Abstract
To explore the functions of human ribonuclease 9 (RNase 9), we constructed a mammalian fusion expression vector pcDNA-hRNase9, prepared recombinant human RNase 9-His fusion protein from HEK293T cells and determined its N-terminal amino acid sequences. According to the determined mature protein, recombinant human RNase 9 was prepared in E. coli. Ribonucleolytic activity and antibacterial activity of recombinant human RNase 9 were detected, and the distribution of human RNase 9 on tissues and ejaculated spermatozoa and in vitro capacitated spermatozoa were analyzed via indirect immunofluorescence assay. The results showed that recombinant human RNase 9 did not exhibit detectable ribonucleolytic activity against yeast tRNA, but exhibited antibacterial activity, in a concentration/time dependent manner, against E. coli. Immunofluorescent analyses showed that the predicted human RNase 9 was present throughout the epididymis, but not present in other tissues examined, and human RNase 9 was also present on the entire head and neck regions of human ejaculated spermatozoa and in vitro capacitated spermatozoa. These results suggest that human RNase 9 may play roles in host defense of male reproductive tract.
Keywords: ribonuclease A superfamily, epididymis, epididymal secretory proteins, spermatozoa, sperm maturation, male reproductive tract, host defense
Introduction
The epididymis has been the subject of physio-logy and biochemistry since Orgebin-Crist 1 discovered that spermatozoa are not capable of fertilizing an ovum when they leave the testis, and must undergo maturational changes in the epididymis to achieve the capa-city. The epididymis is formed by a polarized epithelium composed mostly of principal cells and basal cells, and creates a luminal environment, which promotes both the maturation and survival of spermatozoa until ejaculation. The epithelial principal cells, which exhibit region-specific differences along the epididymal tubule in structure and patterns of protein secretion, regulate the luminal fluid composition. Most proteins present in the luminal fluid are released from the apical surface of the epithelial cells and secreted into the duct lumen, where they come into contact with, and may adsorb to, the surface of spermatozoa, which leads to forward motility and fertilizing capacity of spermatozoa 2, 3. Although many epididymal proteins have been described, the function of the majority of them in sperm physiology remains to be clarified, and this is particularly true in the human.
The RNase A gene superfamily is the only vertebrate-specific family that encodes proteins with enzymatic activity 4. To date, 13 members of the RNase A superfamily have been identified in humans, although the functions of newly identified human RNases 9–13 remains unclear.
All RNase A superfamily members are secretory proteins typically composed of a signal peptide and a mature peptide. Among eight known cloned members, they have three catalytic residues (one lysine and two histidines within a signature CKXXNTF motif) at proper positions and six to eight cysteines that form three to four disulfide bonds 5. Except for these conserved residues, they exhibit diverse expression patterns and possess various catalytic activities against specific RNA substrates. They also exhibit a divergent physiological functions, including degradation of dietary RNA in the digestive gut (RNase 1) 6, angiogenesis (RNase 5) 7, and innate immunity (RNases 2, 3, 7) 8, 9, 10. With the discovery of RNases-9, -10, -11, -12 and -13 11, 12, 13, these requirements have been somewhat relaxed because these genes are clearly the derivatives of the RNase A lineage based on sequence similarity and the presence of the characteristic disulfide bridges, but they lack the active site signature motif and, as such, are unlikely to have ribonucleolytic activity.
Penttinen et al. 13 reported the in silico discovery of mouse RNase 9, a new member of the RNase A superfamily, exclusively expressed in epididymis; nevertheless, the function of mouse RNase 9 remains unknown. With the completion of the human genome sequence, human RNase 9 was identified in the human genome sequence by computational search. It is located in region q11.2 of the human chromosome 14 11. The sequence of human RNase 9 has not been cloned and characterized yet and the function of human RNase 9 remains a mystery. To explore the functions of human RNase 9, here we clone and characterize human RNase 9.
Materials and methods
Tissue and semen sample
Human testes and epididymides were collected from subjects 20–33 years of age, registered in an organ transplantation program, following accidental death. None of them had received prior hormonal treatment or radiotherapy. Other human tissues (heart, liver, spleen, lung, kidney, stomach, pancreas, small intestine, skin, trachea and skeletal muscle) were from surgical samples, with confirmed apparently normal histology, of patients in a local hospital. The procedure was approved by the Ethics Committee of the local hospital with informed, written consent. Semen was obtained from 24–35-year-old healthy donors with normal parameters based on World Health Organization (WHO) criteria 14. Human samples are not accompanied by identifying information and can not be traced to the donors.
