific signaling molecules after endocytosis, we developed a technique to rapidly isolate and separate internal from surface EGFR. Our approach was to use a reversibly biotinylated antibody against the EGFR that would not interfere with ligand binding or receptor function. The mAb 13A9 was chosen because it does not affect EGF binding, receptor internalization, or ability to heterodimerize with HER-2 (Carraway and Cerione, 1993; Lenferink et al., 1998a; Burke, Schooler, and Wiley, unpublished observations). The mAb 13A9 was biotinylated with sulfo-NHS-SS-biotin, yielding Btn-13A9. The disulfide bond in sulfo-NHS-SS-biotin allows us to release the biotin without denaturing the antibody by with the use of 50 mM glutathione. Sulfo-NHS-SS-biotin has been used previously to nonspecifically label cell surface proteins and to follow their internalization (Le Bivic et al., 1990; Schmidt et al., 1997). By first attaching the disulfide-linked biotin to mAb 13A9, we have effectively made the biotinylation reaction specific for the EGFR.
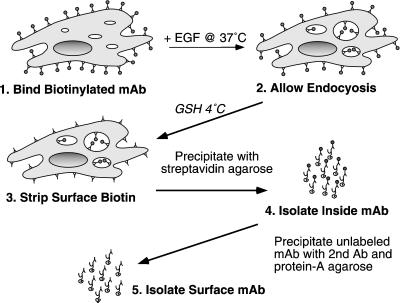
To isolate the EGFR at different points in the endocytic pathway, we took advantage of its occupancy-dependent trafficking. By providing a bolus of exogenous EGF, we can occupy the biotin-tagged EGFR as a cohort and follow its endocytic trafficking as a synchronized wave. After glutathione treatment, only the surface EGFR should be associated with nonbiotinylated mAb 13A9. Streptavidin agarose followed by secondary antibodies coupled to protein A Sepharose can then be used to sequentially separate the two populations of receptors. The general protocol is depicted in Figure 1.
Figure 1.
Protocol for separating internalized vs. surface-associated EGFR. Details are in MATERIALS AND METHODS. GSH, reduced glutathione; Ab, antibody.
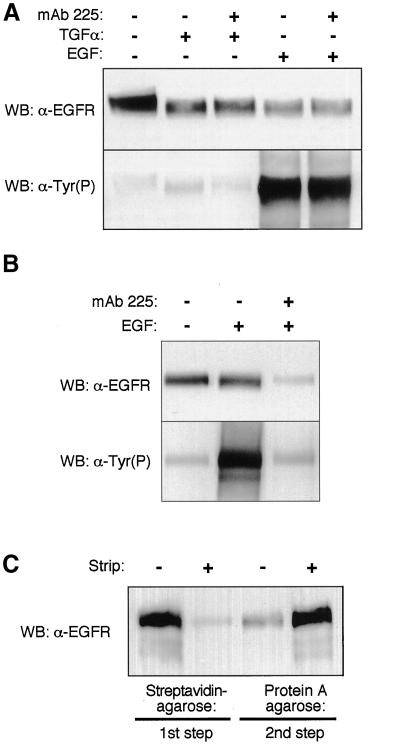
For our protocol to work as planned, both biotinylated and nonbiotinylated 13A9 must remain bound to a particular receptor throughout the course of the isolation steps. To determine the stability of Btn-13A9 binding, we first incubated cells in nonsaturating amounts of Btn-13A9 for 2 h at 37°C. We then added EGF for 10 min, lysed the cells, and isolated Btn-13A9 with streptavidin agarose in the presence of a competing anti-EGFR mAb 225. As shown in Figure 2A, top, competing antibodies did not alter the levels of EGFR isolated with Btn-13A9 (compare lane 2 with lane 3 and lane 4 with lane 5). This is in contrast to biotinylated anti-EGFR mAb 225. As seen in Figure 2B, lane 3, the addition of unlabeled excess 225 mAb blocked recovery of EGFR by streptavidin agarose. This indicates that the unlabeled 225 was able to displace previously bound biotinylated 225 mAb.
Figure 2.
Anti-EGFR mAb 13A9 remains stably associated with the EGFR. (A) Cells were incubated with 100 ng/ml Btn-13A9 for 2 h at 37°C, and they were either left untreated (lane 1) or treated with 50 nM TGF-α (lanes 2 and 3) or EGF (lanes 4 and 5) for 15 min. Extracts were prepared in either the presence (lanes 3 and 5) or absence (lanes 2 and 4) of a 500-fold molar excess of competitive mAb 225. Total EGFR, identified in lane 1, was isolated from parallel cell extracts with the use of 2 μg of Btn-13A9. After precipitation of the Btn-13A9 with streptavidin agarose, Western blots were made and probed with anti-EGFR antibodies (top) or anti-Tyr(P) antibodies (bottom). (B) Cells were incubated with 100 ng/ml Btn-225 for 2 h at 37°C, and they were either left untreated (lane 1) or treated with 2 nM EGF for 15 min (lanes 2 and 3). Extracts were prepared in either the presence (lanes 3) or absence (lanes 1 and 2) of a 100-fold molar excess of competitive mAb 225. Extracts were analyzed as described for A. (C) Efficiency of strip and precipitation steps. Cells were incubated with Btn-13A9 on ice for 2 h. Parallel plates were treated with or without the glutathione strip solution as indicated. Both plates were serially treated with streptavidin agarose (first step) and then rabbit anti-mouse coupled to protein A agarose (second step). Western blots of the precipitates were visualized with anti-EGFR. −, without; +, with.
Further proof of the stability of Btn-13A9 is offered by the divergent results seen when cells are activated with either EGF or TGFα. Because 13A9 selectively blocks the binding of TGFα to EGFR (Winkler et al., 1989; Lenferink et al., 1998a), 13A9 should only be able to precipitate receptors activated by EGF. Thus, the absence of phosphorylated EGFR in Figure 2A, lanes 2 and 3, shows that this is indeed the case. In contrast, the use of biotinylated 225 mAb results in the isolation of phosphorylated EGFR when EGF is added, even though this antibody can bind only to empty receptors. This indicates an exchange between different receptors during the isolation step. The lack of such behavior by Btn-13A9 demonstrates that it remains stably bound to EGFR during the extraction and isolation steps.
To test the efficiency of biotin removal from cell surface-associated Btn-13A9, we first blocked endocytosis by incubating cells at 4°C. Btn-13A9 was bound to the cells, which were then treated either with or without glutathione and lysed in detergent. As shown in Figure 2C, glutathione treatment reduced the ability of streptavidin agarose to pull out 13A9-EGFR complexes by 90–95%. Other investigators who have used sulfo-NHS-SS-biotin, reported similar efficiencies (Le Bivic et al., 1990). The streptavidin agarose incubation step removed ∼85–95% of the total Btn-13A9, based on the inability of secondary antibodies/protein A to bring down any additional EGFR (Figure 2C). Secondary antibodies and protein A Sepharose, however, could efficiently remove the 13A9-EGFR complexes remaining after removal of the biotin (Figure 2C). This demonstrates that the glutathione treatment efficiently removes biotin from surface-associated Btn-13A9. In addition, our data show that we can efficiently isolate both biotinylated and nonbiotinylated 13A9-EGFR complexes.
