Abstract
In this study we evaluate the oncological and functional results of the largest cohort of patients in China treated by laparoscopic radical prostatectomy (LRP) and with at least 3 years of follow-up. 126 inconsecutive patients (range 56–78 years, median 62.5) who had an LRP were retrospectively analyzed. The mean prostate specific antigen level and Gleason score was 13.4 ng mL−1 and 6.4, respectively. Twenty-seven patients had unilateral or bilateral nerve preservation and 29 had pelvic lymphadenectomy. Multivariate analysis was used to adjust for differences in clinical and pathological features when comparing the risk for biochemical progression-free survival (bPFS). Urinary continence was assessed by incontinence questionnaire and erectile function by the Sexual Health Inventory for Men score. The mean operative duration was 250 min and blood loss 354 mL. Five patients received blood transfusion and nine had complications, including rectal injury (two), ureteral injury (one), active bleeding (one), bladder neck stenosis (two), paralytic ileus (one), subcutaneous hematoma (one) and port-site hernia (one). The overall positive surgical margin rate was 20.6% and correlated with pathological stage and Gleason score respectively (P = 0.03, P < 0.001 respectively). All patients had ≥ 3 years of follow-up (range 3–6.75 years, mean 4.6, median 4.75). At 3 years of follow-up, the overall survival rate was 100% and the bPFS was 81.0% in all patients; 124 patients (98.4%) were continent; 22 of 27 patients (81.5%) who underwent nerve preservation retained erectile function. Our series confirms that LRP is an effective, safe and precise technique at Chinese institution.
Keywords: complication, follow-up, laparoscopic surgery, prostate cancer, prostatectomy, survival
Introduction
Prostate cancer (PCa) is one of the most common malignancies in Western countries, but it is relatively rare in China. In an epidemiological study, Yu et al. 1 reported a 26-fold higher rate of PCa in American than in Chinese men, with an intermediate rate in Chinese-American men. Such differences may be due to genetic and/or environmental factors 1.
Although PCa is uncommon in China, some recent reports indicate that its incidence is increasing rapidly 2. Gu 3 evaluated the incidence of PCa at 187 hospitals based in 26 Chinese provinces. The overall incidence rate of PCa in 1997 was 1.5% (1389/95 749). Between 1951 and 1960 the incidence rate of PCa at the Institute of Urology, Beijing University was 0.6%. From 1991 to 1997 this rate increased to 3.4%.
Despite numerous treatment options being currently available, open radical prostatectomy (ORP) remains the standard treatment for patients with clinically localized PCa (cT1–2) and a life expectancy of > 10 years 4. However, during the last decade laparoscopic radical prostatectomy (LRP) techniques have developed dramatically in urology, particularly in Europe. The first LRP was performed in 1997 by Schuessler et al. 5. Since then, LRP has been reported widely and it has gained popularity as a PCa treatment 6.
Many comparative studies have confirmed the advantages of laparoscopic surgery in terms of blood loss, transfusion rates, postoperative pain scores, and the duration of both catheterization and hospitalization, with similar peri-operative complication rates to those of open prostatectomy 7, 8. Some of these peri-operative advantages could be correlated with the reduced invasiveness of the laparoscopic procedure. However, the limited duration of follow-up reported in these comparative studies does not allow a long-term evaluation of biochemical disease-free survival probabilities 9. As for the functional results, at present it is difficult to compare the laparoscopic and open approaches, because of variability in the end points used to evaluate the outcome, and owing to the different lengths of follow-up 7, 9.
In 2000, we performed the first LRP for clinically localized PCa in China. Based on our previous experiences 10, 11, 12, in this study, we evaluated the medium-term oncological and functional results after LRP at our institute.
Materials and methods
Patients selection
From October 2000 to May 2004, 126 patients diagnosed with clinically localized PCa (cT1–2) underwent LRP at our institute. Their medical records were reviewed retrospectively. In principle, indications for LRP were the same as those for open prostatectomy, and patients with clinical stage T1c–2c PCa with a life expectancy of more than 10 years were candidates 4. The selection of LRP was based on a joint decision by surgeons and patients, who were appropriately informed about the surgical procedures and possible complications and provided written informed consent to the surgery and to the use of their clinical data for this study.
Inclusion and exclusion criteria
The study included patients with stage T1c–2cN0M0 localized PCa; those who had had previous hormone therapy, chemotherapy, radiotherapy or surgery for PCa were excluded. Patients suspected of having metastases on CT or MRI of the abdomen and pelvis, or radionuclide bone scan were also excluded. The Human Ethics Review Committee of the Third Affiliated Hospital, Sun Yat-Sen University approved the study protocol.
