Abstract
Benign prostatic hyperplasia (BPH) is an age-related disease of unknown aetiology characterized by prostatic enlargement coincident with distinct alterations in tissue histomorphology. Instead of therapeutic agents that can cause severe side effects, plant extracts are frequently used to treat BPH. In this study, we investigated whether the Melandrium firmum methanolic extract (MFME) improves BPH, using the testosterone propionate (TP)-induced BPH rat model. Castration was performed via the scrotal route under sodium pentobarbital anaesthesia. BPH in castrated rats was generated via daily subcutaneous injections of TP (3 mg kg−1) dissolved in corn oil, for 4 weeks. MFME was administered daily by oral gavage at a dose of 200 mg kg−1 for 4 weeks, along with the TP injections. The control group received injections of corn oil subcutaneously. At the scheduled termination of the experiment, all rats were killed and their prostates weighed; the relative prostate weight (prostate/body weight ratio) was calculated, and histomorphological changes in the prostate were examined. Additionally, we measured the levels of testosterone and dihydrotestosterone (DHT) in the serum and the prostate. Experimentally induced BPH led to marked decreases in the relative prostate weight and the DHT levels in the serum and the prostate. Histologically, BPH was evident in the ventral lobe of the prostate, and MFME treatment suppressed the severity of the lesions. These results indicate that MFME effectively inhibits the development of BPH induced by testosterone in a rat model. Further studies will be needed to identify the compound(s) responsibility for inducing the protective effect against BPH and determine its mechanism of action.
Keywords: benign prostatic hyperplasia, dihydrotestosterone, Melandrium firmum (S. et Z.)ROHRB, prostate, testosterone
Introduction
Benign prostatic hyperplasia (BPH) is extremely common in ageing men, contributing to a pattern of morbidity designated ‘lower urinary tract symptoms' (LUTS) and resulting in significant annual healthcare costs.1 BPH, also known as nodular hyperplasia, benign prostatic hypertrophy or benign enlargement of the prostate, is a hormonal and age-related disease characterized by histological changes in the prostate gland and variable increases in prostate size.
The mechanism underlying the pathogenesis of BPH remains largely unidentified; however, a number of overlapping and complementary theories have been proposed. Ageing and androgens are established risk factors for the development of BPH and benign prostatic enlargement, which may lead to LUTS in elderly men. Androgens and DHT play key roles in BPH development. Several types of therapeutic agent, such as 5α-reductase inhibitors, are currently available for treating BPH.2, 3 However, despite significant efficacy in BPH therapy, the adverse effects of these drugs should not be overlooked. For example, finasteride, a synthetic 5α-reductase inhibitor used to treat BPH,4 triggers adverse effects such as gynaecomastia, the impairment of muscle growth and severe myopathy, owing to structural similarities to steroidal hormones.5 Human BPH is predominantly composed of hyperplastic stromal cells, rather than epithelial cells.
Several sapogenins,6 a saponin,7 flavonoids and triterpenoids8 have been identified in Melandrium firmum and their pharmacological actions have been evaluated. M. firmum is a biennial herbaceous plant that is widely distributed in Korea and commonly used to treat anuria, breast cancer, gonorrhoea and lactation diseases.9 However, the efficacy of M. firmum in treating BPH has not yet to be established. In this investigation, we employ a BPH model (testosterone propionate (TP)-induced BPH rat) to examine the therapeutic effects of M. firmum.
Materials and methods
Preparation of the M. firmum (S. et Z.)ROHRB methanolic extract (MFME)
Whole M. firmum plants were purchased in October 2008 from HMAX (Chungbuk, Korea). A voucher specimen (No. 2009-GO1) has been deposited at the Korea Institute of Oriental Medicine, Daejeon, Korea. These materials have been confirmed taxonomically by Professor Je-Hyun Lee of Dongguk University, Gyeongju, Korea. Fresh M. firmum was washed three times with tap water to remove salts, epiphytes and sand, and stored at −20 °C. Frozen samples were lyophilized and homogenized in a grinder before extraction. Dried whole plants of M. firmum (600 g) were extracted with 70% methanol (MeOH) (6 l, three times) by sonication for 1 h. The extract solution was filtered through filter paper and evaporated to dryness (31.87 g). The yield of the dried extract from the starting crude material was 5.31%. The concentrated extract was freshly dissolved in phosphate-buffered saline prior to use.