Internalization and Trafficking of EGFR Tagged with Btn-13A9
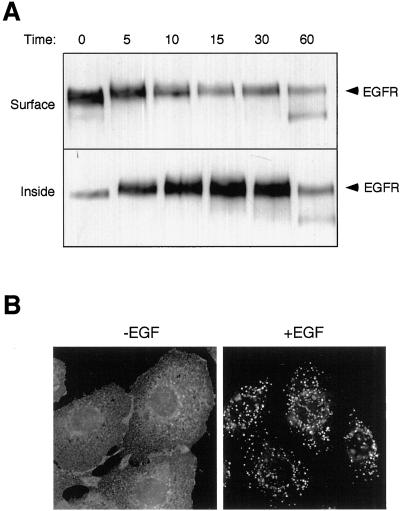
Before we used Btn-13A9 as a “tag” to follow the EGFR through endosomes, we first verified that it did not disrupt the normal trafficking of the EGFR. Btn-13A9 was first bound to the cell surface at 0°C. Cells were then warmed to 37°C in the presence of EGF, and at different times they were cooled and stripped with glutathionine. The surface and internalized EGFR were isolated as described above. The relative levels of the isolated receptors were then determined by Western blot. As shown in Figure 3A, in the absence of EGF and before warming the cells, 90–95% of the EGFR was found on the cell surface. After EGF addition and warming, the Btn-13A9–tagged EGFR rapidly lost its sensitivity to glutathione, indicating internalization. The time necessary to lose half of the Btn-13A9 tag from the cell surface (∼5 min) is the same as the half-life of EGFR internalization in 184A1 cells (Burke and Wiley, 1999), suggesting that Btn-13A9 binding did not alter the kinetics of receptor internalization.
Figure 3.
Trafficking kinetics of EGFR in complex with Btn-13A9. (A) Cells were incubated for 2 h at 4°C in the presence of 500 ng/ml Btn-13A9. Cells were washed and 50 nM EGF in prewarmed medium was added for the indicated number of minutes. Surface-associated biotin was removed with glutathione and internal and surface EGFR were isolated as described in MATERIALS AND METHODS. Shown are Western blots of the precipitates visualized with anti-EGFR. (B) Cells were incubated with 200 ng/ml Btn-13A9 for 60 min at 37°C in the absence (left, −) or presence (right, +) of 50 nM EGF. Cells were then fixed, permeabilized, and stained with Texas Red-labeled streptavidin.
To verify that glutathionine-resistant Btn-13A9 represents internalized EGFR, we incubated cells at 37°C for 1 h with Btn-13A9 in the presence or absence of EGF. The cells were fixed and permeabilized and the distribution of the Btn-13A9 was ascertained with the use of Alexa-594–labeled streptavidin. As shown in Figure 3B, cells treated without EGF displayed a Btn-13A9 distribution predominantly at the cell surface. In the presence of EGF, however, the Btn-13A9 was found in intracellular vesicles. The relative distribution of Btn-13A9 between the cell surface and intracellular compartments matches the sensitivity of Btn-13A9 to glutathione treatment, indicating that glutathione-resistant Btn-13A9 indeed represents internalized EGFR.
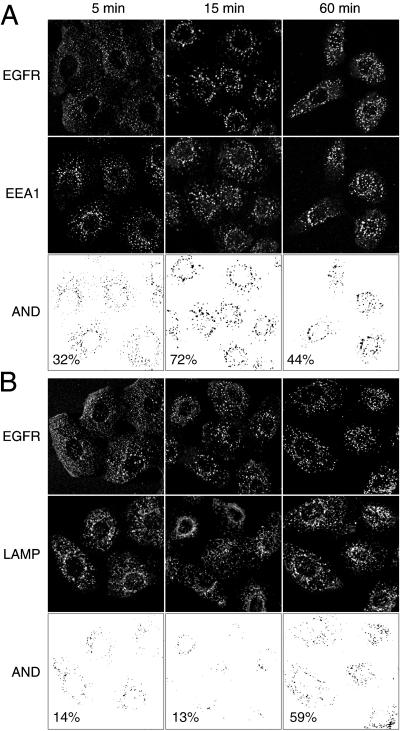
It has previously been shown that the addition of EGF induces the trafficking of the EGFR through early endosomes and into lysosomes (Sorkin and Waters, 1993). To ensure that the Btn-13A9 did not alter the normal trafficking pattern of the EGFR, we followed the trafficking of Btn-13A9–EGFR complexes with the use of Alexa-594 streptavidin and Alexa-488–labeled antibodies against markers of early endosomes (EEA1; Mu et al., 1995) and late endosomes/lysosomes (LAMP-2; Chen et al., 1985). We collected confocal images at different times after EGF addition and determined the extent of colocalization. As shown in Figure 4A, Btn-13A9–EGFR displayed significant colocalization with the EEA1 marker within 5 min. This colocalization peaked at 72% by 15 min and subsequently fell. In contrast, colocalization of Btn-13A9–EGFR with the LAMP-2 marker was low at first but increased to 59% by 1 h (Figure 4B). This indicates that in the presence of EGF the Btn-13A9–EGFR complex passes sequentially through the early endosomes and into the lysosomes with the same kinetics as previously reported for the EGFR (Sorkin and Waters, 1993; Futter et al., 1996).
Figure 4.
Complexes of Btn-13A9–EGFR pass sequentially through early endosomes and into lysosomes. The trafficking of Btn-13A9 was followed with the use of confocal fluorescence microscopy. Cells were incubated with 200 ng/ml Btn-13A9 for 2 h at 37°C before adding EGF for 5, 15, and 60 min. Cells were stained simultaneously with Texas Red streptavidin and Alexa-488–labeled anti-EEA1 (A) or anti-LAMP-2 (B). The images were converted to binary format and a Boolean “AND” operation was used to generate the overlapping pixel pattern. The percentage of total pixels in the EGFR image that colocalized to the appropriate markers is indicated.
Receptor Deactivation Precedes Receptor Degradation
It has been demonstrated that internally localized EGFR can be active and tyrosine phosphorylated. However, how long they remain active is unknown (Wada et al., 1992; Di Guglielmo et al., 1994; Baass et al., 1995). Because EGFR kinase activity is not required for receptor degradation, it seemed possible that the receptor could be deactivated before being degraded (Herbst et al., 1994; Opresko et al., 1995). To investigate the kinetics of assembly of signaling molecules on intracellular EGFR, it was necessary to define the time interval during which internalized EGFR would be active.
