Abstract
We investigated the expression of transient receptor potential canonical 6 (TRPC6) protein in benign and malignant human prostate tissues and in prostate cancer cell lines and the association with the stage, grade and androgen responsiveness of the tumors. Immunohistochemical techniques, Western blot and reverse transcription polymerase chain reaction (RT-PCR) were used to investigate TRPC6 expression. TRPC6 protein was detected in 9 of 20 (45.0%) of benign prostatic hyperplasia (BPH) cases, and there was a significant difference compared with prostate cancer (129 of 149 [86.6%])(P < 0.01). TRPC6 expression was associated with the histological grade and extraprostatic extension (P < 0.01). Tumors of higher stage tended to have a higher frequency of TRPC6 protein staining, but the difference was not significant among T2, T3 and T4. TRPC6 expression difference between androgen-independent (AI) tumors and androgen-dependent (AD) tumors was not statistically significant. TRPC6 was also observed in prostate cancer cell lines. In summary, TRPC6 is detected in benign and malignant human prostate tissues and prostate cancer cell lines and is associated with the histological grade, Gleason score and extraprostatic extension of prostate cancer.
Keywords: benign prostatic hyperplasia, immunohistochemistry, prostate cancer, TRPC6
Introduction
Prostate cancer is one of the leading threats to men's health in Western societies. Following metastasis to bone and lymph nodes, the primary cause of prostate cancer mortality is the progression from androgen-dependent (AD) to androgen-independent (AI) growth. In the early stages of the disease, androgen-ablation therapy can cause tumour regression, and is currently the most successful treatment option. However, once the tumour achieves androgen independence, there is no effective therapy 1. Therefore, new prognostic markers and therapeutic strategies may be of great benefit to prostate cancer patients.
The TRP superfamily can be divided into seven subfamilies, namely, TRPC, TRPV, TRPM, TRPN, TRPA, TRPP and TRPML. These channels have critical roles in physiological processes ranging from sensation to fertilization 2. TRPM8 and TRPV6 are proposed pro-oncogenic factors in prostate cancer, and have an important role in the development of this disease 3, 4, 5, 6. Mammalian TRPC can be divided into three groups on the basis of the amino acid similarity , namely, TRPC2 (pseudogene in human), TRPC3/6/7 and TRPC1/4/5. Recently, it has been shown that transient receptor potential canonical 6 (TRPC6) contributes to the proliferation of AD prostate cancer epithelial cells 7, human epithelial breast cancer cells 8 and human hepatoma cells 9. However, there have been no systematic studies documenting the actual expression of TRPC6 protein in the prostate and its relationship with prostate cancer progression.
On the basis of these observations, we hypothesized that prostate cancer progression is associated with the level of human TRPC6 protein expression. To evaluate this hypothesis, we studied TRPC6 protein expression in benign and malignant human prostate tissues, and correlated TRPC6 protein immunostaining with the stage, grade and androgen responsiveness of the tumours.
Materials and methods
Cell culture
The 22Rv1, DU145 and PC3 prostate cancer cell lines were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). The PC3 cells were maintained in DMEM/F12 medium (GIBCO BRL, Gaithersburg, MD, USA), and the DU145 and 22Rv1 cells were cultured in RPMI 1 640 medium (GIBCO BRL). All cell lines were supplemented with 10% (v/v) foetal calf serum (Invitrogen, Carlsbad, CA, USA) and incubated at 37°C with 5% CO2.