Pre-operative data
The median (range) age of the 126 patients was 62.5 (56–78) years. The mean prostate-specific antigen (PSA) level and Gleason score were 13.4 ng mL−1 and 6.4, respectively (Table 1). All patients had a negative radionuclide bone scan and CT or MRI. All had undergone LRP alone without any kind of neoadjuvant or adjuvant therapy for managing their PCa.
Table 1. Patient demographics and pathological data.
| Characteristic | Mean (range) or n (%) |
|---|---|
| No. of patients | 126 |
| Age, years | 62.5 (56–78) |
| PSA level, ng mL−1 | |
| < 10 | 67 (53.2) |
| 10–20 | 38 (30.2) |
| > 20 | 21 (16.6) |
| Clinical stage | |
| T1c | 22 (17.5) |
| T2a | 61 (48.4) |
| T2b | 30 (23.8) |
| T2c | 13 (10.3) |
| Biopsy Gleason score | |
| 2–6 | 80 (63.5) |
| 7 | 26 (20.6) |
| 8–10 | 20 (15.9) |
| Pathological stage | |
| T2a | 57 (45.2) |
| T2b | 26 (20.1) |
| T2c | 28 (22.2) |
| T3a | 9 (7.1) |
| T3b | 6 (4.4) |
| Pathological Gleason score | |
| 2–6 | 59 (46.8) |
| 7 | 31 (24.6) |
| 8–10 | 36 (28.6) |
Abbreviation: PSA, prostate-specific antigen.
Surgical procedure
LRP was performed using the Montsouris technique, with some modifications by us 10, 11, 12, 13. In brief, after the puboprostatic space is developed, the bilateral endopelvic fascia of the prostate is dissected longitudinally toward the apex and puboprostatic ligament section. The dorsal vein complex is divided and sutured with 2–0 Vicryl. When the bladder neck is transected and its posterior wall opened, the vas deferens and seminal vesicles are dissected carefully to avoid injury to the pelvic plexus. The Denonvilliers' fascia is exposed and opened, and the posterior plane of the prostate is created and extended to the apex of the prostate. The lateral vascular pedicle of the prostate is then dissected using a harmonic scissors. A sharp scissor is used to cut the anterior aspect of the urethra close to the prostate apex. The specimen is entrapped in an extraction bag. The vesicourethral anastomosis is performed using a monofilament 3:0 absorbable suture in a continuous manner. All operations were performed by a single experienced surgeon (XG).
A modified pelvic lymphadenectomy, as described by Stone et al. 14, was used in patients with a PSA level of > 20 ng mL−1 or biopsy Gleason score of > 7. Preservation of the neurovascular bundle was attempted in patients with a PSA level of < 10 ng mL−1 and a primary Gleason score of 3. During LRP the consistency of the local tissue was examined, and if induration was present the ipsilateral neurovascular bundle was excised.
Intra- and peri-operative data acquisition
The operative duration (the time from first incision to closure of the last wound) was recorded. Oral fluids and diet were introduced as tolerated. The drain was removed when the drainage was < 100 mL per 24 h. Patients were discharged home when comfortable.
For the purposes of histopathological analysis specimens were inked, apical and basal shaves taken, and the prostate then sliced in entirety. Whole-mount sections were cut and examined after routine hematoxylin and eosin staining. Positive surgical margins (PSMs) were defined as any cancer cells in contact with ink. All specimens were examined by a single pathologist.
Follow-up
Patients were assessed at 4 weeks after surgery, then at 3-month intervals during the first year and at every 6 months subsequently. Each visit except for the first was preceded by serum PSA measurement, and functional results prospectively recorded by the same reviewing clinician. An interview at the outpatient clinic or a telephone questionnaire was used to evaluate the urinary continence and erectile function.
Biochemical progression was defined as a PSA level of > 0.2 ng mL−1 after LRP. Urinary continence after surgery was defined as being pad-free, according to a validated symptom questionnaire. Erectile function was considered normal after LRP if the Sexual Health Inventory for Men (SHIM) score was > 22 in patients whose previous score was > 22 15.
Statistical analysis
Fisher's exact test was used to compare PSM rates among different pathological stages and Gleason grade groups. Biochemical progression-free survival (bPFS) was analyzed using the Cox proportional hazard model, with multivariate survival analyzed using the stepwise backward procedure to identify whether the variables (including pretreatment PSA, PSM, pathological stage, pathological Gleason grade, seminal vesicle involvement, invasion of the prostate capsule, a nerve-sparing procedure, tumor volume, etc.) had significant independent relationships with survival. The Kaplan–Meier method with the log-rank test was then used to compare the bPFS among different groups. In all analyses, P < 0.05 was considered to indicate statistical significance.