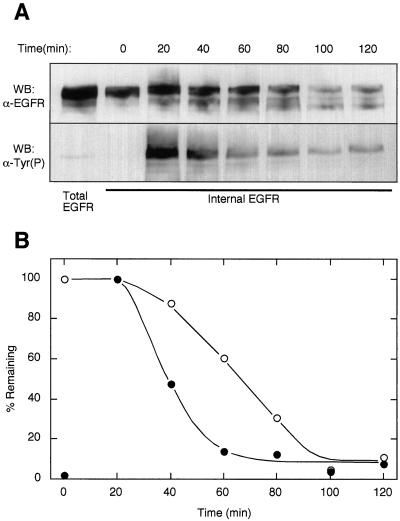
Deactivation and degradation of internally localized EGFR were analyzed directly with the use of our methodology. HMEC were incubated in the presence of Btn-13A9 for 2 h at 37°C. After that time, excess Btn-13A9 was removed and fresh media containing 50 nM EGF were added for various lengths of time. Cell surface-associated biotin was stripped with glutathione, and internally localized EGFR were isolated with the use of streptavidin agarose. EGFR and Tyr(P) were detected by Western blot analysis. Preliminary experiments showed that internalized phosphorylated EGFR reached a maximum between 10 and 20 min (also see Figures 8 and 9). The peak of receptor phosphorylation was followed by a rapid decline such that by 60 min most of the receptor-associated Tyr(P) was gone (Figure 5). In contrast, more than half of the isolated EGFR appeared to be intact at 60 min, and the remainder was lost over the ensuing 40 min. The observed loss of EGFR could be due to either receptor degradation or to breakdown of the Btn-13A9 tag. The appearance of lower molecular weight EGFR-immunoreactive material after 40 min indicates that at least a component of the loss was due to receptor degradation (Figure 5A). It appears, however, that internalized EGFR are deactivated ∼20 min before degradation (Figure 5B). There are at least two mechanisms that could explain why the loss of receptor activity precedes the loss of receptor mass. First, the EGFR could enter a compartment that reduces its activity. Such a compartment, for example, could possess high phosphatase activity or contain other enzymes that covalently modify receptors. Alternately, the ligand could be lost or degraded before receptor degradation. To discriminate between these possibilities, we simultaneously followed total EGFR, activated EGFR, and bound EGF by immunofluorescence.
Figure 8.
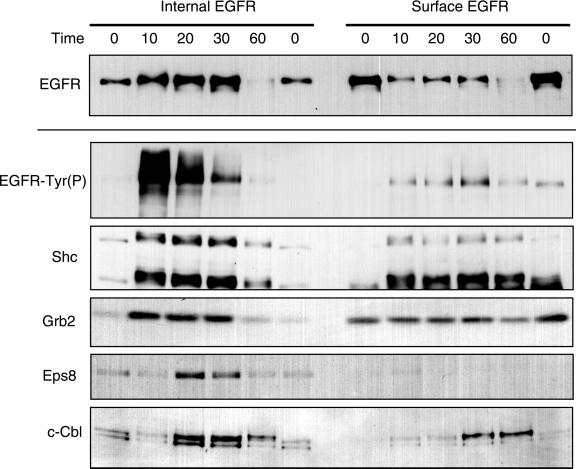
Multiple proteins associate with both surface and internal EGFR in 184A1 cells. Cells incubated with 500 ng/ml Btn-13A9 were treated with 50 nM EGF for the indicated time periods. Internal and surface EGFR were isolated from cells, and the resulting Western blots were visualized with the use of antibodies specific for EGFR, Tyr(P), Shc, Grb2, c-Cbl, and Eps8. All blots are from a single experiment run on the same day. The results are typical based on a minimum of three experiments for each marker.
Figure 9.
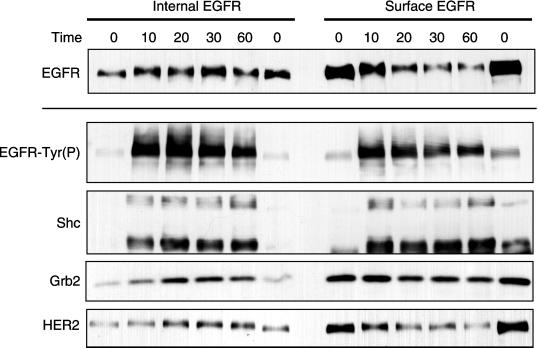
Proteins associated with both surface and internal EGFR in HB2 cells. Cells incubated with 500 ng/ml Btn-13A9 were treated with 50 nM EGF for the indicated time periods. Internal and surface EGFR were isolated from cells, and the resulting Western blots were visualized with the use of antibodies specific for EGFR, Tyr(P), Shc, Grb2, and HER2. All blots are from a single experiment run on the same day.
Figure 5.
Loss of EGFR tyrosine phosphorylation precedes receptor degradation. (A) Btn-13A9 (500 ng/ml) was bound to cells, and 50 nM EGF was added for the indicated periods on time at 37°C. Internal EGFR were isolated as described in MATERIALS AND METHODS, and receptor mass and Tyr(P) levels were determined in parallel Western blots (WB). Total EGFR levels were determined by immunoprecipitation of an extracted parallel plate with 4 μg/ml mAb 225. (B) Bands in A were quantified by densitometry. EGFR (○) bands were normalized to a percentage of the zero-time point. Tyr(P) (●) was normalized to the 20-min time point.
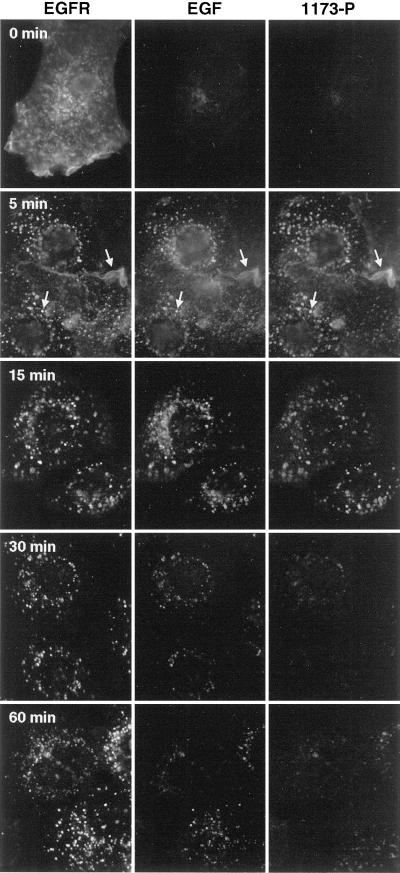
Shown in Figure 6 is the result of treating HMEC for different lengths of time with Texas Red-labeled EGF. Total EGFR was identified by the use of mouse mAb 13A9, whereas activated EGFR were localized by the use of affinity-purified sheep antibodies against a peptide corresponding to the major site of self-phosphorylation of the EGFR (Tyr1173). This antibody recognizes only the phosphorylated EGFR (Figure 6, top). Western blots of cells treated with EGF show only a single band corresponding to the molecular weight of the EGFR, demonstrating that other receptor substrates are not recognized (Schooler and Wiley, unpublished results). At both 5 and 15 min, the pattern of EGF, EGFR, and phosphorylated EGFR (1173-P) are essentially identical, both at membrane ruffles and in intracellular vesicles (Figure 6, top, arrows). Thus, at early time points, virtually all EGF-ligated receptors are phosphorylated, regardless of their cellular location. After ∼30 min, however, the intracellular levels of activated receptors are greatly diminished relative to total receptor mass. There is also a loss of labeled EGF coincident with the loss of phosphotyrosine, although the reduction in phosphorylated EGFR was more extensive (Figure 6, bottom). By 60 min of EGF treatment, loss of phosphorylated EGFR was almost complete, coincident with entry of the EGFR into the late endosome/lysosome compartment (Figure 4). In parallel experiments, the internal 1173-P label colocalized with the EEA1 marker but never colocalized with the Lamp-2 marker (Burke, Schooler, and Wiley, unpublished results). Therefore, the EGFR in the late endosomes/lysosomes do not appear to be active. We conclude that deactivation of the EGFR precedes receptor degradation, most likely because of ligand loss.
