Abstract
Commercial strains of entomopathogenic fungi were evaluated for control of chilli thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae), an invasive pest of ornamental and vegetable plants in the Caribbean and southeastern United States. In laboratory assays, LC50 values against adult S. dorsalis were 5.1 × 104 CFU/mL for Beauveria bassiana GHA, with higher values 3.1 × 105 for Metarhizium brunneum F52 and 3.8 × 105 for Isaria fumosorosea Apopka 97. Second instars were comparatively less susceptible to all isolates, ostensibly due to moulting, with LC50 values of 1.1 × 108, 7.0 × 105, and 9.9 × 105 CFU/spores per mL for GHA, F52, and Apopka 97 strains, respectively. In greenhouse cages, compared with controls, three applications of mycoinsecticides and other biorational insecticides at 7 to 14 day intervals reduced overall S. dorsalis populations on pepper plants Capsicum annuum cv. California Wonder: spinosad reduced populations by 94–99%, M. brunneum F52 by 84–93%, B. bassiana GHA by 81–94%, I. fumosorosea PFR-97 by 62–66%, and different horticultural oils by 58–85%. The proportion of marketable fruit was significantly increased by M. brunneum F52, B. bassiana GHA, and 2% SuffOil-X treatments. Slightly lower levels of control were observed in nursery tests with ornamental rose shrubs, Rosa sp. Red Double Knock Out®, during hot sunny conditions. Four applications reduced thrips populations over 10 weeks: spinosad by an average of 91%, M. brunneum F52 by an average of 81%, B. bassiana GHA by an average of 62%, SuffOil-X by an average of 50%, and I. fumosorosea PFR-97 by an average of 44%. The data show that mycoinsecticides can be used in management strategies for low to moderate populations of S. dorsalis and provide resistance management tools for the limited number of insecticides that are effective against this pest.
Keywords : Beauveria bassiana, Isaria fumosorosea, Metarhizium brunneum, Rosa spp., sweet pepper
Introduction
Chilli thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae), a recent invader in the Caribbean and southeastern United States, has emerged as a significant pest of landscape ornamental plants and poses a risk to several economically important agricultural crops (Seal et al. 2006b, 2010; Arthurs et al. 2009). Unlike many pest thrips, S. dorsalis attacks foliage, with both adults and larvae preferentially feeding on young leaves, buds, and fruits. Feeding causes distortion and discoloration, and severe infestations can lead to defoliation and stunted growth (Venette and Davis 2004). The broad host range and invasiveness of this species is a concern for regulatory agencies involved with USA agriculture and trade. Economically important hosts of S. dorsalis include banana, bean, cashew, castor, corn, citrus, cocoa, cotton, eggplant, grape, kiwi, litchi, longan, mango, melon, onion, passion fruit, peach, peanut, pepper, poplar, rose, sacara, soybean, strawberry, sweet potato, tea, tobacco, tomato, and wild yams (Venette and Davis 2004). S. dorsalis has been reported as a vector of several plant diseases, including tomato spotted wilt virus on peanut (Amin et al. 1981), peanut chlorotic fan virus (Campbell et al. 2005), and tobacco streak virus (Rao et al. 2003).
In 2004, the Florida Cooperative Agricultural Pest Survey program began a survey for S. dorsalis (Silagyi and Dixon 2006). Following its detection in Palm Beach County, Florida, in October 2005, this thrips species has rapidly spread throughout the state (Edwards et al. 2010). Shipments of infested ornamental plants from Florida are suspected to be responsible for recent populations of S. dorsalis reported in Texas (Ludwig and Bográn 2007), Louisiana (Ring 2012), and Georgia (Diffie and Srinivasan 2010). Nietschke et al. (2008) predicted the potential geographic distribution of S. dorsalis in North America would eventually extend north along the western coastal states to the Canadian border, as well as the entire Caribbean region.
A number of studies have evaluated chemical controls for S. dorsalis. Seal et al. (2006a) proposed a tentative management program for pepper production, including the application of chlorfenapyr at the beginning of infestation, followed by spinosad or imidacloprid application in four to seven days, and additional applications of these insecticides as needed at about seven-day intervals. In landscape tests with ornamental plants, Ludwig and Bográn (2007) reported that acephate, imidacloprid, or spinosad, provided control of S. dorsalis. However, in landscapes, exclusive reliance on chemical insecticides is probably not a sustainable option for S. dorsalis control, due to high costs, risks of pesticide resistance, and adverse effects on beneficial organisms and the environment (Reddy et al. 1992; Funderburk et al. 2000; Jensen 2000; Loughner et al. 2005). In Florida, all of these risk factors are enhanced by a long growing season, which allows multiple thrips generations each year.
Several studies have evaluated entomopathogenic fungi for use against western flower thrips, Frankliniella occidentalis Pergande, and other pest thrips (Vestergaard et al. 1995; Jacobson et al. 2001; Sengonca et al. 2006; Thungrabeab et al. 2006; Ansari et al. 2007, 2008). However, there is little information on the effectiveness of entomopathogenic fungi against S. dorsalis. Currently, several mycoinsecticides are registered for thrips control in North America. In this study, the pathogenicity of several commercially produced fungal strains against S. dorsalis life stages were evaluated in the laboratory. Several reduced risk insecticides were compared against infestarions of S. dorsalis in greenhouse and nursery studies.
Materials and Methods
Source of insects and plants
S. dorsalis were obtained from wild populations on ornamental rose bushes in Seminole County, Florida, in 2008, and reared on cotton (Deltapine 493 Conventional) plants in an insectary room maintained at 25 ± 2° C, 70% RH, and under a 12:12 L:D photoperiod. Cotton and pepper plants used in tests were germinated from seed in trays in a pest free greenhouse and transplanted at the sixth to eighth true leaf stage into 15 cm diameter pots using Fafard Growing Mix 2 (Conrad Fafard, Inc., www.fafard.com). Cotton and pepper plants were fertilized weekly with liquid 12-4-8 NPK (Miracle-Gro, Scotts, www.scotts.com) Ornamental roses, Rosa sp. Red Double Knock Out®, were acquired from a local nursery and held for 12 months to eliminate insecticide residues.
