Abstract
Late-onset hypogonadism (LOH) has been considered the most common form of male hypogonadism with a prevalence of approximately 1 in 100 men. Diagnosis of LOH should be made in symptomatic men with unequivocally low serum testosterone (T) levels. However, its clinical presentation is often insidious and difficult to recognize because it is characterized by nonspecific symptoms that make differential diagnosis with physiological ageing problematic. Sexual dysfunction is the most important determinant for medical consultation and the most specific symptom associated with low T. We therefore analysed a consecutive series of 1734 subjects who attended our unit for sexual dysfunction to investigate the associations between low T (different thresholds), sexual parameters, medical history data (delayed puberty, pituitary disease or cryptorchidism) and their physical exam results. Metabolic parameters, in particular waist circumference, display the greatest accuracy in detecting low T. We found that only the association of several symptoms and signs could significantly raise the clinical suspicion of low T. Structured inventories, which cluster together symptoms and signs of hypogonadism, can help clinicians suspect androgen deficiency. In particular, structured interviews, such as ANDROTEST, have been demonstrated to have a greater accuracy when compared to self reported questionnaires in detecting low T levels.
Keywords: late-onset hypogonadism (LOH), male hypogonadism, testosterone, sexual dysfunction
Introduction
The testis (the male gonad) continuously produces sex steroids (mostly testosterone (T)) and sperm soon after the onset of puberty. Testis activity is regulated by intratesticular factors (including T) and by extratesticular trophic factors released from the pituitary (i.e., gonadotropin luteinizing hormone (LH) and follicle-stimulating hormone (FSH)), which are strictly regulated by the hypothalamic peptide gonadotropin-releasing hormone (GnRH). Male hypogonadism is a failure in testis activity that is classically considered to be a partial or total communication breakdown among the hypothalamus, the pituitary and the testis itself. Current definitions of hypogonadism rely on T deficiency more than abnormal sperm production, but from a conceptual point of view, both factors contribute to testicular function and dysfunction.
Classification of male hypogonadism: towards a revision
According to a categorical taxonomy often reported in medical textbooks, male hypogonadism is divided into primary (i.e., caused by an abnormality of the testes) and secondary (i.e., caused by pituitary or hypothalamic dysfunction) hypogonadism. In primary hypogonadism, the testis is dysfunctional and fails to release sex steroids and sperm, even though it is superstimulated by the pituitary gland, whereas in secondary hypogonadism, the testis is normal but is inadequately stimulated by gonadotropins. Hence, the first type of hypogonadism is also defined as hypergonadotropic and the second type as hypogonadotropic. Furthermore, a compensatory form (normal T and elevated LH) of hypogonadism has also been reported.1 A hypogonadism-like syndrome can also result from a reduced sensitivity, or insensitivity, to T and its metabolites (dihydrotestosterone and oestrogens) or from reduced bioavailability of the hormone due to an increase in its carrier protein, sex hormone-binding globulin. Male hypogonadism can be congenital (e.g., Klinefelter's syndrome) or can be acquired later on, during childhood or adult life (e.g., following chemotherapy). This categorical classification is quite useful for selecting the most appropriate therapy for individual patients but is largely unsatisfactory in describing the clinical picture and patient phenotype. With respect to therapy, patients with hypothalamic or pituitary causes of testis failure (hypogonadotropic or secondary hypogonadism) can be successfully treated by removing the precipitating cause (for example, prolactinoma) and/or by appropriate endocrine therapy (i.e., gonadotropins or GnRH for desired fertility, or T for virilisation, if fertility is not the primary goal). However, hypogonadism is more a dimensional disorder rather than a categorical one; in clinical practice, both primary (testis) and secondary (hypothalamus and/or pituitary) failure are often simultaneously present, as in the case of hypogonadism associated with obesity or metabolic syndrome. In addition, signs and symptoms of hypogonadism are quite similar, irrespective of the disease origin; in other words, the clinical phenotype can be identical for primary or secondary hypogonadism.2, 3, 4 However, the clinical phenotype is very different according to the age of hypogonadism onset, which can be used as an alternative classification. Table 1 reports the new age-of-onset-based classification of male hypogonadism. When the problem occurs during early foetal life (very-early-onset hypogonadism), symptoms can be dramatic, from an almost complete female phenotype (complete androgen insensitivity or enzymatic defects blocking androgen synthesis) to various defects in virilisation (micropenis, hypospadias cryptorchidism), as in the case of impaired secretion or activity of GnRH. Differences in the severity of the phenotype arise from the presence or absence of a temporary GnRH-independent/human chorionic gonadotropin-dependent T secretion from the foetal testis. In the case of the peripubertal appearance of hypogonadism (early-onset hypogonadism), because of milder central or peripheral defects (such as in Klinefelter's syndrome), there might be a delay in the onset of puberty with an overall eunuchoidal phenotype, including scant body hair, a high-pitched voice and a small testis, penis and prostate. However, when hypogonadism develops after puberty, and specifically, when it occurs with ageing (late-onset hypogonadism (LOH)), the symptoms will be relatively mild, insidious and difficult to recognize. These symptoms include the following: loss of muscle mass and strength, physical activity and performance decline, decrease in some cognitive abilities, depressive symptoms and sexual dysfunction.4, 5 However, these symptoms are not specific enough to be considered pathognomonic, which makes differential diagnosis between LOH and physiological ageing problematic.
