Abstract
A subset of lupus patients with severe nephritis and anti-nRNP reactivity produces autoantibodies primarily against two major epitopes of the nRNP A (also known as U1A) protein. These sequences span amino acids 44-56 (A3) and amino acids 103-115 (A6). These two epitopes represent structurally different regions of the protein, as both epitopes are located on the surface, but the A6 epitope is functionally masked in vivo by binding between nRNP A and the U1 RNA. Rabbits were immunized with either the A3 or A6 peptides constructed on a branching polylysine backbone. Rabbits immunized with each of these peptides first developed antibodies directed against the peptide of immunization. With boosting, the immune response of rabbits immunized with the A3 peptide spread to other common antigenic regions of nRNP A. These regions of nRNP A bound by A3 immunized rabbits are very similar to common epitopes in human systemic lupus erythematosus. These A3 immunized rabbits also develop antibodies to common antigenic regions of nRNP 70K, nRNP C, Sm B/B′, and Sm D1 proteins, as well as clinical symptoms of systemic lupus erythematosus such as leukopenia and renal insufficiency. On the other hand, rabbits immunized with the A6 peptide only develop antibodies to the peptide of immunization. Anti-A3, but not anti-A6, antibodies are capable of immunoprecipitating native small nuclear ribonucleoprotein complexes. Immunization with the A3 peptide of nRNP A (a surface epitope), but not the A6 peptide (masked), induces an extensive, varied immune response against multiple small nuclear ribonucleoprotein autoantigens similar to that seen in human systemic lupus erythematosus.
Systemic lupus erythematosus (SLE) is a multisystem rheumatic disorder characterized by the production of antibodies against self-antigens. Of particular interest are those autoantibodies in both human patients and animal models that target the small nuclear ribonucleoprotein (snRNP) proteins of the spliceosome complex. These autoantibodies can be separated into two distinct groups by Ouchterlony immunodiffusion: anti-Sm and anti-nRNP. The anti-Sm family of autoantibodies precipitates snRNP complexes containing the U1, U2, U4/U6, and U5 RNAs (1). These antibodies are considered to be directed against one or a combination of eight polypeptides: B, B′, D1, D2, D3, E/F, and G (2, 3). Anti-nRNP antibodies, however, precipitate snRNPs containing only the U1 RNA and exhibit binding to one or more of three major proteins: nRNP 70K (4), nRNP A (5), and nRNP C (6).
Regions of antigenicity of the nRNP A protein have been identified by analysis with maximally overlapping octapeptides by solid-phase ELISA. These studies have revealed that subsets of lupus patient sera exhibit two distinct binding patterns, called patterns I and II (7). The first pattern (pattern I) consists of binding to eight discrete regions of nRNP A (8). The second serological subset (pattern II) consists of patients who make antibodies against two and only two of the eight major regions of antigenicity seen in pattern I (epitopes A3, amino acids 44-56, LVSRSLKMRGQAF; and A6, 103-115, ERDRKREKRKPKS). Greater than 50% of these pattern II patients' total anti-nRNP antibodies were directed specifically against these two peptides. Perhaps the most fascinating aspect of this subset of the anti-nRNP immune response is its preliminary association with severe, unrelenting nephritis in the lupus patients studied (7). Other epitope mapping studies with short synthetic peptides from nRNP A have revealed three major regions of antigenicity in human lupus patients: amino acids 1-11, 35-58, and 257-282 (8).
Contemporary work with monoclonal antibodies recognizing nRNP A has provided some additional insight into the nature of these epitopes. One such anti-nRNP A monoclonal, 12E12, recognizes the A6 epitope of nRNP A and only binds to nRNP A when it is not complexed to the U1 RNA (9, 10). Thus, the properties of 12E12 suggest that inhibition of its binding to nRNP A is due to some conformational change of the protein associated with the protein-RNA binding. The helix immediately preceding the region bound by 12E12 (amino acids 90-98) is very important in nRNP A-U1 RNA interactions (11); solution structures have demonstrated that this helix is in a different orientation when nRNP A protein is bound to U1 RNA and acts as a lid over the RNA binding surface of the protein when bound to U1 RNA (10, 11). This lid action upon RNA binding results in a conformational change that has an effect on the amino acids immediately surrounding the helix. This includes the primary epitope of 12E12 (amino acids 103-115). Thus, when the U1 RNA binds to the nRNP A protein, the 12E12 epitope is inaccessible in the interior of the complex because of this conformational change, and thus it is unable to be bound by antibody (9).
Studies have demonstrated that immunization of animals with certain peptides from Sm B′ are capable of inducing a diverse autoimmune response that is immunologically and clinically similar to human SLE (8, 12, 13). These studies led us to become interested in the ability of the two nRNP A epitopes recognized in both pattern I and pattern II binding (A3 and A6) to produce a human-like autoimmune response in New Zealand White rabbits. Of particular interest was whether there would be a difference in the ability of an epitope that localizes to the surface of native, complexed nRNP A (A3) and one that is masked by U1 RNA in vivo (A6) to trigger an immune response similar to that seen in human SLE.