Experimental treatments
The following fungal preparations were used in tests: Beauveria bassiana (Balsamo-Crivelli) Vuillemin strain GHA containing 4.4×1010 conidia per g (BotaniGard® 22WP, BioWorks, www.bioworksinc.com), Isaria fumosorosea Wise (= Paecilomyces fumosoroseus) Apopka strain 97 containing 109 CFU(blastospore)/g (PFR-97 20% WDG, Certis, certisusa.com), and an oil-based formulation of Metarhizium brunneum Petch strain F52 containing 5×109 conidia/mL (Met52 EC, Novozymes, www.novozymes.com). Germination rates of tested materials were > 80% on PDA after 24 hours at 25° C.
Laboratory bioassay
Fungal preparations were screened against S. dorsalis using a modification of the leaf-disc method of Ugine et al. (2005). All materials were suspended in distilled water with a magnetic stirrer, and the following concentrations were prepared using an improved Neubauer hemocytometer and serial (×10) dilution, 103, 104, 105, 106, 107, 108, and 109 conidia/blastospores per mL. A spreading agent, Tween 80 at 0.05% v/v, was included in all cases, and samples sonicated for 2 minutes to break fungal chains. Fungal suspensions were applied using a Potter spray tower (Burkard Scientific, www.burkardscientific.co.uk) to the abaxial surface of leaf discs (2 cm diameter), which were removed with a sterilized cork borer from six-week-old cotton plants. Spray volumes were calibrated at 2.5 µl per cm2 on the leaf surface, i.e., delivering between 2.5 and 2.5 × 106 conidia/blastopores per cm2 over the range of concentrations. The highest concentrations were filtered through cheesecloth; however, since the larger particulate size of the formulating material for I. fumosorosea (bran) sometimes clogged the spray tower nozzle, the highest concentration was dropped in that treatment. Leaf discs were air-dried and placed individually in 30 mL plastic cups (Solo, www.solocup.com) containing 4 mL of water agar covered with a single 3.5 cm diameter filter paper. This setup preserved high humidity to maintain leaf turgor while also reducing water droplets that could trap insects. Each cup was infested with 10 adults or 20 second instar thrips using an entomological paintbrush. Cups were capped with a tightly fitting lid and incubated at 25 ± 1° C, 80% RH, and with 16:8 L:D photoperiod. Thrips mortality was assessed under a dissecting microscope after seven days. The proportion of cadavers expressing symptomatic development sporulation was noted. There were five cups per fungus concentration/life stage tested, and the study was repeated on three occasions for each fungus species.
Greenhouse cage study
All mycoinsecticides were tested within label rates against artificially established S. dorsalis populations on sweet pepper, Capsicum annuum cv. California Wonder, under greenhouse conditions. Prior to tests, sixweek-old plants (pest free) were infested with 15 adult thrips, followed by an additional 10 after one week (25 thrips/plant total), using the methods of Dogramaci et al. (2011). Since compact plants were easier to manipulate inside cages, uniconazole-P (Sumagic, plant growth regulator, Valent, www.valent.com) was applied at 2 µl/L to limit stem elongation. Plants were maintained inside cages (two plants per cage), consisting of a PVC frame (60 × 60 × 60 cm) covered with nylon mesh (0.36 mm hole size) and fitted with a sleeve for access.
In the first trial, conducted in fall 2010, M. brunneum F52 EC (2.1 ml/L applying 1010 spores/L), PFR-97 WDG (2.1 g/L applying 2.1 × 109 spores/L) and BotaniGard WP (2.4 g/L applying 1011 spores/L), and a highly refined horticultural oil (SuffOil-X, BioWorks) at 2% v/v and spinosad (Conserve® SC, Dow AgroSciences, www.dowagro.com) at 0.47 ml/L were compared. There were six replicate cages per treatment (36 cages total), arranged randomly inside a greenhouse bay. Treatments were applied one week after the second thrips infestation, when eggs and F1 larvae were present. Foliage was sprayed to run-off, using a backpack sprayer (Flo-Master 1101HD, 3.8 L capacity) fitted with a cone nozzle. Concurrently, the soil surface was sprayed around the base of the plant to target any additional nonfeeding stages. An application rate equivalent to 1,000 L/hectare was applied, and a wetting agent (Tween 80 at 0.05% v/v) was added to all treatments. Controls were treated with water and Tween. Plants were isolated for spraying in order to manage drift to adjacent plants and cages. To improve environmental conditions for fungal germination, treatments were applied late in the day (after 18:00) when cooling fans had stopped. Cages were lightly irrigated using overhead misters immediately following spraying, which were noted to maintain > 95% RH in the canopy for approximately 12 hours, but did not result in surface leaf wetness. Two additional applications were made after 7 and 20 days.
Thrips were sampled from the three uppermost terminal leaves (Seal et al. 2006b) at five weekly intervals starting immediately before the first treatments were applied. Plants were scored according to the injury scale described by Kumar et al. (1996): (0) no symptoms; (1) terminal three to four leaves showing tiny eruptions in the inter-veinal area; (2) terminal three to four leaves showing upward curling along leaf margin; (3) severe scarring of terminal and a few basal leaves; (4) stunted plants, leaves severely curled and leaf area greatly reduced; (5) plants with no leaves and only stem remaining. At the end of each experiment, pepper fruits were rated for marketability based on visible insect damage (deformation). The trial was repeated in the spring, with the following modifications: only a single plant was used per cage (infested once with a lower rate of 15 thrips), a different horticultural oil (Year-Round™ Spray Oil, Summit Chemical, www.summitchemical.com) was used at 1% v/v, and evaluations were made over six weeks.