Isolation of cytisoside
The whole plants of M. firmum (10.0 kg) were extracted three times with 80% MeOH (20 l) under reflux for 12 h, then filtered and concentrated to yield the MeOH extract (550.0 g). The MeOH extract was suspended in H2O and extracted with hexane, ethyl acetate (EtOAc) and then BuOH to yield the hexane-soluble fraction (75.9 g), EtOAc-soluble fraction (47.4 g) and butanol (BuOH)-soluble fraction (97.0 g), respectively. The hexane-soluble fraction (50.0 g) was subjected to column chromatography with silica gel (60×12 cm, Silica-gel 70–230 mesh) and eluted using a stepwise gradient of hexane/EtOAc (100∶1→1∶1) to yield 32 subfractions. Fraction 14 (6.5 g) was loaded onto a Sephadex column (4×50 cm, Sephadex LH-20), which was eluted with MeOH to give cytisoside (30.0 mg). The chemical structure of the cytisoside was determined by a comparison of its physicochemical and spectral data with the published values.10
Chromatographic system
The chromatographic analysis was performed using a Shimadzu LC-20A HPLC system (Shimadzu Co., Kyoto, Japan) consisting of a solvent delivery unit, an online degasser, a column oven, an autosampler and a photodiode array (PDA) detector. The data processor employed LC Solution software (Version 1.24; Shimadzu Co.). The Gemini C18 (250×4.6 mm; particle size 5 µm; Phenomenex, Torrance, CA, USA) analytical system was employed. The mobile phases were solvent A (1.0% (v/v), aqueous acetic acid) and solvent B (1.0% (v/v), acetic acid in acetonitrile) with the following gradient flow: (A)/(B)=90∶10 (0 min; hold for 10 min)→(A)/(B)=50∶50 (hold for 30 min)→(A)/(B)=0∶100 (40 min; hold for 5 min)→(A)/(B)=90∶10 (55 min; hold for 15 min). The column temperature was maintained at 40 °C. The analysis was performed at a flow rate of 1.0 ml min−1, with PDA detection from 190 to 400 nm. The injection volume was 10 µl. The identification of the peaks was based on the UV spectrum and retention time of each marker component from M. firmum extract.
Chemicals and reagents
Cytisoside was isolated from M. firmum via a series of chromatography procedures. Vitexin was obtained from Fluka (purity≥96.0% St. Louis, MO, USA). HPLC-grade reagents, methanol, acetonitrile and water were purchased from J.T. Baker (Phillipsburg, NJ, USA). All other chemicals were of analytical grade.
Preparation of standard and sample solutions
Standard stock solutions of cytisoside (500 µg ml−1) and vitexin (500 µg ml−1) were prepared in MeOH and incubated below 4 °C. Working standard solutions were prepared by serial dilutions of stock solutions in methanol. The sample powder was weighed (1.0 g) into a 100 ml flask, and 70% methanol was added to the volumetric mark. The mixture was sonicated for 60 min at room temperature. After extraction, the mixture was filtered through a 0.2 µm membrane filter, and the resultant solution was used for HPLC analysis.
Animals
Specific pathogen-free male Wistar rats aged 10 weeks that were routinely screened serologically for relevant respiratory pathogens were purchased from JungAng Co. Ltd (Seoul, Korea). The rats were maintained in an animal facility under standard laboratory conditions for 1 week prior to the experiments and subjected to a 12-h light–dark cycle in a room with controlled temperature and humidity. All experimental procedures were carried out in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals, and animal handling followed the dictates of the National Animal Welfare Law of Korea.
Experimental BPH
Adult male Wistar rats (12 weeks) weighing 250–350 g were used for in vivo experiments. To exclude the influence of intrinsic testosterone, castration was performed by removing the testicles and epididymal fat through the scrotal sac in all rats. The spermatic cord and blood vessels were ligated with 3-0 sutures and resected. BPH was generated in rats via subcutaneous injections of testosterone (3 mg kg−1) for 4 weeks after castration.11, 12 Castrated rats in this study cohort were divided into four groups (n=6): (A) the castration group, which received PBS administered orally and corn oil injected subcutaneously; (B) the BPH group, which received PBS administered orally and TP (3 mg kg−1 body weight) injected subcutaneously; (C) the MFME group, which received MFME (200 mg kg−1 body weight) administered by oral gavage and TP (3 mg kg−1 body weight) injected subcutaneously; (D) the positive control group, which received finasteride (10 mg kg−1 body weight), a 5α-reductase inhibitor, as a positive control drug administered orally and TP (3 mg kg−1 body weight) injected subcutaneously for 4 weeks after castration.13, 14 The drugs were administered orally once daily for 4 weeks. The rats were weighed weekly during the experiments.
