Abstract
We have observed earlier that testosterone at physiological concentrations can stimulate tissue factor pathway inhibitor (TFPI) gene expression through the androgen receptor in endothelial cells. This study further investigated the impact of testosterone on TFPI levels in response to inflammatory cytokine tumor necrosis factor-alpha (TNF-α). Cultured human umbilical vein endothelial cells were incubated in the presence or absence of testosterone or TNF-α. TFPI protein and mRNA levels were assessed by enzyme-linked immunosorbent assay and quantitative real-time reverse transcription polymerase chain reaction. To study the cellular mechanism of testosterone's action, nuclear factor-kappa B (NF-κB) translocation was confirmed by electrophoretic mobility shift assays. We found that after NF-κB was activated by TNF-α, TFPI protein levels declined significantly by 37.3% compared with controls (P < 0.001), and the mRNA levels of TFPI also decreased greatly (P < 0.001). A concentration of 30 nmol L−1 testosterone increased the secretion of TFPI compared with the TNF-α-treated group. NF-κB DNA-binding activity was significantly suppressed by testosterone (P < 0.05). This suggests that physiological testosterone concentrations may exert their antithrombotic effects on TFPI expression during inflammation by downregulating NF-κB activity.
Keywords: nuclear factor-kappa B, testosterone, tissue factor pathway inhibitor
Introduction
Although male sex has been found to be one of the independent risk factors for cardiovascular diseases 1, it is still controversial whether androgens (testosterone) are detrimental to the cardiovascular system 2. Malkin et al. 3 reported testosterone's protective effect on plaque development in the arterial vessel wall. There is also evidence that one of the factors in thrombotic diseases, as well as myocardial infarction in hypogonadic males, is low baseline fibrinolytic activity 4. Although high-dose intramuscular testosterone may promote thrombosis by causing polycythemia, the direct influence of testosterone on hemostasis is unclear.
The coagulation cascade is initiated as soon as the tissue factor binds activated factor VII, which, in turn, activates factor X, ultimately leading to thrombin formation. Tissue factor may be a major contributor to increased blood thrombogenicity and accelerated thrombogenesis in patients with acute coronary syndrome 5.
Tissue factor pathway inhibitor (TFPI) is a potent inhibitor of tissue factor-induced blood coagulation, which may reduce thrombus formation by inhibiting the activated factor VII/tissue factor complex 6. As such, a decrease in TFPI would induce coagulation 7, 8. Although the function of TFPI has been well determined, its regulation is poorly understood to date.
In addition, inflammation, with the characteristic overactivation of nuclear factor-kappa B (NF-κB), is one of the critical factors involved in arteriosclerosis plaque rupture and acute thrombosis development 9. Many of the transcriptional effects of inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), are mediated by the activation of NF-κB 9.
Our earlier study observed that testosterone at physiological concentrations could increase antigen and mRNA levels of TFPI released from human umbilical vein endothelial cells (HUVECs) 10. However, the detailed cellular mechanism by which testosterone regulates TFPI expression is largely unknown. This study, therefore, was undertaken to investigate the effect of testosterone on NF-κB activity and to study the association between NF-κB activity and TFPI gene expression. On the basis of the findings described here, we discuss further the protective effect of testosterone on inflammation.
Materials and methods
Cell culture
Primary HUVECs, culture medium, growth factors, and supplements were obtained from ScienCell Research Laboratories (San Diego, CA, USA), and the cells were cultured and maintained according to the manufacturer's instructions. All studies were carried out using a third to fourth passage of cultured cells. Enzyme-linked immunosorbent assay analyses were carried out in 96-well plates. Real-time reverse transcription polymerase chain reaction (RT-PCR) and electrophoretic mobility shift assay were carried out in 25-cm2 flasks at a density of 105 cells mL−1.
Assays of TFPI antigen
TFPI total antigens in supernatants were assayed with a commercial enzyme-linked immunosorbent assay (ADI, Stamford, CT, USA). The experimental procedure followed was according to the kit's instructions.