Results
The patient characteristics are summarized in Table 1. All of the 126 LRPs were successfully performed, and there was no conversion to open surgery. Patients were hospitalized for a mean (range) of 6.7 (5–19) days after LRP. Five patients received a blood transfusion and twelve had a fever (≥ 38.5°C). Drains were removed in all patients before discharge. Catheters were removed after a mean (range) of 6.1 (3–15) days, all with no previous cystogram. Twenty-nine patients had a modified pelvic lymphadenectomy. The mean count of removed lymph nodes was 9.2 and none had metastatic nodal disease on final histology. In 27 patients, preservation of the neurovascular bundle was accomplished. A comparison between the first 63 and second 63 cases of the series was made, highlighting the impact of surgical experience. The operative duration decreased significantly with experience (P < 0.05), but the mean blood loss was similar in the first and second cohorts of 63 cases (Table 2).
Table 2. Peri-operative outcomes, with a comparison between the first and second group of 63 patients.
| Outcome | First 63 | Second 63 | Overall, mean (range) |
|---|---|---|---|
| Operative duration, min | 274 | 221* | 250 (110–660) |
| Blood loss, mL | 362 | 349 | 354 (150–1 100) |
| Blood transfusion, n | 3 | 2 | 5 |
| Complications, n | |||
| Rectal injury | 2 | 0 | 2 |
| Ureteral injury | 1 | 0 | 1 |
| Active bleeding | 1 | 0 | 1 |
| Bladder neck stenosis | 1 | 1 | 2 |
| Paralytic ileus | 1 | 0 | 1 |
| Subcutaneous hematoma | 0 | 1 | 1 |
| Port-site hernia | 1 | 0 | 1 |
P < 0.05, compared with the first group of 63 patients.
There were four major complications, all occurring in the first 30 cases of the series. These included rectal injuries in two cases, ureteral injury in one and active bleeding from retropubic vessel complex in one patient. The rectal and ureteral injuries were recognized immediately and repaired laparoscopically during the operation with no sequelae. One continued to be hemodynamically unstable during the surgery, which was controlled by retropubic compression with a balloon catheter through the urethra after the gland had been removed. Minor complications were evenly distributed across the series, and consisted of two patients requiring transurethral incision of bladder neck stenoses, one who developed a paralytic ileus, which resolved spontaneously, one with subcutaneous hematoma, which was cured by conservative treatments, and one who required repair of a port-site hernia (Table 2).
The overall PSM rate was 20.6% and was related to pathological stage and pathological Gleason grade (P = 0.03, P < 0.001, respectively, Table 3). All patients had ≥ 3 years of follow-up (range 3–6.75 years, mean 4.6, median 4.75). The PSA level was < 0.2 ng mL−1 at 6 months after surgery in all patients. The total bPFS decreased with the follow-up time (Figure 1). Figure 2 showed bPFS according to pretreatment PSA value (Figure 2A), PSM (Figure 2B), pathological stage (Figure 2C) and pathological Gleason score (Figure 2D), respectively. Although numerous factors were identified as predictive for bPFS, only pretreatment PSA value, PSM, pathological stage and Gleason score were identified as independent prognostic factors for bPFS in multivariate analysis. At 3 years of follow-up, the overall survival rate was 100% and the bPFS was 81% in all patients, 90% for pT2, 61% for pT3a and 43% for pT3b, respectively. Twenty-four (19%) patients had a biochemical progression at a mean duration of 3.2 (1–5) years after LRP. In one patient, the sign of recurrence was PSA relapse and bone metastasis. In the other 23 patients, PSA relapse was the only sign of recurrence and these 23 patients were started on hormonal therapy.
Table 3. PSM rate according to pathological stage and Gleason score.
| Characteristic | PSM rate (%) | P value |
|---|---|---|
| Pathological stage | 0.03 | |
| T2a | 3/57 (5.3) | |
| T2b | 7/26 (26.9) | |
| T2c | 9/28 (32.1) | |
| T3a | 4/9 (44.4) | |
| T3b | 3/6 (50.0) | |
| Pathological Gleason score | < 0.001 | |
| 2–6 | 4/59 (6.8) | |
| 7 | 6/31 (19.4) | |
| 8–10 | 16/36 (44.4) | |
| Total | 26/126 (20.6) |
Abbreviation: PSM, positive surgical margin.
Figure 1.
