Abstract
This study was performed to assess serum testosterone alterations induced by paradoxical sleep deprivation (PSD) and to verify their attenuation during sleep recovery (SR) based on different durations and ages. Wistar male rats aged 12 weeks for the younger group and 20 weeks for the elder group were randomly distributed into one of the following groups: a control group (cage and platform), 3-day SD, 5-day SD, 7-day SD, 1-day SR, 3-day SR and 5-day SR groups. For PSD, the modified multiple platform method was used to specifically limit rapid eye movement (REM) sleep. Differences in the testosterone and luteinizing hormone levels between the younger group and the elder group according to duration of PSD and SR recovery were analysed. Testosterone continued to fall during the sleep deprivation period in a time-dependent manner in both the younger (P=0.001, correlation coefficient r=−0.651) and elder groups (P=0.001, correlation coefficient r=−0.840). The elder group showed a significantly lower level of testosterone compared with the younger group after PSD. Upon SR after 3 days of PSD, the testosterone level continued to rise for 5 days after sleep recovery in the younger group (P=0.013), whereas testosterone concentrations failed to recover until day 5 in the elder group. PSD caused a more detrimental effect on serum testosterone in the elder group compared to the younger group with respect to decreases in luteinizing hormone (LH) levels. The replenishment of serum testosterone level was prohibited in the elder group suggesting that the effects of SD/SR may be age-dependent. The mechanism by which SD affects serum testosterone and how age may modify the process are still unclear.
Keywords: luteinizing hormone, paradoxical sleep deprivation, recovery, testosterone
Introduction
Sleep deprivation (SD) is considered to be a risk factor that contributes to several disease processes1 via its ability to alter behavioural, hormonal and neurochemical pathways.2, 3, 4, 5 Moreover, SD is known to cause profound changes in the secretory patterns of distinct endocrine axes in humans.6 Several studies have shown that SD reduces circulating androgens in healthy men.7, 8 However, most of the papers focused on hormone alterations induced by stress and/or SD still use only a single measurement at one point in time and, thus, do not elucidate the complex neuroendocrine interactions that might occur in the context of recovery.2 The rebound period is peculiar because although the rats have been allowed to sleep and theoretically recover from the preceding SD, some physiological factors may not have returned to their baseline values. Accordingly, it is of utmost interest to investigate the severity of serum testosterone decreases based on the duration of SD and their replenishment during a period of sleep recovery (SR). Also, to date, there have been no investigations that have reported on the differences in serum testosterone changes seen in paradoxical sleep deprivation (PSD) and recovery by age group. This study was performed to assess changes in serum testosterone level and luteinizing hormone (LH) during different durations of PSD and SR in rats of different ages.
Materials and methods
Animals
Wistar male rats aged 12 weeks for the younger group and 20 weeks for the elder group were used. The rats were housed in standard polypropylene cages (two rats in one cage) in a temperature-controlled (23±1 °C) room with a 12 h∶12 h light–dark cycle (lights on at 07:00 a.m.). All procedures used in this study complied with the Guide for the Care and Use of Laboratory Animals and the experimental protocol of Korea University and were approved by the Korea University Institutional Animal Care and Use Committee.
Experimental procedure
The control group was divided into a cage control group and a platform control group. For the platform control group, the rats were group-housed using a modified multiple platform method without water to investigate whether this group had the same results as the cage control group. For SD, both the younger and elder group were divided into groups based on length of SD as followed: 3, 5 and 7 days (10 rats for each period). After each planned SD, the rats were anesthetized with halothane. Blood was then collected directly from the heart and centrifuged to obtain serum before 10:00 a.m. The testosterone (solid-phase radioimmunoassay, Packard COBRA II gamma counter; Siemens, Hoffman Estates, IL, USA) (ng ml−1) and LH (ng ml−1) (solid-phase radioimmunoassay, Packard COBRA II gamma counter; Biocode, Liege, Belgium) concentrations were measured.