Shade temperature and relative humidity were monitored with a Hobo H8 Pro Series loggers (Onset, www.onsetcomp.com). Conditions inside greenhouse cages averaged 22.8° C (range 14.8–40.1) and 78.5% RH (range 15–100) over the tests.
Nursery study
A simulated nursery study was conducted from late spring through summer in 2011 using ornamental roses, Rosa sp. ‘Red Double Knock Out®, transplanted into 20 L pots containing multipurpose compost and maintained on landscape fabric. Plants were fertilized every other month with 25 g of slow release granules (Scotts Rose & Bloom® 12-4-8) and watered daily (2 L/pot) during the hotter months through drip irrigation. Plants, pruned two weeks prior to encourage new growth, were infested with 30 adult thrips. Ten adults were attached to three separate terminals using previously described methods (Dogramaci et al. 2011). The same treatments used in the greenhouse study were evaluated, but this time set up as a randomized block design. There were six blocks, each one containing one plant for each treatment. Blocks were separated by 10 m, with yellow sticky cards between blocks to monitor thrips movement. Treatments were applied 15 days postinfestation, when S. dorsalis larvae were observed, with additional applications made after 8, 31, and 42 days. Foliage was sprayed to run-off, using a backpack sprayer with a cone nozzle, while an equivalent volume was applied to the soil surface to target nonfeeding stages (equivalent to 1000 L/hectare). A nonionic wetting agent (R-11, Wilbur-Ellis, www.wilburellis.com) was added to all treatments at 0.15% v/v. Controls were treated with water and wetting agent only. Treatments were applied late in the day (after 18:00), and plants were isolated to manage drift. Evaluations were made in situ weekly over 10 weeks, starting shortly prior to spraying. Thrips' life stages were counted with a 10× field magnification visor from three randomly selected terminals per plant. The proportion of new plant terminals with thrips damage was also assessed from 10 terminals per plant.
Data analysis
In laboratory bioassays, mortality estimates averaged for each test date (n = 3) were compared using univariate analysis of variance (ANOVA). Probit analysis of the log10 concentration was used to estimate LC50 along with fiducial limits and slope (SPSS for windows v. 17, IBM, www.ibm.com). In greenhouse and nursery tests, treatments were compared using a repeated measures ANOVA having six treatments and between 5 and 10 time intervals (SPSS). Means were separated using Tukey's HSD tests in the repeated measures model. All data were checked for normality and, if needed, normalized through log (n + 1) or arcsine (proportion data) prior to analysis.
Results
Laboratory assay
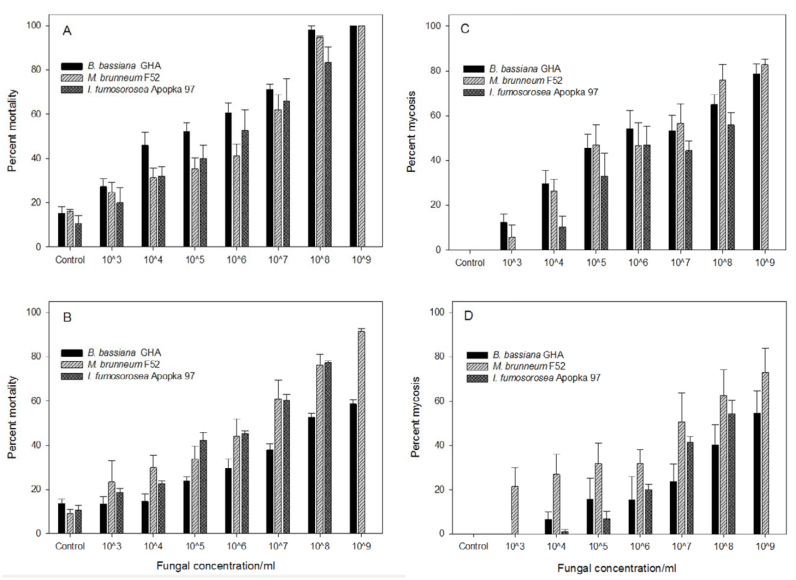
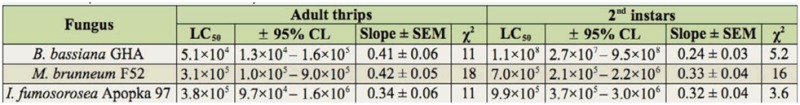
All fungal strains caused significant thrips mortality with a clear dose-response (Figure 1A, B). Adult thrips were more susceptible than larvae (F1, 132 = 9.4, p < 0.005), with a further two-way interaction with fungal species and thrips life stage (F2, 132 = 3.2, p < 0.05). This interaction is explained by comparisons of the LC50, which shows a greater disparity in susceptibility between thrips adults and larvae for B. bassiana (Table 1). The proportion of dead thrips sporulating with symptomatic mycosis followed a doseresponse (Figure 1C, D). Overall, higher rates of sporulation were observed among thrips exposed as adults compared with larvae (F1, 132 = 8.3, p < 0.005), with differences according to fungal species (F2,132 = 5.7, p < 0.005), although the interaction with fungal species and life stage was not significant (F2, 132 = 1.4, p = 0.24).
Figure 1.
Response of Scirtothrips dorsalis to three fungal entomopathogens in leaf disc assays; (A) total mortality of thrips exposed as adults, (B) total mortality of thrips exposed as larvae, (C) proportional mycosis among adults, (D) proportional mycosis among larvae. Data show mean ± SEM of three tests (five replicates per test). High quality figures are available online.
Table 1.