Blood and tissue samples
After a treatment period of 4 weeks and overnight fasting, the rats were anaesthetized with pentobarbital (100 mg kg−1 body weight, i.p.). Blood samples were collected and centrifuged at 200g for 10 min. The whole prostate was immediately removed and weighed. Relative organ weights were calculated as the ratio of the organ weight to body weight. All prostatic specimens in each group were fixed with 10% buffered formalin for 24 h. Subsequently, the tissues were embedded in paraffin, cut into sections of 4 µm thickness, stained with haematoxylin and eosin (H&E) solution (haematoxylin; Sigma MHS-16, and eosin; Sigma HT110-1-32, St. Louis, MO, USA). The tissues were subsequently mounted and coverslipped using Dako mounting medium (DakoCytomation, Denmark, CA, USA).
Measurement of DHT and testosterone levels in the prostate and serum
Prostate tissue was homogenized in lysis buffer containing protease inhibitors (50 mmol l−1 Tris-HCl (pH 7.4), 150 mmol l−1 NaCl, 1 mmol l−1 EDTA, 0.5% NP-40, 0.1% SDS, 1 mmol l−1 EGTA, 100 µg ml−1 PMSF, 10 µg ml−1 pepstatin A and 100 µmol l−1 Na3VO3]. The homogenates were centrifuged at 12 000g for 25 min at 4 °C and protein concentrations in the supernatant fractions determined using Bradford reagent (Bio-Rad, Hercules, CA, USA). Enzyme-linked immunosorbent assay experiments were performed according to the manufacturer's instructions. DHT and testosterone levels in the serum and prostate were measured using enzyme-linked immunosorbent assay kits (Cayman, Ann Arbor, MI, USA). The values were expressed per ml in the serum and per mg protein in the prostate.
Measurement of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in the serum
ALT and AST levels were determined to assess liver and kidney function using commercial kits (Beckman Coulter, Inc., Fullerton, CA, USA) and an auto-analyser (Beckman CX4, Beckman Coulter, Inc.).
Image capture and photomicrography
Photomicrographs were captured using a Photometric Quantix digital camera running a Windows program, and montages were assembled in Adobe Photoshop 7.0 (Adobe, San Jose, CA, USA). The images were cropped and corrected for brightness and contrast but not otherwise manipulated.
Statistical analysis
The data were expressed as the mean±s.d. Statistical significance was determined using analysis of variance (ANOVA). The critical level for significance was set at P<0.05.
Results
Effect of MFME on prostate weight
As shown in Figure 1, at the end of the study, the relative prostate weights of the rats that had been treated with MFME differed from those of the untreated rats with BPH. Specifically, the relative prostate weight in the BPH group was 2.1%±0.4% (castration group vs. BPH group, P<0.05). Upon the administration of 200 mg kg−1 MFME for 4 weeks, the relative prostate weights were lower than those in the BPH group (P<0.05).
Figure 1.
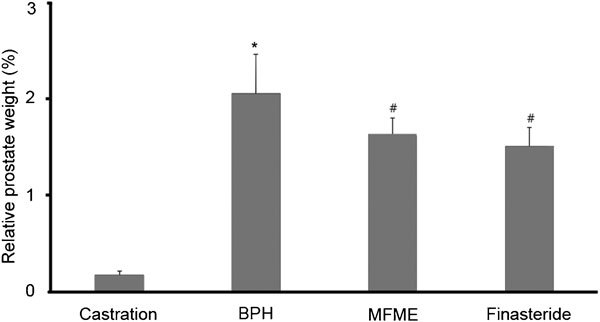
Effects of MFME on prostate weight. The rat model of BPH was created by giving rats testosterone injections for 4 weeks after castration. Castration: corn oil injection (s.c.)+oral administration of PBS; BPH: testosterone (s.c.) injection+oral administration of PBS; MFME: oral administration of MFME (200 mg kg−1)+testosterone (s.c.) injection; finasteride: oral administration of finasteride (10 mg kg−1)+testosterone (s.c.) injection. MFME or finasteride treatment was performed 1 h before testosterone injection. Values are expressed as mean±s.d. *P<0.05, compared with castration group; #P<0.05, compared with BPH group. BPH, benign prostate hyperplasia; MFME, Melandrium firmum methanolic extract; PBS, phosphate-buffered saline.
Effects of MFME on testosterone and DHT levels in the serum
We observed no differences in the serum testosterone levels between the MFME group and BPH group (Table 1). Daily administration of 200 mg kg−1 MFME for 4 weeks induced a decrease in the DHT serum level.