Quantification of TFPI mRNA
HUVECs were divided into four groups for treatment for 48 h: controls, culture medium; testosterone group of a physiological concentration of 30 nmol L−1; 2 h pretreatment with TNF-α group (20 ng mL−1) before testosterone (30 nmol L−1); and TNF-α (20 ng mL−1). Total RNA was isolated from HUVECs according to the manufacturer's recommendations (TaKaRa, Kagosima, Japan). Cellular TFPI mRNA levels were determined by real-time RT-PCR with the following primers: TFPI (109 bp), 5′-GGCTTCTGTATGCCTGCTGCTTA-3′ (forward primer), 5′-TTTCAGTGGTGGCAACTCCGTA-3′(reverse primer) and β-actin (132 bp), 5′-CCACGAAACTACCTTCAACTCC-3′ (forward primer), 5′-GTGATCTCCTTCTGCATCCTGT-3′ (reverse primer). The procedure included a step with DNAse I treatment (TaKaRa). Real-time RT-PCR was performed using the TaKaRa PCR Thermal Cycler Dice Real Time System containing 2 μL cDNA, 12.5 μL SYBR Premix Ex Taq (2 ×), and 0.5 μL Primer F/R in 25 μL final volume. The reactions were carried out in triplicate. Relative TFPI mRNA levels were determined in reference to β-actin mRNA as an internal standard.
Nuclear extraction and electrophoretic mobility shift assay
For the TNF-α and NF-κB pathway study, HUVECs were divided into four groups for treatment for 12 h: controls, culture medium; testosterone group, physiological concentration (30 nmol L−1); 2 h pretreatment with TNF-α group (20 ng mL−1) before testosterone (30 nmol L−1); and TNF-α (20 ng mL−1). The nuclear extract from cells was prepared according to the procedures described by Dignam et al. 11. Nuclear extracts (5 μg μL−1) were mixed with DNA probes (10 ng μL−1) and the mixture was then incubated at 15°C for 30 min. Half of the mixture was loaded onto a 6% polyacrylamide gel and electrophoresed at 120 V in 0.5% Tris-borate EDTA. The sample was then transferred in 0.5% Tris-borate EDTA onto a nylon membrane at 300 mA for 20 min. After transfer, the sample was fixed on the membrane by UV crosslinking.
Statistics
The protein data were expressed as mean ± SD and tested for statistical significance using the unpaired two-tailed t-test. The PCR data were expressed as mean ± SD and tested for statistical significance with the Relative Expression Software Tool (Technical University of Munich, Munich, Germany). P <0.05 was considered statistically significant.
Results
TFPI protein concentration in HUVECs treated with TNF-α and testosterone
We pretreated HUVECs with TNF-α, a known activator of NF-κB, and found that TNF-α significantly suppressed TFPI expression. Figure 1 shows that the antigen levels of TFPI after TNF-α treatment were lower than those of control cells (P < 0.001). The amount of TFPI increased on co-incubation with the combination of testosterone and TNF-α rather than by TNF-α treatment alone (P < 0.05), although this was still less than those of the controls.
Figure 1.
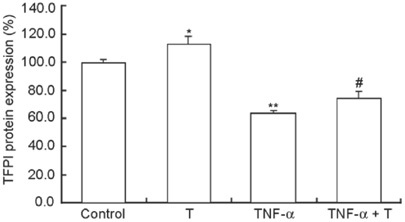
Effect of testosterone (T) with or without tumor necrosis factor-alpha (TNF-α) on tissue factor pathway inhibitor (TFPI) antigen levels in human umbilical vein endothelial cells (HUVECs). Endothelial cells were treated for 48 h with physiological testosterone (30 nmol L−1), 20 ng mL−1 TNF-α or a TNF-α and testosterone combination. TFPI levels in supernatants of TNF-α samples were significantly decreased by 37.3% at 48 h compared with controls. Compared with TNF-α-treated cells, T increased the TFPI antigen level markedly. *P < 0.05, compared with control; **P < 0.001, compared with control; #P < 0.05, compared with TNF-α (n = 4).
TFPI mRNA expression in HUVECs treated with TNF-α and testosterone
We quantified TFPI mRNA expression following TNF-α or testosterone treatment. The constitutive expression of the TFPI gene was noted in untreated HUVECs and was greatly diminished by treatment with TNF-α alone. Compared with TNF-α cultured cells, testosterone treatment resulted in substantial upregulation of the TFPI gene (Figure 2).
Figure 2.
