Abstract
The aims of this study were (a) to determine the prevalence of subjects with semen hyperviscosity (SHV) in a large population of male partners of subfertile couples; (b) to identify any correlation between SHV and infections or inflammation of the genital tract; (c) to assess the effects of therapeutic approaches for treating SHV; and (d) to assess sperm kinetic parameters after successful treatment of SHV. A retrospective study of 1 833 male partners of subfertile couples was conducted. Next, clinical, seminal, bacteriological and ultrasound studies involving 52 subjects suffering from SHV were performed, and the SHV was classified as being mild (length of thread > 2 cm and ≤ 4 cm), moderate (> 4 cm and ≤ 6 cm) or severe (> 6 cm). The prevalence of SHV was observed in 26.2% (480) of the subjects, with 13.2% suffering from mild, 6.6% from moderate and 6.4% from severe SHV. Treatment was completely successful in only 27 subjects (52.0%), primarily in those who had mild basal SHV with a positive semen culture. In these subjects, progressive motility percentage, straight line velocity and linearity were significantly higher than pre-treatment levels. SHV is often found in subjects with subfertility. Pathogenesis was strictly related to infective/inflammatory factors in only 48.0% of cases; therefore, it is possible that biochemical, enzymatic or genetic factors have a role in this condition.
Keywords: genital tract infection, genital tract inflammation, genital tract ultrasound, semen culture, semen hyperviscosity (SHV), sperm motility
Introduction
Semen hyperviscosity (SHV) is a condition that can seriously impair the physical and chemical characteristics of seminal fluid. As such, it deserves greater research attention, as it can have a serious impact on sperm function. Above all, SHV seems to be associated with reduced sperm motility, possibly due to a 'trapping effect' that prevents normal sperm progression through the female genital tract 1, 2.
Semen hyperviscosity also leads to certain technical difficulties in the handling of samples, such as when using Percoll gradients to prepare semen for in vitro fertilization (IVF) programs 3.
Physical and chemical methods have been proposed for the treatment of SHV. The physical method involves forcing the sample through a hypodermic needle 4, whereas the chemical treatment uses mucolytic agents such as α-chymotrypsin 5, 6 and sputolysin 7. However, one cannot ignore the fact that such procedures may damage sperm structure. The pathogenic approach to treat hyperviscosity may, therefore, be more advisable.
The pathogenic aspects of SHV have yet to be fully clarified. Gonzales et al. 8, 9 and Andrade-Rocha 10 showed reduced levels of fructose in SHV, and hypothesized inadequate functioning of the seminal vesicles as the explanation. Carpino and Siciliano 11 studied the possible correlation between SHV and protein secretion of the epididymis, vesicles and prostate. They found that hyperviscosity has no role in the semen coagulation process.
Mendeluk et al. 12 showed that there is no difference in the concentration of total proteins or DNA, or in the percentage of water content in hyperviscous seminal plasma. In addition, Mendeluk et al. 13, 14 observed that lysozyme has no direct role in SHV, although a deficiency in cases of chronic infections could be an aggravating factor from a clinical standpoint. Aydemir et al. 15 point out that increased oxidative damage might be a factor in SHV. In a previous study, we showed that genetic factors can influence the fluidity of semen 16.
One area of particular interest is the possible correlation between SHV and infections and/or inflammation of the genital tract. However, it should be noted that there is a good deal of disagreement in this field. According to Munuce et al. 17, there is no association between SHV and semen culture positivity, leukospermia or the presence of sperm antibodies. In addition, there is no correlation between SHV and human immunodeficiency virus infection 18. However, Wang et al. 19 found that Ureaplasma urealyticum infections were associated with SHV.
The aims of this study are to (a) evaluate the prevalence of subjects with SHV in a large population of male partners of subfertile couples; (b) evaluate the possible correlation between SHV and infections/inflammation of the genital tract; (c) assess the effects of therapeutic approaches in treating SHV; and (d) assess sperm kinetic parameters after successful treatment of SHV.
