Abstract
The hypothesis that addition and removal of cryoprotectants to and from spermatozoa would initiate regulatory volume decrease, and lead to osmolyte loss and reduced sperm function, was tested. Common cryoprotectants, in the absence of freezing and thawing, affected bovine ejaculated spermatozoa by lowering their total and progressive motility in medium, reducing their migration through surrogate cervical mucus, damaging sperm head membranes and inducing sperm tail coiling. Sperm function was slightly better maintained after cryoprotectants were added and removed in multiple small steps rather than in a single step. The intracellular content of the polyol osmolytes, D-sorbitol and myo-inositol, exceeded that of the zwitterion osmolytes, L-carnitine and L-glutamate. Certain cryoprotectants reduced intracellular L-carnitine and L-glutamate concentration but not that of myo-inositol or D-sorbitol. Multistep treatments with some cryoprotectants had advantages over one-step treatments in mucus penetration depending on the original amount of intracellular carnitine and glutamate in the spermatozoa. Overall, sperm quality was best maintained by multistep treatment with glycerol and propanediols that were associated with decreased intracellular glutamate concentration. Bovine spermatozoa seem to use glutamate to regulate cryoprotectant-induced cell swelling.
Keywords: cryoconservation, cryoprotectants, osmotic swelling, regulatory volume decrease
Introduction
Cryoconservation of semen samples is widespread, because it is successful for many species, but for others, problems of cryoinjury make it inapplicable. Attempts to improve cryopreservation procedures have included changing thawing and freezing rates, altering the nature and type of cryoprotective agents (CPAs), modifying the rates and temperatures at which CPAs are added to and removed from the spermatozoa and using vitrification in the absence of cryoprotectants 1.
Until now, emphasis has been on the membrane damage that occurs to cryopreserved cells, but recent observations of a relationship between sperm volume regulation and freezability of cells 2, 3 suggest that other factors may be involved. Before freezing, the volume of spermatozoa initially decreases in face of the high extracellular CPA concentration and then increases when the penetrating protectant enters the cell, causing water influx. After thawing, when the CPAs are removed by dilution, decreasing extracellular osmolality again causes water influx 4. Such swelling would initiate the process of regulatory volume decrease (RVD), in which osmolyte loss through membrane channels drives removal of intracellular water and returns the cell to its original volume 5. The principles and mechanisms whereby spermatozoa achieve this have recently been presented in detail 6. The osmosensitivity of spermatozoa and their inability to regulate their volume are factors related to infertility in certain transgenic mice 7, 8 and lowered fertility rates in domestic species 9, 10, 11, 12.
Several organic osmolytes are involved in volume regulation of somatic cells 4 and many of them (for example, myo-inositol, D-sorbitol, D-glutamate, L-carnitine and taurine) are found in high concentration in epididymal fluid 13. It has been postulated that the epididymis provides these osmolytes to maturing spermatozoa to enable them to survive the osmotic challenges in the female tract 14, as incubation of spermatozoa in hypotonic media containing high concentrations of these osmolytes, which would prevent diffusional loss of intracellular osmolytes, can prevent RVD 15. Organic osmolyte efflux occurs through chloride channels 16, which have been found in spermatozoa from bulls, mice and men 17, 18, 19, 20.
It has also been hypothesized that too much RVD-related osmolyte loss before artificial insemination, such as that which may occur during cryopreservation, could compromise the function of cells post-thaw, because the osmolyte load of cytoplasm-sparse spermatozoa is limited 21. Two lines of evidence support this view. First, spermatozoa that withstand freezing poorly are less able to regulate volume when challenged with increasing hypotonicity than spermatozoa that survive freezing better 22. This could be due to a lower osmolyte content of spermatozoa from the species whose spermatozoa do not survive freezing well. Second, when subjected to addition and removal of several penetrating cryoprotectants, human sperm function is compromised in terms of migration through mucus and flagellar morphology, both of which could reflect volume regulation defects 23, 24, as the velocity of swollen spermatozoa through mucus decreases with the extent of swelling 25. Widiasih et al. 23 showed that multistep addition and removal of CPAs (to limit the extent of osmotic swelling at each step) influenced human sperm function to a lesser extent than a one-step procedure. The quality of post-thaw cryopreserved bovine semen is also improved if a multistep, rather than single-step, dilution is used, but in the study by Correa and Zavos 26, the rate of addition of CP was not altered.
The hypothesis that cell swelling, by activating RVD, reduces osmolyte levels to an extent that influences sperm function is examined here. To examine the hypotheses that (a) addition and removal of CPAs alone is associated with osmolyte loss from spermatozoa and (b) multistep addition is less deleterious than the single-step procedure, multi- and single-step addition and removal of CPAs were carried out on bovine ejaculated spermatozoa and the intracellular organic osmolytes were measured. The effects of CPA treatment on cell volume were monitored indirectly by changes in sperm motility, migration though mucus and flagellar morphology.
Materials and methods
Reagents and media
All chemicals were purchased from Sigma-Aldrich (Tiefenbach, Germany) unless stated otherwise. The CPAs used were glycerol (GLY), 1,1,1-tris(hydroxymethyl)ethane [2-hydroxymethyl-2-methyl-propane-1,3-diol] (THE), 1,1,1-tris(hydroxymethyl)propane [2-ethyl-2-hydroxymethyl-propane-1,3-diol] (THP), ethylene glycol (EG), propane-1,2-diol (PD2), propane-1,3-diol (PD3) and dimethylsulphoxide (DMSO). Each CPA was dissolved in water to a concentration of 2 mol L−1. Media used for the dilution and removal of CPA were modified from Biggers–Whittem–Whittingham medium (BWW 27) containing 20 mmol L−1 Hepes ([4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid]) buffer at pH 7.4 in addition to the bicarbonate buffer, with osmolality adjusted to 290 (BWW290) or 321 (BWW321) mmol kg−1 using NaCl; and bovine serum albumin 4 mg mL−1 was added before use.