Table 1. The effects of MFME on DHT and testosterone levels in serum.
| Group | Dose (mg kg−1) | Testosterone (pg ml−1) | DHT (ng ml−1) |
|---|---|---|---|
| Castration | — | 38.34±0.31 | 6.24±0.50 |
| BPH | — | 204.04±39.07* | 19.16±3.49* |
| MFME | 200 | 171.89±21.27 | 11.95±3.11# |
| Finasteride | 10 | 170.52±28.06 | 14.45±3.61 |
Abbreviations: MFME, Melandrium firmum methanolic extract; DHT, dihydrotestosterone; BPH, benign prostatic hyperplasia.
Means±s.d., n=6; *P<0.05 vs. castration group, #P<0.05 vs. BPH group.
Effect of MFME on the DHT level in the prostate
The prostate DHT level was determined using an enzyme-linked immunosorbent assay. Daily administration of 200 mg kg−1 MFME for 4 weeks induced a decrease in the prostate DHT level, from 52.9±0.4 pg mg−1 protein in the BPH group to 35.5±0.5 pg mg−1 protein in the MFME group (P<0.05) (Figure 2).
Figure 2.
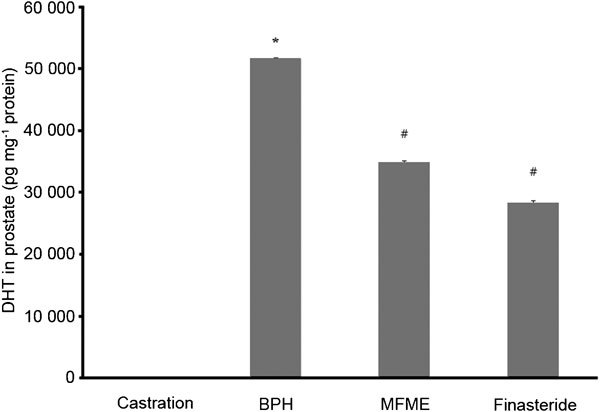
Effects of MFME on the DHT level in the prostate. Castration: corn oil injection (s.c.)+oral administration of PBS; BPH: testosterone (s.c.) injection+oral administration of PBS; MFME: oral administration of MFME (200 mg kg−1)+testosterone (s.c.) injection; finasteride: oral administration of finasteride (10 mg kg−1)+testosterone (s.c.) injection. MFME or finasteride treatment was performed 1 h before testosterone injection. Values are expressed as mean±s.d. *P<0.05, compared with castration group; #P<0.05, compared with BPH group. BPH, benign prostate hyperplasia; DHT, dihydrotestosterone; MFME, Melandrium firmum methanolic extract; PBS, phosphate-buffered saline.
Effect of MFME on the histomorphology of the prostate tissue
In the BPH group, the histoarchitecture of the prostate tissue was disrupted. The amount of connective tissue was well marked with an increased oval shape and size. The epithelial cell layer and stromal cell space in the prostates of BPH-induced animals were larger than those of the castrated rats. Interestingly, histological analyses revealed dramatically altered glandular morphology following MFME group administration (Figure 3). Although weak reductions in stromal cell hyperplasia were observed in the MFME-treated animals, the main decreases compared with BPH group animals were observed in the epithelial layer thickness.
Figure 3.
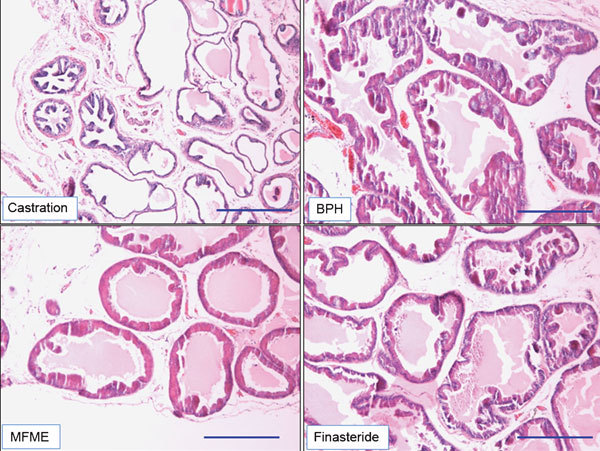
Effects of MFME on prostate hyperplasia. Histological examination of prostate tissue was performed 24 h after the final testosterone injection. Prostate tissues were fixed, sectioned at 4 µm thickness, and stained with H&E solution. Scale bars=49.45 µm. Castration: corn oil injection (s.c.)+oral administration of PBS; BPH: testosterone (s.c.) injection+oral administration of PBS; MFME: oral administration of MFME (200 mg kg−1)+testosterone (s.c.) injection; finasteride: oral administration of finasteride (10 mg kg−1)+testosterone (s.c.) injection. MFME or finasteride treatment was performed 1 h before testosterone injection. BPH, benign prostate hyperplasia; H&E, haematoxylin and eosin; MFME, Melandrium firmum methanolic extract; PBS, phosphate-buffered saline.