Materials and methods
Retrospective study
A retrospective study was conducted to examine data from January 2004 to December 2007 to assess the prevalence of SHV in male partners of subfertile couples referred to the Andrology Unit of Sant'Andrea Hospital (Rome) for semen analysis. In total, 1 833 subjects and 2 587 ejaculates were examined. Subjects included in the analysis were those with completely normal semen parameters and those with one or more altered semen parameters.
Clinical study
The clinical study was conducted in accordance with the hospital's ethics committee guidelines.
To examine for possible involvement of genital tract infections or inflammation as a pathogenic factor in SHV, the study group was comprised of 52 subjects with SHV, aged 25–43 years. A full medical history was taken, and subjects underwent a diagnostic and therapeutic program comprising clinical and seminal examination, semen culture, and ultrasound examination of the testes, seminal vesicles and prostate. All subjects with hormonal alterations, treated cryptorchidism and varicocele with marked venous reflux (time of reflux more than 2 s, detected by color Doppler) 20 were excluded from the study.
The minimum period of couple infertility was 1 year. Semen concentration was ≥ 20 million mL−1 and atypical forms were < 70%. SHV was then confirmed with a second seminal examination 2 months after the first.
All 52 subjects were treated with antiinflammatory and mucolytic agents, 50 mg tetracycline and 5 mg hydrocortisone (Farmaceutici Formenti s.p.a., Milan, Italy) every day for 30 days, and then with 200 mg N-acetyl cysteine (Zambon Svizzera SA, Cadempino, Swisse) every day for the subsequent 2 months. SHV patients who did not respond to therapy were given a second cycle of antiinflammatory drugs. Subjects with a positive semen culture had to first undergo specific antibiotic therapy, according to the antibiogram, at the lowest minimum inhibition concentration for the bacteria identified. The therapy lasted for 5 days.
Standard semen analysis, semen culture and ultrasound examination of the testes, seminal vesicles and prostate were carried out before and after therapy.
Male genital tract infection and inflammation determination
Within our cohort, infections were detected by semen culture. A number of diagnostic methods have been proposed for genital tract inflammation, including examination of ejaculate for peroxidase-positive cells or levels of seminal polymorphonuclear elastase 21. The increase of seminal white blood cells (WBC) in the absence of infection or signs of inflammation affecting the epididymis, seminal vesicles and prostate was taken as a marker of inflammation. Confirmation of inflammation was performed using ultrasound. The subjects were subdivided according to the presence or absence of infection and/or signs of inflammation.
Semen analysis and semen cultures
The semen samples were collected by masturbation after 3–5 days of sexual abstinence. Samples were stored in a controlled incubator (37°C) on a gently moving plate.
After liquefaction, semen samples were analyzed according to World Health Organization (WHO) guidelines 22, 23. The superimposed image analysis system was used to assess sperm motility parameters 24, 25. Progressive motility was defined as follows: class 1—straight line velocity (VSL) ≥ 23 μm s−1 and linearity (LIN) ≥ 0.58; and class 2—VSL > 10 and ≤ 23 μm s−1 and LIN ≥ 0.58 (frame rate: 21 frames s−1) 25. Sperm morphology was studied using the Bryan–Leishman (BL) stain technique 22. Slides were examined at a magnification of × 1 000 with an Olympus CX31 light microscope using a micrometric scale. The BL stain technique is a particularly effective method for distinguishing germinal cells and WBCs 22. Sperm morphology defects were classified according to WHO guidelines 22, 23. For each sample, at least 200 spermatozoa were evaluated.
Viscosity was determined, 1 h after ejaculation, by gently aspirating semen into a 5 mL pipette and then producing semen drops. In cases of abnormal viscosity, the drop formed a thread longer than 2 cm, which was evaluated using a centimeter scale. Hyperviscosity was graded under normal gravity as being mild (length of thread > 2 cm and ≤ 4 cm), moderate (> 4 cm and ≤ 6 cm) or severe (> 6 cm).
Finally, semen culture samples with colonies of > 100 000 UFC mL−1 were considered positive.
Quality control for semen analysis
Internal quality control (QC) (intra- and inter-operator) was carried out every 2 months. External QC was conducted by the National Health Institute.