Bovine ejaculates
Five bulls (Bos indicus) of the Ongole cross breed, more than 2 years old, obtained from local farmers in Yogyakarta, Indonesia, provided several ejaculates by use of a teaser female and an artificial vagina, and the samples in 15 mL tubes were transported to the laboratory. The osmolality of semen from this species is not known, but that of Bos taurus is 341 mmol kg−1 10. Undiluted semen (20 μL) was diluted in 400 μL BWW290 for initial motility assessment and 10 μL was further diluted in a 40-μL WHO fixative 28 for sperm concentration assessment in duplicate. Samples were diluted to 250 × 106 spermatozoa per mL in BWW290 for further processing with CPAs.
Treatment of semen with CPAs
Aliquots of 100 μL diluted semen were treated in three different ways: both addition and removal of CPA in multiple steps (Protocol A) and in a single-step (Protocol B). Restraints of the timed experiments dictated that only one control could be incorporated into the schedule; therefore, a medium containing no CPA was used in the single-step procedure as control (Protocol C). One CPA was tested in each experiment (one ejaculate) and the order of treatments A, B, C was altered when the same CPA was tested on different bulls on different days.
In Protocol A, increasing volumes (14 μL, 19 μL, 27 μL and 40 μL) of 2 mol L−1 CPA were added sequentially at 30 s intervals to 100 μL semen so that there was an increase of 250 mmol L−1 at each step, to provide 200 μL of suspension with a final concentration of 1 mol L−1 CPA. After 30 min incubation at room temperature (RT), the CPA was removed in multiple steps by the sequential addition of 28 μL, 38 μL, 54 μL, 80 μL, 140 μL, 270 μL and 800 μL BWW290 at 30 s intervals up to a total of 1 610 μL BWW290 and 124 mmol L−1 CPA. The sample was then centrifuged for 6 min at 400 × g to pellet the spermatozoa and 1 550 μL supernatant was removed. Spermatozoa were resuspended in the remaining 60 μL of BWW290, and BWW290 was further added in sequential steps of 20 μL, 40 μL and 120 μL at 30 s intervals, so that the final sperm suspension contained 31 mmol L−1 CPA in 240 μL of BWW290 and the final osmolality was 321 mmol kg−1.
In Protocol B, 100 μL of 2 mol L−1 CPA was added to 100 μL semen in one step and after 30 min incubation 1 410 μL of BWW290 was added in one step to dilute the CPA. After centrifugation (400 × g, 6 min) and removal of 1 550 μL supernatant, 180 μL BWW290 was added in one step to the remaining 60 μL sperm suspension (final osmolality was 321 mmol kg−1). In the control Protocol C, 100 μL ejaculate was processed as in B, except that CPA and BWW290 at each step were replaced by 100 μL BWW321 (final osmolality was 321 mmol kg−1).
At the end of the CPA treatment, spermatozoa were assessed for their percentage motility, progressive motility, kinematics, viability, mucus penetration ability, cell swelling status and osmolyte content, as described below.
Motility assessment and analysis of computer-aided kinematic parameters
Spermatozoa were assessed (2 × 100 cells) for their percentage total motility and percentage progressive motility in a 20-μm deep chamber (7 μL suspension under an 18 × 18 mm coverslip) at ambient temperature (28–29°C) with a × 40 phase objective (Olympus BX-51 microscope). The mean of the duplicate results was calculated. For computerized analysis of kinematics, video-recordings were made with pseudo-dark-field optics (×10 phase objective with a Ph3 phase ring). In total, 20 different fields were recorded for 3 s each and the kinematic parameters of at least 200 motile spermatozoa were tracked for 1 s at 25 Hz. The parameters, including averaged path velocity (VAP), straight line velocity (VSL), curvilinear velocity (VCL), amplitude of lateral head displacement, linearity (LIN = 100% × VSL/VCL) and straightness (STR = 100% × VAP/VCL), were analysed with a computer-aided sperm analysis (CASA) system (Hamilton-Thorne IVOS version 10.8), as previously described 24.
Sperm head membrane permeability and sperm tail coiling
A total of 10 μL of the treated sperm suspension was mixed with 10 μL of eosin–nigrosin suspension 28 for 30 s, then smeared, dried and mounted for analysis by bright-field optics at × 400 magnification. A 50-μL aliquot of treated sperm suspension was fixed in 500-μL WHO fixative 28 for 30 min, and spermatozoa were recovered by centrifugation at 600 × g for 5 min and removal of supernatant. A total of 200 spermatozoa in the loosened sperm pellet (5–10 μL) were examined at × 400 magnification for calculating the percentage of straight, coiled and stump (short, apparently folded back) tails.
Mucus penetration test
The irreproducible consistency of bovine cervical mucus 29 makes the use of readily available, uniform and stable macromolecular surrogates attractive. Surrogate mucus for the penetration test was prepared freshly for each experiment by dissolving hyaluronic acid (Sigma H5300 from rooster comb) in BWW290 at a concentration of 6 mg mL−1 and equilibrated for at least 15 min to allow the escape of air bubbles before the mucus columns were prepared. Mucus columns about 8 cm long were prepared by aspirating bubble-free surrogate mucus (see above) into flat capillary tubes (10 cm long, 3 mm deep; Camlab, Cambridge, UK). After sealing 5 mm of the dry end of the capillary with modelling clay and marking the meniscus of the mucus, the column was left standing vertically in 50 μL mucus to prevent drying out of the open end, to permit any remaining fine air bubbles to be lost and to check the tightness of the seal, for at least 15 min before use. The mucus-filled capillary was placed in 100 μL of treated sperm suspension in a 1.5-mL Eppendorf tube and held horizontally in a moist chamber at RT for 2 h. The total number of spermatozoa present in the whole microscopic field focused through the depth of the slide (× 10 phase objective and × 10 eyepiece) at 1 and 3 cm of the column was counted, up to a maximum of 200.