Table 1. Characteristics and prevalence of hypogonadism based on the onset age. Note that conditions reported in italics are only characterized by impaired sperm production and not by abnormal testosterone synthesis and/or activity.
| Hypogonadism class | Clinical onset | Phenotype |
|---|---|---|
| Very-early-onset hypogonadism (VEOH) | Foetal | Incomplete androgenisation → pseudohermaphroditism |
| 1) Decreased testosterone production | ||
| a) Congenital early onset hypothalamic diseases (↓ gonadotrophins, ↓ testosterone) | ||
| I) Kallmann's syndrome (including KAL1, FGFR1, PROK2 and PROKR2 mutations) | ||
| II) GnRH gene deletion | ||
| III) Others | ||
| b) Congenital early onset pituitary diseases (↓ gonadotrophins, ↓ testosterone) | ||
| I) GnRHR mutations | ||
| II) FSHβ and LHβ mutations | ||
| III) Others | ||
| c) Congenital early onset testicular diseases (↑ gonadotrophins±↓ testosterone) | ||
| I) Defects of testosterone biosynthesis | ||
| II) Others | ||
| 2) Congenital early onset decreased testosterone bioactivity (≅↑ gonadotrophins, ≅↑ testosterone) | ||
| I) Androgen receptor alterations (Morris syndrome) | ||
| -Complete/partial androgen insensitivity syndromes | ||
| II) Others | ||
| Early-onset hypogonadism (EOH) | Prepubertal | Delayed puberty→eunuchoidism |
| 1) Decreased testosterone production | ||
| a) Acquired hypothalamic diseases (↓ gonadotrophins, ↓ testosterone) | ||
| I) Hypothalamic tumours | ||
| -Craniopharyngiomas | ||
| -Others | ||
| II) Infiltrative and infective disorders | ||
| -Langerhans' histiocytosis | ||
| -Others | ||
| III) Functional disorders | ||
| -Nutritional | ||
| -Critical illness | ||
| -Excessive exercise (rare) | ||
| b) Acquired pituitary diseases (↓ gonadotrophins, ↓ testosterone) | ||
| I) Pituitary tumours | ||
| II) Infiltrative | ||
| III) Others | ||
| c) Acquired–congenital (late onset) testicular diseases (↑ gonadotrophins±↓ testosterone) | ||
| I) Klinefelter's syndrome | ||
| II) Orchitis (including mumps and autoimmune disorders) | ||
| III) Chemotherapy | ||
| IV) Testicular irradiation | ||
| V) Bilateral torsion/trauma | ||
| VI) Cryptorchidism (including INSL3 and LGR8 mutations) | ||
| Late-onset hypogonadism (LOH) | Adulthood | No specific phenotype |
| 1) Decreased testosterone production | ||
| a) Acquired hypothalamic diseases (↓ gonadotrophins, ↓ testosterone) | ||
| I) Hypothalamic tumours | ||
| II) Infiltrative and infective disorders | ||
| -Langerhans' histiocytosis | ||
| -Sarcoidosis and tuberculosis, syphilis | ||
| -Encephalitis | ||
| III) Head trauma | ||
| IV) Idiopathic | ||
| V) Functional disorders | ||
| -Hyperprolactinaemia (prolactinoma, hypothyroidism, antidopaminergic and serotoninergic drug | ||
| -induced, opiates-induced) | ||
| -Nutritional | ||
| -Critical illness | ||
| -Diabetes mellitus | ||
| -Metabolic syndrome | ||
| -Cushing disease | ||
| b) Acquired pituitary diseases (↓ gonadotrophins, ↓ testosterone) | ||
| I) Pituitary tumours | ||
| -Functional and non-functional adenomas | ||
| -Metastases | ||
| -Others | ||
| II) Infiltrative | ||
| -Primary hypophysitis | ||
| -Sarcoidosis and tuberculosis, syphilis | ||
| -Fungal, parasites, viral | ||
| III) Head trauma | ||
| IV) Empty sella | ||
| V) Vascular | ||
| VI) Drugs | ||
| -GnRH analogs (agonists and antagonists) | ||
| -Oestrogens | ||
| -Anabolic steroids | ||
| -Progestogens (including cyproterone acetate and spironolactone) | ||
| VII) X-irradiation | ||
| c) Testicular diseases (↑ gonadotrophins±↓ testosterone) | ||
| I) Myotonic dystrophy (including type I and II) | ||
| II) Y-chromosome microdeletions | ||
| III) Autosomal translocations | ||
| IV) FSHR mutations | ||
| V) Germinal aplasia (del Castillo syndrome, Sertoli-cell only syndrome) | ||
| VI) Orchitis (including mumps and autoimmune disorders) | ||
| VII) Chemotherapy | ||
| VIII) Testicular irradiation | ||
| IX) Bilateral torsion | ||
| X) Varicocele | ||
| XI) Inhibitors of testosterone synthesis | ||
| -Ketoconazole | ||
| -Aminoglutethimide | ||
| -Mitotane | ||
| -Metyrapone | ||
| XI) Bilateral trauma | ||
| XII) General diseases (including renal failure, liver cirrhosis, diabetes mellitus) | ||
| 2) Decreased testosterone bioactivity (≅↑gonadotrophins, ≅↑testosterone) | ||
| I) Androgen receptor alterations | ||
| -Kennedy syndrome and other extensions of CAG repeats | ||
| II) Drug-induced AR blockade | ||
| -Steroidal anti-androgen (cyproterone acetate, spironolactone) | ||
| -Non-steroidal anti-androgen (flutamide, bicalutamide, nilutamide) | ||
| II) Drug-induced 5α-reductase activity blockade | ||
| -Finasteride (type II) | ||
| -Dutasteride (type I and II) | ||
| III) Drug-induced ER blockade | ||
| -Clomiphene, tamoxifen, raloxifene | ||
| IV) Drug-induced aromatase activity blockade | ||
| -Letrozole, anastrozole, exemestane | ||
| V) Increased sex hormone-binding protein | ||
| -Drug-induced (antiepileptic, oestrogen, thyroid hormones) | ||
| -Hyperthyroidism | ||
| -Liver diseases | ||
| -Ageing | ||
Abbreviations: FGFR-1, fibroblastic growth factor receptor-1; FSHβ, follicle-stimulating hormone β-subunit; FSHR, follicle-stimulating hormone receptor; GnRH, gonadotrophin-releasing hormone; GnRHR, gonadotrophin-releasing hormone receptor; INSL3, insulin-like-3 peptide; KAL-1, anosmin or Kallmann protein; LGR8. leucine-rich repeat-containing, G protein-coupled receptor-8, or insulin-like-3 peptide receptor; LHβ, luteinizing hormone β-subunit; PROK-2, prokineticin-2; PROKR-2, prokineticin-2 receptor.
