Abstract
Digoxin, which is one of the most commonly prescribed drugs for the treatment of heart failure, is mainly eliminated from the circulation by the kidney. P-glycoprotein is well characterized as a digoxin pump at the apical membrane of the nephron. However, little is known about the transport mechanism at the basolateral membrane. We have isolated an organic anion transporter (OATP4C1) from human kidney. Human OATP4C1 is the first member of the organic anion transporting polypeptide (OATP) family expressed in human kidney. The isolated cDNA encodes a polypeptide of 724 aa with 12 transmembrane domains. The genomic organization consists of 13 exons located on chromosome 5q21. Its rat counterpart, Oatp4c1, is also isolated from rat kidney. Human OATP4C1 transports cardiac glycosides (digoxin, Km = 7.8 μM and ouabain, Km = 0.38 μM), thyroid hormone (triiodothyronine, Km = 5.9 μM and thyroxine), cAMP, and methotrexate in a sodium-independent manner. Rat Oatp4c1 also transports digoxin (Km = 8.0 μM) and triiodothyronine (Km = 1.9 μM). Immunohistochemical analysis reveals that rat Oatp4c1 protein is localized at the basolateral membrane of the proximal tubule cell in the kidney. These data suggest that human OATP4C1/rat Oatp4c1 might be a first step of the transport pathway of digoxin and various compounds into urine in the kidney.
Digoxin, which is clinically used as an agent for congestive heart failure and reduces the rate of hospitalizations (1), has a narrow therapeutic window and interacts with several drugs. Because digoxin is mainly eliminated to the urine (2) and it is difficult to control the serum digoxin level in renal failure, it is important to clarify the mechanism of renal tubular handling. The kidney has the essential role for elimination of various compounds from the circulation. This step is mediated by several transporters such as the organic anion transporting polypeptide (OATP) family, organic anion transporter (OAT) family, organic cation transporter (OCT) family, and ATP-binding cassette transporters (reviewed in refs. 3 and 4). P-glycoprotein (Pgp), which is MDR1 gene product and a member of the ATP-binding cassette transporter family, mediates the tubular secretion of digoxin at the apical membrane of the proximal tubule cell. Pgp has been also considered as a potential interaction site of digoxin with drugs such as quinidine, verapamil, and itraconazole (5, 6). To access Pgp, digoxin has to cross the basolateral membrane from the circulation. However, the mechanism of digoxin uptake at the basolateral membrane remains unclear.
The OATP family is involved in the membrane transport of bile acids, conjugated steroids, thyroid hormone, eicosanoids, peptides, and numerous drugs including digoxin in many tissues (7-9). Rat oatp2 transports digoxin and is considered to be involved in the interaction of digoxin with drugs in the liver (10). However, rat oatp2 is not expressed in the kidney. In rat kidney, oatp1 (11) and OAT-K1 (12) are known to be expressed. However both are expressed at the apical membrane and do not transport digoxin. Furthermore, none of the human OATP family members have been reported that transport digoxin and are predominantly expressed in the kidney. Thus, some molecules should be predicted to be involved in the basolateral transport of digoxin in human kidney. Here, we isolated a member of the OATP family termed human OATP4C1, which transports digoxin at the basolateral membrane of the proximal tubule cell in the kidney.
Materials and Methods
The compounds used for uptake assay were purchased from commercial sources. [14C]Pravastatin (588.3 MBq/mmol) and [14C]temocaprilat (536.5 MBq/mmol) are from Sankyo (Tokyo). All other chemicals and reagents were of analytical grade.
Isolation of Human OATP4C1 and Rat Oatp4c1. The GenBank database dbEST was searched with liver-specific transporter (LST)-2/OATP8 (13) by using the tblastn algorithm. One clone that has weak similarity to OATP family was identified (GenBank accession no. BF900225). Based on this sequence, a 5′-RACE reaction was performed with the reverse primer 5′-CCAGGAATTGTTCCTAATAATCGAAGGACC-3′ and adaptor primer by using the human kidney-derived adaptor-ligated Marathon-Ready cDNA library as a template (Clontech). Human kidney cDNA library containing 5 × 105 independent clones, which was constructed with the λ ZAPII (Stratagene), was screened with 1,881 bp of 5′-RACE product as a probe as described (13). Thirty positive clones were isolated, and the plasmids were rescued by in vivo excision. The sequences were determined by using CEQ2000XL sequencer (Beckman Coulter). A rat kidney library containing 5 × 105 independent clones was also screened with 1,881 bp of human 5′-RACE product under the same condition as human library screening, and 20 positive clones were isolated.
Homology Analysis. The hydropathy profile analysis was performed according to the method reported by Kyte and Doolittle (14). Multiple amino acid sequence alignments were carried out by using clustal w (www.ddbj.nig.ac.jp/E-mail/clustalw-e.html). The phylogenetic tree was described by tree view (15).