Figure 6.
Loss of phosphorylated EGFR reflects loss of ligand rather than the receptor. Cells incubated for the indicated amount of time with Texas Red-labeled EGF were fixed, permeabilized, and stained with Alexa-488–labeled mAb 13A9 and affinity-purified sheep antibodies against a peptide corresponding to the major tyrosine phosphorylation site of the EGFR (1173-P). The latter was visualized with an affinity-purified Cy5-labeled anti-sheep antibody. The three fluorescent labels were visualized sequentially with the use of a multiband filter set. The exposure time for each wavelength was constant with the use of the 15-min sample as the standard. The arrows at 5 min indicate a corresponding vesicle and membrane ruffle in the three images. 0 min indicates no treatment with EGF.
EGFR Form Specific Spatially Restricted Signaling Complexes
Our data show that EGFR remain tyrosine phosphorylated for a considerable length of time after internalization. To determine whether these receptors were in complex with other signaling molecules, we treated cells with EGF for varying lengths of time and isolated the intracellular receptors after glutathione stripping. The receptor isolates were separated by gel electrophoresis, transferred to nitrocellulose, and probed with antibodies for phosphotyrosine. Because many proteins that associate with the EGFR are also phosphorylated, nonreceptor proteins that contain phosphotyrosine should represent signaling partners. Because the high levels of phosphorylated EGFR could obscure the detection of minor components, the EGFR-containing sections of Western blots were removed and exposed separately. As shown in Figure 7, multiple tyrosine phosphorylated proteins are associated with internalized EGFR. Additional blots, run in parallel, indicate that the three indicated bands represent Shc. Although the highest levels of substrate association occurs within 10 min, significant association was observed for up to 30 min. These results show that our coimmunoprecipitation protocol allows the detection of multiple EGFR-associated signaling proteins.
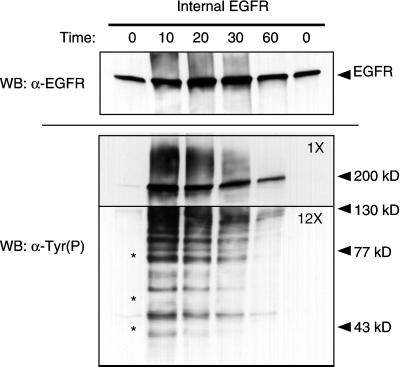
Figure 7.
Internalized EGFR are associated with multiple tyrosine phosphorylated proteins. Btn-13A9 (500 ng/ml) was bound to cells at 37°C, followed by the addition of 50 nM EGF for the indicated periods of time. After removal of surface-associated biotin, internalized Btn-13A9–EGFR complexes were isolated with the use of a 45-min incubation at 0°C. Isolated complexes were separated on 5–10% gradient gels, and levels of EGFR were determined by Western blots (top, WB). Total tyrosine phosphorylated proteins were detected with horseradish peroxidase-conjugated RC-20 antibody (bottom). To prevent the heavily tyrosine-phosphorylated EGFR from obscuring minor proteins, the top part of the gel was exposed for 5 s (1×), whereas the bottom part of the gel was exposed for 1 min (12×). A parallel gel was probed for Shc and the three isoforms ran with the same mobility as the bands marked by asterisks.
To determine whether signaling proteins that associate with internalized EGFR are different from those that associate at the cell surface, we isolated both surface and internal EGFR. Cells were brought to steady state with Btn-13A9, the excess was removed, and EGF was added for different lengths of time, followed by the isolation of internalized and surface-associated EGFR. Western blots of the isolates were then probed with antibodies against a variety of different signaling proteins reported to associate with the EGFR. The results of a typical experiment are shown in Figures 8 and 9. Consistent with previous reports, we found that internalized EGFR were hyperphosphorylated compared with surface EGFR (Wada et al., 1992; Di Guglielmo et al., 1994; Baass et al., 1995). By densitometry, we estimate that the ratio of Tyr(P):EGFR is approximately twice as high for internalized vs. surface EGFR. Also, consistent with other reports, we find that internalized EGFR are associated with Shc and Grb2 (Di Guglielmo et al., 1994). The ratios of Shc:EGFR were nearly equal for surface and internalized EGFR, indicating little preference for the different EGFR populations (Table 1). Grb2, in contrast, showed an approximate twofold bias for surface EGFR.
Table 1.
Relative association of substrates with surface and internal EGFR
| Substrate | 184A1 cells
|
HB2 cells
|
||
|---|---|---|---|---|
| Inside | Surface | Inside | Surface | |
| Shc | 5.3 ± 0.7 | 6.2 ± 1.9 | 1.9 ± 0.3 | 1.8 ± 0.4 |
| Grb2 | 7.0 ± 0.8 | 15 ± 5 | 4.3 ± 1.1 | 7.3 ± 2.3 |
| Eps8 | 2.0 ± 0.8 | 0.6 ± 0.1 | ND | ND |
| HER2 | ND | ND | 2.9 ± 0.7 | 3.8 ± 1.0 |
Shown are the ratios of the substrates and the EGFR isolated from either the surface or intracellular compartment as determined by densitometry of Western blots. Exposures were adjusted to keep the band density within the linear range of the film. Substrates and EGFR were run on same gel and exposed at the same time. Samples were taken between 10 and 30 min after EGF exposure. Error is SEM of at least three separate samples. ND, not determined.
We found that the association of Eps8 with the EGFR was almost exclusively with the internalized receptor (Figure 8). The time that Eps8 is first observed associated with internalized EGFR varies somewhat between experiments. Occasionally we observe Eps8 associating with EGFR at the early 10-min time point (Burke, Schooler, and Wiley, unpublished results). This period appears to correspond to the time at which the EGFR transits through early endosomes. We also found c-Cbl to be predominantly associated with internalized EGFR, although the association appears to occur at about the time that the EGFR exits the early endosomes and enters the late endosomes (Figure 8). Interestingly, the molecular weight of c-Cbl increases at the latter time points, which may represent ubiquitination of the molecule (Joazeiro et al., 1999). The larger molecular weight form of c-Cbl also appears at the cell surface at the later time points, which may be a result of some receptor recycling (Herbst et al., 1994). Our results suggest that the pattern of proteins associated with the EGFR changes during endocytic trafficking.
The results obtained above (Figure 8) were obtained with HMEC displaying a basal phenotype (Taylor-Papadimitriou et al., 1989). Although these cells are very responsive to EGF, they express very low levels of HER2 (<10,000 per cell; Burke and Wiley, unpublished observations). Because HER2 is an important signaling component of the EGFR system, we were interested in determining the location where EGFR and HER2 form heterodimers. For this analysis, we used HB2 cells, which are HMEC that have a pattern of keratin expression that is characteristic of a luminal phenotype (Bartek et al., 1991). These cells express ∼60,000 HER2 molecules per cell (Worthylake et al., 1999). We have previously shown that the trafficking of activated EGFR in HB2 cells is essentially identical to 184A1 cells (Burke and Wiley, 1999).