Ultrasound
Testicular ultrasound was performed with an HDI 4000 (ATL Ultrasound, Bothell, WA, USA) using a probe with a range from 5.5 to 12 MHz. Increased volume of the prostate, seminal vesicles or epididymis, or alterations in echoic patterns were considered as signs of inflammation 26.
Statistics
We used the Shapiro–Wilk test to study progressive motility, VSL and LIN distributions. We used both the Wilcoxon's test and the two-tailed Student's t-test for paired data to evaluate progressive motility, for which the hypothesis of normality cannot be rejected. For VSL and LIN, for which the hypothesis of normality should be rejected, we used only the Wilcoxon's test for paired data, as this test does not require any specific assumptions about data distribution. All the tests were performed using the SPSS statistical package.
Results
Retrospective study
Of the total 2 587 samples, 602 (23.2%) showed some degree of SHV (11.2% mild, 6.2% moderate and 5.8% severe) (Table 1). The prevalence of SHV was 26.2% (480 of 1833 subjects): 13.2% mild, 6.6% moderate and 6.4% severe (Table 1). For those subjects for whom more than one semen analysis was performed, the table shows the viscosity found during the first examination.
Table 1. Prevalence of semen samples and subjects with semen hyperviscosity (SHV) in male partners of subfertile couples.
| Total semen samples (n = 2 587) | Total subjects (n = 1 833) | |
|---|---|---|
| Total SHV | 602 (23.2%) | 480 (26.2%) |
| SHV mild | 291 (11.2%) | 242 (13.2%) |
| SHV moderate | 160 (6.2%) | 121 (6.6%) |
| SHV severe | 151 (5.8%) | 117 (6.4%) |
Clinical study
The 52 subjects involved in the clinical study were divided into three groups according to their base-line diagnostic results (Table 2).
Table 2. Follow-up assessment of 52 subjects (subdivided into 3 groups) who underwent a full diagnostic and therapeutic program.
| Group | SHV | Pre-treatment |
Post-treatment |
|||
|---|---|---|---|---|---|---|
| Normal viscosity | SHV | |||||
| Mild | Moderate | Severe | ||||
| A | mild | 14 | 14 | — | — | — |
| moderate | 1 | 1 | — | — | — | |
| severe | 2 | 2 | — | — | — | |
| 17 | 17 (100%) | — | — | — | ||
| B | mild | 2 | 2 | — | — | — |
| moderate | 4 | 2 | 1 | 1 | — | |
| severe | 6 | — | 3 | 2 | 1 | |
| 12 | 4 (33.3%) | 4 (33.3%) | 3 (25%) | 1 (8.4%) | ||
| C | mild | 6 | 3 | 3 | — | — |
| moderate | 7 | 2 | 1 | 4 | — | |
| severe | 10 | 1 | 3 | 1 | 5 | |
| 23 | 6 (26.1%) | 7 (30.5%) | 5 (21.7%) | 5 (21.7%) | ||
| Total | 52 (100%) | 27 (52.0%) | 11 (21.1%) | 8 (15.4%) | 6 (11.5%) | |
Group A, subjects with positive semen culture. Group B, subjects with > 1 × 106 WBCs and/or inflammatory alterations observed by ultrasound. Group C, subjects negative for the above situations.
Group A (n = 17) included subjects with positive semen culture for Escherichia coli (n = 5), Klebsiella pneumoniae (n = 3), Proteus mirabilis (n = 3), Pseudomonas aeruginosa (n = 1), Enterobacter faecalis (n = 2) or Ureaplasma urealyticum (n = 3).
Group B (n = 12) consisted of subjects with > 1 × 106 seminal WBCs and/or inflammatory signs observed by ultrasound.
Group C (n = 23) included subjects with a negative semen culture and without inflammatory signs observed by ultrasound and 1 × 106 seminal WBC.
Each group was divided into three subgroups on the basis of SHV severity (mild, moderate or severe).