Northern Blot Analysis. Human and rat Multiple Tissue Northern blots containing 2 μg of human poly(A)+ RNA were used (Clontech). Northern blot analysis was performed as described (13). Filters were hybridized with the same probe as that used for the human library screening (1,881 bp of human OATP4C1-coding region; 9-1,889) for human clone and a fragment of rat Oatp4c1 (473 bp of rat Oatp4c1 coding region; 1,655-2,127) for rat clone. Hybridization was performed in a buffer containing 50% formamide, 5× SSC, 5× Denhardt's solution, and 1% SDS at 42°C overnight, washed in 0.1× SSC and 1% SDS at 65°C for 1 h, and exposed to an x-ray film at -80°C for 1-3 days.
Animal experiments were performed in accordance with the Guidelines for Animal Experiments of Tohoku University. Adult Sprague-Dawley rats and Wister Kyoto (WKY) rats (Charles River Breeding Laboratories) were acclimated for 1 wk. Adult male Sprague-Dawley rats were used for 5/6 nephrectomized model (16), and WKY rats were used for anti-glomerular basement membrane (GBM) Ab-injected renal failure model (17). The 5/6 nephrectomized rats and anti-GBM Ab-injected rats were maintained for 2 mo and killed. After kidneys were removed, the total RNA was extracted.
Establishment of the Permanent Cell Lines. The full coding region of human OATP4C1 and rat Oatp4c1 was inserted into pcDNA 3.1(+) (Invitrogen), and the expression construct was transfected into MDCK cells (NBL-2) purchased from the American Type Culture Collection. The expression levels of human OATP4C1 and rat Oatp4c1 mRNA in the cells were analyzed by Northern blot. Endogenous expression of OATP4C1 in MDCK cell was not detected. Localization of OATP4C1 in MDCK cell was confirmed by using GFP-OATP4C1 fusion construct (GFP was linked to the N terminus of human OATP4C1). Fusion protein was predominantly detected at the lateral membrane and, in part, subapical membrane of the MDCK cells, which is comparable to that of the LST-2/OATP8 permanent cell line (18).
Pharmacological Characterization. Cellular uptake of radiolabeled compounds was measured by using monolayer cultures grown in 24-well plates. The cell stably expressing GFP was used as a control. For transport experiments, the culture dishes were washed three times and preincubated with Krebs-Henseleit buffer (19), and uptake was initiated by adding the radiolabeled ligands to the medium. At designated times, uptake was terminated by aspirating uptake buffer and adding ice-cold choline buffer with 1% BSA. The cells were washed three times with BSA-free ice-cold choline buffer and lysed by alkalization. The radioactivity of aliquots was measured. The protein content of solubilized cell was determined by Protein Assay kit (Bio-Rad). ATP-depletion assay was performed as described (20). For efflux study, cells were preincubated for 15 min with [125I]3,5, 3′-triiodo-L-thyronine (T3), and washed three times, and then incubated again in the uptake buffer containing 0.1% BSA in the presence or absence of unlabeled 30 μM T3. The radioactivity remaining in the cells was determined.
Immunohistochemical Analysis. Synthetic peptide containing 11 aa between transmembrane domains 9 and 10 (SPDFEARAGKC) of rat Oatp4c1 was immunized into female rabbits, and the Ab was affinity-purified (13). The rat kidney specimens were stained with this Ab as reported (13). In the control experiment, primary Ab was preabsorbed with antigen peptide overnight before use.
RT-PCR for Rat Oatp4c1 with Microdissected Nephron Segments. The mRNA from microdissected nephron segments were reverse transcribed (12). A set of primers specific for the nucleotide sequence of rat Oatp4c1 was used (forward primer, 5′-GCAAGGTATTGTAGTAAATGGCCTAGT-3′; and reverse primer, 5′-AGACAACACGCAAAAGGAGATGT-3′). For Southern blot analysis, the blot was hybridized with labeled whole rat Oatp4c1 cDNA.
Results
Isolation and Structural Analysis of Human OATP4C1 and Rat Oatp4c1. The isolated cDNA encodes a human organic ion transporter, OATP4C1 (gene symbol SLCO4C1), consisting of 724 aa (Mr 78,944) (Fig. 1). Initially, we deposited it in GenBank as OATP-R (renal type OATP). Based on the database search, the same cDNA was deposited as OATP-H, OATP-X, and OATP4C1. According to the HUGO Gene Nomenclature Committee, we call it OATP4C1 (protein). Hydrophobicity analysis of the predicted human OATP4C1 suggested the presence of 12 transmembrane domains, and it was supposed that the N and C termini were located inside the cell. This feature is in accordance with the topology of other members of the OATP family (8). In human OATP4C1, there are four putative N-glycosylation sites in the predicted extracellular loops and five potential phosphorylation sites in the cytosolic portions. We have also isolated rat counterpart Oatp4c1 from rat kidney. Rat Oatp4c1 consists of 724 aa (Mr 78,651) (Fig. 1). Rat Oatp4c1 was also predicted to have 12 transmembrane domains with similar topology to human OATP4C1. In rat Oatp4c1, there are four putative N-glycosylation sites and five potential phosphorylation sites. In addition, there are two potential putative ATP-binding sites Walker motif A (21) for human OATP4C1 and one for rat Oatp4c1 at the cytosolic portion between transmembrane domain 6 and 7, which does not exist in other OATP family.