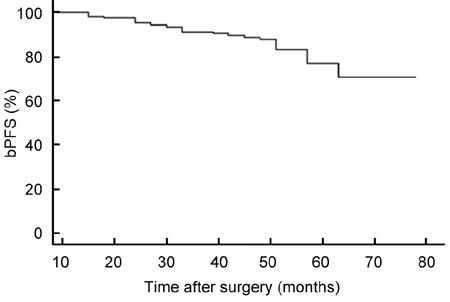
The overall biochemical progression-free survival (bPFS).
Figure 2.
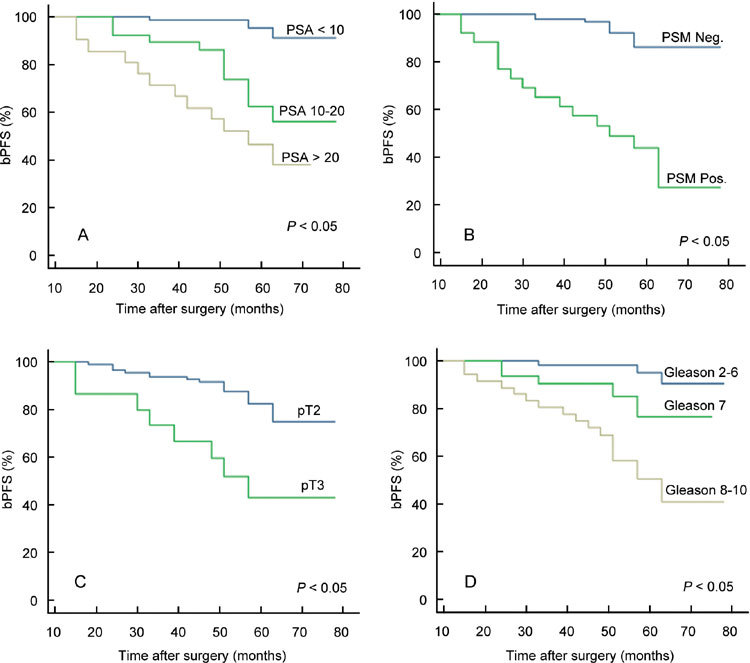
Correlation between biochemical progression-free survival (bPFS) and preoperative PSA level (A), PSM (B), pathological stage (C) and pathological Gleason score (D) (log-rank test, P < 0.05).
Ninety-nine patients (78.6%) were continent after removal of the catheter, 117 patients (92.8%) at 6-month follow-up, 123 patients (97.6%) at 1-year follow-up and 124 patients (98.4%) at ≥ 3-year follow-up. Two patients needed pads when abdominal pressure increased. However, no more than 1 pad / 24 h was needed. Of those patients undergoing nerve preservation, 51.9% (14/27) had satisfactory penile erectile function at 1-year follow-up. At 3-year follow-up, 81.5% (22/27) retained penile erection, and 10 patients were able to have sexual intercourse with the help of phosphodiesterase-5 inhibitor (sildenafil citrate).
Discussion
In Western countries PCa has emerged as one of the most common malignancies after the age of 50 years 16. The wide use of screening programs based on PSA has led to the detection of asymptomatic and early-stage PCa. Indeed, in the United States and Europe PCa is usually asymptomatic at diagnosis, DRE is normal and PCa is diagnosed on the sole basis of increased PSA in more than 60% of cases 17. Conversely, in China, screening for PCa using DRE and PSA is not done in routine practice. Most newly diagnosed PCa cases are symptomatic and at late stage. In a retrospective analysis of 431 consecutive Chinese patients treated for PCa, PCa was suspected because of increased PSA in only 6.2% of cases. Moreover, only 26% of patients had normal DRE 2. These results differ markedly from those in Western reports. Hence, the present series of 126 cases with clinically localized PCa at a single institute is a relatively large series in China, which can provide comprehensive information regarding LRP only for Chinese people.
Laparoscopy provides a much magnified and close-up view of the structures deep within the pelvis, which is usually impossible in conventional open surgery, particularly in Chinese men who have a narrower pelvis than Western men. However, the paucity of medium and longer-term results for LRP, as well as training issues, still prevents the wider acceptance and dissemination of this technique, especially in Asian countries with less workload of PCa.
To our knowledge, this is the largest study to date that represents the longest follow-up of LRP in China. The effectiveness of our technique in removing all prostate tissue was confirmed by the PSA level at 6 months of < 0.2 ng mL−1 in all patients. The malignant potential of organ-confined PCa is usually low and secondary treatments are available for patients with recurrence. As a result, bPFS is frequently used as a surrogate end point. Another surrogate end point for surgical outcome is the margin status.