Osmolyte and cytoplasmic marker measurements
Identical CPA treatments in multiple (Protocol A) and single (Protocol B) steps were run in parallel with those described above for functional studies, except that 300-μL aliquots of diluted semen were used and all volumes of CPA added or removed were adjusted by the same proportion. To obtain sufficient spermatozoa for analysis, duplicate tubes were used for Protocols A, B and C, and at the end of treatment, the duplicate sperm suspensions were pooled. They were layered onto 5 mL of 6% (w/v) Ficoll in BWW321 and centrifuged at 900 g for 20 min to separate spermatozoa from the medium. Sperm pellets were stored for 3–9 months at −20°C and shipped on dry ice to Germany for analysis.
Frozen samples were thawed in 250 μL of 10 mmol L−1 Tris buffer, pH 7.0, mixed by pipetting and vortexing, and transferred to a 1.5-mL Eppendorf tube. Tube and pipette tips were rinsed with 250 μL of buffer to ensure complete transfer of samples. Samples were sonicated thrice for 3 s on ice at 25 W and at an amplitude setting of 30 in a VibraCell sonicator (Sonics and Materials Inc, Zinsser Analytic, Frankfurt, Germany) fitted with a 1.5-mm tip. Samples were centrifuged at 20 000 × g for 10 min at 4°C and the supernatant was stored at −20°C before assay.
The intracellular osmolyte content was determined by end-point fluorimetric assays performed in 96-well plates, as previously described 30, 31 with top standards and sensitivities, respectively, of (μmol L−1): glutamate 20, 0.09; carnitine 500, 14; myo-inositol 600, 11; sorbitol 600, 20. Top standards and assay volumes were adjusted for bovine sperm extracts to ensure that sample results fitted on the linear part of the standard curves. Protein was determined by dye binding from standard curves (to 2 mg mL−1; sensitivity, 0.05 mg mL−1) of bovine serum albumin (BSA) in the Lowry Assay (Bio Rad Laboratories GmbH, Munich, Germany).
As some spermatozoa might have been lost with the supernatants after centrifugation during CPA removal and the Ficoll wash, the number of spermatozoa in the final pellet was not calculated from the initial sperm concentration and volumes. Given that sperm membranes damaged during experimentation could permit loss of cytoplasm (together with its contained osmolytes), but the damaged spermatozoa would still contribute to the extracted proteins, a marker of sperm cytoplasm, glucose-6-phosphate dehydrogenase 32, rather than protein, was measured to reflect the number of intact spermatozoa at the end of treatment.
A fluorimetric kinetic assay for glucose-6-phosphate dehydrogenase was developed, in which glucose-6-phosphate is converted by glucose-6-phosphate dehydrogenase in the sample to 6-phosphogluconate whereby nicotinamide adenine dinucleotide (NAD+) is converted to NADH+; NADH+ oxidase (with flavin adenine dinucleotide (FAD)) reoxidizes NADH+ to NAD+ converting oxygen to H2O2 and finally, Amplex red accepts H2O2 through horseradish peroxidase to produce resorufin, which can be quantified by excitation at 560 nm and emission at 587 nm. Experimentally, 50 μL samples in 30 mmol L−1 KH2PO4 buffer, pH 7.5, containing 0.1% (w/v) BSA, were incubated in 96-well plates with 140 μL reagent containing glucose-6-phosphate (4 mmol L−1), NAD+ (3.33 mmol L−1), NADH+ oxidase (13 mU mL−1), FAD (200 μmol L−1), Amplex red (10 μmol L−1) and horseradish peroxidase (20 mU ml−1), and the readings were taken every 40 s for 10 min. Standard enzyme (up to 20 U mL−1; sensitivity, 1.5 U mL−1) and buffer blanks were included in each plate. Samples were read at 30°C every 40 s for 10 min, and the rate of increase in product compared with the activity (U) of the standard enzyme samples. All measurements were made in a SpectroMax GeminiXS spectrofluorimeter (MDS Analytical Technologies GmbH, Ismaning, Germany).
Data handling and statistics
To eliminate basal differences between ejaculates, the values for Protocols A and B in each experiment were expressed either as a ratio of the control values (A/C, B/C: most values) or as differences from controls (A – C, B – C: percentage of coiled spermatozoa and stump tails) in the same experiment for each parameter before statistical analysis. To compare Protocols A and B, the A/B ratios were calculated.
For multiple comparisons among different CPAs against controls, one-way ANOVA (analysis of variance) was used for data analysis of parameters whose distribution passed the normality and equal variance tests; otherwise, the non-parametric Kruskall–Wallis ANOVA on ranks test was used. A P-value < 0.05 was used to indicate statistical significance; the Holm–Sidak method was used for post hoc comparisons against the control for normally distributed data and Dunn's method when the normality test failed. Linear regression analysis was used for correlation between different osmolytes, and to determine the dependence of sperm migration and motility against intracellular osmolyte levels. SigmaStat (version 3.5) and SigmaPlot (version 10) (Systat Software Inc, Erkrath, Germany) were used for statistical analysis and graphical presentation.
Results
Changes in sperm motility and migration
When expressed as a proportion of the control values (Protocol C), all CPAs reduced total motility in either Protocol A (multistep) or B (single step); most CPAs reduced motility in both Protocols A and B. Similarly, all CPAs, with the exception of PD3, reduced the progressive sperm motility in Protocols A or B. The total number of spermatozoa arriving at the 1- and 3-cm marks within surrogate mucus were significantly reduced when either protocol was applied with DMSO and THP, whereas EG affected only spermatozoa reaching up to 3 cm; for GLY and THE Protocol B reduced sperm migration at both distances (Table 1).