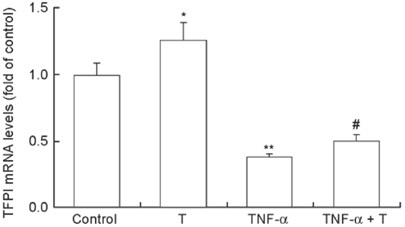
Effect of testosterone (T) with or without tumor necrosis factor-alpha (TNF-α) on tissue factor pathway inhibitor (TFPI) expression in human umbilical vein endothelial cells (HUVECs). Endothelial cells were treated for 48 h with T (30 nmol L−1), 20 ng mL−1 TNF-α or a TNF-α and T combination. TFPI mRNA was quantified by real-time reverse transcription polymerase chain reaction (RT-PCR) analysis. Differences were accounted for by using β-actin mRNA as an internal standard. The data shown are the mean ± SD of three independent experiments. As reported earlier, compared to control cells, TFPI mRNA levels of testosterone-treated cells were significantly increased. TNF-α inhibited TFPI gene expression greatly, and T reversed declines in TNF-α-induced TFPI mRNA levels. *P < 0.05, compared with control; **P < 0.001, compared with control; #P < 0.05, compared with TNF-α.
Testosterone inhibited NF-κB activity in HUVECs
NF-κB is a well-studied trans-cription factor known to be induced by TNF-α during inflammation. We investigated the inhibitory effect of testosterone with electrophoretic mobility shift assays and found that NF-κB activity was less than half of the control cells in the testosterone-treated group (P < 0.05). Compared with the TNF-α group, testosterone and TNF-α co-incubation significantly decreased NF-κB DNA-binding activity (P < 0.05) (Figure 3).
Figure 3.
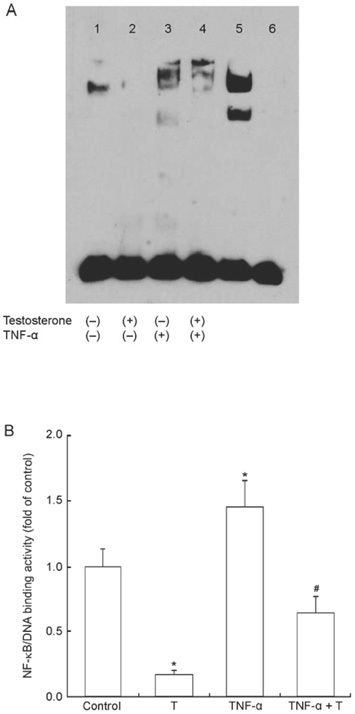
(A): Electrophoretic mobility shift assay (EMSA) result of nuclear factor-kappa B (NF-κB) activity following testosterone (T) incubation or pretreatment of human umbilical vein endothelial cells (HUVECs) with tumor necrosis factor-alpha (TNF-α). Lane 1: control; lane 2: treated with testosterone for 12 h; lane 3: treated with TNF-α for 12 h; lane 4: pretreated with TNF-α for 2 h and then treated with T for 12 h; lane 5: positive sample; lane 6: negative control. (B): NF-κB/DNA-binding activity influenced by T as determined by EMSA. Compared to that of the controls and TNF-α-treated cells, testosterone markedly suppressed NF-κB/DNA-binding activity by nuclear extracts from HUVECs. *P < 0.05, compared with control; #P < 0.05, compared with TNF-α-treated group.
Discussion
Our experiments show that physiological testosterone concentrations significantly decrease TNF-α-induced procoagulant activity in HUVECs by stimulating TFPI expression compared with TNF-α administration at both protein and mRNA levels. This was associated with a marked decrease in NF-κB DNA-binding activity in HUVECs.
Currently, vascular clot formation is characterized as an equilibrium between procoagulant and anticoagulant activities 12. We have reported earlier that physio-logical testosterone concentrations (30 nmol L−1) could activate TFPI release and expression in endothelial cells 10. We assume, therefore, that the upregulation of TFPI expression by testosterone in males may help to inhibit thrombin generation and contributes to reduction in blood coagulation in normal conditions. Inflammation, meanwhile, has been confirmed to be a factor in the promotion of thrombosis 13. In addition, the central role of TNF-α in inflammation has been shown by the ability of agents that block the action of TNF-α to treat a range of inflammatory conditions 14. Therefore, this study aimed to further investigate whether testosterone may be a factor in TFPI expression in response to inflammation.