Despite the unimpressive clinical picture, LOH should not be overlooked. It is important to note that LOH symptoms can place a significant burden on both the patient and the healthcare system. LOH can impact the patient's physical, social, emotional, cognitive and sexual functioning,5, 6 which are all key domains inherent to health-related quality of life.7, 8 Furthermore, male hypogonadism has also recently been associated with an increase in mortality risk.9, 10, 11 A number of symptoms, including fatigue, low mood (which can lead to depression12, 13) and loss of bone density (which may lead to osteoporosis14, 16) have the potential to cause considerable short-term and long-term disabilities with economic consequences.17 LOH is the most common form of male hypogonadism, with even the most conservative estimate recognizing a prevalence of 1–2∶100 adult males (Table 1).4, 18 However, few data on hypogonadism in ageing men are available because the exact criteria for identifying T deficiency in older men lacking pathological hypogonadism have not been determined.1 Figure 1 summarizes the most common clinical illnesses associated with an increased risk of LOH for which the evaluation of T is suggested.
Figure 1.
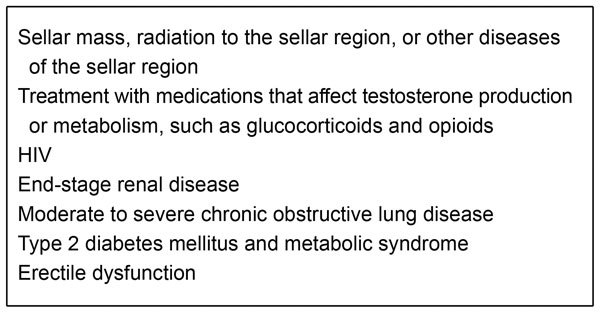
Conditions with a high prevalence of low testosterone levels for which measurement of serum testosterone levels is advisable.
Among the different symptoms, sexual dysfunction is the most important determinant for medical consultation4 and the most specific symptom associated with low T.18 In fact, recent data from the European Male Aging study recognized that a triad of sexual symptoms (low libido as well as reduced spontaneous and sex-related erections) is the only syndromic association with decreased T levels,18 whereas other proposed hypogonadal symptoms did not segregate with androgen deficiency. The lower the T levels are, the higher the number of sexual symptoms describing hypogonadism becomes.
LOH recognition has been greatly helped by the recent consensus among professional societies (see also Table 2).5, 19, 20 Although they differ in their proposed T thresholds for the biochemical definition of hypogonadism,2, 3, 4 all the Andrology Society guidelines recognize that the presence of hypogonadism-related symptoms is the cornerstone to defining a clinically relevant hypogonadism. It has been universally recognized that diagnosis of androgen deficiency should be made only in symptomatic men with unequivocally low serum T levels, as neither marker is consistently reliable alone. For all the guidelines, the total T should be sampled in the morning on at least two separate occasions before making a diagnosis. T substitution should be offered to symptomatic individuals when circulating total T is below 8 nmol l−1 (231 ng dl−1).5 In addition, there is also a general agreement that total T level above 12 nmol l−1 (346 ng dl−1) does not require supplementation. When the total T is between 8 and 12 nmol l−1 in the presence of typical hypogonadal symptoms, a T treatment trial should be considered.5 Measurement of free T (FT) by equilibrium dialysis or calculation (cFT) according to Vermuelen's formula (http://www.issam.ch/freetesto.htm) might help in conditions when sex hormone-binding globulin could be decreased (obesity, acromegaly, hypothyroidism) or increased (ageing, hepatic illnesses, hyperthyroidism, use of anticonvulsants). Conversely, the immunometric method for evaluation of FT has been considered unreliable.2, 3, 4
Table 2. Specifics of available tools for the screening of hypogonadism-related symptoms and signs.
| AMS | ADAM | MMAS | NERI hypogonadism screener | ANDROTEST |
|---|---|---|---|---|
| Low libido (17) | Low libido (1) | Low libido (4–6) | Sexual desire (9) | |
| Decreased sexual performance (15) | Sexual problems (7) | Sexual problems (7) | Reduced sex related erections (3) | Severe erectile dysfunction (5) |
| Frequency of masturbation (7) | ||||
| Feeling during autoerotism (8) | ||||
| History of ejaculate volume reduction (10) | ||||
| History of delayed ejaculation (11) | ||||
| Reduced sleep-related erections (16) | Frequency of morning/spontaneous erections (1–2) | Frequency of nocturnal erections (6) | ||
| Irritability–nervousness (6–7) | Irritability (10) | |||
| Anxiety (8) | Anxiety (9) | |||
| Depression (11) | Decreased enjoyment of life sadness (5–6) | Depression (8) | ||
| Feeling burnt-out (12–13) | ||||
| Increased temper (7) | ||||
| Tiredness (5) | Tiredness after dinner (9) | Tiredness (17–18) | ||
| Sleepy during the day (16) | ||||
| Sleep problems (4) | Sleep quality (4) | Sleep problems (15) | ||
| Lacking vitality (9) | Lack of energy (2) | |||
| Decreased muscular strength (10) | Decrease in strength (3) | |||
| Reduced sport and work performance (8, 10) | Managing ability (8) | |||
| General well-being (1) | ||||
| Sweating (3) | ||||
| Joint pain and muscular ache (2) | ||||
| Difficulty in remembering names | ||||
| Difficulty in remembering things and reading | ||||
| Difficulty in remembering directions | ||||
| Frequently misplacing things (11–14) | ||||
| Reduction of beard growth (14) | ||||
| Headache (6) | ||||
| Loss of height (4) | ||||
| Asthma (3) | ||||
| Diabetes mellitus (2) | ||||
| History of delayed puberty, pituitary diseases, cryptorchidism (2–4) | ||||
| Smoking habit (5) | ||||
| Height and weight (9) | Body mass index (12) | |||
| Age (1) | Age (1) |
Abbreviations: ADAM, Androgen Deficiency in Aging Male scale; AMS, Aging Male Scale; MMAS, scale derived from Massachusetts Male Aging Study; NERI, New England Research Institute.