The oncological data available from comparative studies show that LRP apparently gives results of similar percentages of PSM to those of ORP 18, 19, 20, 21 22, 23, 24, 25, 26, 27, 28, 29, 30 (Table 4). The PSM rates for LRP in pT2 and pT3 disease were 7.4%–27.5% and 17.2%–77.3%, respectively, and for ORP in pT2 and pT3 disease were 3.3%–18.8% and 19.3%–53%, respectively. Our PSM rates (pT2, 17.1%; pT3a, 44.4%; pT3b, 50.0%) were comparable, although we acknowledge the fact that the patients in our series were of better preoperative stage and the follow-up so far has been a brief period of time. Fromont et al. 31 reported a significant reduction of PSM in LRP, and LRP was also reported to have lower PSM rates at apex and multiple sites 32.
Table 4. PSM rates of prostatectomy techniques in previous reports.
| PSM rate (%) |
||||
|---|---|---|---|---|
| Study | No. of patients | pT2 | pT3a | pT3b |
| LRP | ||||
| Guillonneau et al.18 | 1 000 | 15.5 | 30.0 | 34.0 |
| Stolzenburg et al. 19 | 700 | 10.8 | 31.2 | — |
| Rozet et al. 20 | 600 | 14.6 | 26.9 | 22.6 |
| Touijer and Guillonneau 21 | 500 | 8.2 | 17.2 | — |
| Rassweiler et al. 22 | 500 | 7.4 | 25.2 | 42.0 |
| Salomon et al. 23 | 137 | 21.9 | 40.8 | — |
| Hara et al. 24 | 136 | 27.5 | 77.3 | 53.8 |
| Soderdahl et al. 25 | 110 | 13.5 | 50.0 | — |
| El-Feel et al. 26 | 100 | 18.1 | 45.0 | 50.0 |
| Present series | 126 | 17.1 | 44.4 | 50.0 |
| ORP | ||||
| Catalona and Smith 27 | 1 778 | — | 20.9 | — |
| Swindle et al. 28 | 1 209 | 6.8 | 19.3 | — |
| Hull et al. 29 | 1 000 | — | 12.8 | — |
| Pettus et al. 30 | 800 | 3.3 | 53.0 | — |
| Salomon et al. 23 | 145 | 18.8 | 52.7 | — |
Abbreviations: LRP, laparoscopic radical prostatectomy; ORP, open radical prostatectomy; PSM, positive surgical margin.
However, a comparison of the PSM rates between institutes is often difficult because the margin status of the prostate is influenced by factors such as disease extent, surgical skill, preparation of specimen and pathological evaluation. bPFS might offer a better index of oncological outcome. Previous series of LRP reporting bPFS are shown in Table 5 and are compared with large ORP series 18, 22, 23, 24 27, 28, 29. The bPFS rates in the present series at 3-year follow-up, of 90% for pT2, 61% for pT3a and 43.0% for pT3b, respectively, are comparable with both open and laparoscopic series with a similar duration of follow-up. It is not surprising to find that both PSM and bPFS rates were similar for open and laparoscopic procedures, as they essentially used the same approach in removing the organ.
Table 5. bPFS rates of prostatectomy techniques in previous reports.
| bPFS at 3 years (%) |
bPFS at 5 years (%) |
||||||
|---|---|---|---|---|---|---|---|
| Study | No. of patients | pT2 | pT3a | pT3b | pT2 | pT3a | pT3b |
| LRP | |||||||
| Guillonneau et al.18 | 1 000 | 90.4 | 77.1 | 44.1 | — | — | — |
| Rassweiler et al. 22 | 500 | 95.2 | 74.1 | 69.0 | 89.5 | 81.2 | 54.5 |
| Salomon et al. 23 | 137 | 90.4 | 56.8 | — | — | — | — |
| Hara et al. 24 | 136 | 91.8 | 66.8 | 44.9 | — | — | — |
| Present series | 126 | 90.0 | 61.0 | 43.0 | — | — | — |
| ORP | |||||||
| Catalona and Smith 27 | 1 778 | 92.5 | 78.0 | 47.5 | 87.5 | 65.9 | 30.0 |
| Swindle et al. 28 | 1 209 | — | — | — | 93.7 | 80.0 | — |
| Hull et al. 29 | 1 000 | — | — | — | 94.9 | 76.3 | 37.4 |
Abbreviations: bPFS, biochemical progression-free survival; LRP, laparoscopic radical prostatectomy; ORP, open radical prostatectomy.