Estimates of the median lethal concentration (spores/mL) of three entomopathogenic fungi applied against different Scirtothrips dorsalis life stages in leaf disc assays. Data based on three replicate tests (50 adults or 100 larvae/test); all probit estimates were adjusted for control mortality.
Greenhouse cage study
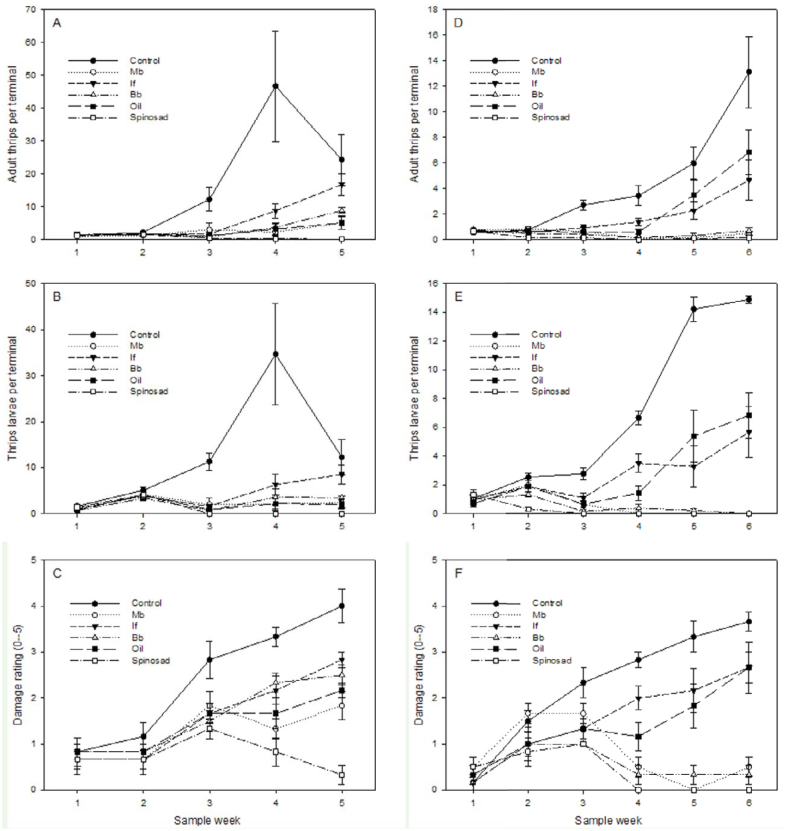
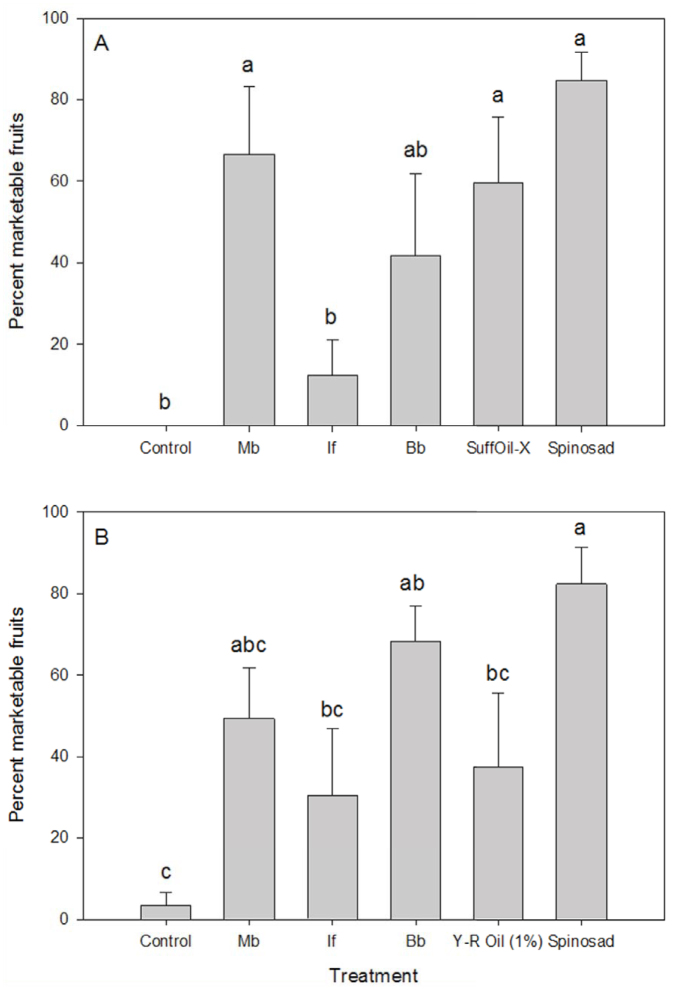
In the fall, thrips developed through at least two complete generations, reaching ≥ 80 individuals per pepper terminal and causing extensive deformation and defoliation of control plants by the end of the study (Figure 2A–C). Insecticide treatment significantly affected both the number of adult thrips and thrips larvae sampled on plant terminals (F5,30 = 16.5, p < 0.0001, and F5,30 = 17.0, p < 0.0001, respectively, in repeated measures ANOVA). Compared with controls, thrips populations were significantly reduced (p < 0.05, Tukey HDS in the repeated measures model) by the following treatments: spinosad (average 94% reduction), followed by SuffOil-X (85%), M. brunneum (84%), B. bassiana (81%), and I. fumosorosea (66%). Intermediate expression of thrips feeding symptoms (rating 1.8–2.8) was recorded in the insecticide treatments, with the exception of spinosad, which remained low (< 0.3) (Figure 2C). Although the initial feeding damage likely impaired fruit set before treatments were first applied, increased levels of marketable fruit in several treatments, notably spinosad, M. brunneum, and SuffOil-X, were observed (Figure 3A).
Figure 2.