Preparation of protein extracts from spermatozoa, testis, epididymal tissues and luminal fluids
Freshly liquefied semen was suspended into Biggers-Whitten-Whittingham (BWW) medium containing 1 mmol L−1 phenylmethylsulphonyl fluoride (PMSF) and spermatozoa were collected by centrifuging at 1 000 × g for 10 min. Protein extracts were prepared by lysing spermatozoa with a solubilization buffer (62.5 mmol L−1 Tris-HCl, 10% glycerol, 1% sodium dodecyl sulfate [SDS], pH 6.8) at 4°C at a final sperm concentration of 1 × 105 spermatozoa mL−1. The suspension was vigorously vortexed for 3 min and then centrifuged at 10 000 × g for 10 min, and the supernatant containing proteins was collected. Protein extracts from testis, epididymal tissues and luminal fluids were prepared according to the methods described by Deng et al. 15. Briefly, testes were minced and the proteins were extracted with the solubilization buffer. The epididymides were isolated and divided into caput, corpus and cauda. Each segment was thoroughly minced and incubated in 2 mL of sperm suspension buffer (50 mmol L−1 Tris, 20 mmol L−1 ethylenediaminetetraacetic acid [EDTA], 1 mmol L−1 p-hydroxy-mercurobenzenzoate, 5 mmol L−1 N-ethylmaleimide and 1 mmol L−1 benzamidine, pH 7.2) to disperse the spermatozoa and allow them to swim out, and the suspension was gently shaken to permit dispersal of the luminal contents. The sperm suspension was centrifuged at 500 × g for 2 min to pellet the tissues, and the epididymal tissues were washed a total of six times with BWW medium containing 1 mmol L−1 PMSF (centrifuged at 500 × g for 2 min) to remove adhering spermatozoa until the sperm concentration in the final supernatant (5 mL) was less than 100 spermatozoa mL−1. The proteins were extracted with the solubilization buffer. After the tissue pieces were allowed to settle, the upper fraction containing spermatozoa and luminal fluid was centrifuged at 1 000 × g for 3min to remove cells. The supernatant obtained was then centrifuged at 3 500 × g for 20 min at 4°C to remove cellular fragments. The proteins in the supernatant were precipitated with three volumes of acetone and recovered in sample buffer. The concentration of protein extracts was determined by using a protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer's instructions.
Amplification of human RNase 9 complementary DNA by reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA of the whole epididymides (from one donor) was isolated using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer's recommendations. Reverse transcription was performed in a volume of 20 μL with Moloney Murine Leukemia Virus reverse transcriptase (Promega Biosciences, WI, USA) and Oligo(dT) primers (Promega) at 42°C for 60 min. Subsequently, 2 μL of the reverse transcription product was used for the human RNase 9 amplification by PCR. Primers were designed according to the complete coding sequence (GenBank accession No. AF382949.1) with EcoRI and KpnI restriction sites: forward primer 5′-CGGAATTCATATGAGAACTCTCATCACAT-3′, reverse primer 5′-CGGGTACCCCTATGACAA ACACC-3′. After an initial denaturation at 95°C for 5 min, PCR was carried out at 95°C for 45 s, 60°C for 1 min and 72°C for 1 min for 30 cycles, with the last extension being performed at 72°C for 8 min.
Construction of mammalian expression vector
The PCR product of human RNase 9 complete coding sequence without stop codon was cloned into pcDNA3.1mycHis(−)B plasmid (Invitrogen) to obtain the recombinant pcDNA-hRNase9 construct with a six-His-tag on the C-terminus. DNA sequencing to confirm fidelity of constructs was performed with an ABI Prism 3700 DNA analyzer (Applied Biosystems, Foster City, CA, USA).
Transfection of HEK293T cells
HEK293T cells (American Type Culture Collection) were plated in 100-mm tissue culture dishes, and were transfected with 8μg of pcDNA–hRNase9 expression vector per dish using lipofectamine 2000 (Invitrogen Life Technologies) according to the manufacturer's recommendations. Twenty-four hours later, the supernatant and HEK293T cells of the cultures were harvested. The recombinant protein was separated using 12.5% SDS polyacrylamide gel electrophoresis (PAGE), confirmed by anti-His reactivity (anti-His antibody, Invitrogen Life Technologies) via Western blot according to the manufacturer's recommendations. The sample was then electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Amersham Biosciences, Little Chalfont, UK), visualized using Coomassie Blue staining and cut from the PVDF membrane for determination of the N-terminal amino acid sequence. The N-terminal amino acid sequencing was analyzed using Edman degradation on a model 491-protein sequencer (Applied Biosystems).
Immunofluorescence staining of transfected HEK293T cells
Coverslips were placed in a 100-mm tissue culture dish. Cell culture and DNA transfection were then carried out as described above. Twenty-fourh after transfection, the coverslips were fixed with 4% formaldehyde in phosphate-buffered saline (PBS) for 15 min, and washed with PBS containing 0.5% Tween-20. The coverslips were blocked with buffer A (2% skim milk powder, 0.5% Tween-20 in PBS) for 30 min before washing and incubating with anti-His antibody (1 : 1 000, Invitrogen Life Technologies) in buffer A for 1h, and then incubated with fluorescein isothiocyanate (FITC) -conjugated anti-rabbit immunoglobulin G (IgG; diluted 1 : 100, Sigma-Aldrich, St. Louis, MO, USA) for 30 min. The coverslips were then washed, mounted in 95% glycerol and analyzed using a confocal microscope (LSM META 510, Carl Zeiss, Jena, Germany).