In this study, bPFS was statistically significantly correlated with PSM, pathological stage, final Gleason grade and preoperative PSA level. This is in accordance with the results reported by Eden et al. 33. They also drew a similar conclusion in their series, but the correlation between PSM and bPFS did not attain statistically significant difference.
Urinary continence was second only to tumor control as an evaluation indicator of the effectiveness of LRP. The reported rates of urinary continence after LRP compare favorably with those in large series of ORP, which have been reported to be 80%–95%. Although the definition of urinary continence varies among the reported series, the incidence of requiring no pads after LRP was 83%–100% at 1 year 22, 23, 24, 25. In a prospective comparison of 70 patients with ORP and 230 with LRP, there was no significant difference in continence at 1 year, but patients undergoing LRP had an earlier return to continence 23. Many investigators have proved the importance of the external urethral sphincter and its related structures to postoperative urinary continence. Based on our initial experience, we discovered that the posterior urethra contributes to the function of the sphincter, and so the posterior urethral stump and its posterior walls should be preserved for as long as possible. Besides, all patients in our study practiced urinary continence training, such as the Kegel exercises or biofeedback therapy, 1 week after catheter removal. The urinary continence rate was 97.6% at 1 year when using these techniques.
When neurovascular bundles were preserved during LRP, the reported penile erectile rates were 33%–85% 18, 19, 20, 21, 22, 23. In this study, it was 51.9% at 1 year and 81.5% at 3 years after surgery. These results are similar to those of Guillonneau 18. In order to decrease the risk of thermal nerve injury, we used titanium clips or Ham-lock instead of bipolar diathermy during the seminal vesicle and neurovascular bundles dissection. In addition, sildenafil citrate given in small dosages during the early recovery stages can stimulate the function of the sexual nerves and prevent sexual neural dystrophy 34.
Notably, the functional outcomes in this study seem to be better than those of several previous reports 22, 23, 24, 25. Although wide disparity exists in the reported continence and erectile function after LRP, which could result from differing definitions of continence and erectile function, surgical techniques and quality-of-life instruments used, there is possible observer–receiver bias when evaluating the functional results by outpatient or telephone interviews. Response rate, which is difficult to determine in outpatient or telephone interviews, is important to estimate the significance of continence or erectile function for patients after LRP 35, 36. All functional results of our patients were prospectively recorded by the same reviewing clinician to minimize the interobserver bias; however, the survey results were probably affected by the observer-expectancy effect. A prospective evaluation with validated quality-of-life questionnaires using mail-in or web survey is the optimal method for evaluating functional outcomes and could be the focus of a future study.
The major obstacle in adopting the laparoscopic procedure is the long operation time. Indeed, laparoscopic surgery in the pelvic cavity is difficult to perform for a surgeon with limited LRP experiences at the beginning. To overcome these difficulties, we learned the laparoscopic technique from other types of laparoscopic surgery, such as laparoscopic ureterolithotomy and nephrectomy, by watching the operations done by experienced surgeons and practicing the skills on experimental pigs. All of these methods help us to shorten the learning curve and accumulate the experience of LRP quickly. For the first case, our operative duration was 660 min. The operative duration decreased significantly with experience. Although there is still much room for improvement, our average operative time of 250 min and blood loss of 354 mL compared favorably with the average operative time of 403 min and blood loss of 856 mL in a larger Asian series 24. The four major complications seen in the first 30 patients of our series are a testament to the technical difficulty of this procedure.
Conclusion
In summary, our series confirms the reproducibility of the results of other centers using LRP for safety and efficacy at the present Chinese institution. This will help to make LRP a more appealing option to the patients. However, our study also confirms that LRP is technically demanding and requires extensive initial experience.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (30772178), Research Fund for the Doctoral Program of Higher Education of China (20060558032), Natural Science Foundation of Guangdong Province (7117362) and the Program of 5010 of SunYat-Sen University (2007028). The authors declare no conflict of interest.