We found that testosterone did influence the activities of many transcription factors through androgen receptor pathways in HUVEC in males, among whom the significant suppression of NF-κB was considered to be especially interesting (data not shown). We subsequently confirmed the results with an electrophoretic mobility shift assay. It is well known that NF-κB is a major transcription factor regulating many target genes, including cellular adhesion molecules, interleukins, and tissue factors 15. TNF-α or low-dose intratracheal lipopolysaccharide-induced NF-κB activation is a prominent feature during inflammation, which has been proven to be crucial in thrombosis formation 16, 17. This study indicates that testosterone may have an anti-inflammatory effect by marked suppression of NF-κB activity. This result, in part, is in accordance with other reports that have shown that testosterone inhibited TNF-α-induced activation of the transcriptional NF-κB during inflammation in human aortic endothelial cells 18, 19. Moreover, some clinical studies have also found that testosterone may suppress the expression of proinflammatory cytokines, including TNF-α and interleukin-6 20.
Although NF-κB predominantly acts as a trans-criptional activator, there is a small but growing list of examples in which it can act as a repressor 9. We hypothesized that NF-κB may act as a negative regulating element on TFPI expression, especially during inflammation, and that physiological testosterone may have a beneficial effect on TFPI at the transcription level, in part by suppressing NF-κB activity. To prove this, we further investigated the effect of testosterone on TFPI protein and mRNA levels of HUVEC preincubated with TNF-α to activate NF-κB. The results, as we had hypothesized, showed that TNF-α significantly decreased TFPI protein and mRNA levels by further reinforcing the inhibiting effects of NF-κB, which is consistent with another investigation of TNF-α-induced TFPI downregulation 21. However, in comparison with the TNF-α-treated group, TFPI levels were higher in the testosterone and TNF-α co-treated group, although they were still lower than those of the controls, indicating that TNF-α-mediated TFPI gene suppression was partially blocked by testosterone. Interestingly, a recent study also revealed a novel repressor element in the promoter of TFPI 22, which we hypothesize might include an NF-κB binding site. Further investigations are needed to confirm this hypothesis.
In conclusion, this study uncovered a mechanism by which testosterone at physiological concentrations could specifically inhibit thrombosis development by promoting TFPI secretion in vascular endothelial cells during inflammation, which may provide a novel insight into the pathogenesis of thrombotic diseases in aging or hypogonadal males. Other testosterone-regulating pathways, including transcription factors such as SP1, are under further investigation in our laboratory.
Acknowledgments
This project was supported by grants from the National Natural Science Foundation of China (No. 30670842) and the Natural Science Foundation of Guangdong Province, China (No. 5300582).
References
- Jones RD, Nettleship JE, Kapoor D, Jones HT, Channer KS. Testosterone and atherosclerosis in aging men: purported association and clinical implications. Am J Cardiovasc Drugs. 2005;5:141–54. doi: 10.2165/00129784-200505030-00001. [DOI] [PubMed] [Google Scholar]
- Gooren L. Androgen deficiency in the aging male: benefits and risks of androgen supplementation. J Steroid Biochem Mol Biol. 2003;85:349–55. doi: 10.1016/s0960-0760(03)00206-1. [DOI] [PubMed] [Google Scholar]
- Malkin CJ, Pugh PJ, Jones RD, Jones TH, Channer KS. Testosterone as a protective factor against atherosclerosis--immunomodulation and influence upon plaque development and stability. Endocrinology. 2003;178:373–80. doi: 10.1677/joe.0.1780373. [DOI] [PubMed] [Google Scholar]
- Winkler UH. Effects of androgens on haemostasis. Maturitas. 1996;24:147–55. doi: 10.1016/s0378-5122(96)82004-4. [DOI] [PubMed] [Google Scholar]
- Toschi V, Gallo R, Lettino M, Fallon JT, Gertz SD, et al. Tissue factor modulates the thrombogenicity of human atherosclerotic plaques. Circulation. 1997;95:594–9. doi: 10.1161/01.cir.95.3.594. [DOI] [PubMed] [Google Scholar]