Clinical specimens
Clinical specimens were collected from the patients registered at Shengjing Hospital of China Medical University (Shenyang, China) between 2004 and 2008 with patients' consent and ethical committee approval. To correlate TRPC6 protein immunostaining with known clinicopathological parameters, patient medical records were retrospectively reviewed to determine age, race, previous therapy and Gleason score at the time when the study sample was obtained. Cancers were considered AI if they were derived from patients who did not initially respond to androgen deprivation or whose disease progressed after an initial response. A rising prostate-specific antigen (PSA) level, the progressive increase in the size of the primary lesion and existing metastatic sites, and the development of additional metastases in the presence of castrate levels of serum testosterone (< 50 ng per 100 mL) were considered as evidences of disease progression. Cancers that responded to androgen deprivation were classified as AD, and specimens were obtained from AD prostate cancer patients before the initiation of androgen-deprivation therapy. The median serum PSA concentration and the prostate size of the benign prostatic hyperplasia (BPH) patients were 2.4 ng mL−1 and 46.7 mL, respectively. AD and AI samples were taken from the prostate. Cancers classified as metastatic included both direct extension and distant metastasis, such as lymph node, bladder, seminal vesicle, peritoneum, testicle and rectum. All the samples used were analysed by two experienced pathologists to ensure that they were correctly identified as either BPH or prostate cancer.
Immunohistochemistry
Samples were derived from either transurethral resection (TUR) or radical prostatectomy specimens. Samples were selected to include BPH and prostate cancers of various histological stages. First, 5-μm sections from formalin-fixed and paraffin-embedded specimens were deparaffinized using xylene and rehydrated in graded ethanol. Samples were then preincubated with 3% H2O2 to inhibit endogenous peroxidase activity. Sections were incubated at room temperature overnight in a 1:400 dilution of the rabbit polyclonal primary anti-TRPC6 antibody (Abcam, Cambridge, UK). A secondary biotinylated goat anti-rabbit IgG was consequently applied and immunoreactivity was visualized using streptavidin-peroxidase along with 3,3′-diaminobenzidine as substrate. In control experiments, the primary antibody was replaced with phosphate-buffered saline.
The TRPC6 in immunohistochemistry score was evaluated by a grading system ranging from 0 to 5 3: 0, 0%–1% of tumour cells positive; 1, 1%–5% of tumour cells positive; 2, 5%–10% of tumour cells positive; 3, 10%–20% of tumour cells positive; 4, 20%–50% of tumour cells positive; and 5, > 50% of tumour cells positive. Tumours were evaluated by random observation of five fields on every section by two investigators. Their averaged categories served as the tumour TRPC6 expression score. The intensity of staining was not considered.
Reverse transcription PCR (RT-PCR) analysis
Total RNA was isolated from cells using Trizol reagent (Invitrogen). It (500 ng) was then reverse transcribed into cDNA using oligo(dT) primers and AMV Reverse Transcriptase (Takara, Shiga, Japan). Next, 10-μL cDNA was used for the PCR reaction in a final reaction volume of 50 μL. For the PCR reaction, specific sense and antisense primers were selected based on GenBank TRPC6 sequences. Primers were synthesized by Takara. Human TRPC6 primer (NM_004621): sense, 5′-GAACTTAGCAATGAACTGGCAGT-3′ antisense, 5′-CATATCATGCCTATTACCCAGGA-3′. Product lengths were 625 bp for TRPC6 and 277 bp for TRPC6γ. β-actin primer (NM_001101): sense, 5′-TGGGCATGGGTCAGAAGGAT-3′ ; antisense 5′-AAGCATTTGCGGTGGACGAT-3′, product length 991 bp. DNA amplification conditions included an initial 5-min denaturation step at 95°C and 30 cycles of 30 sec at 95°C, 30 sec at 58°C, 40 sec at 72°C and a final elongation of 7 min at 72°C. The RT-PCR samples were electrophoresed on a 1.5% agarose gel and stained with ethidium bromide (0.5 μg mL−1); the gels were then photographed under ultraviolet transillumination. This experiment was repeated five times.
Western blotting
Proteins were harvested from prostate cancer cells. Equal amounts of each protein sample (40 μg) were separated by electrophoresis on sodium dodecyl sulphate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Invitrogen) using a semi-dry electroblotter. Immunoblotting was performed using rabbit polyclonal primary anti-TRPC6 antibody (diluted 1:1 000, Abcam) and goat polyclonal primary anti-β-actin (diluted 1:1 000, Santa Cruz, CA, USA), and developed in the enhanced chemiluminescence system (ECL, Amersham, Uppsala, Sweden) using specific peroxidase-conjugated anti-IgG secondary antibodies. This experiment was repeated five times.