Production of recombinant human RNase 9 in E. coli
The cDNA without the signal peptide was obtained by PCR amplification using reverse transcription product as a template. The forward and reverse primers contained NdeI and EcoRI restriction sites to allow in-frame cloning into the pET25b(+) expression vector (Novagen, Nottingham, UK). The resulting recombinant plasmid pET25b(+)-hRNase9 was identified by DNA sequencing and was transformed into E. coli BL21(DE3). A single colony bearing pET25b(+)-hRNase9 was inoculated into LB broth and induced with 100 μmol L−1 isoprobyl-beta-d-thiogalactopyranoside (IPTG, Promega) at 0.5 optical density (O.D. at A600), and 4h later cells were harvested by centrifugation at 4 000 × g for 10 min at 4°C. The cells were sonicated in lysis buffer (50 mmol L−1 Tris-HCl, 1 mmol L−1, EDTA, 100 mmol L−1 NaCl, pH8.0) and cell lysate was centrifugated at 8 000 × g. The recombinant RNase 9 in both supernatant and insoluble pellet fractions was analyzed by 12.5% SDS-PAGE. The recombinant RNase 9 was expressed in forms of inclusion body and soluble protein; we only isolated and purified the soluble recombinant protein. The recombinant RNase 9 was purified using diethylaminoethyl (DEAE) sepharose fast flow column (Amersham Pharmacia, Uppsala, Sweden) according to the manufacturer's protocol. Briefly, supernatant was collected and loaded to DEAE sepharose fast flow column (Pharmacia), which had been equilibrated with 10 mmol L−1 Tris-HCl (pH8.0). The recombinant RNase 9 was eluted from the column with 10 mmol L−1 Tris-HCl (pH8.0) containing 0.2 mol L−1 NaCl, and dialyzed against distilled water and concentrated by freeze-drying. The recombinant RNase 9 was found to be > 95% pure as judged by SDS-PAGE and scanning densitometry, according to an analysis performed using the multi-Analyst software (Bio-Rad Laboratories). The concentration of recombinant RNase 9 was determined by using a protein assay kit (Bio-Rad Laboratories) according to the manufacturer's instructions.
Preparation of RNase 9 specific antiserum
Four- to six-week-old female BALB/c mice were immunized first by hypodermic multisite injection of 25μg of recombinant RNase 9 from E. coli (purity > 95%) emulsified with Freund's complete adjuvant (FCA, Sigma). Two weeks later, the mice were boosted twice at an interval of 2 weeks with RNase 9 mixed with Freund's incomplete adjuvant (FIA, Sigma). An intraperitoneal booster with RNase 9 was given 2 weeks later. Blood was drawn 2 weeks later, and the serum was collected. Titers were determined by indirect enzyme-linked immunosorbent assay (ELISA). The specificity of the antiserum was tested by Western blot.
Indirect ELISA
Purified recombinant RNase 9 from E. coli (purity > 95%) diluted in carbonate/bicarbonate buffer (pH9.6), was coated onto a 96-well microtiter plate (Nunc, Roskilde, Denmark, 0.05 μg/well) and incubated overnight at 4°C. After blocking with 5% bovine serum albumin (BSA) in PBS, plates were incubated with duplicate two-fold serial dilutions of mouse anti-human RNase 9 sera for 1h at 37°C. Horseradish peroxidase-conjugated goat anti-mouse IgG (diluted 1 : 2000, Sigma-Aldrich) was then added for 1 h at 37°C, followed by the addition of the substrate 3,3′,5,5′-tetramethylbenzidine (Bio-Rad). Absorbance was determined at 450 nm using a Bio-Rad microtiter plate reader. An absorbance (optical density) greater than 0.2 was considered a positive result. A negative control was set up by adding PBS instead of primary antibody, and a nonspecific control was set up by adding the 6-histidine fusion protein (10 kDa) expressed by E. coli that was transformed with original pET-25b(+) vector (without any insert) instead of recombinant RNase 9 protein.
Western blot analysis of RNase 9 antiserum
The recombinant RNase 9 from E. coli and protein extracts from spermatozoa, testis, epididymal tissues and luminal fluids (equivalent quantities of protein extract, approximately 100 μg) were separated on 12.5% SDS-PAGE and transferred to nitrocellulose membranes (Amersham Biosciences). Membranes were blocked for 4 h at room temperature with BSA (5% [w/v] in PBS, containing 0.5% Tween-20) and incubated with a 1 : 500 dilution of either mouse anti-RNase 9 antiserum preabsorbed with recombinant RNase 9 from E. coli or the polyclonal anti-RNase 9 antiserum overnight at 4°C followed by horseradish peroxidase-conjugated goat anti-mouse IgG (diluted 1 : 20 000) (Sigma, Aldrich) for 1 h at room temperature. Reactive bands were visualized with 3,3′-diaminobenzidine (40 μg mL−1 in 0.1 mol L−1 Tris, pH 7.5, and 0.01% [v/v] H2O2).
RNase assay
The enzymatic activity of recombinant RNase 9 (purity > 95%) from E. coli was determined against a standard yeast tRNA substrate as described previously 16. Briefly, 40 μg of baker's yeast tRNA (Sigma-Aldrich) was incubated with 10 μg of recombinant RNase 9 in 0.8 mL of 40 mmol L−1 sodium phosphate buffer, pH 7.0 at 37°C. The reaction was stopped at an appropriate time by the addition of 0.5 mL of 20 mmol L−1 lanthanum nitrate with 3% perchloric acid, and insoluble tRNA was removed by centrifugation for 10min at 12000 × g. The amount of solubilized tRNA was determined by measuring the absorbance at 260 nm. In the experiments, we also used 456 pmol of commercially available RNase A (bovine pancreatic ribonuclease, Sigma) as a positive control. The catalytic activity of the RNase was determined as the pmol of RNA digested per second per pmol of RNase. All time points represented averages of triplicate samples.