Materials and Methods
Rabbit Immunizations. Both the A3 peptide (LVSRSLKMRGQAF) and the A6 peptide (ERDRKREKRKPKS) were prepared in 0.1 M synthesis on a polylysine backbone (MAP, Applied Biosystems) by the Oklahoma Molecular Biology Core Facility (14). On day 1, 0.5 mg of immunogen (peptide or control, as indicated) was emulsified with an equal volume of complete Freund's adjuvant and injected s.c. and i.p., as described in detail (12). Boosting with 0.5 mg of immunogen in incomplete Freund's adjuvant into the s.c. tissue and i.p. space was performed on days 26, 53, and 99. The final boost (0.5 mg of immunogen or control) was given intravenously on day 152 without adjuvant. Serial blood samples were collected throughout the protocol.
ELISAs. Standard solid-phase assays were used to measure the antibody reactivity in human and rabbit sera. One microgram of antigen (whole nRNP, A3 MAP peptide, A6 MAP peptide, whole Sm or Ro, Immunovision, Springdale, AR) was coated per well in each of 96 polystyrene wells. Assays were then performed by using a previously described protocol (12).
Solid-Phase Peptide Synthesis and Antibody Assays. The 275 possible overlapping octapeptides of the nRNP A protein were prepared by using solid-phase peptide chemistry and constructed according to the amino acid sequence (5) as described (15). Antipeptide assays were conducted by using a modified ELISA technique testing serum samples at 1:100 dilutions as described (15, 16).
Those epitopes of the nRNP 70K, nRNP A, nRNP C, Sm B/B′, and Sm D1 proteins most commonly bound by autoantibodies from lupus patient sera, along with several commonly nonantigenic peptides from the same proteins, were compiled and used to construct a new limited set of solid-phase octapeptides representing only those epitopes. These include 45 antigenic octapeptides from nRNP 70K, 33 from nRNP A, 24 from nRNP C, 30 from Sm B/B′, and 13 from Sm D1, along with 4 negative control peptides from each protein. These octapeptides were then used as described above to decrease the amount of sera required to scan reactivity with the major nRNP proteins while still providing a maximal amount of human disease-associated epitope specificity.
Western Blots. Affinity-purified nRNP antigen (Immunovision) and HeLa cell extract were subjected to electrophoresis in 12.5% polyacrylamide gels containing 1% SDS (in 0.15 M Tris·HCl, pH 8.8) as described (12). These proteins were then transferred to nitrocellulose and immunoblotted by using rabbit sera at 1:100 dilutions.
Other Autoantibody Assays. Precipitating levels of Ro, La, Sm, and/or nRNP autoantibodies were detected by using double immunodiffusion (17). Rabbit sera were tested for antinuclear antibodies (ANA) by a standard ANA test against Hep-2 cells (INOVA Diagnostics, San Diego) and for autoantibodies binding to native DNA by a Crithidia assay (Protrac Industries, Kerrville, TX) (18, 19).
Sequencing of Rabbit nRNP A. A rabbit liver cDNA library (20) was screened with a random primed 32P-labeled human nRNP A cDNA. Two full-length cDNA clones were isolated and sequenced (GenBank accession no. AY387676). Both strands of the cDNA were sequenced by using Epicentre SequiTherm Long-Read cycle sequencing and Li-Cor automated DNA sequencers. The sequence was compared to the published sequence of the human nRNP A protein (accession no. P09012).
Polyadenylation Assays. RNA transcripts for in vitro polyadenylation reactions were synthesized in the presence of SP6 polymerase, [32P]UTP (Amersham Pharmacia), and SV40 late mRNA as described (21-23). HeLa cell nuclear extracts were prepared as described (24). In vitro polyadenylation reactions contained a final concentration of 58% vol/vol HeLa nuclear extract, 16 mM phosphocreatine (Sigma), 0.8 mM ATP (Amersham Pharmacia), 2.6% polyvinyl alcohol, and 1 × 105 cpm of 32P-labeled substrate RNA (≈50 fmol) in a reaction volume of 12.5 μl. Reactions were allowed to proceed at 30°C for 1 h. Reactions were then extracted with phenol chloroform, precipitated with ethanol, and analyzed on (19:1) 5% polyacrylamide gels containing 8 M urea.
Immunoprecipitations. Immunoprecipitations were performed as described (9). Briefly, aliquots of 293T cell nuclear extract were incubated with protein G-antibody beads (5 μl of sera per 25 μl of Gammabind Plus Sepharose, Amersham Pharmacia) for 30-60 min at 4°C and washed extensively with RSB100. Coprecipitated RNAs were extracted with phenol chloroform and ethanol precipitate. Sera from A3 or A6 immunized animals at 2 weeks, 1 month, and 3 months postimmunization were studied by this method.
Northern blots were performed as described previously except that OHyb contained 100 μg/ml Escherichia coli RNA (9). U1 RNA was detected with a 32P-labeled oligonucleotide (POC74, 5′-ATCTCCCCTGCCACGTAAGTAT-3′) (9) complementary to the 5′ end of U1 RNA.