Statistical analysis
Contingency table and χ2 analyses were performed using the Statistical Package for Social Sciences software (SPSS12.0). P < 0.05 was regarded as statistically significant.
Results
Histopathological and clinical features
We studied 153 prostate cancer samples derived from 142 patients (1 sample in 131 patients, 2 samples in 11 patients) and 20 benign prostate tissue samples derived from BPH patients for TRPC6 protein staining. Patients′ age ranged from 45 to 81 years. The 153 prostate cancer samples consisted of 102 AD, 34 AI and 17 metastatic cancers; 152 of the specimens were adenocarcinomas and 1 was a neuroendocrine carcinoma of the prostate. We also studied palliative TUR specimens from 19 patients with prostate cancer recurrence after androgen-deprivation therapy. The pathological stages, grades and androgen responsiveness of tumour tissues are listed in Table 1.
Table 1. Pathological stages and grades.
| Pathological stage | n | Gleason score | AD | AI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||
| T1 | 13 | 1 | 2 | 4 | 5 | 1 | — | — | — | 13 | — |
| T2 | 25 | — | — | 1 | 10 | 8 | 6 | — | — | 24 | 1 |
| T3 | 71 | — | — | — | 9 | 28 | 20 | 12 | 2 | 63 | 8 |
| T4 | 8 | — | — | — | — | 1 | 3 | 3 | 1 | 2 | 6 |
| Recurrent carcinomas | 19 | ND | 0 | 19 | |||||||
| Metastatic cancer | 17 | ND | |||||||||
Abbreviations: AD, androgen-dependent; AI, androgen-independent; n, number of patients; ND, not determined.
Relationship of TRPC6 protein expression with prostate cancer stage
Positive TRPC6 protein staining was detected in BPH in 9 of 20 cases (45.0%). This staining was localized in areas with relatively hyperplastic epithelium. Tumours of higher stage tended to have a higher frequency of TRPC6 protein staining. Of the 12 T1 cancer samples, 7 (58.3%) stained positively for TRPC6 protein. T2 tumours revealed TRPC6 in 80.0% of cases. Locally advanced tumours (T3) expressed TRPC6 in 63 of 69 (91.3%) cases. In addition, seven of eight (87.5%) high-stage tumours (T4) showed TRPC6 protein staining. Significant expression, that is, more than 20% of tumour cells expressing TRPC6, was noted in 24.0% of T2, 42.0% of T3 and 50.0% of T4 tumours. In total, 88.9% of recurrent carcinomas and 94.1% of metastatic cancer samples were positive for TRPC6 protein staining (Table 2). Statistical analyses revealed a striking difference in TRPC6 protein staining between prostate cancer and BPH (P < 0.01). These staining differences were statistically significant when comparing T1 cancers with T2, T3 and T4 samples (P < 0.05), but there was no difference among T2, T3 and T4 samples. In addition, the amount of TRPC6 expression recorded in metastatic cancers was much higher than that seen in primary tumours (P < 0.01). Images of the staining can be seen in Figure 1.
Table 2. Relationship between tumour pathological stage and TRPC6 protein.
| Pathological stage | n | Evaluable specimens | TRPC6 positive (%) | Specimens classified according to the immunohistochemical TRPC6 score (number [%]) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||||
| BPH | 20 | 20 | 9 (45.0) | 11 (55.0) | 4 (20.0) | 4 (20.0) | 1 (5.0) | 0 | 0 |
| T1 | 13 | 12 | 7 (58.3) | 5 (41.7) | 2 (16.7) | 4 (33.3) | 1 (8.3) | 0 | 0 |
| T2 | 25 | 25 | 20 (80.0) | 5 (20.0) | 1 (4.0) | 5 (20.0) | 8 (32.0) | 6 (24.0) | 0 |
| T3 | 71 | 69 | 63 (91.3) | 6 (8.7) | 4 (5.8) | 15 (21.7) | 15 (21.7) | 23 (33.3) | 6 (8.7) |
| T4 | 8 | 8 | 7 (87.5) | 1 (12.5) | 0 | 2 (25.0) | 1 (12.5) | 2 (25.0) | 2 (25.0) |
| Recurrent carcinomas | 19 | 18 | 16 (88.9) | 2 (11.1) | 1 (5.56) | 6 (33.3) | 6 (33.3) | 3 (16.7) | 0 |
| Metastatic cancer | 17 | 17 | 16 (94.1) | 1 (5.9) | 0 | 5 (29.4) | 6 (35.3) | 3 (17.6) | 2 (11.8) |
Abbreviations: BPH, benign prostatic hyperplasia; TRPC6, transient receptor potential canonical 6.