Table 1. Function and organic osmolyte content of bovine ejaculated spermatozoa processed without cryoprotectants (Protocol C control) and changes relative to the controls, after adding and removing the CPAs listed in Protocols A (multistep) or B (single step).
| CPA | Control (n= 35) | DMSO |
EG |
THP |
GLY |
PD2 |
PD3 |
THE |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protocol | C | A | B | A | B | A | B | A | B | A | B | A | B | A | B | |
| Mean ± SEM. | ||||||||||||||||
| Mean percentages of internal controls (Protocol C: n = 5) | ||||||||||||||||
| Carnitine (nmol) | per U G6PDH | 18.2 ± 2.3 | 67.1 | 102.1 | 76.8 | 94.5 | 15.4* | 21.5* | 99.8 | 99.8 | 36.4 | 42.2 | 41.8 | 45.4 | 48.4 | 53.8 |
| Glutamate (nmol) | per U G6PDH | 8.1 ± 0.8 | 76.7 | 115.9 | 105.3 | 119.8 | 55.5 | 40.4 | 80.7* | 100 | 49.2* | 58.3* | 90.9* | 98.7 | 92.9 | 94.8 |
| Inositol (nmol) | per U G6PDH | 24.0 ± 1.9 | 101.4 | 100.6 | 102.6 | 81.0 | 69.2 | 80.5 | 119.7 | 99.2 | 76.9 | 102.1 | 101.2 | 119.5 | 110.4 | 74.9 |
| Sorbitol (nmol) | per U G6PDH | 23.1 ± 1.6 | 81.3 | 93.5 | 106.9 | 109.6 | 90.1 | 73.7 | 128.9* | 106.6 | 85.5 | 105.3 | 92.5 | 104.2 | 77.0 | 76.8 |
| Total (nmol) | per U G6PDH | 73.4 ± 4.6 | 101.1 | 120 | 96.2 | 94.1 | 44.8* | 45.2* | 96.0 | 84.6 | 86.0 | 107.6 | 72.0 | 82.0 | 78.1 | 69.2 |
| Protein (mg) | per U G6PDH | 0.066 ± 0.004 | 123 | 141 | 134 | 121 | 140 | 160 | 109 | 127 | 97 | 83 | 111 | 105 | 136 | 194 |
| Sperm motility | Total (%) | 72.2 ± 1.4 | 62.1* | 68.0 | 64.2* | 75.1* | 26.0* | 15.7* | 77.3* | 62.6* | 62.4* | 67.1* | 70.8* | 79.7* | 38.6* | 21.0* |
| Progressive (%) | 66.9 ± 2.4 | 31.5* | 29.7* | 29.8* | 34.3* | 53.2 | 11.8* | 33.0* | 36.8* | 41.2* | 48.4* | 54.5 | 55.8 | 22.9* | 50.0 | |
| Sperm migration | 1 cm (N) | 105.3 ± 16.1 | 36.5* | 31.0* | 32.7 | 28.9 | 15.2* | 14.1* | 55.1* | 25.8* | 63.8 | 54.8 | 49.9 | 42.6 | 18.9 | 13.1* |
| 3 cm (N) | 34.8 ± 6.4 | 23.4* | 16.9* | 14.5* | 4.0* | 1.3* | 8.0* | 32.5 | 10.0* | 40.7 | 29.1 | 9.6* | 9.5* | 16.3 | 3.4* | |
| CASA | VAP (μm s−1) | 78.6 ± 3.2 | 51.3 | 40.3* | 51.4 | 44.7* | 32.9* | 22.7* | 63.8 | 56.9* | 72.0 | 66.7* | 75.5 | 66.0* | 40.1* | 25.8* |
| VSL (μm s−1) | 68.0 ± 3.4 | 49.4 | 36.7* | 39.9 | 35.0* | 24.3* | 17.5* | 63.6* | 44.1* | 73.1 | 63.3* | 64.7 | 53.8* | 40.4 | 28.0* | |
| VCL (μm s−1) | 114.7 ± 4.2 | 45.8 | 42.7* | 44.8* | 62.7 | 54.5 | 32.3 | 79.3 | 83.1 | 68.3* | 68.4 | 64.9* | 66.8* | 48.6* | 25.9* | |
| LIN (%) | 59.3 ± 2.3 | 110.6 | 93.1 | 82.1 | 65.6 | 34.2* | 36.5* | 82.4 | 60.1 | 117.3 | 98.4 | 100.2 | 80.7* | 59.0* | 38.7* | |
| Sperm Tail | Straight (%) | 92.7 ± 1.9 | 50.3* | 51.9* | 63.3* | 54.0* | 74.8* | 89.3 | 39.1* | 52.9* | 68.0 | 44.2* | 60.4* | 62.4* | 79.6 | 78.9 |
| Sperm Head | Eosin-neg. (%) | 67.8 ± 3.4 | 77.2* | 88.7 | 69.1 | 70.3* | 31.1* | 16.2* | 83.3 | 73.4* | 58.1* | 62.5* | 63.6* | 71.1 | 44.6* | 28.9* |
| Mean absolute differences from internal controls (Protocol C: n = 5) | ||||||||||||||||
| Sperm tail | Coiled (%) | 4.9 ± 1.6 | 47.5* | 45.6* | 35.9* | 42.4* | 24.6 | 12.5 | 59.4* | 45.9* | 41.6 | 60.4* | 43.8* | 43.3* | 24.9* | 26.1* |
| Stump-tail (%) | 2.0 ± 0.8 | 5.3 | 5.2 | 3.7 | 3.6 | 3.4 | 3.1 | 4.2 | 4.4 | 1.40 | 1.90 | 1.30 | 3.2 | 5.30 | 5.1 | |
| Number of unchanged parameters | 4 | 3 | 5 | 3 | 3 | 2 | 6 | 2 | 7 | 4 | 5 | 3 | 4 | 2 | ||
A, multistep addition and removal of CPA; B single-step addition and removal of CPA; C, control: single-step addition and removal of basal medium alone; CASA, computer-aided sperm analysis; CPA, cryoprotective agent; DMSO, dimethyl sulphoxide; EG, ethylene glycol; THP, 1,1,1-Tris(hydroxymethyl)propane; G6PDH, glucose-6-phosphate dehydrogenase; GLY glycerol; LIN, linearity; PD2, propane-1, 2-diol; PD3, propane-1, 3-diol; THE, 1,1,1-Tris(hydroxymethyl)ethane; VAP, averaged path velocity; VCL, curvilinear velocity; VSL, straight line velocity;
significantly different from C. Treatments EG (A), GLY (A), PD2 (A, B), PD3 (A) are best for maintenance of functional parameters (fewest significant differences from control and better sperm quality).