References
- Yu H, Harris RE, Gao YT, Gao R, Wynder EL. Comparative epidemiology of cancers of the colon, rectum, prostate and breast in Shanghai, China versus the United States. Int J Epidemiol. 1991;20:76–81. doi: 10.1093/ije/20.1.76. [DOI] [PubMed] [Google Scholar]
- Peyromaure M, Debre B, Mao K, Zhang G, Wang Y, et al. Management of prostate cancer in China: a multicenter report of 6 institutions. J Urol. 2005;174:1794–7. doi: 10.1097/01.ju.0000176817.46279.93. [DOI] [PubMed] [Google Scholar]
- Gu F. Epidemiological survey of benign prostatic hyperplasia and prostatic cancer in China. Chin Med J (Engl) 2000;113:299–302. [PubMed] [Google Scholar]
- Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, et al. EAU guidelines on prostate cancer. Eur Urol. 2008;53:68–80. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Schuessler WW, Schulam PG, Clayman RV, Kavoussi LR. Laparoscopic radical prostatectomy: initial short-term experience. Urology. 1997;50:854–7. doi: 10.1016/S0090-4295(97)00543-8. [DOI] [PubMed] [Google Scholar]
- Murphy DG, Challacombe BJ, Costello AJ. Outcomes after robot-assisted laparoscopic radical prostatectomy. Asian J Androl. 2009;11:94–9. doi: 10.1038/aja.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassweiler J, Hruza M, Teber D, Su LM. Laparoscopic and robotic assisted radical prostatectomy--critical analysis of the results. Eur Urol. 2006;49:612–24. doi: 10.1016/j.eururo.2005.12.054. [DOI] [PubMed] [Google Scholar]
- Guazzoni G, Cestari A, Naspro R, Riva M, Centemero A, et al. Intra- and peri-operative outcomes comparing radical retropubic and laparoscopic radical prostatectomy: results from a prospective, randomised, single-surgeon study. Eur Urol. 2006;50:98–104. doi: 10.1016/j.eururo.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Tooher R, Swindle P, Woo H, Miller J, Maddern G. Laparoscopic radical prostatectomy for localized prostate cancer: a systematic review of comparative studies. J Urol. 2006;175:2011–7. doi: 10.1016/S0022-5347(06)00265-5. [DOI] [PubMed] [Google Scholar]
- Gao X, Qiu JG, Cai YB, Zhou XF, Hong LQ. Laparoscopic radical prostatectomy. Chin Med J (Engl) 2004;117:148–9. doi: 10.3901/jme.2004.09.148. [DOI] [PubMed] [Google Scholar]
- Gao X, Qiu JG, Zhang B, Cai YB, Hong LQ. Re: Nerve sparing laparoscopic radical prostatectomy. Asian J Androl. 2003;5:338. [PubMed] [Google Scholar]
- Pu XY, Wang XH, Wu YL, Wang HP. Comparative study of the impact of 3- versus 8-month neoadjuvant hormonal therapy on outcome of laparoscopic radical prostatectomy. J Cancer Res Clin Oncol. 2007;133:555–62. doi: 10.1007/s00432-007-0204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillonneau B, Vallancien G. Laparoscopic radical prostatectomy: the Montsouris experience. J Urol. 2000;163:418–22. doi: 10.1016/s0022-5347(05)67890-1. [DOI] [PubMed] [Google Scholar]
- Stone NN, Stock RG, Unger P. Laparoscopic pelvic lymph node dissection for prostate cancer: comparison of the extended and modified techniques. J Urol. 1997;158:1891–4. doi: 10.1016/s0022-5347(01)64161-2. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Rosen RC. The Sexual Health Inventory for Men (SHIM): a 5-year review of research and clinical experience. Int J Impot Res. 2005;17:307–19. doi: 10.1038/sj.ijir.3901327. [DOI] [PubMed] [Google Scholar]
- Chan JM, Jou RM, Carroll PR.The relative impact and future burden of prostate cancer in the United States J Urol 2004172S13–6.discussion S7. [PubMed] [Google Scholar]
- Murphy AM, McKiernan JM, Olsson CA. Controversies in prostate cancer screening. J Urol. 2004;172:1822–4. doi: 10.1097/01.ju.0000140500.65341.9a. [DOI] [PubMed] [Google Scholar]
- Guillonneau B, el-Fettouh H, Baumert H, Cathelineau X, Doublet JD, et al. Laparoscopic radical prostatectomy: oncological evaluation after 1,000 cases a Montsouris Institute. J Urol. 2003;169:1261–6. doi: 10.1097/01.ju.0000055141.36916.be. [DOI] [PubMed] [Google Scholar]
- Stolzenburg JU, Rabenalt R, Do M, Ho K, Dorschner W, et al. Endoscopic extraperitoneal radical prostatectomy: oncological and functional results after 700 procedures J Urol 20051741271–5.discussion 5. [DOI] [PubMed] [Google Scholar]