The pattern of association of HER2, Grb2, and Shc with surface and internalized EGFR as a function of time is shown in Figure 9. The internalization of the activated EGF was somewhat slower than that observed for 184A1 cells, probably because of normal variation between experiments. As was the case with 184A1 cells, Grb2 showed a preferential association with surface EGFR, whereas Shc displayed no preference (Table 1). Some HER2 was found associated with the EGFR even in the absence of exogenous ligand. The addition of EGF caused the loss of EGFR–HER2 complexes from the cell surface, concomitantly with the loss of surface EGFR. There was a slow accumulation of internal EGFR–HER2 complexes after ∼20–30 min, approximately when EGFR leave the early endosomes. We conclude that EGFR–HER2 complexes initially form at the cell surface but can accumulate in later compartments in the endocytic pathway, consistent with results of previous studies (Worthylake et al., 1999).
DISCUSSION
Endocytosis and lysosomal targeting of the EGFR is a normal consequence of receptor activation. Because degradation will inevitably terminate receptor signaling, trafficking of the EGFR has traditionally been viewed in the context of attenuation. Indeed, inhibition of receptor internalization and degradation will enhance signaling. Although EGFR internalization is the first step in receptor degradation, internalization is not necessarily an attenuation process itself. As demonstrated a number of years ago, most EGF associated with cells at steady state is in an intracellular compartment because of the disparity between rates of receptor internalization and rates of lysosomal targeting (Wiley et al., 1985). In the case of HMEC, the t1/2 of EGF internalization is ∼5 min, but at least 20 min is required to transit the early endosomes. Thus, at a minimum, there will be at least fourfold more EGF inside the cell than at the surface at steady state. The salient question is whether the internalized ligand-receptor complex is still engaged in productive signaling and whether trafficking processes control signaling specificity.
The ability of different signaling molecules to interact with specific domains of phosphorylated receptors has been proposed to control signaling specificity (van der Geer et al., 1994). Recent experiments with the platelet-derived growth factor receptor suggest that SH2 restriction is permissive and functionally weak. Mutations in the platelet-derived growth factor receptor that eliminate specific SH2-binding domains ultimately have little effect on signal specificity (Soler et al., 1994; Fambrough et al., 1999). Other investigators have suggested that trafficking can regulate receptor signaling in that specific recycling rates control receptor half-life and therefore signal duration (Lenferink et al., 1998b; Waterman et al., 1998). Recently, we demonstrated that signaling through the phospholipase C-γ pathway is restricted to the cell surface, whereas signaling through the ras pathway occurs through both the cell surface and intracellular compartments (Haugh et al., 1999a, 1999b). Data from the studies described here also suggest that receptor-ligand trafficking can alter specific receptor-substrate interactions and thereby could potentially regulate signal specificity.
To examine the molecular composition of EGFR-signaling complexes at the cell surface and within cells, we used a reversibly biotinylated anti-EGFR antibody. Although our technique is straightforward, there are some methodological biases that need to be addressed. The first is the stability of the antibody used to label the receptor. The second is the efficiency of the strip protocol used to remove surface-associated tag, and the third is the time bias inherent with serial precipitation steps. We have addressed the first two steps by demonstrating directly that the Btn-13A9 antibody is stably associated with the EGFR and that the biotin moiety can be efficiently removed with a mild glutathione strip. The time bias of serial precipitation steps arises from the longer incubation times needed to remove the surface-associated (nonbiotinylated) antibody from the internalized (biotinylated) antibody. This means that weak interactions between receptors and substrates will be biased toward the internal receptor pool. Despite this concern, we did observe substrates that displayed a preference for the surface EGFR pool, indicating that the association of the EGFR with at least some of it substrates is sufficiently stable to accurately reflect their distribution in situ. In general, however, our technique is probably best suited for the isolation of internalized receptors.
It was important to demonstrate that the antibody used to isolate the internalized EGFR did not alter their behavior. We and others have demonstrated that the association of mAb 13A9 has little effect on the biochemical properties of the EGFR (Winkler et al., 1989; Carraway and Cerione, 1993; Lenferink et al., 1998a). We also found that in the presence of mAb 13A9 EGFR tyrosine kinase activity, EGF-stimulated mitogenesis appear normal (Burke, Schooler, and Wiley, unpublished observations). Results from the current study show that association of Btn-13A9 with the EGFR did not change the trafficking of the EGFR.
To place our biochemical results in context, we simultaneously determined the trafficking pattern of the activated EGFR in our cells. With the use of specific antibodies to phosphorylated EGFR together with fluorescent EGF, we found that internalized receptors were deactivated before degradation. This conclusion is also supported by biochemical data obtained from isolated, internalized EGFR. The loss of receptor activity appears due in large part to the loss of ligand, probably by a combination of processes such as proteolysis, dissociation, and endosomal sorting/recycling (Wiley et al., 1985; French et al., 1995). This conclusion contradicts previous studies that suggest that EGFR and associated ligands are degraded together in lysosomes (McKanna et al., 1979; Futter et al., 1996) but is consistent with previous kinetic data. For example, the degradation t1/2 of activated EGFR in HMEC is ∼2 h, but the t1/2 of ligand internalization is only 5 min (Burke and Wiley, 1999). If the ligand and the receptor were degraded together, then the ratio of EGF between the surface and inside of the cell would be at least 24 (Wiley and Cunningham, 1981). The ratio, however, is usually between 6 and 8 in these cells (Worthylake et al., 1999; Burke and Wiley, unpublished observations). This indicates that EGF is lost three to fourfold faster than the receptor, either by degradation or recycling followed by escape. This is significant because it indicates that the proximal cause of EGFR attenuation and deactivation is ligand removal rather than receptor degradation.
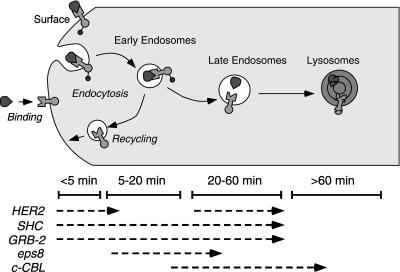
Our results directly demonstrate that internalized EGFR are tyrosine phosphorylated and are associated with numerous phosphorylated proteins. The pattern of phosphorylated proteins was different between the internalized and the surface-associated EGFR. Based on the kinetics of association of the different proteins, it appears that substrates that associate with internalized EGFR are primarily located in EEA1-positive early endosomes. A summary of our results is presented in Figure 10.
Figure 10.
Summary of trafficking and signaling of EGFR in human mammary epithelial cells. Shown is a simplified diagram of the endocytic pathway. Listed below is the time period during which the EGFR traverses the different compartments after EGF addition as determined by colocalization studies. The association of different substrates with the EGFR is based on results from both 184A1 and HB2 cells.