The numbers in parentheses indicate the order of items in the original questionnaires.
How to recognize LOH in men with sexual dysfunction
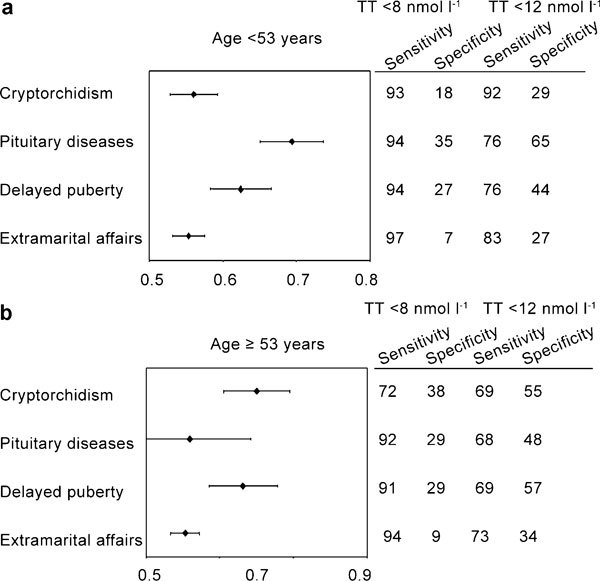
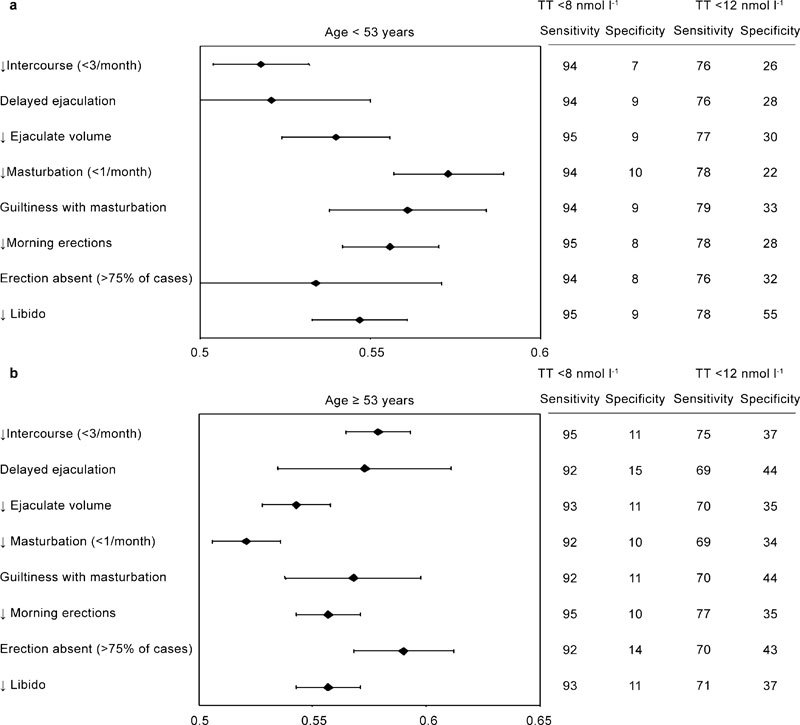
To determine the symptoms and signs that are specifically associated with low T, we retrospectively analysed data of 1734 men that consulted an andrology clinic for sexual dysfunction. All of them were symptomatic by definition because they complained of sexual problems. The results are reported in Figures 2–4, stratified according to the median age of the cohort studied (53 years). Only metrics associated with severe (total T below 8 nmol l−1) or mild (total T below 12 nmol l−1) hypogonadism are depicted and reported as hazard ratio (age-adjusted) in a log scale. By using these criteria, 472 (27.7%) patients exhibited mild hypogonadism, while 128 (7.4%) of the patients had severe hypogonadism. In particular, severe hypogonadism was detected in 53 (6.2%) younger patients and 75 (8.6%) older patients, while mild hypogonadism occurred in 198 (23.2%) younger patients and 274 (31.1%) older patients. Figure 2 shows the medical history-derived information that showed significant association with a severe or milder reduction of total T in our series. As expected, a history of delayed puberty, pituitary disease and cryptorchidism were all associated with severe hypogonadism in both younger and older subjects. The milder form of hypogonadism was only significantly associated with delayed puberty in both cohorts, while cryptorchidism was significant only in the oldest groups, and pituitary diseases were only in the youngest population. In the younger population, self-reported extramarital activity is a negative predictor of mild, but not severe, hypogonadism. In the oldest cohort, any form of low T was associated with several sexual parameters (Figure 3), such as decreased libido, frequency of sexual intercourse, morning and sexual-related erections, and perceived reduced ejaculate volume. Only decreased sexual desire and morning erections predicted any form of hypogonadism in the youngest cohort. A reduced frequency of autoerotism (masturbation) characterized any T deficiency in the youngest, but not in the oldest, cohort. In both populations, a milder form of T deficiency was related to the emotions during masturbation, including the feeling of discomfort or guiltiness during autoeroticism, most likely because hypogonadism reduces male sexual confidence and related aggressiveness. People with a milder reduction in T felt guiltier with autoeroticism than their eugonadal counterpart.
