Abstract
Poor spermatogenesis in patients with inflammation of the genital tract is associated with scrotal hyperthermia. These patients can benefit from acupuncture treatment. We conducted a study to verify whether the influence of acupuncture treatment on sperm output in patients with low sperm density is associated with a decrease in scrotal temperature. The experimental group included 39 men who were referred for acupuncture owing to low sperm output. The control group, which comprised 18 normal fertile men, was used to define a threshold (30.5°C) above which scrotal skin temperature was considered to be high. Accordingly, 34 of the 39 participants in the experimental group initially had high scrotal skin temperature; the other five had normal values. Scrotal skin temperature and sperm concentration were measured before and after acupuncture treatment. The five patients with initially normal scrotal temperatures were not affected by the acupuncture treatment. Following treatment, 17 of the 34 patients with hyperthermia, all of whom had genital tract inflammation, had normal scrotal skin temperature; in 15 of these 17 patients, sperm count was increased. In the remaining 17 men with scrotal hyperthermia, neither scrotal skin temperature nor sperm concentration was affected by the treatment. About 90% of the latter patients suffered from high gonadotropins or mixed etiological factors. Low sperm count in patients with inflammation of the genital tract seems to be associated with scrotal hyperthermia, and, consequently, acupuncture treatment is recommended for these men.
Keywords: acupuncture, poor spermatogenesis, scrotal skin hyperthermia
Introduction
The concept that an elevation of scrotal skin temperature results in impairment of spermatogenesis is widely accepted 1, 2, 3, 4. Several authors believe that scrotal hyperthermia is a modern lifestyle condition associated with tight-fitting, thermally insulating clothing 5, sedentary work (especially driving a vehicle for prolonged periods) 6, obesity 7, exposure to electromagnetic waves 7 and use of a laptop computer 8. Studies performed in bulls showed that dietary energy 9 and gonadotropin-releasing hormone (GnRH) treatment 10 may also increase scrotal skin temperature.
Studies of infertile men with low sperm output have consistently shown that they have higher scrotal temperatures compared with fertile controls 11, 12, 13, 14. Over the years, the inverse correlation between sperm output and hyperthermia has prompted a number of therapeutic options for the reduction of scrotal temperature, such as an evaporative cooling device 14, scrotal cooling by applying ice packs 15 and nocturnal scrotal cooling by periscrotal air circulation 13, 16.
In our earlier controlled study, we showed that acupuncture treatment is an additional therapeutic method that improves sperm output in men suffering from azoospermia or severe oligozoospermia 17. The aim of this study was to verify whether the influence of acupuncture treatment on sperm output is associated with a decrease in scrotal temperature.
Materials and methods
Experimental group
The experimental group comprised 39 primarily infertile men who were referred for acupuncture treatment and who agreed to participate in this study. Their average age was 36.5 ± 3.4 years. Twenty-six of the infertile men were defined as azoospermic (total sperm count = 0), nine others had severe oligozoospermia (0 < sperm concentration < 5 × 106 spermatozoa per mL) and the remaining four were diagnosed as oligozoospermic (5 × 106 ≤ sperm concentration < 20 × 106 spermatozoa per mL, Table 1). A low sperm concentration in each patient was confirmed by at least three consecutive routine semen examinations. The last sperm examination was per-formed at the Bar Ilan Male Fertility Laboratory (Ramat Gan, Israel)not more than one month prior to the commencement of acupuncture treatment.
Table 1. Association between sperm concentration and scrotal skin temperature before and after acupuncture treatment.