Fig. 1.
Alignment of deduced amino acid sequence of human OATP4C1 and rat Oatp4c1. The two sequences are aligned with single letter notation by inserted gaps (-) to achieve maximum homology. The 12 transmembrane domains (boxes) were assigned on the basis of hydrophobicity analysis. Potential N-glycosylation sites (filled triangles) and possible phosphorylation sites (asterisks) are indicated. Possible ATP-binding sites (bar) are also indicated.
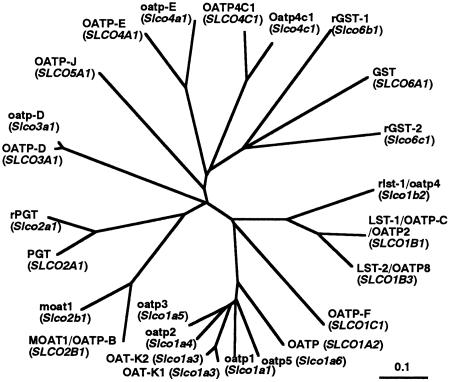
The overall homology between human OATP4C1 and rat Oatp4c1 was 80.4% at the amino acid level (Fig. 1). OATP4C1 and rat Oatp4c1 also have a moderate sequence homology to other OATP family. The overall amino acid sequence identities of human OATP4C1 with other human OATPs were 44.2% for human gonad-specific transporter (GST) (9) and 40.4% for OATP-E (22). Those of rat Oatp4c1 with other rat oatp family were 43.2% for rat oatp-E (22) and 36.4% for GST1 (9). The overall homology of human OATP4C1/rat Oatp4c1 with other OATP family was below 35% (amino acid sequences were reviewed in refs. 7-9). Phylogenetic tree analysis showed that human OATP4C1 and rat Oatp4c1 are positioned near human OATP-E, rat oatp-E, human GST, rat GST1, and GST2, and independent of other OATPs expressed in the kidney such as oatp1 and OAT-K1/K2 (Fig. 2). These data strongly suggest that human OATP4C1/rat Oatp4c1 is a member of the OATP family.
Fig. 2.
Phylogenetic relationship among human OATP4C1, rat Oatp4c1, and the OATP family. Branch lengths are drawn to scale.
Genomic Organization of Human OATP4C1. By using the human OATP4C1 cDNA sequence, a human genome database (GenBank) was searched. Human OATP4C1 gene is located on chromosome 5q21 (data not shown). It is ≈62 kb long and includes 13 exons and 12 introns.
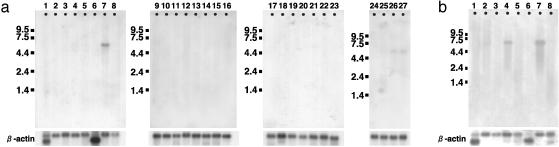
Tissue Distribution of OATP4C1 and Rat Oatp4c1. The tissue distribution of human OATP4C1 was determined by Northern blot analysis. The human OATP4C1 mRNA was predominantly expressed in the kidney (Fig. 3a). The human mRNA was also weakly detected in fetal liver and kidney (Fig. 3a). In all positive tissues, the size of the mRNA was ≈5.6 kb.
Fig. 3.
(a) Human Northern blot analysis. Lanes are as follows: 1, heart; 2, brain; 3, placenta; 4, lung; 5, liver; 6, skeletal muscle; 7, kidney; 8, pancreas; 9, spleen; 10, thymus; 11, prostate; 12, testis; 13, ovary; 14, small intestine; 15, colon; 16, peripheral blood leukocyte; 17, stomach; 18, thyroid; 19, spinal cord; 20, lymph node; 21, trachea; 22, adrenal gland; 23, bone marrow; 24, fetal brain; 25, fetal lung; 26, fetal liver; 27, fetal kidney. (b) Rat Northern blot analysis. Lanes are as follows: 1, heart; 2, brain; 3, spleen; 4, lung; 5, liver; 6, skeletal muscle; 7, kidney; 8, testis.
Tissue expression of rat Oatp4c1 mRNA was also investigated. Rat Oatp4c1 mRNA was detected mainly in the kidney and lung as a single band (5.9 kb), and a faint signal was also detected in the brain (Fig. 3b).