Results
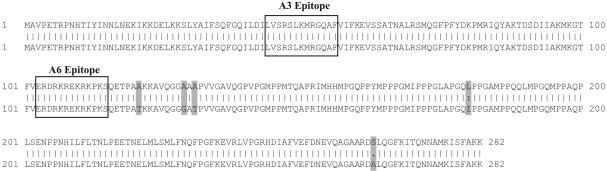
A3 Immunized Animals Produce High Titers of Anti-A3 and Anti-nRNP Antibodies. Analysis of the sequence for the rabbit nRNP A cDNA reveals that there are only five projected amino acid differences between the rabbit and human nRNP A sequences, whereas the A3 and A6 peptides specifically are 100% homologous between human and rabbit (Fig. 1). The rabbits immunized with the A3 peptide were shown to develop high titers of antibodies against the peptide of immunization by ELISA as early as 1 week after initial immunization (Fig. 2). These titers rose quickly for the first few weeks and then peaked and remained at very high levels. At 4-7 weeks after initial immunization, the A3 rabbits also began producing detectable amounts of anti-A6 antibodies. Antibodies to whole Sm developed as early as 3 weeks after initial immunization. Interestingly, antibodies to whole Ro did not reach significant levels until around the 15th week of the protocol. There was some variability observed in the time course from animal to animal. The rate of increase of individual autoantibodies and maximum levels achieved was similar for all of the A3 immunized rabbits.
Fig. 1.
Comparison of rabbit and human nRNP A. The human (lower sequence) and rabbit (upper sequence; GenBank accession no. AY387676) nRNP A gene products are highly conserved (98.2% homology). The areas comprising the A3 and A6 epitopes are boxed, whereas amino acids that differ between the two sequences are shaded in dark gray.
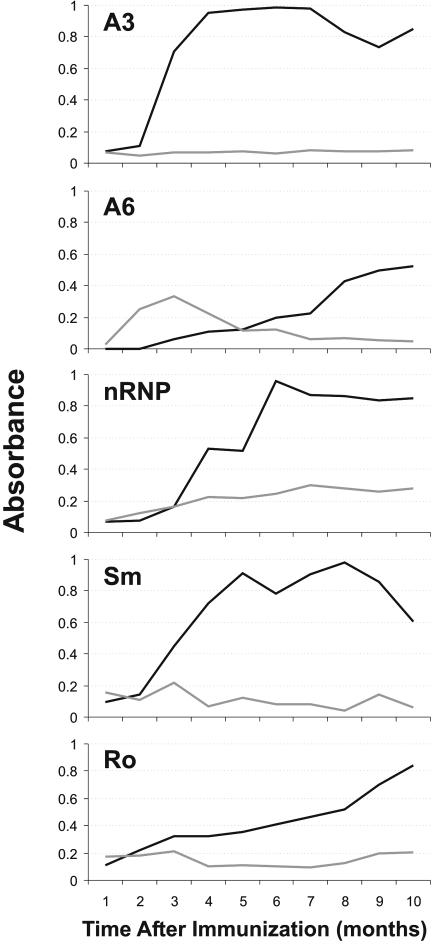
Fig. 2.
Summary of rabbit antibody profiles. Rabbit sera binding by ELISA to the A3 peptide, the A6 peptide, whole nRNP, whole Sm, and whole Ro are shown. Presented binding is the average of rabbits immunized with the A3 peptide (black line) and rabbits immunized with the A6 peptide (light gray line).
The A6 immunized rabbits developed in a remarkably different fashion from those immunized with the A3 peptide. The A6 immunized animals produced only low levels of antibody against the peptide of immunization by ELISA (Fig. 2). This response develops slowly over the first 8-11 weeks and is never equal to more than one-third of that present in the A3 rabbits (and never show a titer >1:500). The A6 immunized rabbits never developed any significant binding to the A3 peptide, whole Sm or whole Ro, and only recognized whole nRNP by ELISA slightly higher than either their own preimmunization samples or sham-immunized animals.
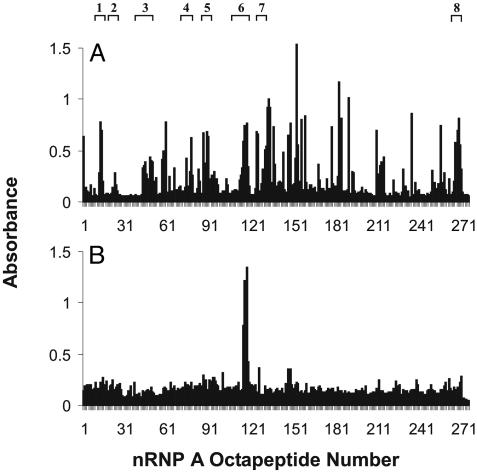
Spreading of A3 Immunized Rabbit Response to Include Additional Regions of nRNP A. The A3 rabbit sera were tested for reactivity with the 275 overlapping octapeptides of nRNP A. This revealed that not only were these animals producing large amounts of antibodies against the peptide of immunization, but they had diversified their response to make antibodies against additional epitopes of the nRNP A protein as well (Fig. 3). This spreading by 6-7 months includes the A6 region, with which these rabbits were not immunized, as well as six of the eight major antigenic regions identified in human lupus patient sera (with pattern I binding). All of the rabbits immunized with the A3 peptide showed similar spreading, although there was some variation among the specific epitopes recognized. Control rabbits immunized with Sm 115-MAP or with adjuvant alone did not show binding to these overlapping octapeptides.