| Before acupuncture treatment |
After acupuncture treatment |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup | Patient | Etiological profile | Temp. category | Scrotal skin temp. | Androl. profile | Sperm concentration per mL | Temp. category | Scrotal skin temp. | Sperm concentration per mL | Treatment success |
| A (n = 5) | 1 | hFSH | N | 30.25 | AZO | 0 | N | 30.23 | 0 | NO |
| 2 | hFSH | N | 30.47 | AZO | 0 | N | 30.17 | 0 | NO | |
| 3 | hFSH | N | 30.00 | AZO | 0 | N | 30.05 | 0 | NO | |
| 4 | hFSH | N | 30.33 | AZO | 0 | N | 30.35 | 0 | NO | |
| 5 | hFSH | N | 30.44 | AZO | 0 | N | 30.42 | 0 | NO | |
| B (n = 17) | 6 | IN+hFSH | H | 30.58 | AZO | 0 | H | 30.69 | 0 | NO |
| 7 | hFSH | H | 31.30 | AZO | 0 | H | 30.76 | 0 | NO | |
| 8 | hFSH | H | 31.15 | AZO | 0 | H | 31.02 | 0 | NO | |
| 9 | IN+hFSH | H | 31.02 | AZO | 0 | H | 31.35 | 0 | NO | |
| 10 | hFSH | H | 31.62 | AZO | 0 | H | 31.33 | 0 | NO | |
| 11 | hFSH | H | 31.51 | AZO | 0 | H | 30.88 | 0 | NO | |
| 12 | IN+V | H | 31.01 | AZO | 0 | H | 30.95 | 0 | NO | |
| 13 | IN | H | 30.74 | AZO | 0 | H | 31.07 | 0 | NO | |
| 14 | IN+V | H | 31.28 | AZO | 0 | H | 31.54 | 0 | NO | |
| 15 | IN+V | H | 31.22 | AZO | 0 | H | 31.09 | 0 | NO | |
| 16 | IN | H | 30.63 | AZO | 0 | H | 30.75 | 40* | NO | |
| 17 | RA | H | 31.10 | AZO | 0 | H | 31.24 | 50* | NO | |
| 18 | hFSH | H | 31.19 | AZO | 0 | H | 31.05 | 80* | NO | |
| 19 | IN+V | H | 30.63 | SO | 2.5 × 106 | H | 30.66 | 2.3 × 106 | NO | |
| 20 | V | H | 30.87 | O | 5 × 106 | H | 31.30 | 4.3 × 106 | NO | |
| 21 | IN+V | H | 31.76 | SO | 0.7 × 106 | H | 31.65 | 4 × 106 | NO | |
| 22 | IN+V | H | 31.54 | O | 7 × 106 | H | 31.06 | 0.8 × 106 | NO | |
| C (n = 17) | 23 | IN | H | 30.59 | AZO | 0 | N | 30.08 | 50MSOME | NO |
| 24 | IN | H | 30.75 | O | 8 × 106 | N | 29.22 | 8.7 × 106 | NO | |
| 25 | IN | H | 30.62 | AZO | 0 | N | 29.67 | 180* | Yes | |
| 26 | IN+V | H | 31.12 | AZO | 0 | N | 29.39 | 180* | Yes | |
| 27 | IN | H | 30.73 | AZO | 0 | N | 29.50 | 350* | Yes | |
| 28 | IN | H | 30.59 | AZO | 0 | N | 30.39 | 500* | Yes | |
| 29 | IN+hFSH | H | 31.00 | AZO | 0 | N | 30.43 | 3 × 103 | Yes | |
| 30 | IN+hFSH | H | 30.77 | AZO | 0 | N | 29.99 | 4 × 104 | Yes | |
| 31 | IN | H | 30.86 | AZO | 50 | N | 30.45 | 3 × 106 | Yes | |
| 32 | IN | H | 30.80 | SO | 1.2 × 106 | N | 29.72 | 48 × 106 | Yes | |
| 33 | IN | H | 31.44 | SO | 1.3 × 106 | N | 30.41 | 28 × 106 | Yes | |
| 34 | IN | H | 30.79 | SO | 1.4 × 106 | N | 30.12 | 9 × 106 | Yes | |
| 35 | IN | H | 30.80 | SO | 2 × 106 | N | 29.89 | 5.2 × 106 | Yes | |
| 36 | IN | H | 30.89 | SO | 2.1 × 106 | N | 29.81 | 10.0 × 106 | Yes | |
| 37 | IN | H | 30.63 | SO | 3 × 106 | N | 29.24 | 15 × 106 | Yes | |
| 38 | IN+V | H | 30.78 | SO | 4.5 × 106 | N | 29.86 | 12.2 × 106 | Yes | |
| 39 | IN | H | 30.75 | O | 9.2 × 106 | N | 30.24 | 45.0 × 106 | Yes | |
Abbreviations: AZO, azoospermia; H, high scrotal temperature; hFSH, high flooicle stimulating hormone level; IN, inflammation of the genital tract; MSOME, Motile Sperm Organelle Morphology Examination; N, normal scrotal temperature; O, oligozoospermia; R, radiation; SO, severe oligozoospermia; V, varicocele.
Number of sperm cells per ejaculate observed by MSOME.
Thirteen participants in the experimental group had more than 106 white blood cells (WBC) in their ejaculates and positive semen culture and were there-fore defined as suffering from an inflammation of the genital tract. Thirteen other patients had high levels of follicle stimulating hormone (FSH) compared with local laboratory standards. A mixed etiology of inflammation of the genital tract and high FSH levels was obtained in four men. One patient suffered from isolated palpable grade II varicocele, and eight other participants in the experimental group had a mixed etiology of palpable grade II or III left varicocele and inflammation of the genital tract. One patient had undergone radiation therapy 10 years earlier (Table 1).
None of the 39 participants in the experimental study group had any surgical pathology or had under-gone any treatment, including antibiotic treatment, for inflammation of the genital tract. All participants said they had never smoked or taken drugs. The mean pregnancy expectation time in the experimental group was 4.3 ± 2.6 years.