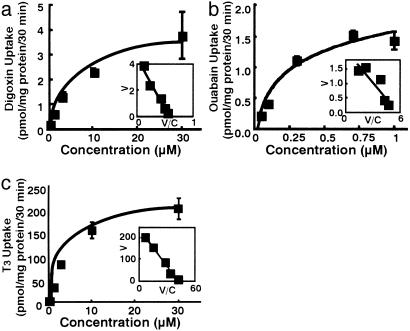
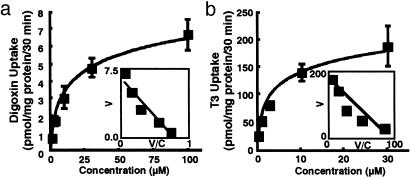
Pharmacological Analyses of Human OATP4C1. By using the human OATP4C1-expressing cell, pharmacological analyses were performed. Digoxin (0.35 ± 0.027 vs. 0.15 ± 0.004 pmol/mg protein/30 min at 0.37 μM), ouabain (2.1 ± 0.13 vs. 0.67 ± 0.07 pmol/mg protein/30 min at 0.1 μM), T3 (118 ± 2.7 vs. 26 ± 1.9 fmol/mg protein/30 min at 0.58 nM), thyroxine (T4) (3.2 ± 0.11 vs. 2.1 ± 0.18 pmol/mg protein/30 min at 0.1 μM), methotrexate (1.8 ± 0.14 vs. 1.0 ± 0.03 pmol/mg protein/30 min at 1.0 μM), and cAMP (0.76 ± 0.08 vs. 0.40 ± 0.03 pmol/mg protein/30 min at 0.23 μM) were significantly transported by human OATP4C1 compared with control (P < 0.01). On the other hand, taurocholate, estradiol 17β-d-glucuronide, prostaglandin E2, p-aminohippurate (PAH), pravastatin, temocaprilat, acetylsalicylic acid, salicylic acid, uric acid, acyclovir, ochratoxin A, benzylpenicillin, cGMP, tetraethylammonium bromide, pravastatin, and temocaprilat were not transported by human OATP4C1. Because the human OATP4C1-mediated T3 (1.0 μM and 30 μM) and digoxin (1.27 μM and 30 μM) uptake was linearly increased up to 30 min (data not shown), further uptake studies were performed within 30 min. The apparent Michaelis-Menten constant (Km) for digoxin, ouabain, and T3 were 7.8 ± 2.0 μM, 0.38 ± 0.1 μM, and 5.9 ± 2.1 μM, respectively (Fig. 4). To further analyze transport property of human OATP4C1, the driving force of human OATP4C1 was analyzed. Sodium, chloride ion, and pH did not affect human OATP4C1-mediated uptake (data not shown). Because human OATP4C1 had ATP-binding sites Walker motif A, an ATP-depletion assay was also performed. In the absence of ATP, T3 uptake was partly inhibited to 40%, indicating that ATP may not be a direct driving force of T3 uptake by human OATP4C1 (data not shown). In addition, to determine the transport direction of human OATP4C1, the efflux of T3 from MDCK/OATP4C1 was further analyzed. T3 was not excreted by means of OATP4C1 with or without extracellular T3 (data not shown). These results indicate that transport direction via OATP4C1 is mainly from outside to inside.
Fig. 4.
Kinetics of human OATP4C1-mediated digoxin (a), ouabain (b), and T3 (c) uptake into transfected MDCK cells. (Inset) Eadie-Hofstee plot. V, velocity; V/C, velocity per concentration of substrate.
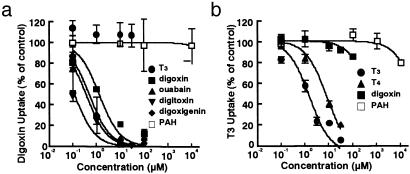
We next performed an inhibition study using digoxin and T3. Although digoxin is a preferential substrate of human OATP4C1, digoxin partially inhibited human OATP4C1-mediated T3 uptake (data not shown). These data suggested that the T3 recognition site and the digoxin recognition site by human OATP4C1 might be different. To clarify this difference, we performed inhibition experiments to estimate the inhibition value (IC50) of various compounds (Fig. 5). At first, the IC50 value of human OATP4C1-mediated digoxin uptake was measured. The estimated IC50 value of digoxin for human OATP4C1-mediated [3H]digoxin uptake was 1.3 μM. T3 and PAH did not inhibit digoxin uptake. The estimated IC50 value of digoxin (1.3 μM) was also in the similar range to the Km value of digoxin (7.8 μM). Compounds structurally related to digoxin were also used as an inhibitor for human OATP4C1-mediated [3H]digoxin uptake. The estimated IC50 values were 0.36 μM for ouabain, 0.12 μM for digitoxin, and 0.49 μM for digoxigenin. The estimated IC50 of ouabain (0.36 μM) was also similar to the Km value of ouabain (0.38 μM).
Fig. 5.
Dose-dependent inhibition by various compounds on [3H]digoxin (a) or [125I]T3 (b) uptake via human OATP4C1. The OATP4C1-mediated 0.1 μM [3H]digoxin or 0.1 μM [125I]T3 uptake was determined in the absence or presence of unlabeled compounds for 30 min.
Next, human OATP4C1-mediated [125I]T3 was inhibited by unlabeled T3,T4, and digoxin. The estimated IC50 values were 1.5 μM for T3, 8.0 μM for T4, and 540 μM for digoxin. On the other hand, PAH did not inhibit T3 uptake. The IC50 value (1.5 μM) and the Km value (5.9 μM) of T3 were also in the same range although the IC50 value of digoxin (540 μM) was 70-fold higher than the Km value of digoxin (7.8 μM).