Antibacterial assay
The antibacterial activity of recombinant human RNase 9 against E. coli XL-I blue was tested using the colony forming unit (CFU) assay as described earlier 17. Briefly, mid-log phase E. coli diluted to approximately 2 × 106 CFU mL−1 in 10 mmol L−1 sodium phosphate (pH7.4) was incubated with varying concentrations of recombinant RNase 9 at 37°C. The assay mixtures were serially diluted, spread on LB agar plates and incubated overnight at 37°C to allow full colony development. The resulting colonies were counted, and antibacterial activity was expressed as percentage of survival using the following formula: percentage of survival = (number of colonies surviving after treatment with the antibacterial protein/number of colonies surviving in absence of antibacterial protein) × 100. BSA (100 μg mL−1 was included in the assays as a control. The limit of detection (1 colony per plate) was equal to 1 × 102 CFU mL−1. Data shown represent median ± SEM of triplicate samples.
Capacitation of ejaculated spermatozoa in vitro
Freshly liquefied semen was aliquoted into 0.5 mL fractions, overlaid with 1 mL of BWW medium and incubated for 30 min at 37°C under 5% CO2 (swim up). The supernatants containing motile spermatozoa were combined and centrifuged at 500 × g for 10 min, resuspended in BWW at 1 × 107 mL−1, and recognized as “non-capacitated spermatozoa”. Aliquots of non-capacitated spermatozoa were incubated in BWW containing 3.5% human serum albumin (HSA, Sigma-Aldrich) 18 for 5h at 37°C under 5% CO2 to achieve capacitation.
Indirect immunofluorescence assay
Epididymides for immunofluorescence analysis were dissected into three regions (caput, corpus and cauda) according to morphological features. All tissues were frozen in liquid nitrogen, and 5–6 mm thick cryosections were prepared and fixed for 1 h with 4% paraformaldehyde in 0.1 mol L−1 PBS (pH7.4), air-dried, and conserved at −20°C until used. Aliquots of non-capacitated and capacitated human spermatozoa were fixed for 1 h at 4°C with 4% paraformaldehyde in PBS, then smeared onto slides and air-dried. Sections and sperm smears were then washed three times in PBS containing 0.5% Tween-20 (PBS-T), blocked in PBS-T containing 5% normal mouse serum and 2.5% BSA at 37°C for 4 h. The sections were then incubated in mouse anti-RNase 9 antiserum (diluted 1 : 200) for 1 h at room temperature. Sperm smears were incubated in Fab of mouse anti-RNase 9 antiserum (mouse anti-RNase 9 was treated with papain, and the Fab was isolated by DEAE chromatography). Parallel controls were incubated with an equal dilution of mouse anti-RNase 9 antiserum preabsorbed with recombinant RNase 9 from E. coli. After washes, the specimens were incubated in goat anti-mouse IgG antibody (diluted 1: 100) conjugated with FITC (Sigma-Aldrich) for 30 min in the dark at room temperature. After repeated washes, the specimens were mounted in glycerol solution under coverslips and viewed under a confocal microscope (LSM META 510, Zeiss).
Results
Cloning of human RNase 9 cDNA
The complete open reading frame (ORF) of the human RNase 9 gene (GenBank accession No. AF382949.1) is composed of 618bp. The human RNase 9 protein has 205 amino acids (without the stop codon) and it is a putative secretory protein with a signal peptide of 26 amino acids at the N terminus by SignalP 3.0 server (http://www.cbs.dtu.dk/services/SignalP) prediction and is a novel member of RNase A superfamily based on its sequence homology. The fusion expression vector pcDNA–hRNase9 was identified by DNA sequencing (one sample of the construct was detected), and the insertion of the designed fragment and the correctness of the orientation were also confirmed. The nucleotide sequence of human RNase 9 cDNA we obtained from human epididymis RNA is identical to the GenBank sequence (GenBank accession No. AF382949.1) except two nucleotide substitutions, A81→G, A576→G, which lead to no substitution of amino acids. The sequence differences may be due to the sequencing errors, RT-PCR mutations or polymorphisms.
Expression of human RNase 9 cDNA in HEK293T cells
The specific expression of human RNase 9 cDNA in HEK293 cells was confirmed by anti-His reactivity (Figure 1). Western blot revealed that the recombinant RNase 9–His fusion protein from HEK293 cells migrated with an apparent molecular weight of about 29 kDa (Figure 2A). The N-terminal amino acid sequences of the mammalian recombinant RNase 9 mature peptide were determined: Gln-Glu-Val-Asp-Thr-Asp-Phe-Asp-Phe-Pro, which are consistent with the prediction by SignalP.
Figure 1.
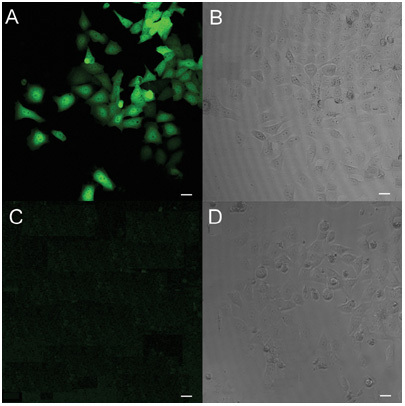
Immunofluorescence staining of HEK293 cells transfected with pcDNA-hRNase9. The matched fluorescent (A, C) and phase-contrast images (B, D) of transfected HEK293 cells immunostained with anti-His antibody (A, B) and normal serum (C, D) are shown. Original magnification × 200. Bars = 20 μm.
Figure 2.