Methods for paradoxical sleep deprivation (Figure 1)
Figure 1.
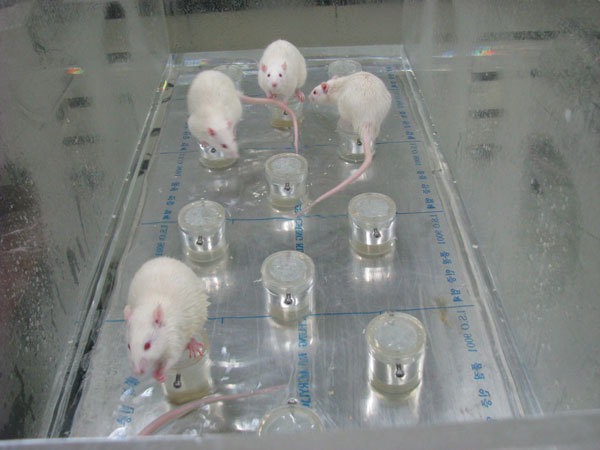
Modified multiple platform method for paradoxical sleep deprivation.
The rats were group housed (five rats in each arena) in modified multiple platform arenas during PSD. The experimental group was submitted to PSD using the modified multiple platform method, which involved placing the rats in an acrylic water tank (123×44×44 cm) containing 14 circular platforms, 6.5 cm in diameter, with water up to 1 cm of their upper surface. Thus, the rats could move around inside the tank by jumping from one platform to another. When they reached the P phase of sleep, muscle atonia set in, and they fell into the water and woke up. Throughout the study, the experimental room was maintained at a controlled temperature (23±1 °C) and light–dark cycle (lights on at 07:00 a.m. and off at 07:00 p.m.). Food and water were provided ad libitum by placing chow pellets and water bottles on a grid located on top of the tank. The water in the tank was changed daily throughout the SD period.
Sleep recovery
After 3 days of PSD, both the younger and elder groups were given a SR period of 1, 3 and 5 days. After each planned SR period, the rats were anesthetized with halothane, and the blood was collected directly from the heart and centrifuged to obtain serum.
Statistical analysis
The Statistical Package for the Social Sciences version 13.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis The Kruskal–Wallis and Mann–Whitney U tests were used. To assess the relationship between the duration of SD or SR and the serum testosterone level, we used the Spearman correlation analysis. P values less than 0.05 were considered to be statistically significant.
Results
Serum testosterone and LH concentrations for rats that underwent PSD for different durations (Table 1 and Figure 2)
Table 1. Serum testosterone and LH concentrations for rats that underwent PSD for different durations.
| Testosterone (ng ml−1) | LH (ng ml−1) | |||
|---|---|---|---|---|
| Younger group | Elder group | Younger group | Elder group | |
| Control | 6.16±2.49 | 4.37±1.66 | 7.42±4.80 | 5.59±4.97 |
| PSD 3 days | 5.10±2.05a | 2.29±0.98a | 3.64±2.02 | 2.15±1.39a,e |
| PSD 5 days | 3.02±1.85a | 0.74±0.28a | 2.62±2.48a | 2.13±1.26a |
| PSD 7 days | 1.31±0.29a | 0.14±0.06a | 2.31±2.35a | 2.01±1.15a |
Abbreviations: LH, luteinizing hormone; PSD, paradoxical sleep deprivation.
P=0.001 compared with the control in the corresponding group.
P=0.001 the degree of decrease compared with that of the younger group.
Figure 2.