Experimental subgroups
Following analysis of the distribution of scrotal skin temperatures in the control group, 30.5°C was defined as the cutoff point between normal and high values for this parameter. Subsequently, the patients included in the experimental study group were subdivided into three subgroups–A, B and C–according to their mean scrotal skin temperatures before and after acupuncture treatment (see the Results section).
Control group
Eighteen normal control volunteers, aged 36.6 ± 5.6 years, were selected according to the following criteria: spontaneous pregnancy achieved within not more than 1 year of pregnancy expectation, with the last pregnancy not more than 1 year prior to examination; no diagnosed varicocele; and no pathology in the urogenital system. All volunteers said they had never smoked or taken drugs.
Acupuncture treatment
Each patient in the experimental group underwent a course of 8–10 acupunctural treatments (two treatments a week). The treatment was finished when, by the criteria of Chinese medicine, an energetic balance was achieved, and, in parallel, the patient experienced a permanent feeling of very heavy testicles.
Sterile disposable stainless steel needles (0.25 mm × 25 mm) were inserted in acupuncture point locations. The depth of needle insertion at each point was determined according to the accepted rules of the acupuncture treatment (the Cooperative Group of Shandong Medical College and Shandong College of Traditional Chinese Medicine, 1982). Rotation of the needle caused caused soreness, numbness or distention around the point. The needles were left in for 25 min and then removed.
In accordance with the principles of traditional acupuncture and syndrome diagnosis 18, acupuncture points appropriate for the deficiency of the kidneys (hormonal imbalance) and damp-heat syndromes (inflammation of the genital tract) were regarded as the main points. Points Sp-6 (Sanyinjiao), Ren-4 (GuanYuan), Lu-7 (Liegue), KI-6 (Zhohai) and ST-30 (Qicong) were used for both syndromes. Four additional main points–KI-3 (Taixi), BL-23 (Shenshu), KI-11 (Henggu) and BL-52 (Zhishi)–were used only for the kidney-yang deficiency syndrome (spermatogenic failure). At all these points, the needles were inserted using the reinforcing method.
Five other main points–Sp-9 (Yinlingquan), Liv-5 (Ligou), Li-11 (Quchi), ST-28 (Shuidao) and Gb-41 (Zuliqi)–were used only for damp-heat in the genital system (inflammation of the genital tract). The needles were inserted at five points using the reducing method. The following acupuncture points, which, according to the principles of traditional acupuncture, are not associated with the kidney-yang deficiency or damp-heat syndromes, were considered secondary points: LI-4 (Hegu), ST-36 (Zusanli), SP-10 (Xuehai), HT-7 (Shenmen), Bl-20 (Pishu), PC-6 (Neiguan), Ren-1 (Huiyin), Ren-2 (Qugu), Ren-6 (Qihai), Du-4 (Mingmen), Du-20 (Baihui), Gb-20 (Fengchi), Liv-3 (Taichong), KI-7 (Fulu) and Gb-27 (Wushu). Specific combinations of main and secondary points were selected for each patient during treatment, according to the principles of traditional acupuncture 18. No more than 12 points were punctured during any single session.
Measurement of femoral and scrotal skin temperatures
Temperature measurement was performed with the Cont-Flex m® system (IPS, Milan, Italy), using the plate in accordance with the manufacturer's instructions. The temperature of the femur and the temperatures of the left and right hemiscrota in the experimental group were evaluated prior to the first acupuncture treatment for the initial values and prior to the last acupuncture treatment for post-treatment values. These evaluations were performed with the patient lying on his back, undressed, with legs spread apart and bent at the knees, after remaining for 10 min at a room temperature of 22–24°C. In accordance with the manufacturer's instructions for identifying the appropriate thermographic plate to be used for obtaining simultaneous images, a plate was placed on the scrotum and the upper interior part of the femur. If it displayed the intermediate colors (green, light blue or violet), the plate had the correct thermal sensitivity for thermographic measurement. We found the thermographic plate RW 29 ST (IPS, Milan, Italy), with color changes at 28.3°C, 29.0°C, 29.5°C, 30.1°C, 30.6°C, 31.3°C, 32.3°C and 33.3°C, to be the one most suitable for our purposes. Indeed, the values obtained with this plate conform to the expected scrotal temperatures of healthy adult men after physiological cooling (28–32°C), according to the manufacturer's instructions. We adapted a digital Nikon E 950 Coolpix 950 photo camera (Nikon Corporation Imaging Products Division Shinagawa-Ku, Tokyo, Japan) instead of the traditionally used Polaroid Image 2 system. The pictures taken by this camera were analyzed using a special computer program developed in our laboratory. The software, which was designed to allow a quantitative analysis of the patient's condition using thermographic data, provides two major components: data extraction and data storage/analysis. The extraction consists of the processing of a digital thermal image, extraction of a temperature average and breakdown of a specified region. This computer program enabled us to calculate the femoral temperature and the temperatures of each hemiscrota.