Figure 2.
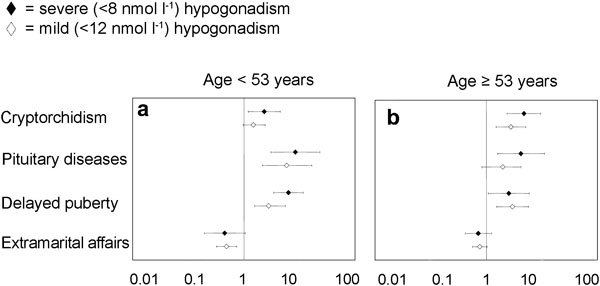
Relevant metrics in patients' medical and sexual histories for detecting mild (<12 nmol l−1) and severe (<8 nmol l−1) hypogonadism in a consecutive series of 1734 patients that attended our unit seeking medical care for sexual dysfunction between 2002 and 2010. Data are reported in a log scale as age-adjusted hazard ratio (95% confidence intervals) stratified by age (<53 years old (a) and ≥53 years old(b)).
Figure 3.
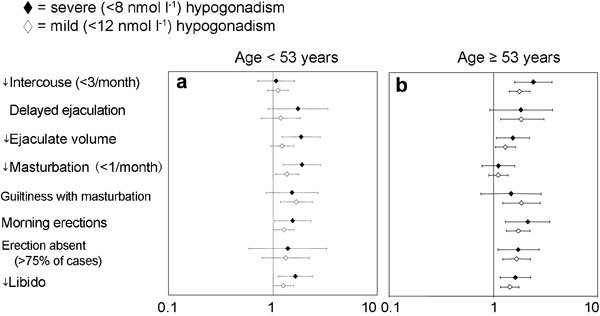
Relevant items in sexual history for detecting mild (<12 nmol l−1) and severe (<8 nmol l−1) hypogonadism in a consecutive series of 1734 patients that attended our unit seeking medical care for sexual dysfunction between 2002 and 2010. Data are reported in a log scale as age-adjusted hazard ratio (95% confidence intervals) stratified by age (<53 years old (a) and ≥53 years old(b)).
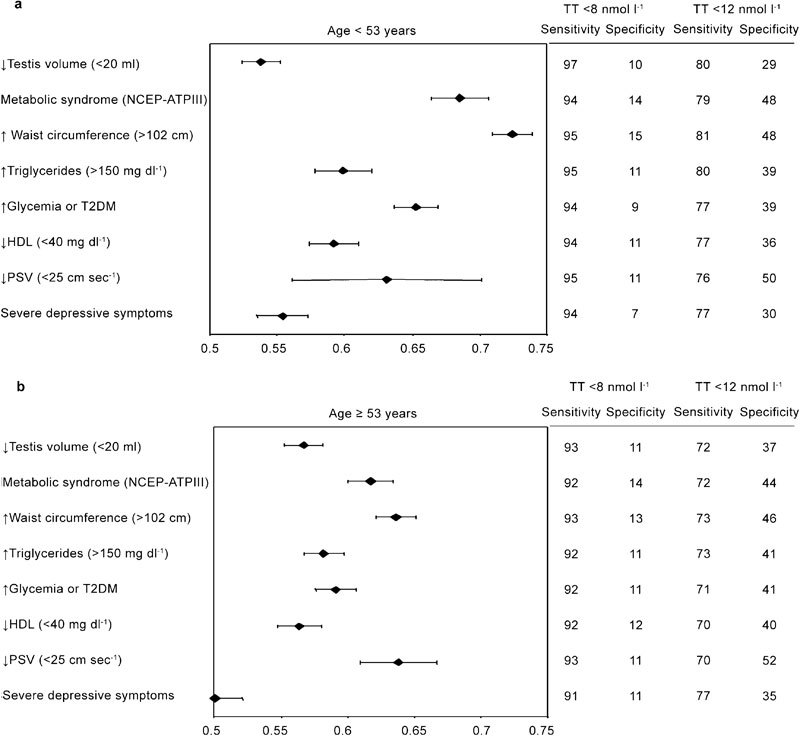
Figure 4.
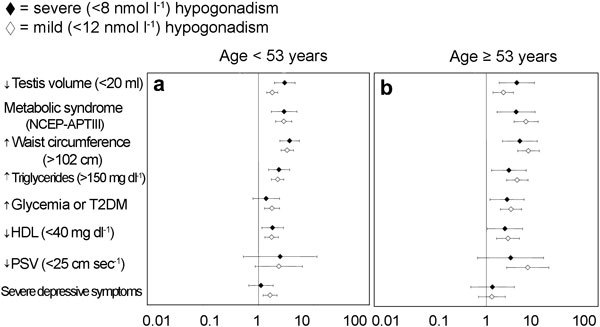
Clinical, metabolic and instrumental metrics for detecting mild (<12 nmol l−1) and severe (<8 nmol −1) hypogonadism in a consecutive series of 1734 patients that attended our unit seeking medical care for sexual dysfunction between 2002 and 2010. Data are reported in a log scale as age-adjusted hazard ratio (95% confidence intervals) stratified by age (<53 years old (a) and ≥53 years old(b)). NCEP-ATPIII, National Cholesterol Education Program's Adult Treatment Panel III; PSV, peak systolic velocity at penile Doppler ultrasound; T2DM, type 2 diabetes mellitus.