Fig. 3.
Antibody reactivity to overlapping octapeptides of nRNP A. (A) Binding of one A3 immunized rabbit with the 275 overlapping octapeptides of the nRNP A protein. Reactivity with the peptide of immunization is seen at epitopes 44-49. All other epitopes recognized have developed over the 4 weeks since initial immunization. (B) Binding of one rabbit immunized with the A6 peptide. Reactivity with the peptide of immunization (A6) is demonstrated at epitopes 103-108. Common epitopes seen in human SLE (ref. 7) are presented at the top.
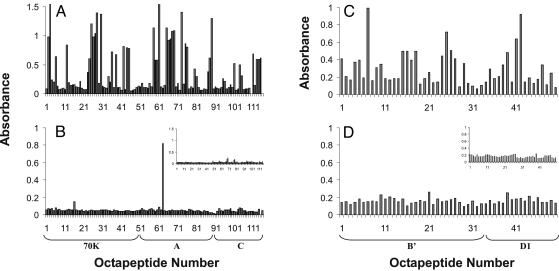
A3 Immunized Animals Produce Antibodies Against Other Components of the Spliceosome. Analysis of A3 rabbit serum was also performed by using overlapping octapeptides from other common lupus autoantigens. These studies revealed that not only did these rabbits show reactivity to many regions of nRNP A commonly recognized by human patients, but they had also spread to regions of nRNP 70K and nRNP C (Fig. 4). Again, the regions of these proteins targeted by autoantibodies were among those seen in the aberrant human lupus immune response (8, 25, 26). The epitope binding profiles of Sm B/B′ and Sm D1 revealed that the immunized animal immune response had spread beyond the nRNP family of proteins to target other snRNP components (Fig. 4). The A3 immunized animals developed antibodies against peptides from Sm B/B′ and Sm D1 that were nonhomologous to the sequences recognized from the nRNP proteins, yet similar to those recognized in human SLE (15, 16).
Fig. 4.
Antibody reactivity to peptides of other autoantigens. Average reactivity of rabbit sera before immunization (B and D Insets) and 4 months after immunization with A3 (A and C) and A6 (B and D) with select overlapping octapeptides of nRNP 70K, A, and C, and Sm B/B′ and Sm D1. These epitopes are areas commonly bound by human lupus patient sera. (A and B) Octapeptides 1-49 are from nRNP 70K, 50-87 are from nRNP A, and 88-115 are from nRNP C. (C and D) Octapeptides 1-34 are from Sm B/B′, whereas 35-50 are from Sm D1.
Additional Autoantibody Profiles of Experimental Animals. In addition to binding short peptides of nRNP by standard ELISA and solid-phase peptide assays, all A3 immunized animals developed antibodies that bind nRNP A by Western blot. Also, these rabbits made antibodies that recognize nRNP 70K, nRNP C, Sm B/B′, and Sm D1 by Western blotting (data not shown). All of the A3 immunized rabbits, but none of the A6, developed ANA during the course of the experiment. Approximately half of the A3 animals also developed anti-double-stranded DNA antibodies (titer ≥1:30) after several weeks. A3 immunized animals developed precipitating levels of anti-nRNP antibodies within a week of initial immunization, followed by precipitating levels of anti-Sm within 3 weeks and anti-Ro within 3 months. None of the A6 immunized animals ever developed precipitating levels of autoantibodies to any of the other antigens tested.
Interestingly, affinity-purified anti-A3 antibodies were also found to positively stain Hep-2 cells in a pattern closely resembling that shown by ANA-positive controls. Neither whole A6 rabbit serum nor affinity-purified anti-A6 antibodies showed any staining when tested against Hep-2 cells, but both were able to recognize denatured nRNP A from HeLa cell extract by Western blot (data summarized in Fig. 5 and Table 1).
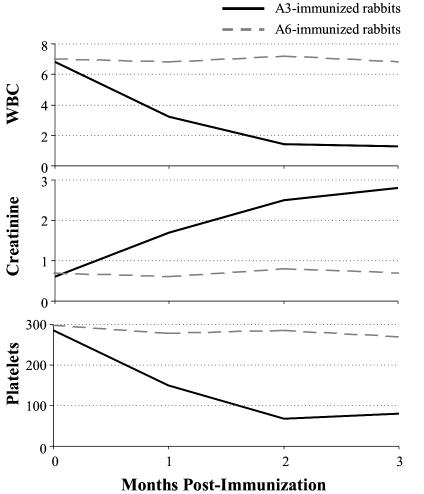
Fig. 5.
Clinical manifestations of immunized animals. A3 immunized animals rapidly develop leukopenia, renal insufficiency, and thrombocytopenia, whereas A6 immunized animals remain at baseline.