No statistical difference was obtained between the skin temperatures of the left and right hemiscrota in any of the studied individuals. The average of these two values was thus considered to be the mean scrotal skin temperature.
To verify the degree of reproducibility in tempera-ture measurement, six control men were examined five times each for their scrotal and femur temperatures, with a time interval of 10 min. The mean values of femur and scrotal temperatures in the 39 measurements were 30.01 ± 0.69°C (SE of mean = 0.08°C) and 30.74 ± 0.29°C (SE of mean = 0.04°C), respectively. The alpha model of reliability analysis showed that the five measures of these two parameters were reproducible (α = 0.92, and α = 0.94, respectively).
Femur and scrotal skin temperatures in the control group were first evaluated in all 18 control men. Only four agreed to repeat this procedure after a month; however, as no significant difference was obtained between the former and the latter measures with regard to each of the initial parameters, one measurement for all 18 controls was considered sufficient.
Sperm concentration
As the cycle of seminiferous epithelium in humans is 16 days, the semen samples from patients with azoospermia were obtained after 17 days of sexual abstinence. Semen samples from patients with severe oligozoospermia and oligozoospermia were obtained after 4 days of sexual abstinence, according to the World Health Organization (WHO) guidelines 19. The samples were collected through masturbation into sterile condoms without spermatocide.
The whole semen sample was first examined for total sperm count using routine light microscopy (LM) 19. When no sperm cells were found by this method (azoospermia), a high-power (× 6 300) examination, using an inverted light microscope equipped with Nomarski optics, enhanced by digital imaging, was performed 20. For this purpose, each milliliter of the native semen was gently placed on a two-layer Sil-Select density gradient, which consists of 1 mL upper (low density) and 0.1 mL lower (high density) layers of silane-coated colloidal silica particles suspended in HEPES-buffered Earle's balanced salt solution (EBSS; Ghent, Belgium). Centrifugation at 375 × g for 15 min at 25°C was performed. The sperm cell pellet was suspended by adding 1 mL of SPERM medium (Medi-Cult, Jyllinge, Denmark) and then re-centrifuged at 375 × g for 10 min at 25°C. The final sperm pellet obtained was gently re-suspended in about 20–50 μL of SPERM medium for observation using a high-power microscope.
The search for sperm cells was conducted in a glass-bottomed Petri dish 21. In all, 2–4 mL of the final sperm suspension was added to each of 4–8 microdroplets of 10 μmL. The SPERM medium was supplemented with 20% human albumin (D-35002; Behring GmbH, Marburg, Germany).
Acupuncture treatment was considered successful if at least one of the following occurred: (a) 100 sperm cells could be obtained by Motile Sperm Organelle Morphology Examination (MSOME) in the ejaculates of initially azoospermic patients; (b) a few spermatozoa could be obtained by LM in the ejaculates of patients in whom sperm cells could initially be observed only by MSOME; (c) severe oligozoospermia was defined in patients who initially had only a few spermatozoa obtained by LM; (d) oligozoospermia was defined in patients who initially suffered from severe oligozoosper-mia; and (e) normal sperm concentration was obtained in patients previously defined as oligozoospermic.
Statistical analysis
All statistical analyses were performed using SPSS for Windows version 10.0 (SPSS Inc., Chicago, IL, USA). Data were presented as mean ± SD. Comparison between the control and infertile groups was performed by one-way analysis of variance (ANOVA) tests. Comparison between pre- and post-treatment conditions in the infertile group was performed using paired t-tests. Because of the abnormal distribution of the scrotal skin temperature in subgroups A and C (see Results section), comparisons within these subgroups were performed by Wilcoxon signed ranks tests using non-parametric statistics. Comparisons between the three subgroups were performed using Mann-Whitney non-parametric statistics.
Results
The average femur temperatures in the fertile control group and the experimental group before and after acupuncture treatment were statistically similar (31.06 ± 0.41°C; 31.07 ± 0.44°C; and 30.96 ± 0.57°C, respectively).
The distribution of the scrotal skin temperature in the control group is shown in Figure 1A. The mean value of this parameter in the fertile men was 29.78 ± 0.36°C, with a median of 29.80°C. As this distribution was normal, the threshold between the normal and elevated scrotal skin temperatures, calculated for the population of this study, was defined to be 30.50°C (mean ± SD). Thus, scrotal skin temperature > 30.50 °C was considered high.
Figure 1.