Internally localized EGFR are associated with Shc and Grb2, as previously described by others (Di Guglielmo et al., 1994; Oksvold et al., 2000), but Grb2 appears to bind preferentially to surface-localized EGFR. We also found more HER2 associated with the surface EGFR at early time points, but this is probably due to most of the HER2 being located at the cell surface. Because HER2 endocytosis is slower than EGFR endocytosis (Baulida et al., 1996; Worthylake et al., 1999), an activating interaction would clearly be biased at the cell surface. At later time points, HER2 was observed to accumulate in an internal compartment. This suggests that EGFR may alter the trafficking of HER2, a conclusion consistent with the demonstrated ability of activated EGFR to induce down-regulation of HER2 (Worthylake and Wiley, 1997).
We found that Eps8 was almost exclusively associated with internalized EGFR. Eps8 is phosphorylated by the EGFR and its overexpression can enhance cell proliferation in response to EGF (Fazioli et al., 1993). Eps8 is found in the perinuclear region and at peripheral cell extensions (Provenzano et al., 1998). It is thought to mediate the transfer of signals between Ras and Rac (Scita et al., 1999). The addition of EGF seems to enhance the association of Eps8 and EGFR, but this association appears to be independent of receptor phosphorylation (Fazioli et al., 1993). Our data confirm that EGF enhances Eps8-EGFR association and suggest that trafficking may regulate the interaction of Eps8 with EGFR. Whether this is due to direct association of Eps8 with the EGFR is currently unclear.
Consistent with previous speculations (Levkowitz et al., 1998; Waterman et al., 1999), we found that c-Cbl associates predominantly with internalized EGFR. c-Cbl is thought to negatively regulate the EGFR by stimulating its degradation (Levkowitz et al., 1998), by acting as an ubiquitin-protein ligase, or E3 (Joazeiro et al., 1999; Yokouchi et al., 1999). Our kinetic data on the association between EGFR and c-Cbl are consistent with the proposed function of c-Cbl and indicate that it associates with EGFR in early endosomes. We also observe a substantial increase in surface-associated c-Cbl at later time points (>20 min), perhaps due to receptor recycling. Interestingly, we observed multiple molecular weight forms of c-Cbl in HMEC. The multiple molecular weights could represent covalently modified forms of c-Cbl. EGFR and other kinases are known to stimulate the tyrosine phosphorylation and ubiquitination of c-Cbl (Wang et al., 1996; Lupher et al., 1998). Internalized EGFR appear to associate well with all molecular weight forms of c-Cbl. The surface-associated c-Cbl appears to predominantly be the larger molecular weight form. It is known that the EGFR undergoes extensive recycling in HMEC (Burke and Wiley, 1999). The association of c-Cbl with recycled EGFR could indicate that, although ubiquitination marks the EGFR for degradation, it does not act as a direct sorting signal. Further studies are needed to clarify these issues.
The question as to whether EGFR signal at the cell surface or from an internal compartment appears to be an oversimplification. It now appears that different patterns of signals arise from surface and internalized receptors. A large number of different signaling molecules have been identified over the last several years that appear to interact with the EGFR. Our data indicate that receptor trafficking could regulate signal specificity. Questions regarding the signaling of EGFR should include the impact of trafficking on individual signaling pathways. The method we have described in this paper should be generally applicable to approaching these questions.
ACKNOWLEDGMENTS
We thank Margaret Woolf for excellent technical assistance and Lee Opresko for critically reading the manuscript. We also thank Martha Stampfer for the 184A1 cells and Joyce Taylor-Papadimitriou for the HB2 cells. This work was supported by grant BES-9421773 from the National Science Foundation Biotechnology Program, Division of Bioengineering and Environmental Systems and National Institutes of Health grant PO1-HD28528. P.M.B. and K.S. are recipients of predoctoral fellowships from the US Army Breast Cancer Research Program.
Abbreviations used:
- Btn-13A9
biotinylated 13A9
- EGF
epidermal growth factor
- EGFR
EGF receptor(s)
- HMEC
human mammary epithelial cell(s)
- Ig
immunoglobulin
- mAb
monoclonal antibody
- PBS
phosphate-buffered saline
- TGF
transforming growth factor
REFERENCES
- Adamson P, Paterson HF, Hall A. Intracellular localization of the P21rho proteins. J Cell Biol. 1992;119:617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Maudsley S, Luttrell LM, Lefkowitz RJ, Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for beta2- adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem. 1999;274:1185–1188. doi: 10.1074/jbc.274.3.1185. [DOI] [PubMed] [Google Scholar]
- Aroian RV, Koga M, Mendel JE, Ohshima Y, Sternberg PW. The let-23 gene necessary for Caenorhabditis elegans vulval induction encodes a tyrosine kinase of the EGF receptor subfamily. Nature. 1990;348:693–699. doi: 10.1038/348693a0. [DOI] [PubMed] [Google Scholar]
- Baass PC, Guglielmo FA, Posner BI, Bergeron JM. Compartmentalized signal transduction by receptor tyrosine kinases. Trends Cell Biol. 1995;5:465–470. doi: 10.1016/s0962-8924(00)89116-3. [DOI] [PubMed] [Google Scholar]
- Band V, Sager R. Distinctive traits of normal and tumor-derived human mammary epithelial cells expressed in a medium that supports long-term growth of both cell types. Proc Natl Acad Sci USA. 1989;86:1249–1253. doi: 10.1073/pnas.86.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartek J, Bartkova J, Kyprianou N, Lalani E-N, Staskova Z, Shearer M, Chang S, Taylor-Papadimitriou J. Efficient immortalization of luminal epithelial cells from human mammary gland by introduction of simian virus 40 large tumor antigen with a recombinant retrovirus. Proc Natl Acad Sci USA. 1991;88:3520–3524. doi: 10.1073/pnas.88.9.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SE, Valverius EM, Ennis BW, Bronzert DA, Sheridan JP, Stampfer MR, Mendelsohn J, Lippman ME, Dickson RB. Expression of the transforming growth factor-alpha/epidermal growth factor receptor pathway in normal human breast epithelial cells. Endocrinology. 1990;126:596–607. doi: 10.1210/endo-126-1-596. [DOI] [PubMed] [Google Scholar]
- Baulida J, Kraus MH, Alimandi M, Di Fiore PP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- Burke PM, Wiley HS. Human mammary epithelial cells rapidly exchange empty EGFR between surface and intracellular pools. J Cell Physiol. 1999;180:448–460. doi: 10.1002/(SICI)1097-4652(199909)180:3<448::AID-JCP16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Carraway K L d, Cerione R A. Inhibition of epidermal growth factor receptor aggregation by an antibody directed against the epidermal growth factor receptor extracellular domain. J Biol Chem. 1993;268:23860–23867. [PubMed] [Google Scholar]