Table 1. Summary of serological and clinical manifestations of immunized animals.
| Preimmune | A3 immunized* | A6 immunized* | |
|---|---|---|---|
| WBC, 103 per ml | 6.8 (±0.7) | 1.4 (±0.4) | 6.6 (±0.7) |
| Serum Creatinine, mg/dl | 0.6 (±0.1) | 2.8 (±0.3) | 0.7 (±0.1) |
| dsDNA | − | 1:30 | − |
| ANA | − | 1:360 | − |
| Purified anti-A3 | − | 1:80‡ | − |
| Purified anti-A6 | − | − | −† |
| Precipitate nRNP A coupled with U1 RNA | − | ++ | − |
| Precipitate free nRNP A | − | ++ | + |
A3 immunized animals develop positive ANA and double-stranded DNA by 3 months after immunization and are capable of precipitating nRNP A, which is complexed to the U1 RNA. A6 immunized sera do neither but are capable of precipitating native nRNP A, which is not complexed to the U1 RNA. Affinity-purified anti-A3 antibodies also test positive for ANA reactivity in standard Hep-2 assays, whereas purified anti-A6 antibodies are not.
As measured 3 months after initial immunization.
Affinity-purified anti-A3 or -A6 antibodies from A3 and A6 immunized animals, respectively, were similarly tested for ANA reactivity.
A6 Immunized Rabbits Make Antibodies Only Against the Peptide of Immunization. As shown in Fig. 2, the A6 immunized animals mounted an immune response solely against the A6 peptide. When tested for reactivity with the overlapping octapeptides of nRNP A, sera from the A6 immunized animals showed significant binding to the A6 epitope but negligible binding to any other regions of nRNP A (Fig. 3). Sera from the A6 immunized rabbits also exhibited no significant binding to any of the regions of nRNP 70K and nRNP C, or Sm B/B′ and Sm D1, which are commonly bound in human lupus sera (Fig. 4). The A6 immunized animals did show binding to nRNP A by Western blot (data not shown) but did not recognize other snRNP proteins by this method, nor are they positive for either ANA or anti-double-stranded DNA antibodies. Negative control animals also failed to make antibodies against any proteins by Western blot and were negative for antibodies against ANA and double-stranded DNA as well.
A6 Immunized Animals Preferentially Recognize Non-snRNP-Associated nRNP A. Previous experiments have demonstrated that the nRNP A protein also exists in human cells in a non-snRNP-associated complex, and this complex plays a role in polyadenylation of pre-mRNAs (9, 27). We used this fact in functional assays to determine whether A6 immunized rabbit sera preferentially bind to the non-snRNP form of nRNP A, as has been demonstrated for the monoclonal 12E12, which recognizes the same epitope (9, 10). We first used sera from A3 and A6 immunized animals to immunoprecipitate protein-RNA complexes from 293T cell nucleoplasm, followed by phenol extraction and ethanol precipitation of the coprecipitated RNAs, and subsequent Northern blotting for the presence of U1 RNA. A6 immunized animals did not precipitate significant levels of U1 RNA-nRNP A complexes at any of the time points tested, whereas A3 immunized animals did precipitate significant amounts of nRNP A containing U1 RNA (Table 1). This is reminiscent of the case with mAb 12E12, which precipitated no U1 RNA, whereas other anti-snRNP mAbs did (9).
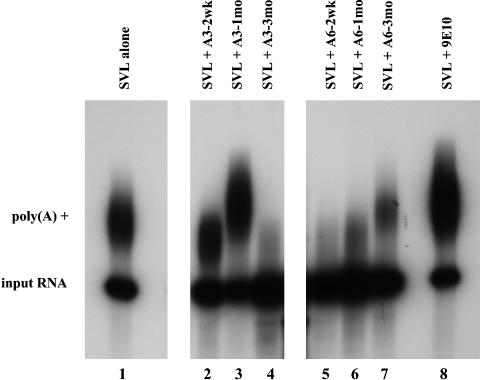
Sera from A3 immunized animals and A6 immunized animals were tested for their ability to affect mRNA polyadenylation in vitro (Fig. 6). The sera from the A6 animals demonstrated significant inhibition of polyadenylation (lanes 5-7, 2 weeks, 1 month, and 3 months, respectively), whereas serum from A3 immunized animals showed no such inhibition of polyadenylation (lanes 2 and 3, 2 weeks and 1 month postimmunization, respectively) until much later in the disease course, when they concurrently begin to develop high titers of anti-A6 antibodies (lane 4, 3 months postimmunization). Taken together, these data suggest that the sera from the A6 immunized animals are likely recognizing the snRNP-free form of nRNP A [U1A] and thereby inhibit pre-mRNA polyadenylation.
Fig. 6.
In vitro polyadenylation reactions reveal differences in specificities of inhibition between sera from the A3 and A6 immunized animals. In vitro polyadenylation reactions were performed by using SV40 late mRNA as substrate RNA (SVL) and HeLa nuclear extract, in the absence (lane 1) or presence (lanes 2-8) of specific antibodies. Lane 1, Polyadenylation reaction with no antibodies; lane 8, polyadenylation reaction in the presence of negative control antibody (anti-myc tag antibody 9E10). The A3 immunized rabbit sera (lanes 2-4) exhibit no inhibition of polyadenylation until late in disease course when anti-A6 antibodies begin to appear. The A6 immunized animal sera (lanes 5-7) exhibit strong inhibition of polyadenylation early in disease, which tapers off as anti-A6 antibody titers decrease over time.