The therapeutic treatment fully resolved SHV in 27 cases (52.0%): 17 of 17 subjects (100%) in group A, 4 of 12 subjects (33.0%) in group B and 6 of 23 subjects (26.1%) in group C. In all these subjects, semen culture became or remained negative, seminal WBC became or remained 1 × 106 and ultrasound no longer showed signs of inflammation.
In contrast, in the other 25 subjects, the treatment was ineffective or only partially effective, as severe hyperviscosity became either moderate or mild. Thus, 25 of the 52 patients treated (48.0%) still had some degree of SHV. Of these, 11 (21.1%) suffered from mild SHV, 8 (15.4%) from moderate and 6 (11.5%) from severe hyperviscosity.
Finally, of the 27 patients who achieved normal viscosity after therapy, progressive motility (Wilcoxon's test P < 0.01 and two-tailed Student's t-test P < 0.005), VSL (Wilcoxon's test P < 0.001) and LIN (Wilcoxon's test P < 0.05) were higher than pre-treatment levels (Table 3, Figure 1). The progressive motility, VSL and LIN improved, respectively, in 21, 25 and 18 subjects out of 27 (Figure 1). Only one subject shifted from asthenozoospermia to normal sperm motility (50% of a+b grades, according to WHO). The mean difference of sperm motility post-treatment vs. pre-treatment was 6.2 + 10.4 (SD) (Table 3).
Table 3. Motility profile in subjects (n = 27) with SHV before treatment and normal viscosity after treatment.
| Pre-treatment (n = 27) | Post-treatment (n = 27) | Differences | |
|---|---|---|---|
| Progressive motility (%) | 20.2 ± 12.6 | 26.4 ± 13.3a,d | 6.2 ± 10.4 |
| VSL (μm s−1) (straight line velocity) | 18.4 ± 6.2 | 25.3 ± 6.8b | 6.8 ± 6.2 |
| LIN (linearity) | 0.76 ± 0.09 | 0.81 ± 0.07c | 0.05 ± 0.11 |
Wilcoxon's test for paired data, aP < 0.01; bP < 0.001; cP < 0.05. Two-tailed Student's t-test for paired data, dP < 0.005.
Figure 1.
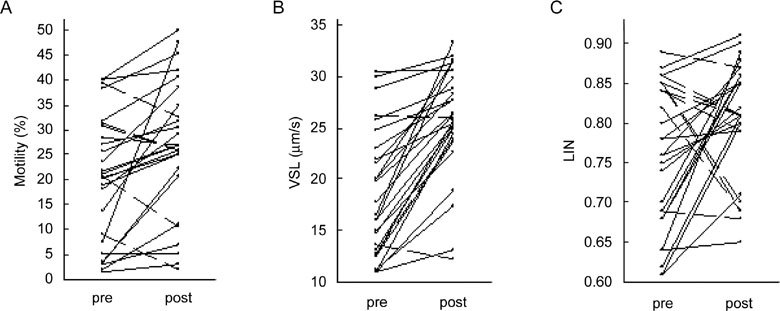
Sperm motility profile in subjects (n = 27) with SHV pre-treatment and normal viscosity post-treatment. Solid and broken lines indicate increment and decrement of kinetic parameters, respectively. (A): Progressive motility percentage (Shapiro–Wilk test pre-treatment P = 0.077, post-treatment P = 0.246; the hypothesis of normality cannot be rejected). Wilcoxon's test for paired data P < 0.01. Two-tailed Student's t-test for paired data P < 0.005. (B): Straight line velocity (VSL) (μm sec−1) (Shapiro–Wilk test pre-treatment P = 0.033, post-treatment P < 0.001; the hypothesis of normality should be rejected). Wilcoxon's test for paired data P < 0.001. (C): Linearity (LIN) (Shapiro–Wilk test pre-treatment P = 0.043, post-treatment P = 0.017; the hypothesis of normality should be rejected). Wilcoxon's test for paired data P < 0.05.
Discussion
The purpose of this research was to evaluate the prevalence of SHV in a large population of male partners of subfertile couples, and to evaluate the possible correlation between infections/inflammation of the genital tract and SHV. In addition, we assessed the effects of therapeutic approaches for the treatment of SHV and evaluated sperm kinetic parameters after successful treatment.