- Chen JW, Murphy TL, Willingham MC, Pastan I, August JT. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985;101:85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SS, Caron MG, Lefkowitz RJ. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo GM, Baass PC, Ou WJ, Posner BI, Bergeron JJ. Compartmentalization of SHC, GRB2 and mSOS, and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. EMBO J. 1994;13:4269–4277. doi: 10.1002/j.1460-2075.1994.tb06747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Opresko LK, Dempsey PJ, Lauffenburger DA, Coffey RJ, Wiley HS. Metalloprotease-mediated ligand release regulates autocrine signaling through the epidermal growth factor receptor. Proc Natl Acad Sci USA. 1999;96:6235–6240. doi: 10.1073/pnas.96.11.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D, McClure K, Kazlauskas A, Lander ES. Diverse signaling pathways activated by growth factor receptors induce broadly overlapping, rather than independent, sets of genes. Cell. 1999;97:727–741. doi: 10.1016/s0092-8674(00)80785-0. [DOI] [PubMed] [Google Scholar]
- Fazioli F, Minichiello L, Matoska V, Castagnino P, Miki T, Wong WT, Di Fiore PP. Eps8, a substrate for the epidermal growth factor receptor kinase, enhances EGF-dependent mitogenic signals. EMBO J. 1993;12:3799–3808. doi: 10.1002/j.1460-2075.1993.tb06058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore PP, Gill GN. Endocytosis and mitogenic signaling. Curr Opin Cell Biol. 1999;11:483–488. doi: 10.1016/s0955-0674(99)80069-6. [DOI] [PubMed] [Google Scholar]
- Fowler KJ, Walker F, Alexander W, Hibbs ML, Nice EC, Bohmer RM, Mann GB, Thumwood C, Maglitto R, Danks J A, et al. A mutation in the epidermal growth factor receptor in waved-2 mice has a profound effect on receptor biochemistry that results in impaired lactation. Proc Natl Acad Sci USA. 1995;92:1465–1469. doi: 10.1073/pnas.92.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French AR, Tadaki DK, Niyogi SK, Lauffenburger DA. Intracellular trafficking of epidermal growth factor family ligands is directly influenced by the pH sensitivity of the receptor/ligand interaction. J Biol Chem. 1995;270:4334–4340. doi: 10.1074/jbc.270.9.4334. [DOI] [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill GN, Kawamoto T, Cochet C, Le A, Sato JD, Masui H, McLeod C, Mendelsohn J. Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binding and antagonists of epidermal growth factor binding and antagonists of epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984;259:7755–7760. [PubMed] [Google Scholar]
- Haugh JM, Huang AC, Wiley HS, Wells A, Lauffenburger DA. Internalized epidermal growth factor receptors participate in the activation of p21(ras) in fibroblasts. J Biol Chem. 1999a;274:34350–34360. doi: 10.1074/jbc.274.48.34350. [DOI] [PubMed] [Google Scholar]
- Haugh JM, Schooler K, Wells A, Wiley HS, Lauffenburger DA. Effect of epidermal growth factor receptor internalization on regulation of the phospholipase C-gamma1 signaling pathway. J Biol Chem. 1999b;274:8958–8965. doi: 10.1074/jbc.274.13.8958. [DOI] [PubMed] [Google Scholar]
- Herbst JJ, Opresko LK, Walsh BJ, Lauffenburger DA, Wiley HS. Regulation of postendocytic trafficking of the epidermal growth factor receptor through endosomal retention. J Biol Chem. 1994;269:12865–12873. [PubMed] [Google Scholar]
- Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD, Huang CM, Gill GN, Wiley HS, Cavenee WK. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem. 1997;272:2927–2935. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- Joazeiro CA, Wing SS, Huang H, Leverson JD, Hunter T, Liu YC. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- Kallal L, Gagnon AW, Penn RB, Benovic JL. Visualization of agonist-induced sequestration and down-regulation of a green fluorescent protein-tagged beta2-adrenergic receptor. J Biol Chem. 1998;273:322–328. doi: 10.1074/jbc.273.1.322. [DOI] [PubMed] [Google Scholar]
- Kaplan KB, Swedlow JR, Varmus HE, Morgan DO. Association of p60c-src with endosomal membranes in mammalian fibroblasts. J Cell Biol. 1992;118:321–333. doi: 10.1083/jcb.118.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A, Sambuy Y, Mostov K, Rodriguez-Boulan E. Vectorial targeting of an endogenous apical membrane sialoglycoprotein and uvomorulin in MDCK cells. J Cell Biol. 1990;110:1533–1539. doi: 10.1083/jcb.110.5.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenferink AE, De Roos AD, Van Vugt MJ, Van de Poll ML, Van Zoelen EJ. The linear C-terminal regions of epidermal growth factor (EGF) and transforming growth factor-alpha bind to different epitopes on the human EGF receptor. Biochem J. 1998a;336:147–151. doi: 10.1042/bj3360147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenferink AE, Pinkas-Kramarski R, van de Poll ML, van Vugt MJ, Klapper LN, Tzahar E, Waterman H, Sela M, van Zoelen EJ, Yarden Y. Differential endocytic routing of homo- and hetero-dimeric ErbB tyrosine kinases confers signaling superiority to receptor heterodimers. EMBO J. 1998b;17:3385–3397. doi: 10.1093/emboj/17.12.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupher ML, Jr, Rao N, Lill NL, Andoniou CE, Miyake S, Clark EA, Druker B, Band H. Cbl-mediated negative regulation of the Syk tyrosine kinase: a critical role for Cbl phosphotyrosine-binding domain binding to Syk phosphotyrosine 323. J Biol Chem. 1998;273:35273–35281. doi: 10.1074/jbc.273.52.35273. [DOI] [PubMed] [Google Scholar]
- McKanna JA, Haigler HT, Cohen S. Hormone receptor topology and dynamics: morphological analysis using ferritin-labeled epidermal growth factor. Proc Natl Acad Sci USA. 1979;76:5689–5693. doi: 10.1073/pnas.76.11.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu FT, Callaghan JM, Steele-Mortimer O, Stenmark H, Parton RG, Campbell PL, McCluskey J, Yeo JP, Tock EP, Toh BH. EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J Biol Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- Oksvold MP, Skarpen E, Lindeman B, Roos N, Huitfeldt HS. Immunocytochemical localization of Shc and activated EGF receptor in early endosomes after EGF stimulation of HeLa cells. J Histochem Cytochem. 2000;48:21–33. doi: 10.1177/002215540004800103. [DOI] [PubMed] [Google Scholar]
- Opresko LK, Chang C-P, Will BH, Burke PM, Gill GN, Wiley HS. Endocytosis and lysosomal targeting of epidermal growth factor receptors are mediated by distinct sequences independent of the tyrosine kinase domain. J Biol Chem. 1995;270:4325. doi: 10.1074/jbc.270.9.4325. [DOI] [PubMed] [Google Scholar]
- Provenzano C, Gallo R, Carbone R, Di Fiore PP, Falcone G, Castellani L, Alema S. Eps8, a tyrosine kinase substrate, is recruited to the cell cortex and dynamic F-actin upon cytoskeleton remodeling. Exp Cell Res. 1998;242:186–200. doi: 10.1006/excr.1998.4095. [DOI] [PubMed] [Google Scholar]