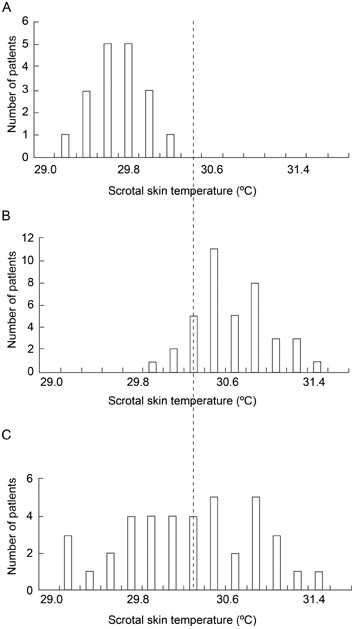
Scrotal skin temperature in the control and experimental groups: (A): control group; (B): experimental group before acupuncture treatment; (C): experimental group after acupuncture treatment.
Prior to the acupuncture treatment, the experimental group had a significantly higher average scrotal skin temperature compared with the control group (30.87 ± 0.37°C vs. 29.78 ± 0.36°C, F = 125.0, P < 0.01, Table 2, Figures 1A and 1B). After treatment, the average scrotal skin temperature in the experimental group was significantly reduced compared with the initial measurement (30.45 ± 0.63°C vs. 30.88 ± 0.39°C, t = 4.8, P < 0.01, Table 2, Figure 1C). Nonetheless, it was still significantly higher than the average scrotal skin temperature in the normal control group (30.45 ± 0.63°C vs. 29.84 ± 0.33°C, F = 9.0, P < 0.01, Table 2, Figure 1C).
Table 2. Scrotal skin and femoral temperatures in the controlfertile group, the experimental group and the three subgroups before and after acupuncture treatment.
| Scrotal skin | Femur | |
|---|---|---|
| Temperature (°C) | Temperature (°C) | |
| Control (n = 18) | 29.78 ± 0.36 | 31.06 ± 0.41 |
| Experimental study groups (n = 39) | ||
| Pre | 30.87 ± 0.37a | 31.07 ± 0.44 |
| Post | 30.45 ± 0.63a,b | 30.96 ± 0.57 |
| Subgroups of the experimental study group | ||
| A (n = 5) | ||
| Pre | 30.32 ± 0.20c | 31.13 ± 0.41 |
| Post | 30.04 ± 0.28e | 30.90 ± 0.73 |
| B (n = 17) | ||
| Pre | 31.16 ± 0.30d | 31.00 ± 0.48 |
| Post | 31.10 ± 0.27 | 31.02 ± 0.48 |
| C (n = 17) | ||
| Pre | 30.81 ± 0.23 | 31.07 ± 0.16 |
| Post | 29.90 ± 0.40b | 31.00 ± 0.63 |
Significantly higher compared with the scrotal skin temperature in the control group (P < 0.01)
Significantly lower compared with the pre-treatment condition (P < 0.01)
Significantly lower compared with the pre-treatment condition in subgroup C (P < 0.01)
Significantly higher compared with the pre-treatment condition in subgroup C (P < 0.01)
Significantly higher compared with the post-treatment condition in subgroup C (P < 0.01).
Using the calculated threshold, 34 of 39 (87.1%) members in the experimental group were defined as having a high scrotal temperature prior to treatment, and the other five had normal values of this parameter. When the same threshold was applied to the post-treatment scrotal skin temperature values in the experimental group, the patients could be divided into three subgroups: subgroup A–five patients with normal scrotal skin temperature before and after acupuncture treatment (patients 1–5, Table 1, Figure 2A); subgroup B–17 men with elevated scrotal skin temperature before and after treatment (patients 6–22, Table 1, Figure 2B); and subgroup C–17 patients who had scrotal hyperthermia before treatment and normal scrotal skin temperature after treatment (patients 23–39, Table 1, Figure 2C).
Figure 2.
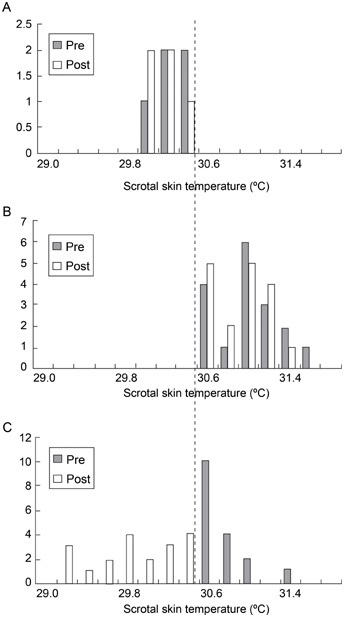
Scrotal skin temperature before (Pre) and after (Post) acupuncture treatment in the three subgroups: (A): subgroup A; (B): subgroup B; (C): subgroup C.