Clinical Symptoms of Immunized Animals. Animals immunized with the A3 peptide also develop renal toxicity, as evidenced by significant increases (up to 4-fold, t test, P = 0.005) in serum creatinine levels by 4 months after initial immunization. Furthermore, they develop significant (P = 0.003) leukopenia with white counts in the 1,000-2,000 per mm3 range. Animals immunized with either adjuvant alone or with the A6 peptide exhibited no leukopenia and no increase in serum creatinine, nor any other symptoms common to human SLE (Fig. 5 and Table 1).
Discussion
Immunization with one specific epitope of nRNP A, A3 (which is clearly significant in human disease), can elicit a diversified, mature autoimmune response against other portions of the parent molecule. In addition, this response expands to include other snRNP proteins that are known to associate with that molecule as members of the spliceosome in vivo. The fact that the A3 epitope is able to induce such a global response is very interesting. Previous work in lupus autoimmunity has shown that specific epitopes from the sequences of the autoantigens Sm B′ or 60-kDa Ro were able to trigger lupus-like autoimmune development (12, 13, 28-31), whereas in the MRL model of lupus autoimmunity, the first detectable anti-snRNP autoantibodies are directed against the nRNP A protein (32). However, the presence of antibodies against nRNP is not necessarily indicative or causative of additional anti-snRNP autoantibodies in human autoimmune disease (33, 34).
This begs the question, then, why this particular epitope of nRNP A induces an immune response against multiple components of the spliceosome along with clinical features of SLE, as compared to the failure of the clearly antigenic A6 epitope to produce a similar response. The A6 epitope sequence (ERDRKREKRKPKS) provides little insight. Sequences with multiple lysine residues have been shown to be very antigenic in the nRNP 70K molecule, whereas epitopes containing multiple arginine residues have been demonstrated to be highly antigenic in the Sm D1 protein. Additionally, a high isoelectric point has proven to be a fair indicator of autoantigenicity of epitopes in the snRNP spliceosomal system (35), and both the A3 and A6 peptides have relatively basic pIs (12.0 and 11.0, respectively). The difference is not due to species specificity, because we have demonstrated that the sequences of the epitopes in question are identical between human and rabbit. Similar to A6, certain peptides from the sequences of other autoantigens such as 60-kDa Ro and Sm B/B′ have also been shown to be relatively nonimmunogenic in animal models (31, 36). It would seem, then, that the observed process of triggering an immune response coupled with spreading is not due simply to instigation of stress on host immunity but rather is due to properties of specific peptide sequences and the mechanism of their interaction with the host immune system.
One possible avenue to explain this phenomenon arises from the three-dimensional structure of nRNP A. The first 95 aa of nRNP A have been crystallized, and amino acids 1-117 have been solved by NMR (37). The A3 epitope is postulated to be on the surface of the molecule in vivo, and as such is likely to be freely available to be bound by specific antibodies. This conclusion is further supported by our finding that anti-A3 antibodies are capable of reacting with native snRNP in vivo as demonstrated by polyadenylation and immunoprecipitation studies, and by producing an ANA binding pattern similar to that seen in human SLE. These findings with the A3 peptide support earlier conclusions that the B cells involved in binding this epitope of nRNP A may exhibit more than the capacity to produce antibodies that bind the A3 peptide on the animal's own snRNP. They may also process it and present it on MHC class II molecules, along with other peptide components of the spliceosome, to T cells as has been described (38, 39). This process, along with proper immune signaling, would then lead to Ig epitope spreading and autoimmunity as seen in this model.
The lack of response to the A6 peptide seems likely to be due to the cryptic nature of this epitope in vivo when bound to the U1 RNA. A monoclonal anti-nRNP A antibody, 12E12, recognizes the A6 epitope and binds to U1A only when the protein is part of a non-snRNP complex that does not contain U1 RNA (9, 10, 40). Our polyadenylation data demonstrate that the anti-A6 antibodies from immunized animals react in a similar fashion, suggesting that they recognize the snRNP-free nRNP A protein and thereby prevent the binding of U1 RNA to nRNP A and therefore inhibit polyadenylation. Furthermore, anti-A6 antibodies were incapable of immunoprecipitating nRNP A coupled to U1 RNA, showed no staining in an ANA assay, and do not precipitate or bind native nRNP in immunodiffusion assays. Anti-A3 antibodies, however, are demonstrated to do all of these. These data suggest that antibodies to the A6 peptide lack the ability to stimulate the immune response through promoting the binding and processing of complexed snRNPs, because of the fact that the target epitope for these autoantibodies is not freely available to the immune system in vivo. Once anti-nRNP A immunity is instigated to an epitope that is freely available on the surface of the protein (such as A3), then the whole complex could be bound by autoreactive B cell-surface Ig, phagocytosed, and processed, with the subsequent presentation of additional epitopes (eventually including A6) through MHC class II molecules for immune targeting. The A3 sequence, because of its accessible surface position, could potentially be presented to the immune system in myriad situations, including apoptosis or other cell lysis where nuclear protein complexes would be released into tissue or blood. Data demonstrating that the major epitopes of other common autoantibodies, such as those targeting the Sm proteins and others, also localize to the surface of the snRNP complex further suggest that this may be a common theme in human autoimmune disease (35, 41-44).