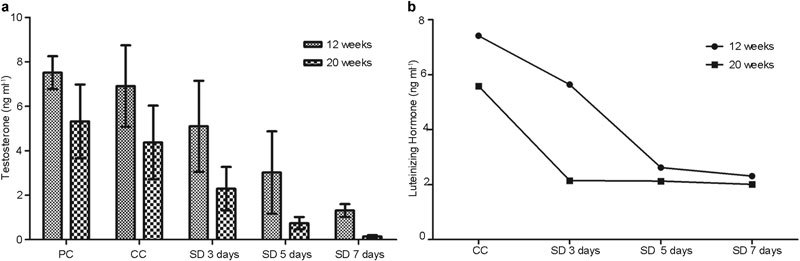
Changes in the testosterone (a) and LH concentrations (b) according to duration of sleep deprivation. The testosterone concentration continued to fall in all sleep deprivation periods in a time-dependent manner in both the younger (P=0.001, correlation coefficient r=−0.651) and elder groups (P=0.001, correlation coefficient r=−0.840). CC, cage control; LH, luteinizing hormone; PC, platform control; SD, sleep deprivation.
There were no statistical differences in serum testosterone level between the platform control and the cage control groups in both the 12-week-old younger group and 20-week-old elder group.
On Kruskal–Wallis testing, both the younger and elder group showed statistically significant differences in testosterone based on the duration of SD (i.e., 3, 5 and 7 days, P=0.001 for all). In the younger group, the serum testosterone levels based on the number of days of SD (i.e., 3, 5 and 7 days) were 5.10±2.05, 3.02±1.85 and 1.31±0.29 ng ml−1, respectively. In the elder group, the serum testosterone levels based on the number of days of SD (i.e., 3, 5 and 7 days) were 2.29±0.98, 0.74±0.28 and 0.14±0.06 ng ml−1, respectively. The elder group showed a stronger negative correlation between the duration of SD and serum testosterone level. The testosterone concentrations continued to fall in all SD periods in a time-dependent manner in both the younger (P=0.001, correlation coefficient r=−0.651) and elder groups (P=0.001, correlation coefficient r=−0.840).
In the younger group, the serum LH levels after SD for 3, 5 and 7 days were 3.64±2.02, 2.62±2.485 and 2.31±2.35 ng ml−1, respectively. In elder group, the serum LH levels after SD for 3, 5 and 7 days were 2.15±1.39, 2.13±1.26 and 2.01±1.15 ng ml−1, respectively. The effect of PSD on serum LH started earlier and the degree was greater in the elder group (P=0.001) compared with the younger group (Figure 2b).
Serum testosterone and LH concentrations based on the duration of SR after PSD (Table 2 and Figure 3)
Table 2. Serum testosterone and LH concentrations according to duration of sleep recovery after 3 days of sleep deprivation.
| Testosterone (ng ml−1) | LH (ng ml−1) | |||
|---|---|---|---|---|
| Younger group | Elder group | Younger group | Elder group | |
| PSD 3 days | 5.10±2.05 | 2.29±0.98 | 3.64±2.02 | 2.15±1.39 |
| SR 1 day | 3.90±2.07 | 1.28±0.66 | 3.46±1.97 | 2.37±1.18 |
| SR 3 days | 6.83±1.53 | 1.97±0.44 | 9.28±0.98a | 4.11±3.19 |
| SR 5 days | 8.36±1.64a | 0.71±0.17 | 9.85±1.45a | 4.35±1.10 |
Abbreviations: LH, luteinizing hormone; PSD, paradoxical sleep deprivation; SR, sleep recovery.
P<0.05 compared with the control of the corresponding group.
Figure 3.
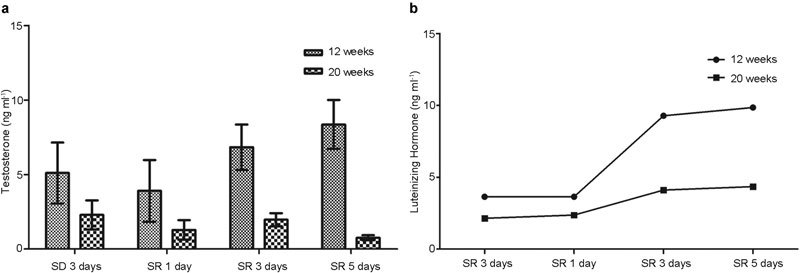
Changes in the testosterone (a) and luteinizing hormone (b) concentrations according to duration of sleep recovery after 3 days of paradoxical sleep deprivation. In the younger group, a strong positive correlation was noted between the duration of sleep recovery and serum testosterone concentrations based on Spearman correlation analysis (P=0.001, correlation coefficient r=0.758). SD, sleep deprivation; SR, sleep recovery.