The single-step protocol (B), but not the multistep protocol (A), significantly decreased VAP and VSL compared with controls for four CPAs (DMSO, EG, PD2 and PD3; Table 1), THP and THE reduced VAP in both protocols, whereas THP and GLY affected VSL in both Protocols A and B, but THE affected only VSL in Protocol B. VCL was affected differently: THE and PD3 were effective at reducing VCL in Protocols A and B, PD2 and EG were effective in Protocol A, whereas DMSO affected VCL only in Protocol B. LIN was reduced by THP and THE in both protocols and by PD3 in Protocol B (Table 1).
Changes in sperm vitality and tail morphology
All seven CPAs in Protocols A and B increased head staining by eosin relative to controls (THP, PD2 and THE in both Protocols A and B; EG and GLY in Protocol B; and DMSO and PD3 in Protocol A; Table 1). The most drastic effect was found with THP, which reduced the percentage of eosin-free spermatozoa to between 31% and 16%. The percentage of straight tails was reduced by DMSO, EG, GLY and PD3 in both protocols, and by THP and PD2 only in Protocols A and B, respectively. Conversely, the percentage of coiled tails relative to controls was increased by DMSO, EG, GLY, PD3 and THE in both Protocols A and B, and by PD2 only in Protocol B. Stump tails were few, and were little affected by CPA treatment.
From the sperm parameters listed in Table 1, with the exception of the stump-tail that did not show any changes with any of the CPAs, it may be inferred that CPAs differed in their ability to maintain sperm function, as judged from the number of parameters that were unchanged from the controls. For each CPA, the number of unchanged parameters was higher for Protocol A than for Protocol B. Among all CPAs, the treatments that could best maintain sperm quality and attain a higher number of unchanged parameters were PD2 (seven parameters), GLY (six) and PD3 and EG (five) (Table 1).
Relationships between intracellular osmolytes
Irrespective of the bull or the CPA treatment, there were high-positive linear relationships between the cellular content of carnitine and glutamate, and between myo-inositol and sorbitol (Figure 1), and there was more sorbitol and inositol than glutamate and carnitine (Figure 2). The content of the cyclic polyol inositol (median 22.9 nmol U−1 G6PDH) was no different from that of the linear polyol sorbitol (20.5 nmol U−1), but both were significantly higher than intracellular contents of the zwitterions, carnitine (9.8 nmol U−1) and glutamate (5.8 nmol U−1), which differed significantly from each other. Overall, there was no significant difference in the total organic osmolytes between controls (Protocol C: 73.4 ± 4.6 nmol U−1) and Protocols A (60.2 ± 4.2 nmol U−1) and B (63.0 ± 4.3 nmol U−1). When results from all CPA treatments (Protocols A and B) were pooled, there was a significantly lower total osmolyte content (61.7 ± 3.0 nmol U−1) than in the controls (73.4 ± 4.6 nmol U−1), reflecting the significant decrease in carnitine (see Figure 2).
Figure 1.
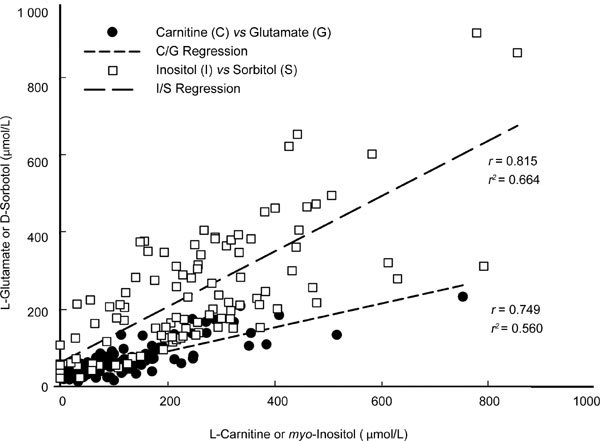
Relationships between the concentrations of L-carnitine, myo-inositol, L-glutamate and D-sorbitol in sperm extract after treatments will Protocols A, B and C. Note the positive linear relationship between the zwitterions, carnitine and glutamate, and between the polyols, inositol and sorbitol.
Figure 2.
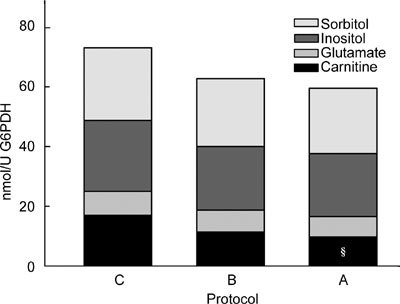
Total organic osmolyte content (L-carnitine + L-glutamate + myo-inositol + D-sorbitol) expressed per unit glucose-6-phosphate dehydrogenase (G6PDH) activity in spermatozoa treated by control Protocol C (C), A (A) and B (B), each partitioned into the percentages occupied by sorbitol (□), inositol (□), glutamate (□), carnitine (□). §, Proportion of carnitine significantly lower than that in Protocol C.
Among the individual osmolytes, the content of carnitine was significantly lower in Protocols A (9.4 ± 1.2 nmol U−1) and B (11.5 ± 1.3 nmol U−1) than in the controls (Protocol C: 18.2 ± 2.3 nmol U−1). Similarly, the proportions of each osmolyte in the control samples, myo-inositol (35%), sorbitol (32%) and glutamate (10%), were little affected by CPA treatment; only the relative amount of carnitine declined significantly from 22% to 12% with Protocol A (pooled from all CPAs).
Among the CPAs tested, the amount of intracellular carnitine per U G6PDH was significantly reduced in both Protocols A and B by THP and the glutamate content per U G6PDH was reduced in both protocols by PD2, but only in Protocol A by both GLY and PD3. No decreases in intracellular myo-inositol or sorbitol were found on using any CPA in either Protocol.
Total osmolytes measured per U G6PDH were only significantly reduced by THP in both protocols, reflecting more the depletion of carnitine than glutamate (Table 1). However, this decrease in carnitine was only due to THP, which seemed to be toxic on the basis of the severely increased percentages of eosin-stained heads and decreased percentages of motile cells (Table 1).
On the other hand, glutamate was the osmolyte that was depleted after CPA treatments and that caused least damage to sperm quality (namely, in Protocol A using GLY, PD2 and PD3).