- Ross AH, Baltimore D, Eisen HN. Phosphotyrosine-containing proteins isolated by affinity chromatography with antibodies to a synthetic hapten. Nature. 1981;294:654–656. doi: 10.1038/294654a0. [DOI] [PubMed] [Google Scholar]
- Rothberg PG, Harris TJ, Nomoto A, Wimmer E. O4-(5′-uridylyl)tyrosine is the bond between the genome-linked protein and the RNA of poliovirus. Proc Natl Acad Sci USA. 1978;75:4868–4872. doi: 10.1073/pnas.75.10.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozakis-Adcock M, McGlade J, Mbamalu G, Pelicci G, Daly R, Li W, Batzer A, Thomas S, Brugge J, Pelicci P G, et al. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992;360:689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hannah MJ, Huttner WB. Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor. J Cell Biol. 1997;137:445–458. doi: 10.1083/jcb.137.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scita G, Nordstrom J, Carbone R, Tenca P, Giardina G, Gutkind S, Bjarnegard M, Betsholtz C, Di Fiore PP. EPS8 and E3B1 transduce signals from Ras to Rac. Nature. 1999;401:290–293. doi: 10.1038/45822. [DOI] [PubMed] [Google Scholar]
- Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- Soler C, Beguinot L, Carpenter G. Individual epidermal growth factor receptor autophosphorylation sites do not stringently define association motifs for several SH2-containing proteins. J Biol Chem. 1994;269:12320–12324. [PubMed] [Google Scholar]
- Sorkin A, Waters CM. Endocytosis of growth factor receptors. Bioessays. 1993;15:375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- Stampfer MR. Isolation and growth of human mammary epithelial cell. J Tissue Culture Methods. 1985;9:107–115. [Google Scholar]
- Stampfer MR, Pan CH, Hosoda J, Bartholomew J, Mendelsohn J, Yaswen P. Blockage of EGF receptor signal transduction causes reversible arrest of normal and immortal human mammary epithelial cells with synchronous reentry into the cell cycle. Exp Cell Res. 1993;208:175–188. doi: 10.1006/excr.1993.1236. [DOI] [PubMed] [Google Scholar]
- Stern DF, Kamps MP. EGF-stimulated tyrosine phosphorylation of p185neu: a potential model for receptor interactions. EMBO J. 1988;7:995–1001. doi: 10.1002/j.1460-2075.1988.tb02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PB, Kim SK. Signaling specificity: the RTK/RAS/MAP kinase pathway in metazoans. Trends Genet. 1999;15:145–149. doi: 10.1016/s0168-9525(99)01694-7. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J, Stampfer M, Bartek J, Lewis A, Boshell M, Lane EB, Leigh IM. Keratin expression in human mammary epithelial cells cultured from normal and malignant tissue: relation to in vivo phenotypes and influence of medium. J Cell Sci. 1989;94:403–413. doi: 10.1242/jcs.94.3.403. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris R C, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- van der Geer P, Hunter T, Lindberg RA. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol. 1994;10:251–337. doi: 10.1146/annurev.cb.10.110194.001343. [DOI] [PubMed] [Google Scholar]
- Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- Wada I, Lai WH, Posner BI, Bergeron JJ. Association of the tyrosine phosphorylated epidermal growth factor receptor with a 55-kD tyrosine phosphorylated protein at the cell surface and in endosomes. J Cell Biol. 1992;116:321–330. doi: 10.1083/jcb.116.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yeung YG, Langdon WY, Stanley ER. c-Cbl is transiently tyrosine-phosphorylated, ubiquitinated, and membrane-targeted following CSF-1 stimulation of macrophages. J Biol Chem. 1996;271:17–20. doi: 10.1074/jbc.271.1.17. [DOI] [PubMed] [Google Scholar]
- Waterman H, Levkowitz G, Alroy I, Yarden Y. The RING finger of c-Cbl mediates desensitization of the epidermal growth factor receptor. J Biol Chem. 1999;274:22151–22154. doi: 10.1074/jbc.274.32.22151. [DOI] [PubMed] [Google Scholar]
- Waterman H, Sabanai I, Geiger B, Yarden Y. Alternative intracellular routing of ErbB receptors may determine signaling potency. J Biol Chem. 1998;273:13819–13827. doi: 10.1074/jbc.273.22.13819. [DOI] [PubMed] [Google Scholar]
- Wells A, Welsh JB, Lazar CS, Wiley HS, Gill GN, Rosenfeld MG. Ligand-induced transformation by a noninternalizing epidermal growth factor receptor. Science. 1990;247:962–964. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]
- Wiley HS, Cunningham DD. A steady state model for analyzing the cellular binding, internalization and degradation of polypeptide ligands. Cell. 1981;25:433–440. doi: 10.1016/0092-8674(81)90061-1. [DOI] [PubMed] [Google Scholar]
- Wiley HS, Herbst J, Walsh BJ, Lauffenburger DA, Rosenfeld MG, Gill GN. Role of tyrosine kinase activity in endocytosis, compartmentation and downregulation of the EGF receptor. J Biol Chem. 1991;266:11083–11094. [PubMed] [Google Scholar]
- Wiley HS, VanNostrand W, McKinley DN, Cunningham DD. Intracellular processing of epidermal growth factor and its effect on ligand-receptor interactions. J Biol Chem. 1985;260:5290–5295. [PubMed] [Google Scholar]
- Wiley HS, Woolf MF, Opresko LK, Burke PM, Will B, Morgan JR, Lauffenburger DA. Removal of the membrane-anchoring domain of epidermal growth factor leads to intracrine signaling and disruption of mammary epithelial cell organization. J Cell Biol. 1998;143:1317–1328. doi: 10.1083/jcb.143.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler ME, O'Connor L, Winget M, Fendly B. Epidermal growth factor and transforming growth factor alpha bind differently to the epidermal growth factor receptor. Biochemistry. 1989;28:6373–6378. doi: 10.1021/bi00441a033. [DOI] [PubMed] [Google Scholar]
- Worthylake R, Opresko LK, Wiley HS. ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors. J Biol Chem. 1999;274:8865–8874. doi: 10.1074/jbc.274.13.8865. [DOI] [PubMed] [Google Scholar]
- Worthylake R, Wiley HS. Structural aspects of the epidermal growth factor receptor required for transmodulation of erbB-2/neu. J Biol Chem. 1997;272:8594–8601. doi: 10.1074/jbc.272.13.8594. [DOI] [PubMed] [Google Scholar]
- Xie W, Paterson AJ, Chin E, Nabell LM, Kudlow JE. Targeted expression of a dominant negative epidermal growth factor receptor in the mammary gland of transgenic mice inhibits pubertal mammary duct development. Mol Endocrinol. 1997;11:1766–1781. doi: 10.1210/mend.11.12.0019. [DOI] [PubMed] [Google Scholar]
- Xue L, Lucocq J. ERK2 signaling from internalized epidermal growth factor receptor in broken A431 cells. Cell Signal. 1998;10:339–348. doi: 10.1016/s0898-6568(98)00011-4. [DOI] [PubMed] [Google Scholar]
- Yokouchi M, Kondo T, Houghton A, Bartkiewicz M, Horne WC, Zhang H, Yoshimura A, Baron R. Ligand-induced ubiquitination of the epidermal growth factor receptor involves the interaction of the c-Cbl RING finger and UbcH7. J Biol Chem. 1999;274:31707–31712. doi: 10.1074/jbc.274.44.31707. [DOI] [PubMed] [Google Scholar]