In the younger group, after 3 days of PSD, the testosterone concentrations after SR for 1, 3 and 5 days were 3.90±2.07, 6.83±1.53 and 8.36±1.64 ng ml−1, respectively. The testosterone concentration in the younger group showed a time-dependent increase during SR for 1, 3 and 5 days (P=0.013). A Spearman correlation analysis showed a strong positive correlation between the duration of SR and serum testosterone concentration (P=0.001, correlation coefficient r=0.758). However, in the elder group, on Kruskal–Wallis test, the testosterone concentration did not begin to recover until day 5 of SR (P=0.004). In addition, LH failed to recover in the elder group during SR, whereas LH began to recover on day 2 of SR in the younger group (Figure 3).
Discussion
A relationship between PSD and testosterone levels has been previously reported.9 To date, this is the first study to compare the effects of different durations of SD and SR on serum testosterone levels in rats of different ages. We found that SD had more detrimental effects on serum testosterone levels in the elder group compared with the younger group and that the attenuation of such effects was inhibited in the elder group during SR.
In this study, we used the modified multiple platform method for PSD. PSD using the modified multiple platform method was developed to minimize the intervening stress variables present on a single platform method, such as movement restriction.10 In our study, animals were raised together from weaning, and habituation within the tank for 3 days was performed to establish a socially stable group, thereby obviating other possible stress variables. This procedure is known to be important, as PSD-induced hypothalamic–pituitary–adrenal axis (HPA axis) variation is exacerbated under socially unstable conditions.11, 12 Therefore, reduced hormone concentrations may be due to both psychological stress and SD and may indicate hypogonadism, as has been proposed.13
Our results demonstrated a time-dependent decrease in serum testosterone concentrations in both the younger and elder groups. Other studies demonstrated that 96 h of PSD led to a reduction in testosterone concentrations and enhanced levels of progesterone,14 and corticosterone.15 Our finding confirmed previous reports of significantly decreased serum testosterone concentrations with PSD after 24 h of wakefulness, and this reduction was further enhanced with longer durations of SD. Singer and Zumoff8 reported a marked reduction in serum testosterone levels in sleep-deprived healthy male medical interns compared with other hospital personnel.
The degree of serum testosterone suppression was further enhanced in the elder group falling down to almost nadir levels (Table 1). Andersen et al.14 also demonstrated that SD reduced serum testosterone levels in groups of both younger and older rats. However, our experiment was the first to compare the effect of the duration of SD on the serum testosterone concentration. The enhanced effect of SD on serum testosterone levels in the elder group might be due to the vulnerability of the elder group to SD-associated stress and their reduced ability to accommodate the HPA axis derangements that accompany SD, thus allowing them to return to a normally functioning HPA axis during periods of SR. These changes might be more obvious in an even older group than the one used in this study. However, as the age that we chose for the elder group may not be old enough, this finding may be due to differences in developmental processes of aging.
In the SR period, the young group showed a time-dependent recovery in serum testosterone concentrations after 5 days of SR. In contrast, serum testosterone concentrations failed to recover until day 5 of SR in the elder group. The time needed for recovery of serum testosterone to normal levels was at least 5 days, which is in accordance with previous reports that serum testosterone remained low until 96 h of SR.2 The mechanisms responsible for the inability to return to baseline testosterone values during SR until day 5 were unclear. Three days of SD caused a reduction in peripheral testosterone, and testosterone levels did not return to baseline in the recovery period of 5 days in the elder group. This suggested that longer periods of SD may have long-term effects2 that may become harmful and might even lead to hormone imbalances in the elder group. Longer periods of SR might have enabled the elder group to eventually return to baseline serum testosterone levels.