Effect of MFME on ALT and AST levels in serum
AST and ALT kits were used to measure AST and ALT in the serum, according to the manufacturer's protocols. MFME did not affect the activity of either of these serum toxicity marker enzymes, indicating normal liver function (Figure 4).
Figure 4.
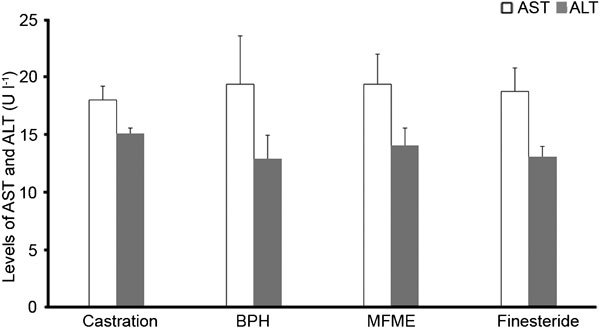
Levels of AST and ALT in the serum. Serum was collected from rats 48 h after the final testosterone injection. The samples were analysed using commercial kits (Beckman Coulter, Inc., Fullerton, CA, USA). Castration: corn oil injection (s.c.)+oral administration of PBS; BPH: testosterone (s.c.) injection+oral administration of PBS; MFME: oral administration of MFME (200 mg kg−1)+testosterone (s.c.) injection; finasteride: oral administration of finasteride (10 mg kg−1)+testosterone (s.c.) injection. MFME or finasteride treatment was performed 1 h before testosterone injection. Values are expressed as mean±s.d. ALT, alanine aminotransferase; AST, aspartate aminotransferase; MFME, Melandrium firmum methanolic extract; PBS, phosphate-buffered saline.
HPLC analysis
A three-dimensional chromatogram was obtained using an HPLC-PDA detector. Sample analysis using mobile phases consisting of solvents A (1.0% (v/v), aqueous acetic acid) and B (1.0% (v/v), acetic acid in acetonitrile) under optimized chromatography conditions led to the elution of two compounds before 30 min: cytisoside and vitexin, both flavonoids. The standard curves for cytisoside and vitexin were y=12440.16x−10752.52 (R2=1.0000) and y=18570.68x−16085.85 (R2=1.0000), respectively. The contents of cytisoside and vitexin were 0.82±0.008 mg g−1 and 0.89±0.004 mg g−1, respectively. The three-dimensional HPLC chromatogram of the M. firmum extract is presented in Figure 5.
Figure 5.
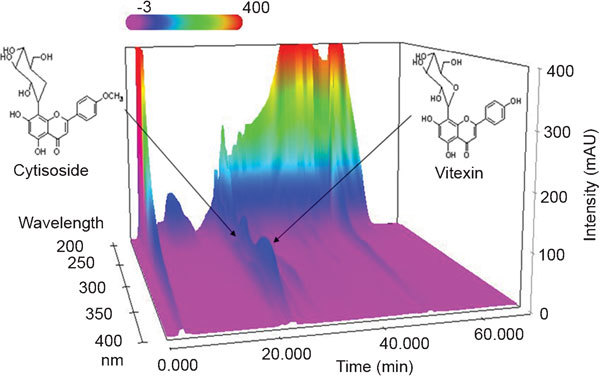
Three-dimensional chromatograms of MFME analysed using HPLC-PDA. MFME, Melandrium firmum methanolic extract; HPLC-PDA, high-performance liquid chromatography-photo diode array detector.
Discussion
BPH and prostate cancer are the most common proliferative disorders affecting older men. Androgens and oestrogens significantly influence the development of BPH. However, the prostate needs androgens for normal physiological functioning. An alternative pharmacological approach in patients with BPH is to inhibit the androgens responsible for prostatic hyperplasia.