With regard to the first point, the study of a large population of male partners of subfertile couples showed that the prevalence of SHV was 26.2%, confirming the relatively high frequency of the condition. This prevalence was considerably lower than that found by Gonzales et al. 8, 9, but considerably higher when compared with that found by Gopalkrishnan et al. 27 and Esfandiari et al. 28. This may be because of differences in patient populations, and may also be because of variations in the standardization of SHV measurement.
With regard to the causes and pathogenic aspects of SHV, they have yet to be fully clarified. This study investigated the correlation between hyperviscosity and classic pathogenic factors proposed in the literature, such as infection or inflammation of the male genital tract. This study showed a pervasiveness of mild SHV in group A (positive semen culture), whereas it showed a predominance of severe SHV in group B and, surprisingly, in group C, which was the group without any sign of inflammation.
With regard to SHV treatment, in the era before the availability of IVF techniques, Amelar and Dubin 4 stated that SHV should be treated only when post-coital test results were poor. Treatment to reduce SHV by indirect measures, such as over hydration of patients, prostatic massages or administration of parenteral hyaluronidase, was not effective 4. They also proposed using the first portion of the ejaculate (if viscosity was lower than that of the entire ejaculate) or the total ejaculate, after forcing it through a hypodermic needle several times, for intrauterine insemination (IUI) 4.
The introduction of IVF and the use of ever more sophisticated methods of semen preparation have underlined the importance of doing more in cases of SHV. In fact, mucolytic agents have been proposed for in vitro treatment. Among the most important studies in this area, Honea et al. 6 stated that limited proteolysis using α-chymotrypsin, in cases in which it improved sperm penetration assay results, could represent a useful pre-treatment of hyperviscous semen for IUI or IVF programs. In addition, according to Zavos et al. 5, the limited proteolysis of hyperviscous semen specimens using α-chymotrypsin assisted in the recovery of a greater number of higher quality spermatozoa that could be used in various assisted reproductive techniques.
However, it cannot be ruled out that such procedures may damage sperm structure. As a result, the pathogenic approach to treat hyperviscosity may be more promising.
Munuce et al. 17 employed treatment with antibiotics in subjects with infections of the genital tract, with and without SHV. Therapy was based on the respective microorganism and antibiogram. They concluded that there was no association between SHV and positivity in semen cultures, number of species isolated in semen cultures, leukospermia or the presence of sperm antibodies.
In this study, we evaluated the effects of not only antibiotic treatment but also antiinflammatory and mucolytic agents in vivo. Analysis of the results showed that the treatment was successful in approximately half of the cases, primarily in those with mild SHV with a positive semen culture. In the remaining patients, the treatment either had no effect or simply reduced the hyperviscosity from severe to moderate or mild. On the basis of these observations, it is possible to hypothesize that cases of mild SHV may result from easily treatable infection and inflammation. On the other hand, severe SHV may represent something quite different. In a previous study, we hypothesized that certain cases of idiopathic SHV could be considered as minimal clinical expressions of cystic fibrosis, as the CFTR gene sequence variations may constitute the genetic basis for this disease 16. In spite of the negative impact of severe SHV on the ability to reproduce, and although it would be of great importance to understand the underlying molecular mechanisms, no other studies on the genetic basis of idiopathic SHV are found in the literature.
Therefore, in cases in which antiinflammatory treatment is unsuccessful, a genetic origin of SHV may be suspected. In these cases, it may be useful to conduct genetic analysis, primarily for mutations in the CFTR gene.
Finally, the deleterious effect of SHV on sperm motility seems to be further shown by the improved kinetics seen in successfully treated patients. This is in agreement with Elzanaty et al. 2 and Curi et al. 29, and confirms the important 'trapping effect', which is naturally more evident in severe SHV.
In conclusion, the results of the study allow us to make the following observations: SHV is often found in subfertile men and negatively influences seminal kinetic parameters; specific antibiotic and antiinflammatory therapy can successfully treat mild SHV, in which the condition seems to be the result of infection or inflammation; and hyperviscosity seems not to be due to a single pathogenic factor, but rather due to several (biochemical, enzymatic and genetic) factors that act in synergy. These factors require further study to better understand their role in the pathogenesis of SHV.