Correlation of sperm function with intracellular osmolytes
The 'initial' intracellular content of both carnitine and glutamate (per U G6PDH), taken as that measured after subjecting the semen samples to control Procotol C, was related to the relative efficacy of the multistep treatment A in maintaining migration of spermatozoa through surrogate mucus better than that of the single-step treatment B. When EG was used as the CPA, the migration within mucus up to 3 cm was greater in Protocol A than in B when the cells initially had more carnitine (Figure 3A) or glutamate (Figure 3B). Similarly, when PD2 was used as the CPA, there were more spermatozoa migrating up to 1 cm within mucus when they had been subjected to Protocol A than to Protocol B, when more carnitine (Figure 3C) and glutamate (Figure 3D) were initially present in the cells.
Figure 3.
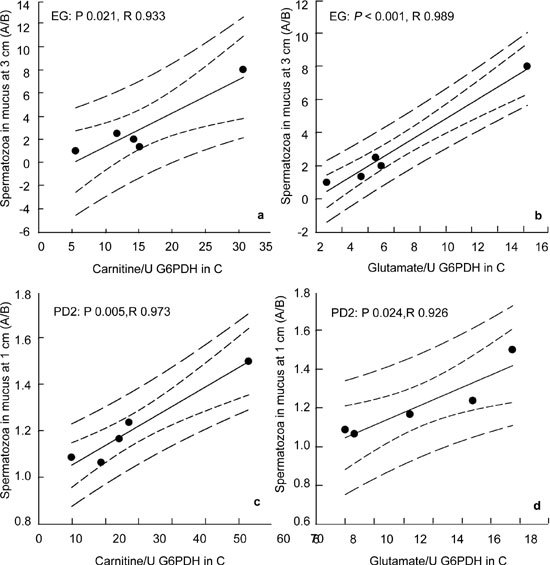
Relationship between sperm content (per U G6PDH activity) of L-carnitine (A, C) and L-glutamate (B, D) of the control sample (Protocol c: abscissa) and the relative effectiveness of Protocol A over Protocol B (A/B ratio of sperm numbers, ordinate) in maintaining sperm migration into surrogate mucus at the 3 cm mark (A, B) and the 1 cm mark (C, D) after addition and removal of ethylene glycol (EG: A, B) and propane-1, 2-diol (PD2: C, D).
Discussion
In 1984, Philips and Kalay 33 observed that a major component of cryoprotectants, glycerol, causes flagellar angulation at the site of the cytoplasmic droplet and induces backwards swimming in bovine spermatozoa. That this occurred even before freezing and thawing was indicative of another, so far unappreciated, action of the polyol. As bovine flagellar angulation can be caused by osmotic swelling 34, 35, it has been suggested that the highly permeant glycerol induces cell swelling that is countered by RVD through loss of intracellular osmolytes 22. In this study, this hypothesis was tested by observing cell function and measuring intracellular osmolytes in bovine spermatozoa that were subjected to a range of cryoprotectants applied in two protocols aimed at providing two different extents of volume change.
Each of the seven CPAs tested in this study affected sperm function after they had been added to, incubated with and removed from bovine ejaculated spermatozoa. Whether the absolute values of functional parameters, or the relative changes from the controls were examined, decreases in sperm motility (total and progressive motility, kinematic parameters and migration through mucus) and increases in damage to sperm head membranes (eosin-stained cells) were observed. The incidence of sperm tail coiling was increased, confirming the published effects of glycerol and extending them to several other permeating cryoprotectants. As the cells were not frozen and thawed, these effects are solely because of the CPAs themselves. Furthermore, because sperm tail coiling is indicative of cell swelling, it may result from inadequate volume regulation caused by insufficient intracellular osmolytes.
Although not markedly obvious, Protocol A was apparently superior to Protocol B with regard to maintenance of sperm function, as it was associated with more parameters unchanged from the controls, and certain CPAs exhibited less damage in Protocol A than in B (PD2 (for seven parameters) > GLY (for six) > PD3 (for five) = EG (for five)
The organic osmolytes myo-inositol, L-glutamate, L-carnitine and D-sorbitol were measurable in the ejaculated spermatozoa and there was more of the uncharged hexitols, inositol and sorbitol (accounting for two thirds of the total osmolytes measured), than the zwitterions, carnitine and glutamate. After carrying out the sperm processing necessary for normal cryoypreservation procedure (addition, incubation, removal of CPA and centrifugation), the cellular content of the sum of the four osmolytes measured was significantly reduced when all CPAs were considered, although statistical significance could only be achieved for some CPAs (GLY, PD2, PD3) and only for carnitine and glutamate, when considered individually. The decrease in osmolyte load was substantial, about one-third, and in cases in which osmolyte loss was observed, there was a selective loss of carnitine as a proportion of the total osmolyte content and significant loss of glutamate from intact cells. Kulkarni et al. 36 demonstrated the loss of an inorganic ion, K+, from bovine spermatozoa undergoing RVD in hypotonic medium in the absence of cryoprotectants.
The osmolyte load was expressed per U G6PDH and a decrease in this was considered to indicate a specific loss of osmolyte, unrelated to the bulk loss of cellular cytoplasm, possibly lost through channels related to RVD. However, there was little correlation between the intracellular contents of the organic osmolytes measured and the sperm functions monitored. The greatest loss of carnitine was caused by THP, which seemed to be toxic to the cells, as judged by reduced sperm motility and migration, VSL and high eosin stainability, and lower cell coiling compared with other CPAs.
On the other hand, glutamate was significantly reduced in those treatments (PD2, GLY and PD3 in Protocol A) that maintained sperm function better. The fact that the intracellular content of glutamate was the lowest of the four osmolytes measured, suggests that only for this osmolyte were the osmotic stresses provided sufficient to cause measurable loss of intracellular content. Therefore, for bovine spermatozoa, glutamate is more likely than carnitine, myo-inositol and sorbitol, to be involved in efflux for CPA-induced volume regulation. For the infertile c-ros KO mice, whose caudal spermatozoa express flagellar angulation and increased cell volume in the female tract 7, 8, the glutamate content of the caudal spermatozoa is also reduced 37.