Thrips counts and plant damage in greenhouse tests with caged pepper plants; (A–C) adults, larvae, and plant damage in fall test, (D–F) adults, larvae, and plant damage in spring test. Data are mean ± SEM from plants in six replicate cages. High quality figures are available online.
Figure 3.
Final proportion of marketable pepper fruits (mean ± SEM) in greenhouse studies: (A) fall, (B) spring tests. High quality figures are available online.
Broadly similar findings occurred in the spring test, although thrips populations were somewhat lower compared with the fall, and reduced effectiveness was observed with the different horticultural oils (Figure 2D–F, 3B). Overall, insecticide treatments were highly significant (F5, 30 = 45.5, p < 0.0001), with thrips populations significantly reduced by the following: spinosad (average 99% reduction compared with control plants), B. bassiana (94%), M. brunneum (93%), I. fumosorosea (62%), and 1% oil (58%).
Nursery study
Thrips developed through three to four generations, with populations peaking in weeks five and six on control plants and gradually declining over the remainder of the study (Figure 4). Repeated measures ANOVA revealed that treatment applied, including controls, significantly affected both the number of adult thrips (F5, 30 = 37.2, p < 0.0001) and thrips larvae (F5, 30 = 42.1, p < 0.0001) sampled on plant terminals. Compared with control plants, thrips (adults and larvae combined) were significantly reduced (p < 0.05, Tukey HDS in the repeated measures model) by the following treatments: spinosad (average 91% reduction with respect to control plants), M. brunneum (81%), B. bassiana (62%), SuffOil-X (50%), and I. fumosorosea (44%). Thrips damage, observed on > 95% of new rose terminals on untreated plants (Figure 3C), was also affected by treatments (F5, 30 = 51.7, p < 0.0001). Although posthoc tests revealed that less damage occurred on all treated plants compared with controls (p < 0.05 in the repeated measures model), a relatively high proportion of terminals were still affected, with only spinosad and M. brunneum having 50% of terminals damaged by the end of the study. The predator Orius insidiosus Say was observed throughout all plots during the study, although its impact was not quantified. Conditions remained generally hot and sunny (up to 1030 W/m2) with some afternoon showers; average hourly measurements ranged from 13.2 to 37.3° C, 28 to 96% RH, with a total of 30.4 cm rainfall during the study.
Figure 4.
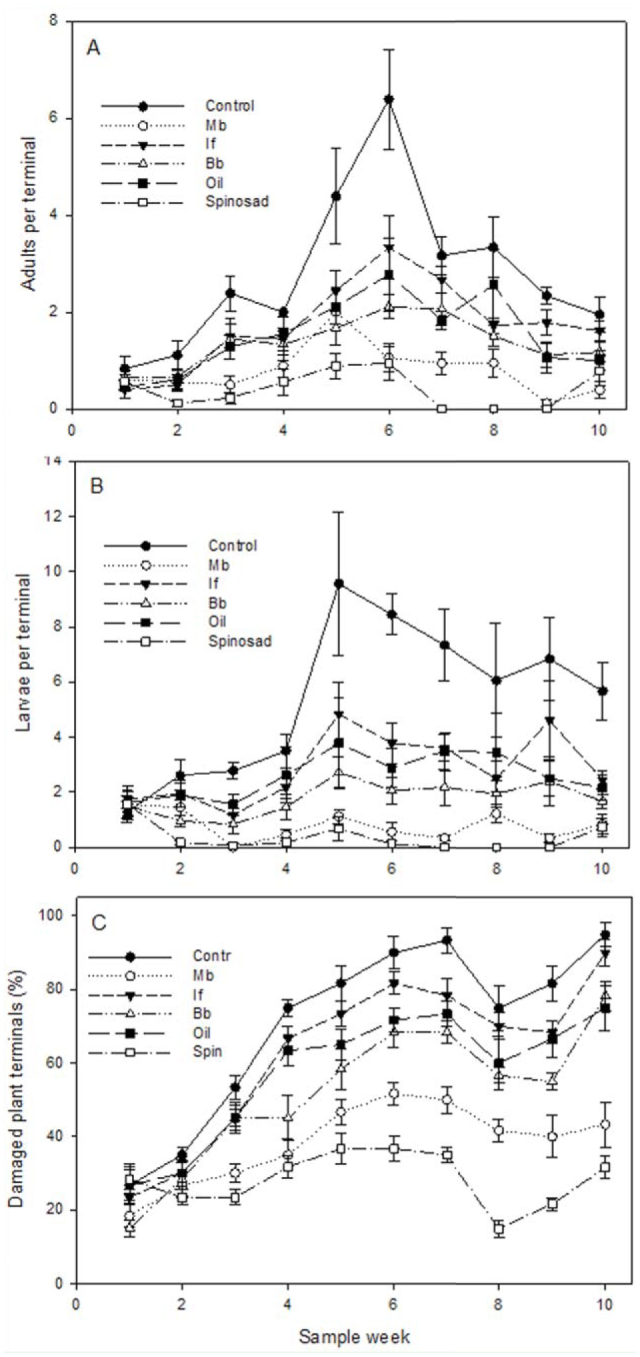
Weekly thrips counts, (A) adults and (B) larvae, and (C) damaged plant terminals from knockout roses treated with mycoinsecticides and other biorational materials in nursery study. Data are mean ± SEM of six replicate shrubs. High quality figures are available online.