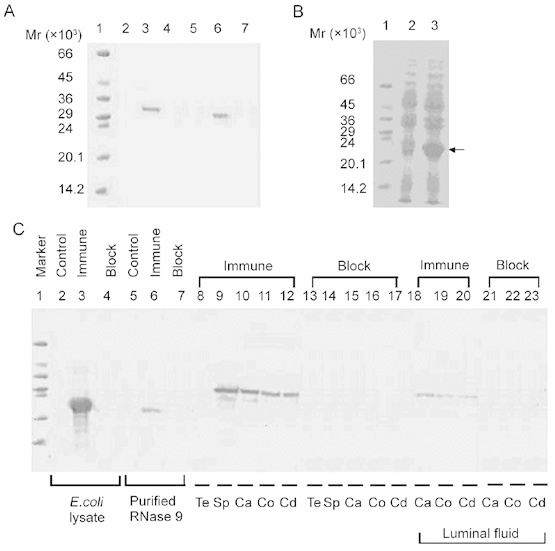
(A): Western blot of mammalian recombinant human RNase 9. Total lysate (lanes 2–4, 10 μg of total protein each lane) and culture supernatant (lanes 5–7, 10 μg of total protein each lane) of HEK293T cells transfected with pcDNA–hRNase9 was probed with normal serum (lanes 2, 5), anti-His antibody (lanes 3, 6) or anti-His antibody that had been preincubated with recombinant RNase 9 (lanes 4, 7). Protein marker on lane 1. (B): Expression of human RNase9 in pET25b (+)-hRNase9 transformed E. coli BL21(DE3). Lane 1, protein marker; lanes 2–3, total cell lysate (50 μg of total protein each lane) of pET25b (+)-hRNase9 transformed E. coli BL21(DE3) without isoprobyl-beta-d-thiogalactopyranoside (IPTG) induction or with IPTG induction for 4 h. (C): Western blot of human RNase 9 antiserum. Total cell lysate protein of pET25b (+)-hRNase9 transformed E. coli (lanes 2–4, 50 μg of total protein each lane), purified recombinant RNase 9 from E. coli (lanes 5–7, 500 ng each lane), and protein extracts from testis (Te), ejaculated sperm (Sp), caput (Ca), corpus (Co), cauda (Cd), epididymal luminal fluid were probed with preimmune serum (control), immune serum or immune serum that had been preincubated with recombinant RNase 9 (block). Equivalent quantities of protein extract (approximately 100 μg) were loaded on lanes 8–23. Protein marker on lane 1.
Production and identification of recombinant human RNase 9 from E. coli
The recombinant RNase 9 from E.coli was present with an apparent molecular weight of approximately 21kDa, similar to the calculated molecular weight of the RNase 9 mature protein, in SDS-PAGE (Figure 2B).
Preparation of RNase 9 specific antiserum and Western blot analysis
Titration of collected mouse serum by indirect ELISA indicated a titer of 1 : 3 × 104 for anti-RNase 9. The antiserum displayed a strong and specific reactivity with recombinant RNase 9 (Figure 2C) and RNase 9 protein extracted from ejaculated human spermatozoa, epididymal tissues and luminal fluids. Western blot analysis also indicated that RNase 9 was expressed in epididymal tissues and testis did not present detectable levels of RNase 9 expression.
Ribonucleolytic activity of recombinant human RNase 9
We examined ribonucleolytic activity against yeast tRNA of recombinant RNase 9 from bacteria, but no ribonucleolytic activity was detected; whereas the catalytic activity of the commercially available RNase A (bovine pancreatic ribonuclease) was 4.15 s−1.
Antibacterial activity of recombinant human RNase 9 protein
Recombinant RNase 9 was bactericidal against E. coli in a concentration/time dependent manner (Figure 3). Though recombinant RNase 9 at a concentration of 0.5 μmol L−1 only caused the death of the minority of bacteria, even after 8h incubation, bacterial survival was reduced by half in response to 1.0 μmol L−1 recombinant RNase 9 after 90min incubation. When the concentration of recombinant RNase 9 was increased to 8 μmol mL−1, virtually all bacteria were killed after 2h incubation. BSA was included in the assays as a negative control; it did not exhibit any bactericidal activity at the 100 μg mL−1 concentration even after 8 h incubation.
Figure 3.
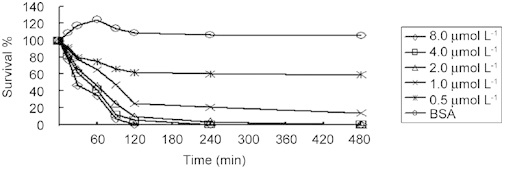
Antibacterial activity of recombinant RNase 9. The antibacterial activity of recombinant RNase 9 was tested against E. coli XL-I blue by colony forming unit (CFU) assay. The experiments were repeated three times, and the median ± SEM values are shown. BSA, bovine serum albumin.
Unique expression pattern of human RNase 9 in human adult tissues
RNase 9 was detected in epididymis (Figure 4A, B, C), but not in any other human tissues examined, including testis (Figure 4I), heart, liver, spleen, lung, kidney, stomach, pancreas, small intestine, skin, trachea and skeletal muscle (data not shown). RNase 9 was found throughout the caput (Figure 4A), corpus (Figure 4B) and cauda (Figure 4C) epididymis, a strong positive immune staining was found mainly in the epithelial cells lining the epididymal lumen and in the luminal contents (containing spermatozoa). The results were consistent with those revealed by Western blot analysis of RNase 9 protein extracted from testis, epididymal tissues and luminal fluids.