Despite the consistent differences between the glutamate content of control and CPA-treated spermatozoa for GLY, PD2 and PD3 in Protocol A, there was no consistent difference between the osmolyte content of spermatozoa subjected to Protocol A and Protocol B, in which differences would be anticipated from the hypothesis that the multistep technique brings less osmotic challenge to the cells. This may reflect the fact that the total driving force for osmolyte loss depends on the total osmotic challenge, which was the same for both protocols. Nevertheless, treatments that could best maintain sperm quality were found on using the multistep Protocol A, in which a reduction in osmolytes was observed. This shows that the multistep technique was less damaging to the cells than the single-step procedure.
This osmolyte loss may be a demonstration of RVD that helps to maintain sperm function in this Protocol. In somatic cells, the nature of the osmolyte expended during RVD depends on the extent of the osmotic challenge 38, 39. By comparing the relative efficacy of Protocol A over Protocol B on sperm migration through mucus, a positive correlation was found with the presumed initial osmolyte content of carnitine and glutamate (that is, in control cells, subjected to Procotol C, which should not have experienced an osmotic challenge) with both EG and PD2. For both CPAs, the cells migrated further when they originally contained more glutamate or carnitine, suggesting that these zwitterionic organic osmolytes can better protect the spermatozoa when the osmotic challenge is provided in small multiple steps than in a single large osmotic insult.
In conclusion, the mere addition and removal of permeating CPAs can damage bovine sperm cell morphology (inducing flagellar bending) and function (reducing motility and migration) in association with a reduction in the glutamate content. These observations are consistent with a cryoprotectant-induced reduction in osmolytes that renders volume regulation inadequate. Such compromised volume-regulating activity could impair the fertilizing capacity of those spermatozoa that survived the freeze-thawing process per se. If use of the CPAs promoting osmolyte efflux cannot be avoided, one way to overcome their effect would be to increase the osmolyte load of the cells before the cryopreservation process.
Acknowledgments
This work was funded by the Alexander von Humboldt Foundation, Grant No. DEU/1004684 and the Deutsche Forschungsgemeinschaft CO248/12–1.
References
- Leibo SP, Picton HM, Gosden RG., Cryopreservation of human spermatozoa. In: , Vayena E, Rowe PJ, Griffin PD.editors. Current Practices And Controversies In Assisted Reproduction Geneva: World Health Organization 2002152–65.
- Petrunkina AM, Gropper B, Gunzel-Apel AR, Töpfer-Petersen E. Functional significance of the cell volume for detecting sperm membrane changes and predicting freezability in dog semen. Reproduction. 2004;128:829–42. doi: 10.1530/rep.1.00296. [DOI] [PubMed] [Google Scholar]
- Petrunkina AM, Gropper B, Töpfer-Petersen E, Gunzel-Apel AR. Volume regulatory function and sperm membrane dynamics as parameters for evaluating cryoprotective efficiency of a freezing extender. Theriogenology. 2005;63:1390–406. doi: 10.1016/j.theriogenology.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Gao DY, Liu J, Liu C, McGann LE, Watson P, et al. Prevention of osmotic injury to human spermatozoa during addition and removal of glycerol. Hum Reprod. 1995;10:1109–122. doi: 10.1093/oxfordjournals.humrep.a136103. [DOI] [PubMed] [Google Scholar]
- Lang F. The diversity of volume regulatory mechanisms. Cell Physiol Biochem. 1998;8:1–45. doi: 10.1159/000016269. [DOI] [PubMed] [Google Scholar]
- Petrunkina AM. Fundamental aspects of gamete cryobiology. J Reproduktionsmed Endokrinol. 2007;4:78–91. [Google Scholar]
- Yeung CH, Sonnenberg-Riethmacher E, Cooper TG. Infertile spermatozoa of c-ros tyrosine kinase receptor knockout mice show flagellar angulation and maturational defects in cell volume regulatory mechanisms. Biol Reprod. 1999;61:1062–9. doi: 10.1095/biolreprod61.4.1062. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Anapolski M, Cooper TG. Measurement of volume changes in mouse spermatozoa using an electronic sizing analyser and a flow cytometer: validation and application to an infertile mouse model. J Androl. 2002;23:522–8. [PubMed] [Google Scholar]
- Leidinger H, Reiner G, Krapoth J, Dzapo V. Beziehungen zwischen Spermatozoenvolumen und Eberfruchtbarkeit. Arch Tierz Dummerstorf. 1998;41:65–73. [Google Scholar]
- Petrunkina AM, Petzoldt R, Stahlberg S, Pfeilsticker J, Beyerbach M, et al. Sperm-cell volumetric measurements as parameters in bull semen function evaluation: correlation with nonreturn rate. Andrologia. 2001;33:360–7. doi: 10.1046/j.1439-0272.2001.00457.x. [DOI] [PubMed] [Google Scholar]
- Khalil AA, Petrunkina AM, Sahin E, Waberski D, Toepfer-Petersen E. Enhanced binding of sperm with superior volume regulation to oviductal epithelium. J Androl. 2006;27:754–65. doi: 10.2164/jandrol.106.000232. [DOI] [PubMed] [Google Scholar]
- Druart X, Gatti JL, Huet S, Dacheux JL, Humblot P. Hypotonic resistance of boar spermatozoa: sperm subpopulations and relationship with epididymal maturation and fertility. Reproduction. 2009;137:205–13. doi: 10.1530/REP-08-0225. [DOI] [PubMed] [Google Scholar]
- Cooper TG.Epididymis. In: Neill JD, Knobil E, editors. Encyclopedia of Reproduction San Diego: Academic Press 1998. p1–17.