The relative prostate weight is used as one important marker of BPH development.15, 16 In previous studies, animals with BPH have shown an increased relative prostate weight. Finasteride or other agents used to treat BPH decrease the relative prostate weight. In the present study, the animals with BPH showed an increased relative prostate weight compare to castration group (P<0.05). In contrast, the animals treated with MFME showed a reduction in relative prostate weight compared to BPH group (P<0.05). These results indicate that MFME attenuated the prostatic enlargement induced by testosterone. Additionally, these findings were supported by histological examination of the prostate tissue. The animals with BPH experienced stromal proliferation and glandular hyperplasia in the prostate, whereas the animals with BPH that had been treated with MFME showed mild glandular hyperplasia. These results suggested that MFME inhibits the progression of BPH induced by testosterone.
In the present investigation, we established that the administration of MFME to rats with induced BPH had a positive effect on the prostate mass, which was decreased after 4 weeks of treatment (P<0.05). The two main classes of drug employed for BPH treatment are inhibitors of α1-adrenoceptor antagonists that inhibit smooth muscle cell contraction17 and inhibitors of type II 5α-reductase, an enzyme responsible for the conversion of testosterone to the more potent androgen dihydrotestosterone (DHT).18 Finasteride, a type II 5α-reductase inhibitor, suppresses both the plasma and intraprostatic DHT concentrations, leading to reductions in glandular size, epithelial cell height and the synthetic and proliferative activities of DHT. Finasteride also enhances the apoptotic index of epithelial cells19, 20, 21, 22 and is thus employed as a positive control. The local conversion of testosterone to its metabolite, DHT, catalysed by the steroid enzyme 5α-reductase is implicated as a causative factor in BPH. In our experiments, MFME suppressed the serum DHT level (P<0.05) and relative prostate weight (P<0.05) compare to the BPH group. These results indicate that MFME has important antiproliferative effects and possibly interferes with androgen action. MFME suppressed the DHT level, either via inhibition of the action of 5α-reductase or other, more complicated mechanisms involving tissue androgen receptors that remain to be determined.
The development of BPH has been associated with antioxidants. In BPH, increased lipid peroxidation and decreased levels of antioxidants have been observed.23, 24, 25 Various herbal extracts reduce oxidative stress in testosterone-induced BPH rats.26, 27, 28, 29 In addition, saw palmetto extract, which is commonly used to treat BPH, has shown antioxidant effects.30 Therefore, antioxidants can be considered good candidates to suppress the development of BPH. In this study, we analysed two flavonoids, cytisoside and vitexin, contained in MFME by using HPLC. Of these, vitexin is the most likely to possess antioxidant properties, according to multiple studies.31, 32 These compounds might underlie the reduced development of BPH in response to MFME; however, the available data do not clearly confirm this hypothesis. Therefore, more studies are needed on the relationship between the antioxidant effects of MFME and its constituents and the development of BPH.
M. firmum extracts contain saponins, steroids and flavonoids.33, 34, 35, 36 Among the isolated components, steroids and saponins predominate, including α-spinasterol and melandrioside A.33, 34 However, these compounds are difficult to analyse by HPLC because they are comparatively weak UV chromophores compared to the flavonoids. Thus, we performed HPLC analysis for two flavonoids in MFME, cytisoside and vitexin. We achieved good separation of the MFME. However, activity tests for the major components of MFME need to be performed.
In conclusion, MFME administered orally at a dose of 200 mg kg−1 d−1 appears effective in reducing established prostate hyperplasia.
Author contributions
MYL designed and performed the experiments, analysed the data and wrote the manuscript. ISS helped design the study and conduct the experiments. NHL and HKH helped conduct the experiments. CSS performed the extraction and HPLC of MFME. HKS conceived the idea of this study and supervised the team. All authors read and approved the final version of the manuscript.
Acknowledgments
This research was supported by a grant ‘Evidence-based medicine for herbal formulae' (No. K11030) from Korea Institute of Oriental Medicine.
The authors declared that no competing financial interests exist.