- Francischetti IM, Mather TN, Ribeiro JM. Penthalaris, a novel recombinant five-Kunitz tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick vector of Lyme disease, Ixodes scapularis. Thromb Haemost. 2004;91:886–98. doi: 10.1160/TH03-11-0715. [DOI] [PubMed] [Google Scholar]
- Tang H, Ivanciu L, Popescu N, Peer G, Hack E, et al. Sepsis-induced coagulation in the baboon lung is associated with decreased tissue factor pathway inhibitor. Am J Pathol. 2007;171:1066–77. doi: 10.2353/ajpath.2007.070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroney SA, Cooley BC, Sood R, Weiler H, Mast AE. Combined tissue factor pathway inhibitor and thrombomodulin deficiency produces an augmented hypercoagulable state with tissue specific fibrin deposition. J Thromb Haemost. 2008;6:111–7. doi: 10.1111/j.1538-7836.2007.02817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn RH, Deming CB, Johns DC, Champion HC, Bian C, et al. Regulation of endothelial thrombomodulin expression by inflammatory cytokines is mediated by activation of nuclear factor-kappa B. Blood. 2005;105:3910–7. doi: 10.1182/blood-2004-03-0928. [DOI] [PubMed] [Google Scholar]
- Jin H, Lin J, Fu L, Mei YF, Peng G, et al. Physiological testosterone stimulates tissue plasminogen activator and tissue factor pathway inhibitor and inhibits plasminogen activator inhibitor type 1 release in endothelial cells. Biochem Cell Biol. 2007;85:246–51. doi: 10.1139/O07-011. [DOI] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–89. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirshahi F, Vasse M, Tedgui A, Li H, Merval R, et al. Oncostatin M induces procoagulant activity in human vascular smooth muscle cells by modulating the balance between tissue factor and tissue factor pathway inhibitor. Blood Coagul Fibrinol. 2002;13:449–55. doi: 10.1097/00001721-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Cai H, Song C, Endoh I, Goyette J, Jessup W, et al. Serum amyloid A induces monocyte tissue factor. J Immunol. 2007;178:1852–60. doi: 10.4049/jimmunol.178.3.1852. [DOI] [PubMed] [Google Scholar]
- Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–60. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- Cirillo P, Pacileo M, DE Rosa S, Calabrò P, Gargiulo A, et al. Neopterin induces pro-atherothrombotic phenotype in human coronary endothelial cells. J Thromb Haemost. 2006;4:2248–55. doi: 10.1111/j.1538-7836.2006.02125.x. [DOI] [PubMed] [Google Scholar]
- Speiser W, Kapiotis S, Kopp CW, Simonitsch I, Jilma B, et al. Effect of intradermal tumor necrosis factor-alpha-induced inflammation on coagulation factors in dermal vessel endothelium. An in vivo study of human skin biopsies. Thromb Haemost. 2001;85:362–7. [PubMed] [Google Scholar]
- Fox EA, Kahn SR. The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb Haemost. 2005;94:362–5. doi: 10.1160/TH05-04-0266. [DOI] [PubMed] [Google Scholar]
- Hatakeyama H, Nishizawa M, Nakagawa A, Nakano S, Kigoshi T, et al. Testosterone inhibits tumor necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in human aortic endothelial cells. FEBS Lett. 2002;530:129–32. doi: 10.1016/s0014-5793(02)03440-3. [DOI] [PubMed] [Google Scholar]
- Norata GD, Tibolla G, Seccomandi PM, Poletti A, Catapano AL. Dihydrotestosterone decreases tumor necrosis factor -alpha and lipopolysaccharide induced inflammatory response in human endothelial cells. J Clin Endocrinol Metab. 2006;91:546–54. doi: 10.1210/jc.2005-1664. [DOI] [PubMed] [Google Scholar]
- Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–8. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhou S, Heng CK. Impact of serum amyloid A on tissue factor and tissue factor pathway inhibitor expression and activity in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:1645–50. doi: 10.1161/ATVBAHA.106.137455. [DOI] [PubMed] [Google Scholar]
- Amini Nekoo A, Iles D. Analysis of a T-287C polymorphism in the tissue factor pathway inhibitor gene and identification of a repressor element in the promoter. Thromb Res. 2008;121:813–9. doi: 10.1016/j.thromres.2007.08.012. [DOI] [PubMed] [Google Scholar]