Figure 4 reports the association of hypogonadism with clinical signs and laboratory findings. Irrespective of age, the presence of a reduced testis volume strongly suggests a reduction of total T. Similar results were obtained when the diagnosis of metabolic syndrome was considered (National Cholesterol Education Program-Adult Treatment Panel III criteria). When individual components of metabolic syndrome were analysed, increased waist circumference and elevated triglycerides were the most relevant features associated with any form of hypogonadism. No association was found with systolic hypertension in both younger and older cohorts (data not shown). A milder form of hypogonadism was associated with a decreased peak systolic velocity in penile vessels only in the oldest cohort, as detected by colour Duplex ultrasound. The presence of depressive symptoms, as assessed by the Middlesex Hospital Questionnaire,21 was associated with hypogonadism only in younger subjects.
Receiver-operating characteristic (ROC) is a graphical plot of the sensitivity, or true positive rate vs. false positive rate (1—specificity or 1—true-negative rate), for a binary classifier system, as its discrimination threshold is varied. ROC curve analysis was used to determine the sensitivity and specificity of all of the aforementioned symptoms and signs at the accepted biochemical thresholds (below 8 and 12 nmol l−1). The results are reported for each cohort (i.e., youngest and oldest) as the area under the curve, which essentially recapitulates the accuracy of each parameter (true specificity+true sensitivity) (Figures 5–7). Interestingly, all the analysed parameters, when individually considered, showed a lack of specificity but a good sensitivity in any age band and at any biochemical threshold. Quite unexpectedly, increased waist circumference was the most specific and most accurate hypogonadism-associated sign. This suggests that metabolic parameters could better identify hypogonadal patients than ‘specific' sexual symptoms. In fact, the latter do not appear to be specific at all; none of them has specificity greater than 15%. Sexual symptoms, when individually analysed, showed a rather low accuracy in diagnosing low T. Because the specificity measures the proportion of negatives that are correctly identified (e.g., the percentage of healthy people who are correctly identified as not having hypogonadism), the lack of specificity warrants further testing (i.e., measuring blood androgens). Hence, only the association of several symptoms and signs could significantly raise the clinical suspicion of low T. This is the advantage of structured inventories, which cluster symptoms and signs of hypogonadism together to help clinicians detect hypogonadism.
Figure 5.
Area under the curve (95% confidence intervals) derived from receiver-operating characteristic (ROC) curves for severe (total testosterone (TT)<8 nmol l−1) or mild (TT<12 nmol −1) hypogonadism compared to different metrics derived from patients' medical and sexual history. Data are stratified by age (<53 years old (a) and ≥53 years old(b)).
Figure 6.
Area under the curve (95% confidence intervals) derived from receiver-operating characteristic (ROC) curves for severe (total testosterone (TT)<8 nmol −1) or mild (TT<12 nmol l−1) hypogonadism compared to different metrics derived from sexual history. Data are stratified by age (<53 years old (a) and ≥53 years old(b)).
Figure 7.
Area under the curve (95% confidence intervals) derived from receiver-operating characteristic (ROC) curves for severe (total testosterone (TT)<8 nmol l−1) or mild (TT<12 nmol l−1) hypogonadism compared to different measurements derived from clinical, metabolic and instrumental items. Data are stratified by age (<53 years old (a) and ≥53 years old(b)). NCEP-ATPIII, National Cholesterol Education Program's Adult Treatment Panel III; PSV, peak systolic velocity; T2DM, type 2 diabetes mellitus.
Structured inventories for detecting LOH
A self-reported, validated questionnaire is a research instrument that consists of a series of questions and other prompts to gather information from respondents. It is the most common form of structured inventory. Although it should be noted that validated questionnaires cannot replace a detailed history and physical examination, they can be very useful for collecting systematic anamnestic data, and they can help guide a correct collection of symptoms in clinical practice. In addition, they can be used as screening tools for outpatients who need to be more carefully studied. To help recognize hypogonadal stigmata, four different questionnaires have been developed: the St. Louis University Androgen Deficiency in Aging Males (ADAM),22 the Aging Male Scales (AMS),23 the questionnaire of the Massachusetts Male Aging Study (MMAS)24 and, very recently, the New England Research Institute Hypogonadism screener (see also Table 2).25 All these instruments have demonstrated good sensitivity in cross-sectional surveys;25 however, their relative specificity is variable.25 For instance, in five different clinical settings, the sensitivity of the ADAM in detecting low T was good (81%–97%), but the specificity was poor (19.7%–32%).26, 27, 28, 29, 30 Similar figures were reported for the AMS and MMAS.27 Moreover, 6 months of oral T supplementation had no significant effect on scores of the ADAM and AMS questionnaires in a group of elderly men with low-normal T levels.31 These questionnaires are designed for screening the general population for hypogonadism and are not specifically targeted towards individuals with sexual dysfunction.
As discussed before, self-reported questionnaires have demonstrated important limits in the evaluation of patients with suspected hypogonadism. An alternative structured inventory is the structured interview (also known as a standardized interview or a researcher-administered survey), which is a quantitative research method commonly employed in survey research. Structured interviews allow the physician to explain the technical terms used, thus reducing the risk of misunderstandings. The question is read to respondents, who are asked to rephrase it into their own words and then answer. Each interview is presented with exactly the same questions in the same order. This ensures that answers can be reliably aggregated and that comparisons can be made with confidence between sample subgroups or survey periods. Questions that are likely to lead to dishonesty, either because they are embarrassing or are considered too private to discuss even with physicians, can be identified by signs of discomfort in respondents and can therefore be rephrased while reassuring the patients of their confidentiality. In addition, the structured interview allows the patient to provide a complete and accurate answer, which is then rated by the interviewer, instead of forcing a choice among a limited number of fixed answers. A further advantage of the structured interview over the self-reported questionnaire is that a face-to-face interview facilitates a virtuous, intimate physician–patient relationship.32 In fact, structured interviews have been found to have a greater diagnostic value than self-reported questionnaires in other contexts when involving possibly sensitive issues.33 We have recently developed a brief (12 items) structured interview (ANDROTEST) specifically designed for the screening of hypogonadism (total T <10.4 nmol l−1) in subjects with sexual dysfunction (see also Figure 1).34 In the validation sample, we demonstrated that ANDROTEST had almost 70% sensitivity and specificity in detecting low total or FT with ROC curves. Although ANDROTEST could help identify patients who should undergo T assessment, the determination of T remains mandatory to confirm a diagnosis of hypogonadism and/or to start any substitutive therapy according to accepted clinical guidelines.