References
- Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005;173:1256–61. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride long-term efficacy and safety Study Group. N Engl J Med. 1998;338:557–63. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- Foley CL, Kirby RS. 5 alpha-reductase inhibitors: What's new. Curr Opin Urol. 2003;13:31–7. doi: 10.1097/00042307-200301000-00006. [DOI] [PubMed] [Google Scholar]
- Vaughan D, Imperato-McGinley J, McConnell J, Matsumoto AM, Bracken B, et al. Long-term (7 to 8-year) experience with finasteride in men with benign prostatic hyperplasia. Urology. 2002;60:1040–4. doi: 10.1016/s0090-4295(02)01971-4. [DOI] [PubMed] [Google Scholar]
- Uygur MC, Gur E, Arik AI, Altug U, Erol D. Erectile dysfunction following treatments of benign prostatic hyperplasia: a prospective study. Andrologia. 1998;30:5–10. doi: 10.1111/j.1439-0272.1998.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Chang IS, Han YB, Woo WS, Kang SS, Lotter H, et al. 1989. Sapogenins from Melandrium firmum. Planta Med. 1989;55:544–7. doi: 10.1055/s-2006-962090. [DOI] [PubMed] [Google Scholar]
- Woo EH, Woo WS, Chmurny GN, Hilton BD. Melandrioside a saponin from Melandrium firmum. J Nat Prod. 1992;55:786–94. doi: 10.1021/np50084a013. [DOI] [PubMed] [Google Scholar]
- Zhen MS, Hwang NK, Kim DH, Moon TC, Song JK, et al. Chemical constituents of Melandrium firmum Rohrbach and their anti-inflammatory activity. Arch Pharm Res. 2008;31:318–22. doi: 10.1007/s12272-001-1158-9. [DOI] [PubMed] [Google Scholar]
- Perry LM.Medicinal Plants of East and Southeast Asia, Attributed Properties and Uses CambridgeCA/London; MIT Press; 1980. p74. [Google Scholar]
- Sharaf M, el-Ansari MA, Matlin SA, Saleh NA. Four flavonoid glycosides from Peganum harmala. . Phytochemistry. 1997;44:533–6. doi: 10.1016/s0031-9422(96)00531-6. [DOI] [PubMed] [Google Scholar]
- Naslund MJ, Coffey DS. The differential effects of neonatal androgens, estrogen and progesterone on adult rat prostate growth. J Urol. 1986;136:1136–40. doi: 10.1016/s0022-5347(17)45239-6. [DOI] [PubMed] [Google Scholar]
- Guo QL, Ding GL, Wu ZQ. Effect of baicalein on experimental prostatic hyperplasia in rats and mice. Biol Pharm Bull. 2004;27:333–7. doi: 10.1248/bpb.27.333. [DOI] [PubMed] [Google Scholar]
- di Salle E, Giudici D, Biagini L, Cominato C, Briatico G, et al. Effects of 5 alpha-reductase inhibitors on intraprostatic androgens in the rat. J Steroid Biochem Mol Biol. 1995;53:381–5. doi: 10.1016/0960-0760(95)00083-c. [DOI] [PubMed] [Google Scholar]
- Huynh H. Induction of apoptosis in rat ventral prostate by finasteride is associated with alteration in MAP kinase pathways and Bcl-2 related family of proteins. Int J Oncol. 2002;20:1297–303. [PubMed] [Google Scholar]
- Arruzazabala ML, Mas R, Molina V, Noa M, Carbajal D, et al. Effect of D-004, a lipid extract from the cubal royal palm fruit, on atypical prostate hyperplasia induced by phynylephrine. Drug R D. 2006;7:233–41. doi: 10.2165/00126839-200607040-00003. [DOI] [PubMed] [Google Scholar]
- Veeresh Babu, SV, Veeresh B, Patill AA, Warke YB. Lauric acid and myristic acid prevent testosterone induced prostatic hyperplasia in rats. Eur J Pharmacol. 2010;625:262–5. doi: 10.1016/j.ejphar.2009.09.037. [DOI] [PubMed] [Google Scholar]
- Furuya S, Kumamoto Y, Yokoyama E, Tsukamoto T, Izumi T, et al. Alphaadrenergic activity and urethral pressure in prostatic zone in benign prostatic hypertrophy. J Urol. 1982;128:836–9. doi: 10.1016/s0022-5347(17)53216-4. [DOI] [PubMed] [Google Scholar]
- Griffiths K, Denis LJ. Exploitable mechanisms for the blockade of androgenic action. Prostate Suppl. 2000;10:43–51. doi: 10.1002/1097-0045(2000)45:10+<43::aid-pros9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Rittmaster RS, Manning AP, Wright AS, Thomas LN, Whitefield S, et al. Evidence for atrophy and apoptosis in the ventral prostate of rats given the 5 alpha-reductase inhibitor finasteride. Endocrinology. 1995;136:741–8. doi: 10.1210/endo.136.2.7835306. [DOI] [PubMed] [Google Scholar]