- Cooper TG, Yeung CH. Acquisition of volume regulatory response of sperm upon maturation in the epididymis and the role of the cytoplasmic droplet. Microsc Res Tech. 2003;61:28–38. doi: 10.1002/jemt.10314. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Barfield JP, Cooper TG. Physiological volume regulation by spermatozoa. Mol Cell Endocrinol. 2006;250:98–105. doi: 10.1016/j.mce.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Strange K, Jackson PS. Swelling-activated organic osmolyte efflux: a new role for anion channels. Kidney Int. 1995;48:994–1003. doi: 10.1038/ki.1995.381. [DOI] [PubMed] [Google Scholar]
- Petrunkina AM, Harrison RA, Ekhlasi-Hundrieser M, Töpfer-Petersen E. Role of volume-stimulated osmolyte and anion channels in volume regulation by mammalian sperm. Mol Hum Reprod. 2004;10:815–23. doi: 10.1093/molehr/gah106. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Barfield JP, Cooper TG. The role of anion channels and Ca2+ in addition to K+ channels in the physiological volume regulation of murine spermatozoa. Mol Reprod Dev. 2005;71:368–79. doi: 10.1002/mrd.20261. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Barfield JP, Cooper TG. Chloride channels in physiological volume regulation of human spermatozoa. Biol Reprod. 2005;73:1057–63. doi: 10.1095/biolreprod.105.044123. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Yeung CH. Involvement of potassium and chloride channels and other transporters in volume regulation by spermatozoa. Curr Pharm Des. 2007;13:3222–30. doi: 10.2174/138161207782341240. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Barfield JP. Utility of infertile male models for contraception and conservation. Mol Cell Endocrinol. 2006;250:206–11. doi: 10.1016/j.mce.2005.12.047. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Barfield JP, Yeung CH. The tonicity of murine epididymal spermatozoa and their permeability towards common cryoprotectants and epididymal osmolytes. Reproduction. 2008;153:625–33. doi: 10.1530/REP-07-0573. [DOI] [PubMed] [Google Scholar]
- Widiasih D, Yeung CH, Junaidi A, Cooper TG. Multi-step and single-step treatment of human sperm with cryoprotectants. Fertil Steril. 2009;92:382–9. doi: 10.1016/j.fertnstert.2008.05.046. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Cooper TG. Effects of the ion-channel blocker quinine on human sperm volume, kinematics and mucus penetration, and the involvement of potassium channels. Mol Hum Reprod. 2001;7:819–28. doi: 10.1093/molehr/7.9.819. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Anapolski M, Depenbusch M, Zitzmann M, Cooper TG. Human sperm volume regulation. Response to physiological changes in osmolality, channel blockers and potential sperm osmolytes. Hum Reprod. 2003;18:1029–36. doi: 10.1093/humrep/deg204. [DOI] [PubMed] [Google Scholar]
- Correa JR, Zavos PM. Frozen-thawed bovine spermatozoa diluted by slow or rapid dilution method: measurements on occurrence of osmotic shock and sperm viability. Theriogenology. 1995;44:963–71. doi: 10.1016/0093-691x(95)00283-e. [DOI] [PubMed] [Google Scholar]
- Biggers JD, Whittem WK, Whittingham DG.The culture of mouse embryos in vitro. In: Daniel JC, editor. Methods in Mammalian Embryology San Francisco: Freeman 1971. p86–116.
- WHO. Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge: Cambridge University Press; 1999
- Lorton SP, Kummerfield HL, Foote RH. Polyacrylamide as a substitute for cervical mucus in sperm migration tests. Fertil Steril. 1981;35:222–5. [PubMed] [Google Scholar]
- Pruneda A, Pinart E, Bonet S, Yeung CH, Cooper TG. Study of the polyol pathway in the porcine epididymis. Mol Reprod Dev. 2006;73:859–65. doi: 10.1002/mrd.20481. [DOI] [PubMed] [Google Scholar]
- Pruneda A, Yeung CH, Bonet S, Pinart E, Cooper TG. Concentrations of carnitine, glutamate and myo-inositol in epididymal fluid and spermatozoa from boars. Anim Reprod Sci. 2007;97:344–54. doi: 10.1016/j.anireprosci.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Aitken R, Krausz C, Buckingham D. Relationships between biochemical markers for residual sperm cytoplasm, reactive oxygen species generation, and the presence of leukocytes and precursor germ cells in human sperm suspensions. Mol Reprod Dev. 1994;39:268–79. doi: 10.1002/mrd.1080390304. [DOI] [PubMed] [Google Scholar]
- Phillips DM, Kalay D. Mechanisms of flagellar motility deduced from backward-swimming bull sperm. J Exp Zool. 1984;231:109–16. doi: 10.1002/jez.1402310114. [DOI] [PubMed] [Google Scholar]
- Lindahl PE, Drevius LO. Observations on bull spermatozoa in a hypotonic medium related to sperm mobility mechanisms. Exp Cell Res. 1964;36:632–46. doi: 10.1016/0014-4827(64)90319-2. [DOI] [PubMed] [Google Scholar]
- Drevius LO. Permeability coefficients of bull spermatozoa for water and polyhydric alcohols. Exp Cell Res. 1971;69:212–6. doi: 10.1016/0014-4827(71)90327-2. [DOI] [PubMed] [Google Scholar]
- Kulkarni SB, Sauna ZE, Somlata V, Sitaramam V. Volume regulation of spermatozoa by quinine-sensitive channels. Mol Reprod Dev. 1997;46:535–50. doi: 10.1002/(SICI)1098-2795(199704)46:4<535::AID-MRD12>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Anapolski M, Setiawan I, Lang F, Cooper TG. Effects of putative epididymal osmolytes and the ion channel blocker quinine on sperm volume regulation of fertile and infertile transgenic mice. J Androl. 2004;25:216–33. doi: 10.1002/j.1939-4640.2004.tb02781.x. [DOI] [PubMed] [Google Scholar]
- Kinne RK. Mechanisms of osmolyte release. Contrib Nephrol. 1998;123:34–49. doi: 10.1159/000059927. [DOI] [PubMed] [Google Scholar]
- MacLeod RJ, Hamilton JR. Increases in intracellular pH and Ca2+ are essential for K+ channel activation after modest 'physiological' swelling in villus epithelial cells. J Membr Biol. 1999;172:47–58. doi: 10.1007/s002329900582. [DOI] [PubMed] [Google Scholar]