Figure 1.

Expression of TRPC6 in BPH, prostate cancer and metastatic cancer (brown denotes positive). (A): Control group of BPH ( × 400) showing no staining when the primary antibody was omitted. (B): Immunohistochemistry staining of TRPC6 in BPH (×400). Arrow indicates faint staining. (C): Control group of prostate cancer ( × 400) showing no staining when the primary antibody was omitted. (D): Immunohistochemistry staining of TRPC6 in well-differentiated prostate cancer (×400). (E): Immunohistochemistry staining of TRPC6 in moderately differentiated prostate cancer (×400). (F): Immunohistochemistry staining of TRPC6 in poorly differentiated prostate cancer ( × 400). (G): Immunohistochemistry staining of TRPC6 in peritoneal metastasis of prostate cancer ( × 100). (H): Immunohistochemistry staining of TRPC6 in bladder metastasis of prostate cancer ( × 100). (A)–(F): Scale bars = 50 μm; (G) and (H): Scale bars = 200 μm.
Relationship of TRPC6 protein expression to prostate cancer grade
Irrespective of pathological stage, TRPC6 expression correlated with histological grade. The staining differences among G1, G2 and G3–4 tumours were statistically significant (P < 0.01) (Table 3).
Table 3. Relationship between histological grade and TRPC6 protein.
| Histological grade (Gleason score) | n | Evaluable specimens | TRPC6 positive (%) | Specimens classified according to the immunohistochemical TRPC6 score (number [%]) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||||
| G1 (2–4) | 3 | 3 | 1 (33.3) | 2 (66.7) | 0 | 1 (33.3) | 0 | 0 | 0 |
| G2 (5–6) | 29 | 28 | 20 (71.4) | 8 (28.6) | 3 (10.7) | 7 (25.0) | 5 (17.9) | 5 (17.9) | 0 |
| G3–4 (7–10) | 85 | 83 | 76 (91.6) | 7 (8.4) | 4 (4.8) | 18 (21.7) | 20 (24.1) | 26 (31.3) | 8 (9.6) |
Abbreviations: TRPC6, transient receptor potential canonical 6.
Relationship of TRPC6 protein expression to prostate cancer androgen responsiveness
Increasing levels of TRPC6 were detected in AI tumours when compared with AD tumours, but the difference was not statistically significant. However, when compared with BPH, TRPC6 expression differences in AD and AI were statistically significant (P < 0.01) (Table 4).
Table 4. Relationship between tumour androgen responsiveness and TRPC6 protein.
| Androgen responsiveness | n | Evaluable specimens | TRPC6 positive (%) |
|---|---|---|---|
| BPH | 20 | 20 | 8 (40.0) |
| AD | 102 | 99 | 82 (82.8) |
| AI | 34 | 33 | 29 (87.9) |
Abbreviations: AD, androgen-dependent; AI, androgen-independent; BPH, benign prostatic hyperplasia; TRPC6, transient receptor potential canonical 6.
TRPC6 expression in prostate cancer cell lines
We found the expression of TRPC6 mRNA transcripts in the three human prostate cancer cell lines that we examined. Moreover, we also observed the expression of the TRPC6γ splice variant. Using western blotting, we found that the TRPC6 proteins were expressed in all three human prostate cancer cell lines (Figures 2, 3).
Figure 2.