- Lobaccaro JM, Boudon C, Lechevallier E, Mottet N, Rebillard X, et al. Effect of finasteride (Proscar) on the proliferation of cultured epithelial and stromal cells from normal and hyperplastic human prostates. Cell Mol Biol. 1996;42:511–8. [PubMed] [Google Scholar]
- Yamashita A, Hayashi N, Sugimura Y, Cunha GR, Kawamura J. Influence of diethylstilbestrol, Leuprolelin (a luteinizing hormone-releasing hormone analog), Finasteride (a 5 alpha-reductase inhibitor), and castration on the lobar subdivisions of the rat prostate. Prostate. 1996;29:1–14. doi: 10.1002/(SICI)1097-0045(199607)29:1<1::AID-PROS1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Huynh H. Induction of apoptosis in rat ventral prostate by finasteride is associated with alteration in MAP kinase pathways and Bcl-2 related family of proteins. Int J Oncol. 2002;20:1297–303. [PubMed] [Google Scholar]
- Aryal M, Pandeya A, Gautam N, Baral N, Lamsal M, et al. Oxidative stress in benign prostatic hyperplasia. Nepal Med Coll J. 2007;9:222–4. [PubMed] [Google Scholar]
- Aydin A, Arsova-Sarafinovska Z, Sayal A, Eken A, Erdem O, et al. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin Biochem. 2006;39:176–9. doi: 10.1016/j.clinbiochem.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Mchedlidze MG, Shioshvili TI. Influence of antioxidants on the development of benign prostatic hyperplasia. Georgian Med News. 2006;140:23–7. [PubMed] [Google Scholar]
- Siddiqui IA, Raisuddin S, Shukla Y. Protective effects of black tea extract on testosterone induced oxidative damage in prostate. Cancer Lett. 2005;227:125–32. doi: 10.1016/j.canlet.2004.10.046. [DOI] [PubMed] [Google Scholar]
- Prasad S, Kalra N, Singh M, Shukla Y. Protective effects of lupeol and mango extract against androgen induced oxidative stress in Swiss. Asian J Androl. 2008;10:313–8. doi: 10.1111/j.1745-7262.2008.00313.x. [DOI] [PubMed] [Google Scholar]
- Lopez E, Molina V, Illnait J, Oyarzabal A, Fernandez LC, et al. Antioxidant effects of D-004, a lipid extract from the Roystonea regia fruit, on the plasma of healthy men. Asian J Androl. 2009;11:385–92. doi: 10.1038/aja.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevesi BT, Houghton PJ, Habtemariam S, Kery A. Antioxidant and anti-inflammatory effect of Epilobium parviflorum Schreb. Phytother Res. 2009;23:719–24. doi: 10.1002/ptr.2725. [DOI] [PubMed] [Google Scholar]
- Belostottskaia LI, Nikitchenko IuB, Gomon N, Chaika LA, Bordar VV, et al. Effect of biologically active substances of animal and plant origin on prooxidant-antioxidant balance in rats with experimental prostatic hyperplasia. Eksp Klin Farmakol. 2006;4:66–8. [PubMed] [Google Scholar]
- Ying XX, Li HB, Chu ZY, Zhai YJ, Leng AJ, et al. HPLC determination of malondialdehyde in ECV304 cell culture medium for measuring the antioxidant effect of vitexin-4″-O-glucoside. Arch Pharm Res. 2008;31:878–85. doi: 10.1007/s12272-001-1241-2. [DOI] [PubMed] [Google Scholar]
- Li HB, Ying XX, Lu J. The mechanism of vitexin-4″-O-glucoside protecting ECV-304 cells against tertbutyl hydroperoxide induced injury. Nat Prod Res. 2010;24:1695–703. doi: 10.1080/14786410902853847. [DOI] [PubMed] [Google Scholar]
- Zheng MS, Hwang NK, Kim DH, Moon TC, Son JK, et al. Chemical constituents of Melandrium firmum Rohrbach and their anti-inflammatory activity. Arch Pharm Res. 2008;31:318–22. doi: 10.1007/s12272-001-1158-9. [DOI] [PubMed] [Google Scholar]
- Woo EH, Woo WS. Melandrioside A, a saponin from Melandrium firmum. . J Nat Prod. 1992;55:786–94. doi: 10.1021/np50084a013. [DOI] [PubMed] [Google Scholar]
- Besson E, Besset A, Bouillant ML, Chopin J, Brederode J, et al. Genetically controlled 2″-O-glycosylation of isovitexin in the petals of Melandrium album. . Phytochemistry. 1979;18:657–8. [Google Scholar]
- Darmograi VN. Flavonoids from certain species of the genus Melandrium. . Khimiya Prirodnykh Soedinenii. 1977;1:115–6. [Google Scholar]