Functional Analysis of Rat Oatp4c1. We also investigated the transport activity of rat Oatp4c1 expressed in MDCK cell. Rat Oatp4c1 also transports digoxin (2.2 ± 0.01 vs. 1.1 ± 0.01 pmol/mg protein/30 min at 3 μM) and T3 (220 ± 2.3 vs. 60 ± 1.1 fmol/mg protein/30 min at 1 nM) significantly compared with control (P < 0.01), and rat Oatp4c1-mediated T3 uptake was linear up to 30 min (data not shown). The apparent Km values for digoxin and T3 were 8.0 ± 2.2 μM and 1.9 ± 0.6 μM, respectively (Fig. 6). The Km values of rat Oatp4c1 were similar to those of human OATP4C1-mediated uptake. In addition, rat Oatp4c1-mediated uptake was also sodium- and chloride-independent (data not shown).
Fig. 6.
Rat Oatp4c1-mediated transport in transfected MDCK cell. Kinetics of rat Oatp4c1-mediated digoxin (a) and T3 (b) uptake into transfected MDCK cells. (Inset) Eadie-Hofstee plot. V, velocity; V/C, velocity per concentration of substrate.
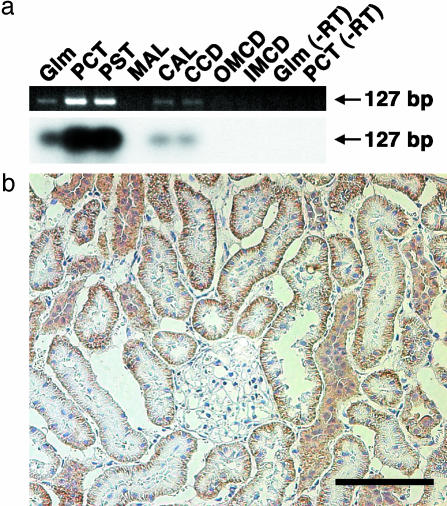
RT-PCR for Rat Oatp4c1 with Microdissected Nephron Segments. To obtain information about the localization of rat Oatp4c1 mRNA in the kidney, RT-PCR was performed by using microdissected nephron segments (Fig. 7a). A band with 127 bp was detected in the glomeruli, proximal convoluted tubule, proximal straight tubule, cortical thick ascending limb, and cortical collecting duct. A Southern blot assay also identified that the band was generated from rat Oatp4c1.
Fig. 7.
(a) RT-PCR along rat dissected nephron segment. The PCR product amplified was electrophoresed and stained with ethidium bromide (Upper). Subsequently, gels were transferred and hybridized with [32P]dCTP-labeled rat Oatp4c1 cDNA (Lower). Glm, glomerulus; PCT, proximal convoluted tubule; PST, proximal straight tubule; MAL, medullary thick ascending limb; CAL, cortical thick ascending limb; CCD, cortical collecting duct; OMCD, outer medullary collecting duct; IMCD, inner medullary collecting duct. (b) Immunohistochemistry of rat Oatp4c1 in rat kidney. The rat Oatp4c1 immunoreactivity was significantly detected at the basolateral membrane of the proximal tubule cell. (Scale bar = 100 μm.)
Immunohistochemical Analysis of Rat Oatp4c1 in the Kidney. By using polyclonal Ab, rat Oatp4c1 immunoreactivity was significantly detected at the basolateral membrane of the proximal tubule cell (Fig. 7b). The specificity of the Ab was determined by the preabsorption of the Ab with excess antigen peptide (data not shown).
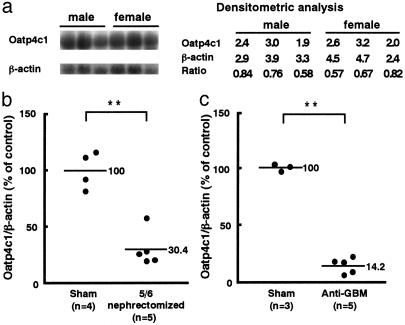
The Gender Difference of mRNA Level of Rat Oatp4c1 in Rat Kidney. Because gender difference has been known for some transporters expressed in the kidney such as oatp1 (23, 24) and OCT 2 (25), we performed Northern blot analysis with rat male and female kidney. Northern blot analysis revealed that there is no difference of the rat Oatp4c1 mRNA level between the male and female kidney (Fig. 8a).
Fig. 8.
Northern blot analysis of the rat Oatp4c1 mRNA (total RNA 20 μg per lane) in the intact male and female Sprague-Dawley rat kidneys (a), male 5/6 nephrectomized rat kidneys (b), and male anti-GBM Ab-injected WKY rat kidneys (c). **, P < 0.01, 5/6 nephrectomized rat or anti-GBM injected rat vs. sham control.