- Rozet F, Galiano M, Cathelineau X, Barret E, Cathala N, et al. Extraperitoneal laparoscopic radical prostatectomy: a prospective evaluation of 600 cases. J Urol. 2005;174:908–11. doi: 10.1097/01.ju.0000169260.42845.c9. [DOI] [PubMed] [Google Scholar]
- Touijer K, Guillonneau B. Laparoscopic radical prostatectomy: a critical analysis of surgical quality. Eur Urol. 2006;49:625–32. doi: 10.1016/j.eururo.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Rassweiler J, Schulze M, Teber D, Marrero R, Seemann O, et al. Laparoscopic radical prostatectomy with the Heilbronn technique: oncological results in the first 500 patients. J Urol. 2005;173:761–4. doi: 10.1097/01.ju.0000153486.94741.e5. [DOI] [PubMed] [Google Scholar]
- Salomon L, Levrel O, de la Taille A, Anastasiadis AG, Saint F, et al. Radical prostatectomy by the retropubic, perineal and laparoscopic approach: 12 years of experience in one center Eur Urol 200242104–10.discussion 10–1. [DOI] [PubMed] [Google Scholar]
- Hara I, Kawabata G, Tanaka K, Kanomata N, Miyake H, et al. Oncological outcome of laparoscopic prostatectomy. Int J Urol. 2007;14:515–20. doi: 10.1111/j.1442-2042.2007.01773.x. [DOI] [PubMed] [Google Scholar]
- Soderdahl DW, Diaz JI, Rabah DM, Schellhammer PF, Fabrizio MD. Laparoscopic radical prostatectomy: evaluation of specimen pathologic features to critically assess and modify surgical technique. Urology. 2005;66:552–6. doi: 10.1016/j.urology.2005.03.094. [DOI] [PubMed] [Google Scholar]
- El-Feel A, Davis JW, Deger S, Roigas J, Wille AH, et al. Positive margins after laparoscopic radical prostatectomy: a prospective study of 100 cases performed by 4 different surgeons. Eur Urol. 2003;43:622–6. doi: 10.1016/s0302-2838(03)00148-9. [DOI] [PubMed] [Google Scholar]
- Catalona WJ, Smith DS. Cancer recurrence and survival rates after anatomic radical retropubic prostatectomy for prostate cancer: intermediate-term results. J Urol. 1998;160:2428–34. doi: 10.1097/00005392-199812020-00012. [DOI] [PubMed] [Google Scholar]
- Swindle P, Eastham JA, Ohori M, Kattan MW, Wheeler T, et al. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 2005;174:903–7. doi: 10.1097/01.ju.0000169475.00949.78. [DOI] [PubMed] [Google Scholar]
- Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, et al. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002;167:528–34. doi: 10.1016/S0022-5347(01)69079-7. [DOI] [PubMed] [Google Scholar]
- Pettus JA, Weight CJ, Thompson CJ, Middleton RG, Stephenson RA. Biochemical failure in men following radical retropubic prostatectomy: impact of surgical margin status and location. J Urol. 2004;172:129–32. doi: 10.1097/01.ju.0000132160.68779.96. [DOI] [PubMed] [Google Scholar]
- Fromont G, Guillonneau B, Validire P, Vallancien G. Laparoscopic radical prostatectomy. Preliminary pathologic evaluation. Urology. 2002;60:661–5. doi: 10.1016/s0090-4295(02)01855-1. [DOI] [PubMed] [Google Scholar]
- Brown JA, Garlitz C, Gomella LG, Hubosky SG, Diamond SM, et al. Pathologic comparison of laparoscopic versus open radical retropubic prostatectomy specimens. Urology. 2003;62:481–6. doi: 10.1016/s0090-4295(03)00387-x. [DOI] [PubMed] [Google Scholar]
- Eden CG, Moon DA. Laparoscopic radical prostatectomy: minimum 3-year follow-up of the first 100 patients in the UK. BJU Int. 2006;97:981–4. doi: 10.1111/j.1464-410X.2006.06090.x. [DOI] [PubMed] [Google Scholar]
- Ward JF, Zincke H, Bergstralh EJ, Slezak JM, Myers RP, et al. The impact of surgical approach (nerve bundle preservation versus wide local excision) on surgical margins and biochemical recurrence following radical prostatectomy. J Urol. 2004;172:1328–32. doi: 10.1097/01.ju.0000138681.64035.dc. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Cooper KL, Wei JT, Sarma AV, Raghunathan TE, et al. Use of multiple imputation to correct for nonresponse bias in a survey of urologic symptoms among African-American men. Am J Epidemiol. 2002;156:774–82. doi: 10.1093/aje/kwf110. [DOI] [PubMed] [Google Scholar]
- Mond JM, Rodgers B, Hay PJ, Owen C, Beumont PJ. Mode of delivery, but not questionnaire length, affected response in an epidemiological study of eating-disordered behavior. J Clin Epidemiol. 2004;57:1167–71. doi: 10.1016/j.jclinepi.2004.02.017. [DOI] [PubMed] [Google Scholar]