The animals immunized with the A3 epitope develop some symptoms common in human SLE, including leukopenia, thrombocytopenia, and an increase in serum creatinine levels (Fig. 5 and Table 1). Indeed, these animals actually meet SLE diagnostic criteria. The link between anti-snRNP antibodies and clinical findings has not been proven, but the sequence of events in the A3 immunization model described here seems to reiterate that autoimmune processes can be causative of clinical symptoms, whether directly or indirectly. However, the potential effects of three-dimensional structure and function on availability and immunogenicity of self-peptides in the development of autoimmunity is becoming more clear. Certainly, current data suggest that the humoral autoimmune response in human SLE is largely propagated by epitopes that localize to the surface of complexed autoantigens. Hopefully, further analysis of models such as the one presented herein will provide additional insight into the relationships between and mechanisms behind both the autoimmune processes and the clinical manifestations associated with them.
Acknowledgments
We thank Mei Zhu, Amber Davis, Jama Shoemaker, Janeen Arbuckle, Monica Kirby, and the Oklahoma Molecular Biology Core Facility. This work was supported by National Institutes of Health Grants AR45451, AR45231, AR47575, AR48940, and AR48045, Arthritis Investigator Award 2AI-LUT-A-5, and American Cancer Society Grant RPG-00-265-01-GMC.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SLE, systemic lupus erythematosus; snRNP, small nuclear ribonucleoprotein; ANA, antinuclear antibodies.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY387676).
References
- 1.van Venrooij, W. J. (1987) J. Rheumatol. 14, 78-82. [PubMed] [Google Scholar]
- 2.Hardin, J. A. (1989) in Primer on the Rheumatic Diseases, ed. Schumacher, H. R. (Arthritis Foundation, Atlanta), pp. 32-38.
- 3.Lehmeier, T., Foulaki, K. & Luhrmann, R. (1990) Nucleic Acids Res. 18, 6475-6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spritz, R. A., Strunk, K., Surowy, C. S., Hotch, S. O., Barton, D. E. & Franke, U. (1987) Nucleic Acids Res. 15, 10373-10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sillekens, P. T. G., Habets, W. J., Beijer, R. P. & Venrooij, W. J. (1987) EMBO J. 6, 3841-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sillekens, P. T. G., Beijer, R. P., Habets, W. J. & Venrooij, W. J. (1988) Nucleic Acids Res. 16, 8307-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James, J. A. & Harley, J. B. (1996) J. Immunol. 156, 4018-4026. [PubMed] [Google Scholar]
- 8.Barakat, S., Brand, J. P., Abuaf, N., Van Regenmortel, M. H. V. & Muller, S. (1991) Clin. Exp. Immunol. 86, 71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Connor, J. P., Alwine, J. C. & Lutz, C. S. (1997) RNA 3, 1444-1455. [PMC free article] [PubMed] [Google Scholar]
- 10.Lutz, C. S., McClain, M. T., Harley, J. B. & James, J. A. (2002) J. Mol. Recognit. 15, 163-170. [DOI] [PubMed] [Google Scholar]
- 11.Avis, J. M., Allain, F. H. T., Howe, P., Varani, G., Nagai, K. & Neuhaus, D. (1996) J. Mol. Biol. 257, 398-411. [DOI] [PubMed] [Google Scholar]
- 12.James, J. A., Gross, T., Scofield, R. H. & Harley, J. B. (1995) J. Exp. Med. 181, 453-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James, J. A. & Harley, J. B. (1998) J. Immunol. 160, 502-508. [PubMed] [Google Scholar]
- 14.Tam, J. P. (1988) Proc. Natl. Acad. Sci. USA 85, 5409-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James, J. A. & Harley, J. B. (1992) J. Immunol. 148, 2074-2079. [PubMed] [Google Scholar]
- 16.James, J. A., Mamula, M. J. & Harley, J. B. (1994) Clin. Exp. Immunol. 98, 419-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattioli, M. & Reichlin, M. (1971) J. Immunol. 107, 1281-1290. [PubMed] [Google Scholar]