Alteration of mRNA Level in the Renal Failure Model. The 5/6 nephrectomized rat and anti-GBM Ab-injected WKY rat were used as renal failure models to investigate the alteration of Oatp4c1 mRNA level in the pathologic condition. To confirm renal insufficiency, blood urea nitrogen level was measured. The blood urea nitrogen level of 5/6 nephrectomized rat was 62.5 ± 5.58 mg/dl (sham operation rat, 12.3 ± 1.52 mg/dl) and that of anti-GBM Ab-injected WKY rat was 66.0 ± 9.77 mg/dl (sham operation rat, 18.5 ± 1.66 mg/dl). Northern blot analysis revealed that renal expression level of Oatp4c1 was significantly decreased in both 5/6 nephrectomized (30.4 ± 7.79%; P < 0.01) and anti-GBM Ab-injected WKY rat kidney (14.2 ± 3.18%; P < 0.01) (Fig. 8 b and c).
Discussion
The kidney has an important role in the elimination of various compounds from circulation into the urine. By using anionic (PAH, etc.) and cationic compounds (tetraethylammonium bromide, etc.), tubular secretion has been classified into two systems: organic anion and cation transport system (3, 4). Recently, molecules responsible for anion transport, the OAT family (OAT1 and OAT3), and cation transport, the OCT family (OCT2), have been isolated and pharmacologically characterized (3, 4). The OAT and OCT families transport relatively small hydrophilic ionic compounds at the basolateral membrane of the proximal tubule. However, the OAT and OCT families do not transport uncharged and charged hydrophobic compounds such as digoxin and thyroid hormone (2, 26).
Tubular secretion of digoxin seems to be a major route of eliminating into urine (2). In the past, glomerular filtration was considered as a main elimination pathway because digoxin excretion is correlated with glomerular filtration rate. However, coadministration of several drugs with digoxin (e.g., verapamil, quinidine, amiodarone, etc.) decreases the renal excretion of digoxin without affecting glomerular filtration rate (reviewed in ref. 2). Because digoxin secretion system is reported to be different from OAT and OCT systems (2, 26), these data further suggest that other type(s) of molecule(s) should be involved in the basolateral transport of digoxin.
Here, we have isolated human OATP4C1 and revealed that it transports cardiac glycosides (digoxin and ouabain), thyroid hormones (T3 and T4), cAMP, and methotrexate. Human OATP4C1 is the first member of the OATP family predominantly expressed in the kidney. The amino acid homology of rat Oatp4c1 to human OATP4C1 is considerably high and also transports digoxin and T3 with similar Km value. These data and the phylogenetic tree analysis clearly indicate that human OATP4C1/rat Oatp4c1 is a member of the OATP family. Because other members of the OATP family are neither expressed at the basolateral membrane of the kidney nor capable of transporting digoxin, human OATP4C1/rat Oatp4c1 is the primary molecule of digoxin transport at the basolateral membrane in the kidney.
At the apical side of the nephron, it is well known that ATP-dependent efflux pump, Pgp, mediates digoxin secretion (27, 28). When digoxin is coadministrated with Pgp inhibitor (5, 6), serum digoxin level is increased by reduction of renal excretion (2). In addition, decreased renal elimination of digoxin is also reported in Pgp knockout mouse (29). Thus, Pgp has an important role in the renal secretion of digoxin in the apical membrane. The colocalization of human OATP4C1/rat Oatp4c1 and Pgp at the proximal tubule suggests the concerted transport of digoxin in the nephron. Furthermore, the expression level of rat Oatp4c1 is decreased in the renal failure. This reduction may be one of the mechanisms of impaired urinary excretion of digoxin in renal failure (30) because an unchanged expression level of Pgp has been reported (31).
We have also revealed that human OATP4C1 transports ouabain. Ouabain not only is one of the plant-derived compounds but also is known as a new class of steroid hormone produced in the adrenal gland (32). Ouabain should be a typical substrate of human OATP4C1 because its Km value (0.38 μM) is one order lower than the Km values of human OATP4C1-mediated T3 (5.9 μM) and digoxin (7.8 μM) uptake, and three orders lower than those of other OATP family (oatp1, 1.7-3.0 mM; oatp2, 470 μM, oatp3, 1.6 mM; OATP-A, 5.5 mM). Because human OATP4C1 transports ouabain in a highly specific manner, human OATP4C1 might participate in the regulation of its membrane transport.
We have clarified that human OATP4C1 transports thyroid hormone. Thyroid hormone plays an important role in the maintenance of kidney function by activation of target gene transcription via its nuclear receptor (33). To activate this pathway, thyroid hormone must cross the cell membrane. However, thyroid hormone fails to cross the plasma membrane by diffusion because of its polar amino acid side chain (34). Recently, we have identified that the OATP family is involved in thyroid hormone transport (35, 36). Other transporters are also involved in thyroid hormone transport, e.g., Na+/taurocholate cotransporting polypeptide (37) and l-amino acid transporter 1-4F2 heavy chain complex (38). However, these transporters are not expressed in the kidney. Our findings suggest that human OATP4C1/rat Oatp4c1 may have an important role in regulation of thyroid hormone concentration in the proximal tubule cell. In addition, the proximal tubule cell expresses type I 5′-deiodinase, which is a major enzyme producing active thyroid hormone by converting T4 to T3 (39). Colocalization of human OATP4C1/rat Oatp4c1 and 5′-deiodinase might be functionally concerted in the regulation of thyroid hormone metabolism in situ.