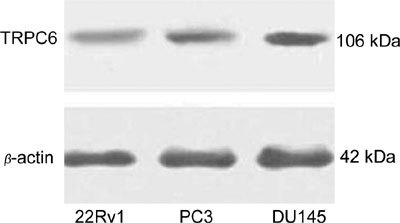
TRPC6 protein expression in prostate cell lines.
Figure 3.

TRPC6 mRNA expression in prostate cell lines.
Discussion
Through our systematic study of the TRPC6 protein in benign and malignant prostate tissues, we found that TRPC6 was expressed in BPH, prostate cancer and, in the DU145, PC3 and 22Rv1 prostate cancer cell lines, and was associated with the cancer's histological grade, Gleason score and extraprostatic extension status.
Some studies show a correlation between TRPV6 and TRPM8 expression and the development of prostate cancer 10, 11. There is also one study showing the involvement of the TRPC6 channels in the proliferation of epithelial human prostate cancer cells in primary culture 7. Our results clearly showed that only low levels of TRPC6 were detected in BPH, whereas, in contrast, the prostate cancer specimens revealed a significant overexpression of TRPC6. Moreover, TRPC6 was expressed at the mRNA and protein levels in the DU145, PC3 and 22Rv1 prostate cancer cell lines. No earlier studies have compared the actual expression of TRPC6 protein with tumour stages, grades and androgen responsiveness. Our research showed that higher-stage prostate cancer tended to have increased TRPC6 protein staining, although the difference was not significant among stage T2, T3 and T4 cancers. In addition, the difference in TRPC6 expression between AI tumours and AD tumours was not statistically significant. We also illustrated that the increased staining seen in malignant samples was associated with the histological grade, Gleason score and extraprostatic extension status of the cancer. Fixemer et al. 3 previously found that TRPV6 is downregulated in AI tumours undergoing androgen deprivation, and that, although androgen-sensitive LNCaP expresses detectable levels of TRPV6, the androgen-insensitive PC3 and DU145 cell lines do not, suggesting that TRPV6 expression may be AD. Some studies show that TRPC6 is undetectable in the LNCaP cell line 12, 13. In our analysis of another androgen-sensitive cell line, 22Rv1, as well as of androgen-insensitive PC3 and DU145 cell lines, we found that TRPC6 mRNA and proteins were expressed in all three cell lines. The different androgen-sensitive cell lines might yield differential expression of TRPC6. Thus, we suggest that TRPC6 may be a novel target for therapeutic intervention against prostate cancer, especially in the case of AI prostate cancer, for which no current therapy is effective.
TRPC6 has been characterized as a Ca2+-selective cation channel. Plasma membrane Ca2+ channels are the key players in the regulation of numerous physiological cellular functions, and there is growing evidence that calcium signalling is also involved in cell proliferation and cell death. Some findings show that hepatocyte growth factor (HGF) upregulates TRPC6 expression in Huh-7 cells 9, and that HGF exploits TRPC6 to induce the growth and migration of renal tubular cells 14. Nakashiro et al. 15, 16 have found that HGF, produced by prostate-derived stromal cells, is a paracrine growth factor that stimulates the growth of AI prostate cancer cell lines. This group also suggests that progression from the AD to the AI phenotype is associated with an adaptive switch in support of mechanism from paracrine to autocrine. Some findings also show that HGF is elevated in the serum of patients with carcinoma of the prostate, and that this elevation is related to the stage of malignancy and is independent of age 17, 18. As such, we believe that HGF may induce the TRPC6 expression in prostate cancer, and that TRPC6 may be involved in the prostate cancer proliferation and phenotypic switch. Future studies are required to further elucidate the role of TRPC6 in prostate cancer progression in vivo and in vitro and to clarify the role of HGF in this process.
Acknowledgments
We thank James Martin Brauer for his assistance in manuscript preparation and revision.