- 18.Gonzales, E. N. & Rothfield, N. F. (1966) N. Engl. J. Med. 271, 1333-1338. [DOI] [PubMed] [Google Scholar]
- 19.Arden, A., de Groot, E. R. & Fletkamp, T. E. W. (1975) Ann. N.Y. Acad. Sci. 254, 505-512. [DOI] [PubMed] [Google Scholar]
- 20.White, R. V., Kaufman, K. M., Letson, C. S., Platteborze, P. L. & Sodetz, J. M. (1994) J. Immunol. 152, 2501-2508. [PubMed] [Google Scholar]
- 21.Natalizio, B. J., Muniz, L. C., Arhin, G. K., Wilusz, J. & Lutz, C. S. (2002) J. Biol. Chem. 277, 42733-42740. [DOI] [PubMed] [Google Scholar]
- 22.Faig, O. Z. & Lutz, C. S. (2003) Scand. J. Immunol. 57, 79-84. [DOI] [PubMed] [Google Scholar]
- 23.Lutz, C. S. & Alwine, J. C. (1994) Genes Dev. 8, 576-586. [DOI] [PubMed] [Google Scholar]
- 24.Moore, C. L. (1990) Methods Enzymol. 181, 49-74. [DOI] [PubMed] [Google Scholar]
- 25.James, J. A. & Harley, J. B. (1995) Clin. Exp. Rheumatol. 13, 299-305. [PubMed] [Google Scholar]
- 26.James, J. A., Scofield, R. H. & Harley, J. B. (1994) Scand. J. Immunol. 39, 557-566. [DOI] [PubMed] [Google Scholar]
- 27.Lutz, C. S., Murthy, K. G. K., Schek, N., O'Connor, J. P., Manley, J. L. & Alwine, J. C. (1996) Genes Dev. 10, 325-337. [DOI] [PubMed] [Google Scholar]
- 28.Scofield, R. J., Williams, H. E., Kurien, B. T., James, J. A. & Harley, J. B. (1996) J. Immunol. 156, 4059-4066. [PubMed] [Google Scholar]
- 29.Topfer, F., Gordon, T. & McCluskey, J. (1995) Proc. Natl. Acad. Sci. USA 92, 875-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tseng, C. E., Chan, E. K., Miranda, E., Gross, M., Di Donato, F. & Buyon, J. P. (1997) Arthritis Rheum. 40, 936-944. [DOI] [PubMed] [Google Scholar]
- 31.McClain, M. T., Scofield, R. H., Kurien, B. T., Gross, T. F. & James, J. A. (2002) Scand. J. Immunol. 56, 399-407. [DOI] [PubMed] [Google Scholar]
- 32.Fatenejad, S., Brooks, W., Schwartz, A. & Craft, J. (1994) J. Immunol. 152, 5523-5531. [PubMed] [Google Scholar]
- 33.Amigues, J. M., Cantagrel, A., Abbal, A. & Mazieres, B. (1996) J. Rheumatol. 23, 2055-2062. [PubMed] [Google Scholar]
- 34.Fisher, D. E., Reeves, W. H., Wisniewolski, R., Lahita, R. G. & Chiorazzi, N. (1985) Arthritis Rheum. 28, 1348-1355. [DOI] [PubMed] [Google Scholar]
- 35.McClain, M. T., Ramsland, P. A., Kaufman, K. M. & James, J. A. (2002) J. Immunol. 168, 2054-2062. [DOI] [PubMed] [Google Scholar]
- 36.Scofield, R. H., Kaufman, K. M., Baber, U., James, J. A., Harley, J. B. & Kurien, B. T. (1999) Arthritis Rheum. 42, 1017-1024. [DOI] [PubMed] [Google Scholar]
- 37.Nagai, K., Oubridge, C., Jessen, T. H., Li, J. & Evans, P. R. (1990) Nature 348, 515-520. [DOI] [PubMed] [Google Scholar]
- 38.Paisansinsup, T., Vallejo, A. N., Luthra, H. & David, C. S. (2001) J. Immunol. 167, 4083-4090. [DOI] [PubMed] [Google Scholar]
- 39.Paisansinsup, T., Deshmukh, U. S., Chowdhary, V. R., Luthra, H. S., Fu, S. M. & David, C. S. (2002) J. Immunol. 168, 5876-5884. [DOI] [PubMed] [Google Scholar]
- 40.Milcarek, C., Martincic, K., Chung-Ganster, L. H. & Lutz, C. S. (2003) Mol. Immunol. 39, 809-814. [DOI] [PubMed] [Google Scholar]
- 41.Kinoshita, G., Keech, C. L., Sontheimer, R. D., Purcell, A., McCluskey, J. & Gordon, T. P. (1998) Lupus 7, 7-11. [DOI] [PubMed] [Google Scholar]
- 42.Gordon, T. P., Kinoshita, G., Cavill, D., Keech, C., Farris, A. D., Kaufman, K., McCluskey, J. & Purcell, A. W. (2002) Scand. J. Immunol. 56, 168-173. [DOI] [PubMed] [Google Scholar]
- 43.Miller, S. D., Katz-Levy, Y., Nevilee, K. L. & Vanderlugt, C. L. (2001) Adv. Virus Res. 56, 199-217. [DOI] [PubMed] [Google Scholar]
- 44.Brouwer, R., Vree Egberts, W. T. M., Hengstman, G. J. D., Raijmakers, R., van Engelen, B. G. M., Seelig, H. P., Renz, M., Mierau, R., Genth E., Pruijn, G. J. M., et al. (2002) Arthritis Res. 4, 134-138. [DOI] [PMC free article] [PubMed] [Google Scholar]