Inhibition study revealed that human OATP4C1-mediated digoxin uptake is inhibited by not only digoxin but also compounds structurally related to digoxin such as ouabain, digitoxin, and digoxigenin. However, OATP4C1-mediated digoxin uptake was not inhibited by T3. On the other hand, T3 uptake was not inhibited by digoxin and vice versa. Although both T3 and digoxin are substrate for human OATP4C1, these data suggest that digoxin and T3 recognition sites might be different from each other. A similar phenomenon has been reported in oatp2 and OAT-K1/K2 (40). Because of the existence of the several substrate recognition sites, human OATP4C1 could transport various structurally unrelated compounds and may keep the transport activity by minimizing the mutual inhibition of substrates.
Acknowledgments
We thank Dr. Kazuo Nunoki for discussions. This work was supported in part by research grants from the Ministry of Education, Science and Culture of Japan, the Takeda Foundation, and the Sankyo Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GBM, glomerular basement membrane; GST, gonad-specific transporter; OATP, organic anion transporting polypeptide; OCT, organic cation transporter; OAT, organic anion transporter; Pgp, P-glycoprotein; T3, 3,5,3′-triiodo-L-thyronine; T4, thyroxine; WKY, Wister Kyoto; PAH, p-aminohippurate.
Data deposition: The sequences in this paper have been deposited in the GenBank database [accession nos. AF401643 (human OATP4C1) and AF454761 (rat Oatp4c1)].
References
- 1.The Digitalis Investigation Group (1997) N. Engl. J. Med. 336, 525-533. [DOI] [PubMed] [Google Scholar]
- 2.Koren, G. (1987) Clin. Pharmacokinet. 13, 334-343. [DOI] [PubMed] [Google Scholar]
- 3.Inui, K., Masuda, S. & Saito, H. (2000) Kidney Int. 58, 944-958. [DOI] [PubMed] [Google Scholar]
- 4.Dresser, M. J., Leabman, M. K. & Giacomini, K. M. (2001) J. Pharm. Sci. 90, 397-421. [DOI] [PubMed] [Google Scholar]
- 5.Woodland, C., Ito, S. & Koren, G. (1998) Ther. Drug Monit. 20, 134-138. [DOI] [PubMed] [Google Scholar]
- 6.Takara, K., Kakumoto, M., Tanigawara, Y., Funakoshi, J., Sakaeda, T. & Okumura, K. (2002) Life Sci. 70, 1491-1500. [DOI] [PubMed] [Google Scholar]
- 7.Hagenbuch, B. & Meier, P. J. (2003) Biochim. Biophys. Acta 1609, 1-18. [DOI] [PubMed] [Google Scholar]
- 8.Abe, T., Suzuki, T., Unno, M., Tokui, T. & Ito, S. (2002) Trends Endocrinol. Metab. 13, 215-220. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki, T., Onogawa, T., Asano, N., Mizutamari, H., Mikkaichi, T., Tanemoto, M., Abe, M., Satoh, F., Unno, M., Nunoki, K., et al. (2003) Mol. Endocrinol. 17, 1203-1215. [DOI] [PubMed] [Google Scholar]
- 10.Kodawara, T., Masuda, S., Wakasugi, H., Uwai, Y., Futami, T., Saito, H., Abe, T. & Inui, K. (2002) Pharm. Res. 19, 738-743. [DOI] [PubMed] [Google Scholar]
- 11.Bergwerk, A. J., Shi, X., Ford, A. C., Kanai, N., Jacquemin, E., Burk, R. D., Bai, S., Novikoff, P. M., Stieger, B., Meier, P. J., et al. (1996) Am. J. Physiol. 271, G231-G238. [DOI] [PubMed] [Google Scholar]
- 12.Masuda, S., Saito, H., Nonoguchi, H., Tomita, K. & Inui, K. (1997) FEBS Lett. 407, 127-131. [DOI] [PubMed] [Google Scholar]
- 13.Abe, T., Unno, M., Onogawa, T., Tokui, T., Kondo, T. N., Nakagomi, R., Adachi, H., Fujiwara, K., Okabe, M., Suzuki, T., et al. (2001) Gastroenterology 120, 1689-1699. [DOI] [PubMed] [Google Scholar]
- 14.Kyte, J. & Doolittle, R. F. (1982) J. Mol. Biol. 157, 105-132. [DOI] [PubMed] [Google Scholar]
- 15.Page, R. D. (1996) Comput. Appl. Biosci. 12, 357-358. [DOI] [PubMed] [Google Scholar]