Within subgroups A and B, the average values of the scrotal skin temperature before and after treatment were statistically similar (Table 2, Figures 2A and 2B), whereas within subgroup C, the pre-treatment scrotal skin temperature was significantly higher than the post-treatment value (30.81 ± 0.23°C vs. 29.90 ± 0.41°C, Z = 3.6; P < 0.01, Table 2, Figure 2C). Comparison between subgroup C and the two other subgroups with regard to their pre-treatment average scrotal skin temperatures showed that the initial value of this parameter in subgroup C was significantly higher than that in subgroup A (30.81 ± 0.23°C vs. 30.32 ± 0.20°C: Z = 2.9; P < 0.01) and significantly lower than that in subgroup B (30.81 ± 0.25°C vs. 31.16 ± 0.30°C: respectively, Z = −3.3; P < 0.01, Table 2). Comparison of the post-treatment average scrotal skin temperatures showed that the value of this parameter in subgroup C was statistically similar to that in subgroup A and significantly lower than that in subgroup B (29.90 ± 0.41°C vs. 31.10 ± 0.27°C, Z = 3.3, P < 0.01, Table 2).
With respect to the etiological profile of the three subgroups, it was found that all five patients in subgroup A had high FSH levels only (Table 1). In subgroup B, nine patients suffered from isolated etiological factors, including five patients with high FSH levels (patients 7, 8, 10, 11, and 18); two patients with inflammation of the genital tract (patients 13 and 16); one man with varicocele (patient 20); and one man who had been treated with radiation 10 years previously (patient 17, Table 1). The other eight patients in subgroup B had mixed etiological factors, including two patients with high FSH levels and inflammation of the genital tract (patients 6 and 9) and six patients with inflammation of the genital tract and varicocele. In subgroup C, all 17 patients had inflammation of the genital tract, including two men who additionally had high FSH levels (patients 29 and 30) and two men with additionally diagnosed varicocele (patients 26 and 38).
With respect to the success of the acupuncture treatment in increasing sperm output, none of the patients in the A and B subgroups had improvement in their sperm concentration following acupuncture treatment (Table 1). However, 15 (88.2%) of the 17 men in subgroup C had an increased sperm concen-tration following acupuncture treatment. Of the seven initially azoospermic men, four had ≥ 100 sperm cells in their ejaculates (patients 25–28) and three others became severely oligozoospermic (patients 28–31, Table 1). Of the seven initially severe oligozoospermic patients, two had normal sperm concentration (patients 32–33) and five became oligozoospermic (patients 34–38, Table 1) following acupuncture treatment. The last oligozoospermic man also had normal sperm concentration (patient 39, Table 1).
Discussion
This study confirms previous statements by other authors that the mean scrotal skin temperature in infertile men is significantly higher than that observed in fertile men 12 and that patients with low sperm output usually exhibit a scrotal temperature that is markedly higher than their body temperature 11, 12, 13, 14, 16.
To define hyperthermia, we related the scrotal temperature to the femoral one, because it enabled us to obtain simultaneous thermographic images of the goal and the reference.
The major findings of this study were that 100% of the men with inflammation of the genital tract had scrotal hyperthermia and that the initially elevated scrotal skin temperature was normalized only in patients with this etiology, including 86.7% of the men with isolated inflammation. These findings are in full agreement with the studies of Setchell 22, 23, who showed that, in mammals, testicular inflammation causes elevation of the testicular/scrotal temperature. Setchell 22, 23 assumed that high scrotal temperature leads to an increased metabolism and oxygen demand that cannot be met by the limited blood flow and thereby results in hypoxia, generation of reactive oxygen species (ROS), and, consequently, decreased sperm output. If this is the case, we can then assume that acupuncture treatment directed to the testis may enhance blood supply in the testicular artery 24, decrease the testicular temperature by heat exchange in the pampiniform plexus and interrupt the perioxidation process 25. In all probability, acupuncture does not directly kill the pathogenic micro-organisms but rather stimulates the body's immune response. Indeed, existing laboratory research corroborates the systemic immunoregulatory actions of acupuncture 26. The fact that 15 of the 17 men with normalized scrotal skin temperature had an increased sperm concentration supports this assumption.
One may argue that the men who responded to the acupuncture treatment simply experienced a coincidental increase in sperm density. However, in our earlier study 17, this possibility was excluded by using a control group of untreated patients with low sperm density.
An additional conclusion of this study fits that of Jung et al. 12 and Hjollund et al. 13, who claimed that levels of testosterone, FSH and luteinizing hormone (LH) were not associated with scrotal hyperthermia. Indeed, all five men with an isolated etiology of high FSH levels had normal scrotal temperature.
We also found that the femoral (corporal) temper-ature of the patients with low sperm density was statistically similar to that of the normal fertile men. Moreover, this parameter was not changed following acupuncture treatment. We conclude, therefore, that there is no relationship between corporal temperature and sperm production, and that the performed acupuncture treatment was specific only to the scrotum.