Discussion
This study highlights the potential of mycoinsecticides for management of S. dorsalis in vegetables or ornamental plants. Both BotaniGard and PFR-97 are registered in North America for greenhouse and nursery use against thrips and other soft bodied pests, including aphids and whiteflies. Metarhizium strain F52 is approved for non-food use in greenhouses, nurseries, and limited outdoor sites. In particular, respectable rates of control on pepper (> 80% population reduction) were obtained from B. bassiana and M. brunneum applied within label rates in greenhouses. The increasing numbers of thrips in all treatments at the end of this study may have been exacerbated by adults migrating out of control cages after destruction of host plants, since thrips were intercepted on sticky cards hung between cages at this time (at < 2mm, adult S. dorsalis can fit though most insect screens). It would need to be confirmed whether the humidity inside greenhouse cages (elevated approximately 3% compared with outside) enhanced efficacy of fungal treatments compared with an open crop.
Slightly less effective control was achieved on roses maintained outside during summer conditions. Given that S. dorsalis primarily feeds on developing foliage and unopened flower buds, it is likely that higher UV levels were detrimental to the fungal persistence in these tests (Braga et al. 2001; Fernandes et al. 2007; Zimmermann 2008). However, the possibility of thrips movement between control and treated plants in the nursery study cannot be excluded; hence, better control might be expected under operational conditions where reinfestation from untreated plants is minimized.
Although application strategy was not quantified, the spraying of soil surfaces may be beneficial by targeting the developing prepupal and pupal stages. Application of entomopathogenic fungi to container plants though soil drenches or pre-mixing with potting compost is an effective strategy against F. occidentalis (Brownbridge 1995; Helyer et al. 1995; Ansari et al. 2008). The species/strain of fungus may influence the level of control achieved against thrips. In laboratory tests, Ansari et al. (2008) reported that M. brunneum strains V275 and ERL700 were the most effective, causing 85 to 96% mortality of F. occidentalis larvae and pupae 11 days after inoculation in the soil, compared with 51 to 84% mortality in four other M. brunneum strains, 54 to 84% mortality from two B. bassiana strains, 63 to 75% mortality from two I. fumosorosea strains, and 15 to 54% for the insecticide Fipronil.
There are few reports of entomopathogenic fungi being used against S. dorsalis. However, compared with our data, Seal and Kumar (2010) reported relatively less effective control of S. dorsalis treated with B. bassiana GHA. In insecticide screening tests with Jalapeño peppers under greenhouse conditions, label rates of BotaniGard applied to the foliage reduced S. dorsalis larvae by about 50% at 5 days after treatment, but not at 10 days after treatment (Seal and Kumar 2010). Differences in experimental procedures may explain these differences, since in the latter tests the soil surface was not apparently treated, and pepper plants were maintained adjacent to infested cotton plants, which may have encouraged more rapid reinfestation.
Fungal pathogens have been recovered from S. dorsalis in its native range. Two strains of Fusarium spp. and Neozygites floridana were isolated from cadavers of S. dorsalis in chilli pepper fields in India (Mikunthan and Manjunatha 2006). The authors cultured both Fusarium isolates on media and subsequently obtained LC50 value of 2.7 × 107 and 7.6 × 107 spores/mL for Fusarium incarnatum (formerly semitectum) (Desm.) Sacc and Fusarium sp. isolate GM 15, respectively, applied against second instar S. dorsalis. In further tests, the researchers applied oilformulation of the more virulent F. incarnatum strain against S. dorsalis in field-grown chilli peppers at 35, 50, and 70 days post-transplanting (Mikunthan and Manjunatha 2008). The authors attempted to improve the micro-environmental conditions for fungal infection and transmission through intercropping chilli peppers with taller companion plants. Although the lack of unsprayed controls prevented the impact of the fungus being addressed directly, the authors observed improvements in overall yield of chilli peppers in treated plots planted with chilli-sorghum, chilli-cotton-chilli, and chilli-red gram, and suggested that chilli-cotton-chilli cropping systems are favorable environmental conditions for managing S. dorsalis and broad mites using F. incarnatum.
Relatively more species and strains of entomopathogenic fungi have been tested against F. occidentalis. Brownbridge (1995) tested more than 150 isolates and concluded that M. brunneum, B. bassiana, and Lecanicillium lecanii R. Zare and W. Gams had the highest level of insecticidal activity. Sengonca et al. (2006) tested 41 isolates from 25 species and 11 genera of entomopathogenic fungi in Thailand against first instar F. occidentalis on bean leaves. Among the 14 most virulent isolates, LC50 values of Beauveria spp. ranged from 2.4 × 104 to 5.9 × 106 conidia/mL, Metarhizium spp. from 2.0 × 104 to 5.0 × 105 conidia/mL, and Isaria spp. from 3.9 × 104 to 5.5 × 106 conidia/mL. The latter two values reported by Sengonca et al. (2006) compare with our laboratory-based estimates for second instar S. dorsalis reared on cotton (i.e., within 95% CL from Table 1). However, higher LC50 values were observed among S. dorsalis larvae exposed to B. bassiana in the present study, possibly due to its shorter developmental period compared with F. occidentalis. The difference in host plant used in the present laboratory studies and those of Sengonca et al. (2006) may have influenced the results. Thungrabeab et al. (2006) reported that F. occidentalis reared on cotton were significantly less susceptible to infection by B. bassiana compared with those reared on bean plants, ostensibly due to sequestered gossypol and/or other allelochemicals in the cotton plants (Poprawski and Jones 2000).
The observation that S. dorsalis larvae were less susceptible to mycosis than adults is consistent with the inoculum being shed with the exuvium during ecdysis. Vestergaard et al. (1995) also reported lower susceptibility of F. occidentalis larvae compared with adults exposed to M. brunneum V275 (27% versus 100% mortality). Evidence that ecdysis may provide a resistance mechanism to fungal infection was provided by Ugine et al. (2005), who demonstrated that early second instar F. occidentalis became progressively less susceptible to B. bassiana as they aged within the instar. It is hypothesized that a slower germination of B. bassiana on the S. dorsalis larval cuticle may have reduced its efficacy compared with other fungal strains.