- 16.Wada, M., Nagano, N., Furuya, Y., Chin, J., Nemeth, E. F. & Fox, J. (2000) Kidney Int. 57, 50-58. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki, K., Yaoita, E., Yamamoto, T. & Kihara, I. (1992) Kidney Int. 41, 1517-1526. [DOI] [PubMed] [Google Scholar]
- 18.Cui, Y., Konig, J. & Keppler, D. (2001) Mol. Pharmacol. 60, 934-943. [DOI] [PubMed] [Google Scholar]
- 19.Kouzuki, H., Suzuki, H., Ito, K., Ohashi, R. & Sugiyama, Y. (1998) J. Pharmacol. Exp. Ther. 286, 1043-1050. [PubMed] [Google Scholar]
- 20.Saito, H., Masuda, S. & Inui, K. (1996) J. Biol. Chem. 271, 20719-20725. [DOI] [PubMed] [Google Scholar]
- 21.Walker, J. E., Saraste, M., Runswick, M. J. & Gay, N. J. (1982) EMBO J. 1, 945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujiwara, K., Adachi, H., Nishio, T., Unno, M., Tokui, T., Okabe, M., Onogawa, T., Suzuki, T., Asano, N., Tanemoto, M., et al. (2001) Endocrinology 142, 2005-2012. [DOI] [PubMed] [Google Scholar]
- 23.Li, N., Hartley, D. P., Cherrington, N. J. & Klaassen, C. D. (2002) J. Pharmacol. Exp. Ther. 301, 551-560. [DOI] [PubMed] [Google Scholar]
- 24.Kato, Y., Kuge, K., Kusuhara, H., Meier, P. J. & Sugiyama, Y. (2002) J. Pharmacol. Exp. Ther. 302, 483-489. [DOI] [PubMed] [Google Scholar]
- 25.Urakami, Y., Nakamura, N., Takahashi, K., Okuda, M., Saito, H., Hashimoto, Y. & Inui, K. (1999) FEBS Lett. 461, 339-342. [DOI] [PubMed] [Google Scholar]
- 26.Milton, A., Odlind, B. & Dencker, L. (1986) Acta Physiol. Scand. 127, 9-16. [DOI] [PubMed] [Google Scholar]
- 27.Tanigawara, Y., Okamura, N., Hirai, M., Yasuhara, M., Ueda, K., Kioka, N., Komano, T. & Hori, R. (1992) J. Pharmacol. Exp. Ther. 263, 840-845. [PubMed] [Google Scholar]
- 28.Ernest, S., Rajaraman, S., Megyesi, J. & Bello-Reuss, E. N. (1997) Nephron 77, 284-289. [DOI] [PubMed] [Google Scholar]
- 29.Kawahara, M., Sakata, A., Miyashita, T., Tamai, I. & Tsuji, A. (1999) J. Pharm. Sci. 88, 1281-1287. [DOI] [PubMed] [Google Scholar]
- 30.Marcus, F. I., Peterson, A., Salel, A., Scully, J. & Kapadia, G. G. (1966) J. Pharmacol. Exp. Ther. 152, 372-382. [PubMed] [Google Scholar]
- 31.Laouari, D., Yang, R., Veau, C., Blanke, I. & Friedlander, G. (2001) Am. J. Physiol. 280, F636-F645. [DOI] [PubMed] [Google Scholar]
- 32.Schoner, W. (2002) Eur. J. Biochem. 269, 2440-2448. [DOI] [PubMed] [Google Scholar]
- 33.Azuma, K. K., Balkovetz, D. F., Magyar, C. E., Lescale-Matys, L., Zhang, Y., Chambrey, R., Warnock, D. G. & McDonough, A. A. (1996) Am. J. Physiol. 270, C585-C592. [DOI] [PubMed] [Google Scholar]
- 34.Lai, C. S., Korytowski, W., Niu, C. H. & Cheng, S. Y. (1985) Biochem. Biophys. Res. Commun. 131, 408-412. [DOI] [PubMed] [Google Scholar]
- 35.Abe, T., Kakyo, M., Sakagami, H., Tokui, T., Nishio, T., Tanemoto, M., Nomura, H., Hebert, S. C., Matsuno, S., Kondo, H., et al. (1998) J. Biol. Chem. 273, 22395-22401. [DOI] [PubMed] [Google Scholar]
- 36.Abe, T. Kakyo, M., Tokui, T., Nakagomi, R. Nishio, T., Nakai, D., Nomura, H., Unno, M., Suzuki, M., Naitoh, T., et al. (1999) J. Biol. Chem. 274, 17159-17163. [DOI] [PubMed] [Google Scholar]
- 37.Friesema, E. C., Docter, R., Moerings, E. P., Stieger, B., Hagenbuch, B., Meier, P. J., Krenning, E. P., Hennemann, G. & Visser, T. J. (1999) Biochem. Biophys. Res. Commun. 254, 497-501. [DOI] [PubMed] [Google Scholar]
- 38.Friesema, E. C., Docter, R., Moerings, E. P., Verrey, F., Krenning, E. P., Hennemann, G. & Visser, T. J. (2001) Endocrinology 142, 4339-4348. [DOI] [PubMed] [Google Scholar]
- 39.Lee, W. S., Berry, M. J., Hediger, M. A. & Larsen, P. R. (1993) Endocrinology 132, 2136-2140. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi, A., Masuda, S., Saito, H., Abe, T. & Inui, K. (2001) J. Pharmacol. Exp. Ther. 299, 261-267. [PubMed] [Google Scholar]