Two results of this study are unclear:
(a) One would expect that, in patients with varicocele, where there is a ROS-mediated damage to the sperm count 27, 28, acupuncture would interrupt the perioxidation process and thereby improve sperm output. In fact, the acupuncture treatment was useful for only two of the eight patients with varicocele. To verify this unsuitability, further measurement of ROS levels before and after treatment should be performed in varicocele cases.
(b) We cannot yet explain why two mixed cases with inflammation and high FSH levels were not affected by the acupuncture treatment, whereas two others showed normalization of their scrotal skin temperature and an increase in their sperm concentration. This phenomenon may be related to the question of which of the mixed etiologies is the dominant one and will require further investigation. On the other hand, the result of the acupuncture treatment in the unsuccessful subgroup B may have been affected by the initially higher scrotal skin temperature than that in the successful subgroup C. Perhaps prolonged acupuncture treatment could be beneficial for mixed cases.
This study did not deal with the possible association between sperm quality and scrotal skin temperature. However, other authors came to a conclusion that acupuncture has a positive effect on sperm integrity 29, 30. Our previous investigations also showed that sperm activity (including percentage of viability and motility), as well as the integrity of the axoneme, was significantly increased on treatment 31. The question of whether this influence is associated with a decrease in scrotal temperature should be further verified.
In conclusion, low sperm count in patients with inflammation of the genital tract is associated with scrotal hyperthermia, and, consequently, these men can benefit from acupuncture treatment.
Footnotes
Edited by Dr Premendu Prekash Mathur
References
- Jung A, Schuppe HC. Influence of genital heat stress on semen quality in humans. Andrologia. 2007;39:203–15. doi: 10.1111/j.1439-0272.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- Lue YH, Lasley BL, Laughlin LS, Swerdloff RS, Hikim AP, et al. Mild testicular hyperthermia induces profound transitional spermatogenic suppression through increased germ cell apoptosis in adult cynomolgus monkeys (Macaca fascicularis) J Androl. 2000;23:799–805. [PubMed] [Google Scholar]
- Dada R, Gupta NP, Kucheria K. Spermatogenic arrest in men with testicular hyperthermia. Teratogen Carcinogen Mutagen. 2003. pp. 235–43. [DOI] [PubMed]
- Wang C, Cui YG, Wang XH, Jia Y, Sinha Hikim A, et al. Transient scrotal hyperthermia and levonorgestrel enhance testosterone-induced spermatogenesis suppression in men through increased germ cell apoptosis. J Clin Endocrinol Metab. 2007;92:3292–304. doi: 10.1210/jc.2007-0367. [DOI] [PubMed] [Google Scholar]
- Jung A, Leonhardt F, Schill WB, Schuppe HC. Influence of the type of undertrousers and physical activity on scrotal temperature. Hum Reprod. 2005;20:1022–7. doi: 10.1093/humrep/deh697. [DOI] [PubMed] [Google Scholar]
- Bujan L, Daudin M, Charlet JP, Thonneau P, Mieusset R. Increase in scrotal temperature in car drivers. Hum Reprod. 2000;15:1355–7. doi: 10.1093/humrep/15.6.1355. [DOI] [PubMed] [Google Scholar]
- Jung A, Schill WB.Male infertility Current life style could be responsible for infertility MMW Fortschr Med 200014231–3.in German]. [PubMed] [Google Scholar]
- Sheynkin Y, Jung M, Yoo P, Schulsinger D, Komaroff E. Increase in scrotal temperature in laptop computer users. Hum Reprod. 2005;20:452–5. doi: 10.1093/humrep/deh616. [DOI] [PubMed] [Google Scholar]
- Coulter GH, Cook RB, Kastelic JP. Effects of dietary energy on scrotal surface temperature, seminal quality, and sperm production in young beef bulls. J Anim Sci. 1997;75:1048–52. doi: 10.2527/1997.7541048x. [DOI] [PubMed] [Google Scholar]
- Gábor G, Kastelic JP, Cook RB, Sasser RG, Brito LF, et al. Effects of GnRH treatment on scrotal surface temperatures in bulls. Can J Vet Res. 2001;65:60–3. [PMC free article] [PubMed] [Google Scholar]