Figure 4.
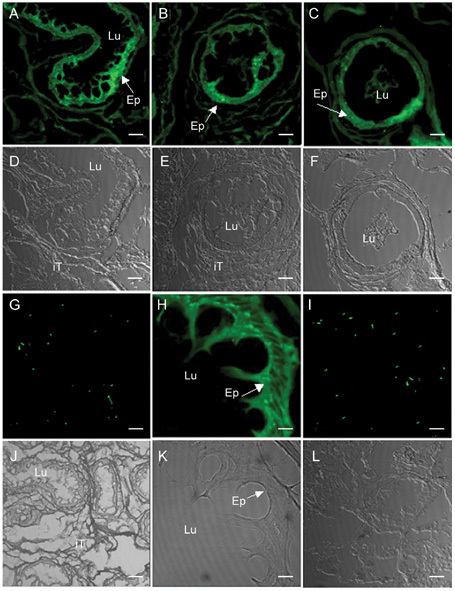
Immunofluorescent localization of RNase 9 in human adult epididymis and testis. Matched fluorescent and phase-contrast images of cryosections of epididymis (A–H, J, K) and testis (I, L) are shown, the green fluorescent fluoroscein isothiocyanate (FITC) images are shown in A–C (caput, corpus, cauda), G–I, and the corresponding phase-contrast images are shown in D–F, J–L. G shows no staining image of epididymis probed with immune serum that had been preincubated with recombinant RNase 9, and I and L show cryosections of testis. The images shown are photographed with original magnification × 200 except H and K of epididymis cryosections with magnification × 1 000 (panel H is a magnification of results presented in panel A). Ep, epididymal epithelium; iT, intertubular tissue; Lu, lumen. Bars = 100 μm (except H, K, bars = 500μm.).
Immunofluorescent localization of human RNase 9 on non-capacitated and capacitated human spermatozoa
Immunofluorescent stain showed that RNase 9 protein was restricted to the head and neck regions on non-capacitated spermatozoa surface (Figure 5A, B), and was still present on head and neck regions after capacitation (Figure 5E, F).
Figure 5.
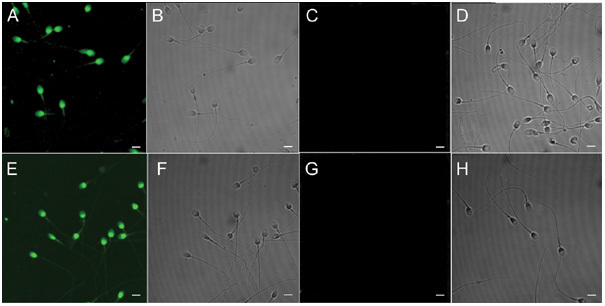
Immunofluorescent localization of RNase 9 on non-capacitated and capacitated human spermatozoa. Smears of non-capacitated (A–D) and capacitated (E–H) human spermatozoa were probed with Fab of mouse anti-RNase 9 antiserum (A, B, E, F), mouse anti-RNase 9 antiserum that had been preabsorbed with recombinant RNase 9 from E. coli (C, D, G, H). The fluorescent images are shown in A, C, E and G and the corresponding phase-contrast images are shown in B, D, F and H. Micrographs are representatives of spermatozoa observed from five different males. Original magnification × 1 000. Bars = 500 μm.
Discussion
In the present study, we describe the cloning and characterization of human RNase 9, a newly identified member with unknown functions of the RNase A superfamily. This is the first report of cloning and characterization of human RNase 9. The human RNase 9 gene is located in the region q11.2 of human chromosome 14 that encodes all other known cloned members 19, 20 (human RNases 1–8) and recently reported new members (human RNases 9–13) 11, 12, 13 of the RNase A superfamily, forming a cluster of ∼550 kb. Figure 6 shows the amino acid sequence alignment of human RNases 1–13. Although the amino acid sequences of RNases 9–13 are only 15%–30% identical to the canonical RNases (RNases 1–8), several characteristics suggest that all these proteins share a common ancestry. First, the RNase genes are all closely linked on the chromosome, an indication of origin by tandem gene duplication. Second, for all the RNase genes, the entire ORF is contained within a single exon. Third, most of the 6 to 8 cysteine residues, and also several other residues that are important for folding and structure in canonical RNases, are conserved in RNases 9–13. Fourth, the ORF of these proteins indicate that they have a signal peptide at the N-terminus and that the mature proteins have a two-domain structure with different N-terminal domains (with insertions of 40–50 residues in RNases 9, 10 and 11), but similar C-terminal domains of about 100 amino acid residues with four conserved disulfide bonds in most of them.
Figure 6.