Acknowledgments
This work was supported by a Telethon grant (GGP06199).
References
- Tjioe DY, Oentoeng S. The viscosity of human semen and the percentage of motile spermatozoa. Fertil Steril. 1968;19:562–5. doi: 10.1016/s0015-0282(16)36728-0. [DOI] [PubMed] [Google Scholar]
- Elzanaty S, Malm J, Giwercman A. Visco-elasticity of seminal fluid in relation to the epididymal and accessory sex gland function and its impact on sperm motility. Int J Androl. 2004;27:94–100. doi: 10.1046/j.1365-2605.2003.00455.x. [DOI] [PubMed] [Google Scholar]
- Esfandiari N, Burjaq H, Gotlieb L, Casper RF. Seminal hyperviscosity is associated with poor outcome of in vitro fertilization and embryo transfer: a prospective study. Fertil Steril. 2008;90:1739–43. doi: 10.1016/j.fertnstert.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Amelar RD, Dubin L.Special problems in managementIn: Amelar RD, Dubin L, Walsh PC, editors. Male Infertility. Philadelphia: WB Saunders Company; 1977p191–214.
- Zavos PM, Zarmakoupis-Zavos PN, Correa JR. Effect of treatment of seminal viscosity difficulties with α-chymotrypsin on the recovery of spermatozoa for assisted reproductive technologies: comparison between the SpermPrep filtration and Percoll gradient centrifugation methods. Middle East Fertil Soc J. 1997;2:223–9. [Google Scholar]
- Honea KL, Houserman VL, Merryman DC, Free DA, Stringfellow SE. Effect of limited proteolysis with α-chymotrypsin on semen with an abnormal sperm penetration assay and possible application for in vitro fertilization or intrauterine insemination. J Assist Reprod Genet. 1993;10:255–60. doi: 10.1007/BF01204938. [DOI] [PubMed] [Google Scholar]
- Upadhyaya M, Hibbard BM, Walker SM. Use of Sputolysin form liquefaction of viscid human semen. Fertil Steril. 1981;35:657–61. doi: 10.1016/s0015-0282(16)45560-3. [DOI] [PubMed] [Google Scholar]
- Gonzales GF, Kortebani G, Mazzolli AB. Hyperviscosity and hypofunction of the seminal vesicles. Arch Androl. 1993;30:63–8. doi: 10.3109/01485019308988370. [DOI] [PubMed] [Google Scholar]
- Gonzales GF. Function of seminal vesicles and their role on male fertility. Asian J Androl. 2001;3:251–8. [PubMed] [Google Scholar]
- Andrade-Rocha FT. Physical analysis of ejaculate to evaluate the secretory activity of the seminal vesicles and prostate. Clin Chem Lab Med. 2005;43:1203–10. doi: 10.1515/CCLM.2005.208. [DOI] [PubMed] [Google Scholar]
- Carpino A, Siciliano L. Unaltered protein pattern/genital tract secretion marker levels in seminal plasma of highly viscous human ejaculates. Arch Androl. 1998;41:31–5. doi: 10.3109/01485019808988543. [DOI] [PubMed] [Google Scholar]
- Mendeluk GR, Gonzales Flecha FL, Castello PR, Bregni C. Factors involved in the biochemical etiology of human seminal plasma hyperviscosity. J Androl. 2000;21:262–7. [PubMed] [Google Scholar]
- Mendeluk GR, Blanco AM, Bregni C. Rheology of human seminal fluid: role of lysozyme. Acta Farm Bonaerense. 1996;15:163–8. [Google Scholar]
- Mendeluk GR, Blanco AM, Bregni C. Viscosity of human seminal fluid: role of lysozyme. Arch Androl. 1997;38:7–11. doi: 10.3109/01485019708988526. [DOI] [PubMed] [Google Scholar]
- Aydemir B, Onaran I, Kiziler AR, Alici B, Akyolcu MC. The influence of oxidative damage on viscosity of seminal fluid in infertile men. J Androl. 2008;29:41–6. doi: 10.2164/jandrol.107.003046. [DOI] [PubMed] [Google Scholar]