References
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Ann Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fixemer T, Wissenbach U, Flockerzi V, Bonkhoff H. Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: a novel prognostic marker for tumor progression. Oncogene. 2003;22:7858–61. doi: 10.1038/sj.onc.1206895. [DOI] [PubMed] [Google Scholar]
- Lehen'kyi V, Flourakis M, Skryma R, Prevarskaya N. TRPV6 channel controls prostate cancer cell proliferation via Ca2+/NFAT-dependent pathways. Oncogene. 2007;26:7380–5. doi: 10.1038/sj.onc.1210545. [DOI] [PubMed] [Google Scholar]
- Zhang L, Barritt GJ. TRPM8 in prostate cancer cells: a potential diagnostic and prognostic marker with a secretory function. Endocr Relat Cancer. 2006;13:27–38. doi: 10.1677/erc.1.01093. [DOI] [PubMed] [Google Scholar]
- Yang ZH, Wang XH, Wang HP, Hu LQ. Effects of TRPM8 on the proliferation and motility of prostate cancer PC-3 cells. Asian J Androl. 2009;11:157–65. doi: 10.1038/aja.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thebault S, Flourakis M, Vanoverberghe K, Vandermoere F, Roudbaraki M, et al. Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Cancer Res. 2006;66:2038–47. doi: 10.1158/0008-5472.CAN-05-0376. [DOI] [PubMed] [Google Scholar]
- Guilbert A, Dhennin-Duthille I, Hiani YE, Haren N, Khorsi H, et al. Expression of TRPC6 channels in human epithelial breast cancer cells. BMC Cancer. 2008;8:125. doi: 10.1186/1471-2407-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Boustany C, Bidaux G, Enfissi A, Delcourt P, Prevarskaya N, et al. Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology. 2008;47:2068–77. doi: 10.1002/hep.22263. [DOI] [PubMed] [Google Scholar]
- Bodding M, Wissenbach U, Flockerzi V. Characterisation of TRPM8 as a pharmacophore receptor. Cell Calcium. 2007;42:618–28. doi: 10.1016/j.ceca.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Prevarskaya N, Zhang L, Barritt G. TRP channels in cancer. Biochim Biophys Acta. 2007;1772:937–46. doi: 10.1016/j.bbadis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Yang SL, Cao Q, Zhou KC, Feng YJ, Wang YZ. Transient receptor potential channel C3 contributes to the progression of human ovarian cancer. Oncogene. 2009;28:1320–8. doi: 10.1038/onc.2008.475. [DOI] [PubMed] [Google Scholar]
- Thebault S, Roudbaraki M, Sydorenko V, Shuba Y, Lemonnier L, et al. Alpha1-adrenergic receptors activate Ca2+-permeable cationic channels in prostate cancer epithelial cells. J Clin Invest. 2003;111:1691–701. doi: 10.1172/JCI16293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampino T, Gregorini M, Guidetti C, Broggini M, Marchini S, et al. KCNA1 and TRPC6 ion channels and NHE1 exchanger operate the biological outcome of HGF/scatter factor in renal tubular cells. Growth Factors. 2007;25:382–91. doi: 10.1080/08977190801892184. [DOI] [PubMed] [Google Scholar]
- Nakashiro K, Hara S, Shinohara Y, Oyasu M, Kawamata H, et al. Phenotypic switch from paracrine to autocrine role of hepatocyte growth factor in an androgen-independent human prostatic carcinoma cell line, CWR22R. Am J Pathol. 2004;165:533–40. doi: 10.1016/s0002-9440(10)63318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiro K, Okamoto M, Hayashi Y, Oyasu R. Hepatocyte growth factor secreted by prostate-derived stromal cells stimulates growth of androgen-independent human prostatic carcinoma cells. Am J Pathol. 2000;157:795–803. doi: 10.1016/s0002-9440(10)64593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem M, Essam T. Hepatocyte growth factor as a tumor marker in the serum of patients with prostate cancer. J Egypt Natl Canc Inst. 2005;17:114–20. [PubMed] [Google Scholar]
- Yasuda K, Nagakawa O, Akashi T, Fujiuchi Y, Koizumi K, et al. Serum active hepatocyte growth factor (AHGF) in benign prostatic disease and prostate cancer. Prostate. 2009;69:346–51. doi: 10.1002/pros.20890. [DOI] [PubMed] [Google Scholar]