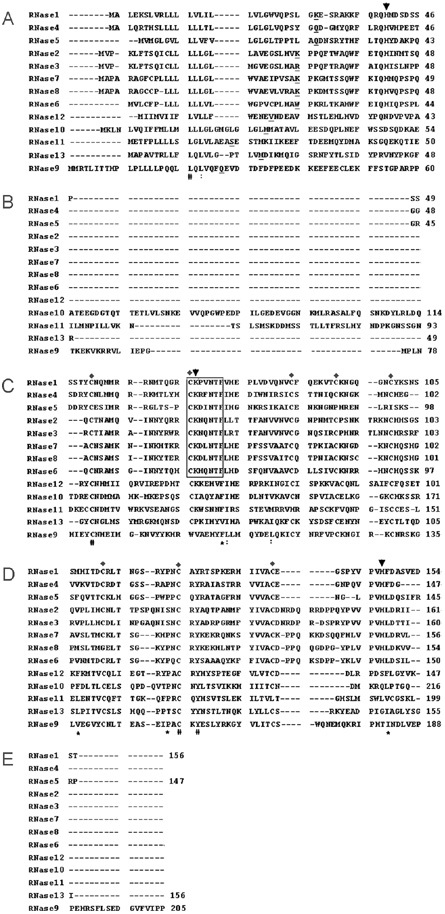
Amino acid sequence alignment of human members of the RNase A superfamily. The alignment was generated by CLUSTAL W (version 1.83). Gaps are indicated by dashes. The starting residues of the mature RNase A proteins are underlined. The conserved CKXXNTF motif are boxed, with three catalytic residues indicated by “↓” and eight conserved cysteines indicated by “♦”. “#”, the residues in that column are identical in all sequences. “*”, conserved substitution; “:”, semi-conserved substitution. GenBank accession numbers: RNase 1 (NP_937878), RNase 2 (NP_002925), RNase 3 (NP_002926), RNase 4 (NP_919411), RNase 5 (NP_001136), RNase 6 (NP_005606), RNase 7 (NP_115961), RNase 8 (NP_612204), RNase 9 (AAQ02792), RNase 10 (AAV87183), RNase 11 (AAV87184), RNase 12 (AAV87185), RNase 13 (AAV87186).
Human RNase 9 is distantly related to the RNase A superfamily based on its lower amino acid sequence homology; it is most similar to human RNase 7 (30% sequence identity) of the eight known members, and most similar to human RNase 12 (31% sequence identity) of the five new members. The mature protein of human RNase 9 has a calculated isoelectric point (pI) of 5.9 and a calculated molecular weight of 21kDa. Human RNase 9 possesses seven conserved cysteines at proper positions, but lacks the catalytic triad and signature motif of CKXXNTF, and these sequence features suggest that human RNase 9 may be unlikely to have ribonucleolytic activity.
The most noticeable difference between RNase 9 and the other RNase A proteins is a 30-amino acid long sequence that lies between a putative 26-residue signal peptide predicted by SignalP and the rest of the protein. The eight established members of the RNase A superfamily also have a signal peptide, but none has this intervening stretch of amino acids. However, the actual start of the mature RNase 9 needs experimental verification 21. In this study, we determined the start of the mature protein, which was consistent with the prediction by SignalP.
As expected from the amino acid sequence, no ribonucleolytic activity was detected. The absence of enzymatic activity of recombinant human RNase 9 found in this study confirmed that the key amino acids are essential for ribonucleolytic activity. On the other hand, it is likely that bacterial recombinant human RNase 9 without post-translational processing is inactive of itself.
Interestingly, we found that recombinant human RNase 9 exhibited bactericidal activity against E. coli. The presence of positively charged residues (Arg and Lys, 14%) and the forming of an amphipathic alpha-helix conformation (29.05%) in mature RNase 9 protein enable it to adsorb and permeate the negatively charged micro-organism's cell membrane, thereby causing increased permeability and a loss of barrier function, resulting in the leakage of cytoplasmic components and cell death.
The tissue-specific expression of human RNase 9 may provide some clues for its in vivo physiological function. We show here that human RNase 9 was expressed uniquely in the epididymis while remaining undetectable in twelve other tissues examined. It is consistent with the expression of rhesus monkey Esc461 22 (homolog of human RNase 9) and mouse RNase 9 13. This indicates that human RNase 9 may have functions related to male reproduction system.
Immunofluorescent stain revealed human RNase 9 in the apical part of the human epididymal epithelia throughout the epididymis, corresponding to the supranuclear region and stereocilia of principal cells, and in the lumen, and not in the testis. These results suggest that human RNase 9 may be synthesized and secreted by principal cells of the epididymis, and may bind to spermatozoa when they are passing by. Therefore, human RNase 9 may be a human sperm maturation-related protein.
The various changes that occur to mammalian spermatozoa when they come in contact with the female reproductive tract are known collectively as capacitation. A variety of physiological changes take place in spermatozoa during capacitation, including membrane lipid bilayer modulation, increased phosphorylation, intracellular ion fluctuations and loss of some surface coats 23. The epididymis has been shown to alter the sperm plasma membrane by addition of numerous proteins to promote the maturation of spermatozoa; epididymal proteins added to the sperm surface may be lost or redistribute during capacitation. The outcomes of sperm conjugated proteins of epididymal origin during capacitation may provide some clues with respect to function 24. Immunofluorescent stain revealed human RNase 9 was found on ejaculated non-capacitated spermatozoa and in vitro capacitated spermatozoa. These results also indicate that human RNase 9 may not only bind to spermatozoa during their passage in epididymis, but also accompany spermatozoa through the whole male reproductive tract. We also found that recombinant human RNase 9 exhibited antibacterial activity. Antibacterial protein bound to the sperm surface may promote fertility by protecting spermatozoa against attack by bacteria in the male and female reproductive tracts. Taken together, human RNase 9 may have a role in male reproductive tract host defense.
In conclusion, human RNase 9, a new member of the RNase A superfamily, secreted by the epididymis, exhibits antibacterial activity, binds to human ejaculated non-capacitated spermatozoa and is present on capacitated spermatozoa, and may be involved in host defense of the male reproductive tract.