- Rossi T, Grandoni F, Mazzilli F, Quattrucci S, Antonelli M, et al. High frequency of (TG)mTn variant tracts in the cystic fibrosis transmembrane conductance regulator gene in men with high semen viscosity. Fertil Steril. 2004;82:1316–22. doi: 10.1016/j.fertnstert.2004.03.065. [DOI] [PubMed] [Google Scholar]
- Munuce MJ, Bregni C, Carizza C, Mendeluk G. Semen culture, leukocytospermia, and the presence of sperm antibodies in seminal hyperviscosity. Arch Androl. 1999;42:21–8. doi: 10.1080/014850199263002. [DOI] [PubMed] [Google Scholar]
- Dondero F, Rossi T, D'Offizi G, Mazzilli F, Rosso R, et al. Semen analysis in HIV seropositive men and in subjects at high risk for HIV infection. Hum Reprod. 1996;11:765–8. doi: 10.1093/oxfordjournals.humrep.a019251. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liang CL, Wu JQ, Xu C, Qin SX, et al. Do Ureaplasma urealyticum infections in the genital tract affect semen quality. Asian J Androl. 2006;8:562–8. doi: 10.1111/j.1745-7262.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- Winkelbauer FW, Ammann ME, Karnel F, Lammer J. Doppler sonography of varicocele: long-term follow-up after venography and trancatheter sclerotherapy. J Ultrasound Med. 1994;23:953–8. doi: 10.7863/jum.1994.13.12.953. [DOI] [PubMed] [Google Scholar]
- Henkel R, Maass G, Jung A, Haidl G, Schill WB, et al. Age-related changes in seminal polymorphonuclear elastase in men with asymptomatic inflammation of the genital tract. Asian J Androl. 2007;9:299–304. doi: 10.1111/j.1745-7262.2007.00270.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Laboratory manual for the examination of human semen and sperm-cervical mucus interaction3rd edn. New York: Cambridge University Press; 1992 [Google Scholar]
- World Health Organization. Laboratory manual for the examination of human semen and sperm-cervical mucus interaction4th edn. New York: Cambridge University Press; 1999 [Google Scholar]
- Mazzilli F, Rossi T, Sabatini L, Dondero F. Superimposed image analysis system (SIAS) software: a new approach to sperm motility assessment. Fertil Steril. 1995;64:653–6. doi: 10.1016/s0015-0282(16)57810-8. [DOI] [PubMed] [Google Scholar]
- Mazzilli F, Rossi T, Delfino M, Nofroni I. Application of upgraded image superimposition System (SIAS) to the assessment of sperm kinematics. Andrologia. 1999;31:187–194. doi: 10.1046/j.1439-0272.1999.00276.x. [DOI] [PubMed] [Google Scholar]
- Kimura A, Kurooka Y, Hirasawa K, Kitamura T, Kawabe K. Accuracy of prostatic volume calculation in transrectal ultrasonography. Int J Urol. 1995;2:252–6. doi: 10.1111/j.1442-2042.1995.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Gopalkrishnan K, Padwal V, Balaiah D. Does seminal fluid viscosity influence sperm chromatin integrity. Arch Androl. 2000;45:99–103. doi: 10.1080/014850100418783. [DOI] [PubMed] [Google Scholar]
- Esfandiari N, Gotlieb L, Casper RF. Seminal hyperviscosity is associated with poor outcome of controlled ovarian stimulation and intrauterine insemination: a prospective study. Int J Fertil Wom Med. 2006;51:21–7. [PubMed] [Google Scholar]
- Curi SM, Ariagno JI, Chenlo PH, Mendeluk GR, Pugliese MN, et al. Asthenozoospermia: analysis of a large population. Arch Androl. 2003;49:343–9. doi: 10.1080/01485010390219656. [DOI] [PubMed] [Google Scholar]