In conclusion, mycoinsecticides may be used to manage S. dorsalis and provide resistance management tools for spinosad or other insecticides. Mycoinsecticides may be most effective in pest managements programs integrating beneficial arthropods, or in greenhouse crops where favorable environmental conditions (high humidity and low UV exposure) can be manipulated (Jacobson et al. 2001; Down et al. 2009). Additional research to optimize entomopathogenic fungi, for example through formulation, is warranted. Other candidate entomopathogens include entomopathogenic nematodes (Jagdish and Purnima 2011), while cultural methods include reduced fertilizer inputs and removing pupations substrates (Duraimurugan and Jagadish 2004).
Acknowledgments
We are grateful to Brett Highland, Mike Dimmock, Jarrod Leland, and Chris Hayes for providing materials and ideas. Robert Leckel provided technical support, and USDA's IR4 Biopesticide Project #88085 provided funding.
References
- Amin PW, Reddy DVR, Ghanekar AM. Transmission of tomato spotted wilt virus, the causal agent of bud necrosis of peanut, by Scirtothrips dorsalis and Frankliniella schultzei. Plant Disease. 1981;65:663–665. [Google Scholar]
- Ansari MA, Brownbridge M, Shah FA, Butt TM. Efficacy of entomopathogenic fungi against soil-dwelling life stages of western flower thrips, Frankliniella occidentalis, in plant-growing media. Entomologia Experimentalis et Applicata. 2008;127:80–87. [Google Scholar]
- Ansari MA, Shah FA, Whittaker M, Prasad M, Butt TM. Control of western flower thrips (Frankliniella occidentalis) pupae with Metarhizium anisopliae in peat and peat alternative growing media. Biological Control. 2007;40:293–297. [Google Scholar]
- Arthurs S, McKenzie CL, Chen J, Doğramaci M, Brennan M, Houben K, Osborne L. Evaluation of Neoseiulus cucumeris and Amblyseius swirskii (Acari: Phytoseiidae) as biological control agents of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) on pepper. Biological Control. 2009;49:91–96. [Google Scholar]
- Braga GUL, Flint SD, Miller CD, Anderson AJ, Roberts DW. Variability in response to UV-B among species and strains of Metarhizium isolated from sites at latitudes from 61° N to 54° S. Journal of Invertebrate Pathology. 2001;78:98–108. doi: 10.1006/jipa.2001.5048. [DOI] [PubMed] [Google Scholar]
- Brownbridge M. Prospects for mycopathogens in thrips management. In: Parker BL, Skinner M, Lewis T, editors. Thrips Biology and Management. Plenum Press; 1995. pp. 281–295. [Google Scholar]
- Campbell LR, Robb KL, Ullman DE. The complete tospovirus resource guide. Kansas State University; 2005. Available online: http://www.oznet.ksu.edu/tospovirus/tospo_list.htm. [Google Scholar]
- Diffie S, Srinivasan R. Occurrence of Leucothrips furcatus, Scirtothrips dorsalis and Tenothrips frici (Thysanoptera: Thripidae) previously unreported from Georgia. Journal of Entomological Science. 2010;45:394–396. [Google Scholar]
- Doğramaci M, Chen J, Arthurs S, McKenzie CL, Irizarry F, Houben K, Brennan M, Osborne L. Mini-aspirator: a new device for collection and transfer of small arthropods to plants. Florida Entomologist. 2011;94:22–27. [Google Scholar]
- Down RE, Cuthbertson AGS, Mathers JJ, Walters KFA. Dissemination of the entomopathogenic fungi, Lecanicillium longisporum and L. muscarium, by the predatory bug, Orius laevigatus, to provide concurrent control of Myzus persicae, Frankliniella occidentalis and Bemisia tabaci. Biological Control. 2009;50:172–178. [Google Scholar]
- Duraimurugan P, Jagadish A. Nonchemical management of Scirtothrips dorsalis Hood damaging rose flowers. Journal of Applied Zoological Research. 2004;15:163–165. [Google Scholar]
- Edwards GB, Hodges G, Dixon W. Chilli thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) a new pest thrips for Florida. Florida Department of Agriculture and Consumer Services; Division of Plant Industry Pest Alert: 2010. Available online: http://www.freshfromflorida.com/pi/pest-alerts/scirtothrips-dorsalis.html. [Google Scholar]
- Fernandes EKK, Rangel DEN, Moraes AML, Bittencourt VREP, Roberts DW. Variability in tolerance to UV-B radiation among Beauveria spp. isolates. Journal of Invertebrate Pathology. 2007;96:237–243. doi: 10.1016/j.jip.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Funderburk J, Stavisky J, Olson S. Predation of Frankliniella occidentalis (Thysanoptera : Thripidae) in field peppers by Orius insidiosus (Hemiptera : Anthocoridae). Environmental Entomology. 2000;29:376–382. [Google Scholar]
- Helyer NL, Brobyn PJ, Richardson PN, Edmondson RN. Control of western flower thrips (Frankliniella occidentalis Pergande) pupae in compost. Annals of Applied Biology. 1995;127:405–412. [Google Scholar]
- Jacobson RJ, Chandler D, Fenlon J, Russell KM. Compatibility of Beauveria bassiana (Balsamo) Vuillemin with Amblyseius cucumeris Oudemans (Acarina: Phytoseiidae) to control Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) on cucumber plants. Biocontrol Science and Technology. 2001;11:391–400. [Google Scholar]
- Jagdish EJ, Purnima AP. Evaluation of selective botanicals and entomopathogens against Scirtothrips dorsalis Hood under polyhouse conditions on rose. Journal of Biopesticides. 2011;4:81–85. [Google Scholar]