Figure 8 shows results obtained in a larger population (1734 subjects) of male subjects with sexual dysfunction studied at the University of Florence. We now demonstrate that ANDROTEST can also detect overt hypogonadism, as recently defined (total T below 8 nmol l−1 or cFT below 225 pmol l−1), with similar sensitivity and specificity to those reported previously.34 However, the diagnostic threshold should be decreased by two points from what was originally reported. When a threshold of <6 was chosen, the sensitivity and specificity for detecting low total T (<8 nmol l−1, n=1734) were 67% and 64%, respectively; for low measured (<37 pmol l−1, n=608, analogue method), they were 70% and 63%, respectively; and for calculated (<225 pmol l−1, n=1467, Vermilion formula) FT, the values were 67% and 67%, respectively, with an accuracy of 0.663±0.027, 0.721±0.02 and 0.668±0.021 (all P<0.0001). ANDROTEST therefore remains a versatile, quick, patient- and physician-friendly, easy-to-administer hypogonadism screener that can detect low T with an acceptable accuracy at any desired threshold. It facilitates the detection of male hypogonadism, a disease often present but seldom diagnosed in patients with sexual dysfunction.
Figure 8.
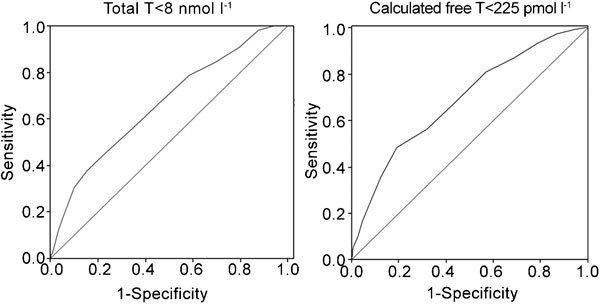
ROC curves for severe hypogonadism (total testosterone (T)<8 nmol l−1 or calculated free T according to Vermuelen's formula <225 pmol l−1) in relation to ANDROTEST score. All data were derived from a consecutive series of subjects attending our unit from 2002 to 2010 seeking medical care for sexual dysfunction. Total T and calculated free T were available for 1734 and 1467 subjects, respectively.3
Conclusions
In contrast to other forms of male hypogonadism, LOH is a very common condition, often present in subjects consulting for sexual dysfunction.2 Unfortunately, LOH has no pathognomonic signs or symptoms. In contrast to the general population,18 subjects with sexual dysfunction are not suspected by their clinicians to have hypogonadism based on the presence of sexual symptoms because of a lack of specificity, especially in cases of severe hypogonadism. Other clinical signs, such as increased waist circumference, might help identify hypogonadism, but only the clustering of several symptoms and signs, such as those reported in ANDROTEST, results in a reliable screen for this condition. Using ANDROTEST in subjects with sexual dysfunction might identify a severe form of male hypogonadism with acceptable sensitivity and specificity (almost 70%).
References
- Tajar A, Forti G, O'Neill TW, Lee DM, Silman AJ, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810–8. doi: 10.1210/jc.2009-1796. [DOI] [PubMed] [Google Scholar]
- Buvat J, Maggi M, Gooren L, Guay AT, Kaufman J, et al. Endocrine aspects of male sexual dysfunctions. In: Montorsi F, Basson R, Adaikan G, Becher E, Clayton A, Giuliano G, Khoury S, Sharlip I, editors. Sexual Medicine, Sexual Dysfunctions in Men and Women. Proceedings of the 3rd International Consultation on Sexual Medicine Paris; Health Publication Ltd; 2010. p681. [DOI] [PubMed] [Google Scholar]
- Morelli A, Corona G, Filippi S, Ambrosini S, Forti G, et al. Which patients with sexual dysfunction are suitable for testosterone replacement therapy. J Endocrinol Invest. 2007;30:880–8. doi: 10.1007/BF03349232. [DOI] [PubMed] [Google Scholar]
- Corona G, Rastrelli G, Forti G, Maggi M. Update in testosterone therapy for men. J Sex Med. 2011;8:639–54. doi: 10.1111/j.1743-6109.2010.02200.x. [DOI] [PubMed] [Google Scholar]
- Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol. 2008;159:507–14. doi: 10.1530/EJE-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak A, Brod M, Elbers J. Andropause and quality of life: findings from patient focus groups and clinical experts. Maturitas. 2002;43:231–7. doi: 10.1016/s0378-5122(02)00274-8. [DOI] [PubMed] [Google Scholar]
- EuroQol Group EuroQol: a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166:1660–5. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- Corona G, Monami M, Boddi V, Cameron-Smith M, Fisher AD, et al. Low testosterone is associated with an increased risk of MACE lethality in subjects with erectile dysfunction J Sex Med 2010(4 Pt 1); 1557–64. [DOI] [PubMed]
- Corona G, Rastrelli G, Vignozzi L, Mannucci E, Maggi M. Testosterone, cardiovascular disease and the metabolic syndrome. Best Pract Res Clin Endocrinol Metab. 2011;25:337–53. doi: 10.1016/j.beem.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Burris AS, Banks SM, Carter CS, Davidson JM, Sherins RJ. A long-term, prospective study of the physiologic and behavioral effects of hormone replacement in untreated hypogonadal men. J Androl. 1992;13:297–304. [PubMed] [Google Scholar]
- Corona G, Ricca V, Bandini E, Mannucci E, Petrone L, et al. Association between psychiatric symptoms and erectile dysfunction. J Sex Med. 2008;5:458–68. doi: 10.1111/j.1743-6109.2007.00663.x. [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Klibanski A, Neer RM, Greenspan SL, Rosenthal DI, et al. Osteoporosis in men with idiopathic hypogonadotropic hypogonadism. Ann Intern Med. 1987;106:354–61. doi: 10.7326/0003-4819-106-3-. [DOI] [PubMed] [Google Scholar]