Acknowledgments
The authors would like to thank Mr Shou-Xin Zhang and other members of the Research Center, Yuhuangding Hospital (Yantai, China) for technical assistance.
Footnotes
Edited by Dr Trevor G. Cooper and Dr Russell C. Jones
References
- Orgebin-Crist MC. Sperm maturation in rabbit epididymis. Nature. 1967;216:816–8. doi: 10.1038/216816a0. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Nixon B, Lin M, Koppers AJ, Lee YH, et al. Proteomic changes in mammalian spermatozoa during epididymal maturation. Asian J Androl. 2007;9:554–64. doi: 10.1111/j.1745-7262.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Frenette G, Girouard J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J Androl. 2007;9:483–91. doi: 10.1111/j.1745-7262.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–21. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Beintema JJ, Kleineidam RG. The ribonuclease A superfamily:general discussion. Cell Mol Life Sci. 1998;54:825–32. doi: 10.1007/s000180050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard EA. Biological function of pancreatic ribonuclease. Nature. 1969;221:340–4. doi: 10.1038/221340a0. [DOI] [PubMed] [Google Scholar]
- Fett JW, Strydom DJ, Lobb RR, Alderman EM, Bethune JL, et al. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985;24:5480–6. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis. 1998;177:1458–64. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol. 2001;70:691–8. [PubMed] [Google Scholar]
- Harder J, Schroder JM. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J Biol Chem. 2002;277:46779–84. doi: 10.1074/jbc.M207587200. [DOI] [PubMed] [Google Scholar]
- Cho S, Beintema JJ, Zhang J. The ribonuclease A superfamily of mammals and birds: identifying new members and tracing evolutionary histories. Genomics. 2005;85:208–20. doi: 10.1016/j.ygeno.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Castella S, Fouchecourt S, Teixeira-Gomes AP, Vinh J, Belghazi M, et al. Identification of a member of a new RNase A family specifically secreted by epididymal caput epithelium. Biol Reprod. 2004;70:319–28. doi: 10.1095/biolreprod.103.022459. [DOI] [PubMed] [Google Scholar]
- Penttinen J, Pujianto DA, Sipila P, Huhtaniemi I, Poutanen M. Discovery in silico and characterization in vitro of novel genes exclusively expressed in the mouse epididymis. Mol Endocrinol. 2003;17:2138–51. doi: 10.1210/me.2003-0008. [DOI] [PubMed] [Google Scholar]
- World Health Organization . New York: Cambridge University Press; 1999. WHO Laboratory Manual for the Examination of Human Semen and Semen Cervical Mucous Interaction; p. p60. [Google Scholar]
- Deng X, He Y, Martin-DeLeon PA. Mouse Spam1 (PH-20): evidence for its expression in the epididymis and for a new category of spermatogenic-expressed genes. J Androl. 2000;21:822–32. [PubMed] [Google Scholar]
- Rosenberg HF. Recombinant human eosinophil cationic protein. Ribonuclease activity is not essential for cytotoxicity. J Biol Chem. 1995;270:7876–81. doi: 10.1074/jbc.270.14.7876. [DOI] [PubMed] [Google Scholar]
- Yenugu S, Hamil KG, Birse CE, Ruben SM, French FS, et al. Antibacterial properties of the sperm-binding proteins and peptides of human epididymis 2 (HE2) family: salt sensitivity, structural dependence and their interaction with outer and cytoplamic membranes of Escherichia coli. Biochem J. 2003;372:473–83. doi: 10.1042/BJ20030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focarelli R, Rosati F, Terrana B. Sialylglycoconjugate release during in vitro capacitation of human spermatozoa. J Androl. 1990;11:97–104. [PubMed] [Google Scholar]
- Zhang J, Dyer KD, Rosenberg HF. RNase 8, a novel RNase A superfamily ribonuclease expressed uniquely in placenta. Nucleic Acids Res. 2002;30:1169–75. doi: 10.1093/nar/30.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Dyer KD, Rosenberg HF. Human RNase 7: a new cationic ribonuclease of the RNase A superfamily. Nucleic Acids Res. 2003;31:602–7. doi: 10.1093/nar/gkg157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor EJ, Moffat-Wilson KA, Galbraith JJ. LOC 390443 (RNase 9) on chromosome 14q11.2 is related to the RNase A superfamily and contains a unique amino-terminal preproteinlike sequence. Human Biology. 2004;76:921–35. doi: 10.1353/hub.2005.0016. [DOI] [PubMed] [Google Scholar]
- Liu Q, Hamil KG, Sivashanmugam P, Grossman G, Soundararajan R, et al. Primate epididymis-specific proteins: characterization of ESC42, a novel protein containing a trefoil-like motif in monkey and human. Endocrinology. 2001;142:4529–39. doi: 10.1210/endo.142.10.8422. [DOI] [PubMed] [Google Scholar]
- De Lamirande E, Leclerc C, Gagnon C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol Hum Reprod. 1997;3:175–94. doi: 10.1093/molehr/3.3.175. [DOI] [PubMed] [Google Scholar]
- Cuasnicu PS, Ellerman DA, Cohen DJ, Busso D, Morgenfeld MM, et al. Molecular mechanisms involved in mammalian gamate fusion. Arch Med Res. 2001;32:614–8. doi: 10.1016/s0188-4409(01)00321-6. [DOI] [PubMed] [Google Scholar]