- Jensen SE. Insecticide resistance in the western flower thrips, Frankliniella occidentalis. Integrated Pest Management Reviews. 2000;5:131–146. [Google Scholar]
- Kumar NKK, Aradya M, Deshpande AA, Anand N, Ramachandar PR. Initial screening of chilli and sweet pepper germplasm for resistance to chilli thrips, Scirtothrips dorsalis Hood. Euphytica. 1996;89:319–324. [Google Scholar]
- Loughner RL, Warnock DF, Cloyd RA. Resistance of greenhouse, laboratory, and native populations of western flower thrips to spinosad. HortScience. 2005;40:146–149. [Google Scholar]
- Ludwig S, Bográn C. Chilli thrips a new pest in the home landscape. Texas Cooperative Extension EEE-00041; 2007. [Google Scholar]
- Mikunthan G, Manjunatha M. Mycopathogens associated with pests of chilli and their pathogenicity against thrips (Scirtothrips dorsalis) and mites (Polyphagotarsonemus latus). Tropical Agricultural Research. 2006;18:1–10. [Google Scholar]
- Mikunthan G, Manjunatha M. Impact of habitat manipulation on mycopathogen, Fusarium semitectum to control Scirtothrips dorsalis and Polyphagotarsonemus latus of chilli. Biocontrol. 2008;53:403–412. [Google Scholar]
- Nietschke BS, Borchert DM, Magarey RD, Ciomperlik MA. Climatological potential for Scirtothrips dorsalis (Thysanoptera: Thripidae) establishment in the United States. Florida Entomologist. 2008;91:79–86. [Google Scholar]
- Poprawski TJ, Jones WJ. Host plant effects on activity of the mitosporic fungi Beauveria bassiana and Paecilomyces fumosoroseus against two populations of Bemisia whiteflies (Homoptera: Aleyrodidae). Mycopathologia. 2000;151:11–20. doi: 10.1023/a:1010835224472. [DOI] [PubMed] [Google Scholar]
- Rao RD, Reddy AS, Reddy SV, Reddy K, Thirumala-Devi SC, Rao VM, Kumar K, Subramaniam TY, Reddy SN, Reddy DVR. The host range of Tobacco streak virus in India and transmission by thrips. Annals of Applied Biology. 2003;142:365–368. [Google Scholar]
- Reddy GPV, Prasad VD, Rao RS. Relative resistance in chilli thrips, Scirtothrips dorsalis Hood populations in Andhra Pradesh to some conventional insecticides. Indian Journal of Plant Protection. 1992;20:218–222. [Google Scholar]
- Ring D. Chilli thrips threaten Louisiana Knock Out Roses. Louisiana Agriculture Magazine; 2012. available http://www.lsuagcenter.com/en/communications/publications/agmag/archive/2012/winter/chilli-thrips-threaten-louisiana-knock-out-roses.htm. [Google Scholar]
- Seal DR, Ciomperlik M, Richards ML, Klassen W. Comparative effectiveness of chemical insecticides against the chilli thrips, Scirothrips dorsalis Hood (Thysanoptera : Thripidae), on pepper and their compatibility with natural enemies. Crop Protection. 2006a;25:949–955. [Google Scholar]
- Seal DR, Ciomperlik MA, Richards ML, Klassen W. Distribution of chilli thrips, Scirtothrips dorsalis (Thysanoptera : Thripidae), in pepper fields and pepper plants on St. Vincent. Florida Entomologist. 2006b;9:311–320. [Google Scholar]
- Seal DR, Klassen W, Kumar V. Biological parameters of Scirtothrips dorsalis (Thysanoptera: Thripidae) on selected hosts. Environmental Entomology. 2010;39:1389–1398. doi: 10.1603/EN09236. [DOI] [PubMed] [Google Scholar]
- Seal DR, Kumar V. Biological response of chilli thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae), to various regimes of chemical and biorational insecticides. Crop Protection. 2010;29:1241–1247. [Google Scholar]
- Sengonca C, Thungrabeab M, Blaeser P. Potential of different isolates of entomopathogenic fungi from Thailand as biological control agents against western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Journal of Plant Diseases and Protection. 2006;113:74–80. [Google Scholar]
- Silagyi AJ, Dixon WN. Assessment of chilli thrips, Scirtothrips dorsalis, in Florida. Florida Cooperative Agricultural Pest Survey; 2006. Program Report No. 2006-08-SDS-01. [Google Scholar]
- Thungrabeab M, Blaeser P, Sengonca C. Effect of temperature and host plant on the efficacy of different entomopathogenic fungi from Thailand against Frankliniella occidentalis (Pergande) and Thrips tabaci Lindeman (Thysanoptera : Thripidae) in the laboratory. Journal of Plant Diseases and Protection. 2006;113:181–187. [Google Scholar]
- Ugine TA, Wraight SP, Brownbridge M, Sanderson JP. Development of a novel bioassay for estimation of median lethal concentrations (LC50) and doses (LT50) of the entomopathogenic fungus Beauveria bassiana, against western flower thrips Frankliniella occidentalis. Journal of Invertebrate Pathology. 2005;89:210–218. doi: 10.1016/j.jip.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Venette RC, Davis EE. Chilli thrips/yellow thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) mini risk assessment. University of Minnesota; St. Paul, MN: 2004. [Google Scholar]
- Vestergaard S, Gillespie AT, Butt TM, Schreiter G, Eilenberg J. Pathogenicity of the hyphomycete fungi Verticillium lecanii and Metarhizium anisopliae to the western flower thrips, Frankliniella occidentalis. Biocontrol Science and Technology. 1995;5:185–192. [Google Scholar]
- Zimmermann G. The entomopathogenic fungi Isaria farinosa (formerly Paecilomyces farinosus) and the Isaria fumosorosea species complex (formerly Paecilomyces fumosoroseus): biology, ecology and use in biological control. Biocontrol Science and Technology. 2008;18:865–901. [Google Scholar]