- Zorgniotti AW, Sealfon AI. Measurement of intrascrotal temperature in normal and subfertile men. J Reprod Fertil. 1988;82:563–6. doi: 10.1530/jrf.0.0820563. [DOI] [PubMed] [Google Scholar]
- Jung A, Schill WB, Schuppe HC. Improvement of semen quality by nocturnal scrotal cooling in oligozoospermic men with a history. Int J Androl. 2005;28:93–8. doi: 10.1111/j.1365-2605.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- Hjollund NH, Storgaard L, Ernst E, Bonde JP, Olsen J. Impact of diurnal scrotal temperature on semen quality. Reprod Toxicol. 2002;16:215–21. doi: 10.1016/s0890-6238(02)00025-4. [DOI] [PubMed] [Google Scholar]
- Zorgniotti AW, Sealfon AI, Toth A. Chronic scrotal hypothermia as a treatment for poor semen. Lancet. 1980;1:904–6. doi: 10.1016/s0140-6736(80)90839-9. [DOI] [PubMed] [Google Scholar]
- Mulcahy JJ. Scrotal hypothermia and the infertile man. J Urol. 1984;132:469–70. doi: 10.1016/s0022-5347(17)49694-7. [DOI] [PubMed] [Google Scholar]
- Jung A, Eberl M, Schill WB. Improvement of semen quality by nocturnal scrotal cooling and moderate beha-vioural change to reduce genital heat stress in men with oligoasthenozoospermia. Reproduction. 2001;121:595–603. doi: 10.1530/rep.0.1210595. [DOI] [PubMed] [Google Scholar]
- Siterman S, Eltes F, Wolfson V, Lederman H, Bartoov B. Does acupuncture treatment affect sperm density in males with very low sperm count? A pilot study. Andrologia. 2000;32:31–9. [PubMed] [Google Scholar]
- Maciocia G, Kaptchuk TJ. New York: Elsevier Churchill Livingstone; 1998. Obstetrics and Gynecology in Chinese Medicine; pp. p317–32.pp. 738–9. [Google Scholar]
- World Health Organization (WHO) Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction 4thedn.Cambridge: Cambridge University Press; 1999 [Google Scholar]
- Bartoov B, Berkovitz A, Eltes F, Kogosowski A, Menezo Y, et al. Real-time fine morphology of motile human sperm cells is associated with IVF-ICSI outcome. J Androl. 2002;23:1–8. doi: 10.1002/j.1939-4640.2002.tb02595.x. [DOI] [PubMed] [Google Scholar]
- Bartoov B, Berkovitz A, Eltes F, Kogosovsky A, Yagoda A, et al. Pregnancy rates are higher with intracytoplasmic morphologically selected sperm injection than with conventional intracytoplasmic injection. Fertil Steril. 2003;80:1413–9. doi: 10.1016/j.fertnstert.2003.05.016. [DOI] [PubMed] [Google Scholar]
- Setchell BP. Ithaca: Cornell Univer-sity Press; 1978. The Mammalian Testis. [Google Scholar]
- Setchell BP. The Parkes Lecture Heat and the testis. J Reprod Fertil. 1998;114:179–94. doi: 10.1530/jrf.0.1140179. [DOI] [PubMed] [Google Scholar]
- Litscher G. Bioengineering assessment of acupuncture, part 3: Ultrasound. Crit Rev Biomed Eng. 2006;34:295–326. doi: 10.1615/critrevbiomedeng.v34.i4.20. [DOI] [PubMed] [Google Scholar]
- Yu Y, Kang J. Clinical studies on treatment of chronic prostatitis with acupuncture and mild moxibustion. J Tradit Chin Med. 2005;25:177–81. [PubMed] [Google Scholar]
- Kavoussi B, Ross BE. The neuroimmune basis of anti-inflammatory acupuncture. Integr Cancer Ther. 2007;6:251–7. doi: 10.1177/1534735407305892. [DOI] [PubMed] [Google Scholar]
- Goldstein M, Eid JF. Elevation of intratesticular and scrotal skin surface temperature in men with varicocele. J Urol. 1989;142:743–5. doi: 10.1016/s0022-5347(17)38874-2. [DOI] [PubMed] [Google Scholar]
- Barbieri ER, Hidalgo ME, Venegas A, Smith R, Lissi EA. Varicocele-associated decrease in antioxidant defenses. J Androl. 1999;20:713–7. [PubMed] [Google Scholar]
- Gurfinkel E, Cedenho AP, Yamamura Y, Srougi M. Effects of acupuncture and moxa treatment in patients with semen abnormalities. Asian J Androl. 2003;5:345–8. [PubMed] [Google Scholar]
- Pei J, Strehler E, Noss U, Abt M, Piomboni P, et al. Quantitative evaluation of spermatozoa ultrastructure after acupuncture treatment for idiopathic male infertility. Fertil Steril. 2005;84:141–7. doi: 10.1016/j.fertnstert.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Siterman S, Eltes F, Wolfson V, Zabludovsky N, Bartoov B. Effect of acupuncture on sperm parameters of males suffering from subfertility related to low sperm quality. Arch Androl. 1997;39:155–61. doi: 10.3109/01485019708987914. [DOI] [PubMed] [Google Scholar]