- Greenspan SL, Neer RM, Ridgway EC, Klibanski A. Osteoporosis in men with hyperprolactinemic hypogonadism. Ann Intern Med. 1986;104:777–82. doi: 10.7326/0003-4819-104-6-777. [DOI] [PubMed] [Google Scholar]
- Stepan JJ, Lachman M, Zverina J, Pacovsky V, Baylink DJ. Castrated men exhibit bone loss: effect of calcitonin treatment on biochemical indices of bone remodeling. J Clin Endocrinol Metab. 1989;69:523–7. doi: 10.1210/jcem-69-3-523. [DOI] [PubMed] [Google Scholar]
- Maggi M, Schulman C, Quinton R, Langham S, Uhl-Hochgraeber K. The burden of testosterone deficiency syndrome in adult men: economic and quality-of-life impact. J Sex Med. 2007;4:1056–69. doi: 10.1111/j.1743-6109.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–35. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- Petak SM, Nankin HR, Spark RF, Swerdloff RS, Rodriguez-Rigau LJ. American Association of Clinical Endocrinologists American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients-2002 update. Endocr Pract. 2002;8:440–56. [PubMed] [Google Scholar]
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- Crown S, Crisp AH. A short clinical diagnostic self-rating scale for psychoneurotic patients. The Middlesex Hospital Questionnaire (M.H.Q.) Br J Psychiatry. 1966;112:917–23. doi: 10.1192/bjp.112.490.917. [DOI] [PubMed] [Google Scholar]
- Morley JE, Charlton E, Patrick P, Kaiser FE, Cadeau P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49:1239–42. doi: 10.1053/meta.2000.8625. [DOI] [PubMed] [Google Scholar]
- Heinemann LA, Saad F, Heinemann K, Thai DM. Can results of the Aging Males' Symptoms (AMS) scale predict those of screening scales for androgen deficiency. Aging Male. 2004;7:211–8. doi: 10.1080/13685530400004223. [DOI] [PubMed] [Google Scholar]
- Smith KW, Feldman HA, McKinlay JB. Construction and field validation of a self-administered screener for testosterone deficiency (hypogonadism) in ageing men. Clin Endocrinol (Oxf) 2000;53:703–11. doi: 10.1046/j.1365-2265.2000.01152.x. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Araujo AB, Connor MK, Gerstenberger EP, Morgentaler A, et al. The NERI Hypogonadism Screener: psychometric validation in male patients and controls. Clin Endocrinol. 2011;74:248–56. doi: 10.1111/j.1365-2265.2010.03925.x. [DOI] [PubMed] [Google Scholar]
- Tancredi A, Reginster JY, Schleich F, Pire G, Maassen P, et al. Interest of the androgen deficiency in aging males (ADAM) questionnaire for the identification of hypogonadism in elderly community-dwelling male volunteers. Eur J Endocrinol. 2004;151:355–60. doi: 10.1530/eje.0.1510355. [DOI] [PubMed] [Google Scholar]
- Morley JE, Perry HM, 3rd, Kevorkian RT, Patrick P. Comparison of screening questionnaires for the diagnosis of hypogonadism. Maturitas. 2006;53:424–9. doi: 10.1016/j.maturitas.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Chu LW, Tam S, Kung AW, Lam TP, Lee A, et al. A short version of the ADAM Questionnaire for androgen deficiency in Chinese men. Gerontol A Biol Sci Med Sci. 2008;63:426–31. doi: 10.1093/gerona/63.4.426. [DOI] [PubMed] [Google Scholar]
- Blümel JE, Chedraui P, Gili SA, Navarro A, Valenzuela K, et al. Is the Androgen Deficiency of Aging Men (ADAM) questionnaire useful for the screening of partial androgenic deficiency of aging men. Maturitas. 2009;63:365–8. doi: 10.1016/j.maturitas.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Martínez-Jabaloyas JM, Queipo-Zaragozá A, Rodríguez-Navarro R, Queipo-Zaragozá JA, Gil-Salom M, et al. Relationship between the Saint Louis University ADAM questionnaire and sexual hormonal levels in a male outpatient population over 50 years of age. Eur Urol. 2007;52:1760–7. doi: 10.1016/j.eururo.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Emmelot-Vonk MH, Verhaar HJ, Nakhai-Pour HR, Grobbee DE, van der Schouw YT. Low testosterone concentrations and the symptoms of testosterone deficiency according to the Androgen Deficiency in Ageing Males (ADAM) and Ageing Males' Symptoms rating scale (AMS) questionnaires. Clin Endocrinol (Oxf) 2011;74:488–94. doi: 10.1111/j.1365-2265.2010.03954.x. [DOI] [PubMed] [Google Scholar]
- Corona G, Jannini EA, Maggi M. Inventories for male and female sexual dysfunctions. Int J Impot Res. 2006;18:236–50. doi: 10.1038/sj.ijir.3901410. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Peveler RC, Davies B, Mann JI, Mayou RA. Eating disorders in young adults with insulin dependent diabetes mellitus: a controlled study. BMJ. 1991;303:17–20. doi: 10.1136/bmj.303.6793.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona G, Mannucci E, Petrone L, Balercia G, Fisher AD, et al. ANDROTEST: a structured interview for the screening of hypogonadism in patients with sexual dysfunction. J Sex Med. 2006;3:706–15. doi: 10.1111/j.1743-6109.2006.00262.x. [DOI] [PubMed] [Google Scholar]