The exact mechanism whereby SD induces a reduction in serum testosterone concentrations in PSD has not yet been determined. Prolonged PSD induces alterations in several classical stress indices, which reflect the function of the HPA axis.10 Marked reductions in serum testosterone concentrations could be characterized as an indicator of the stress response.16 Testosterone reduction may be related to the increase in HPA-axis activity, especially corticosterone, which has been shown to reduce testosterone production in Leydig cells and to induce apoptosis in these cells, thereby directly suppressing testicular functions.17, 18, 19 Reduced testosterone is presumed to be associated with an LH-mediated process20 in accordance with our results. Here, we found that PSD induced reductions in LH in association with reductions in serum testosterone levels. Also, the suppressive effect of PSD on LH and serum testosterone was more pronounced in the elder group as compared with the younger group. These findings may be due to the fact that the elderly may be more vulnerable to stress or that the function of their Leydig cells may be more prone to suppression by the effect of PSD. However, as serum testosterone failed to recover until day 5 of SR with an increase in LH, the decrease in the serum testosterone level cannot be fully explained by the effect of decreased LH levels. Although we did not investigate serotonin as another possible factor for a decrease in testosterone, others have reported that serotonin inhibited testosterone production,21 and some animal studies have shown that serotonin concentrations were increased during SD.22, 23 Thus, the decreased testosterone levels associated with SD may be, in part, due to the serotonin-related inhibition of testosterone production.24 Although SD by itself could reduce testosterone,2 SD involves some degree of stress. Cortisol is a major stress hormone and is known to play an important neuroendocrine role in many physiological processes under the regulation of the HPA axis.25 Several studies have reported a negative relationship between sleep and cortisol levels,26, 27 and this hormone is known to contribute to the aetiology or pathophysiology of other medical problems. It is more likely that other important alterations, along with stress, contribute to the decrease in serum testosterone levels seen during SD,2 as it is known that sleep deprivation produces a reliable syndrome that includes increased energy expenditure and metabolic rate, decreased body temperature, etc.28 Also, based on a report demonstrating the influence of sleep disturbances on steroid 5α-reductase mRNA levels in the rat brain, rapid eye movement SD enhances the expression of the 5α-reductase gene and, hence, the production of neuroactive 5α-reductase steroid metabolites in the brainstem, thus providing further evidence for the possible involvement of 5α-reduced neurosteroids in the physiological mechanisms regulating the sleep–wake cycle.29
SD caused time-dependent serum testosterone and LH decreases in both the younger and elder groups. During SR, the testosterone level failed to recover in the elder group, indicating a more detrimental effect of SD on the elder group.
In summary, our results indicated that SD causes time-dependent serum testosterone and LH decreases in both the younger group and elder groups. However, during the SR phase, the testosterone level failed to recover in the elder group, suggesting that the effects of SD/SR may be age-dependent. The mechanism by which SD affects serum testosterone and how age may modify the process are still unclear.
Author contributions
MMO performed the statistical analysis and drafted the manuscript. MHJ carried out the experimental procedures and participated in its design and coordination. DGM participated in the design of the study JWK and JJK conceived of the study, and helped to draft the manuscript.
There is no conflict of interest or any competing financial interest to declare.
References
- Miller NE, Bartus RT. Sleep, sleep pathology, and psychopathology in later life: a new research frontier. Neurobiol Aging. 1982;3:283–6. doi: 10.1016/0197-4580(82)90016-1. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Martins PJ, D'Almeida V, Bignotto M, Tufik S. Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. J Sleep Res. 2005;14:83–90. doi: 10.1111/j.1365-2869.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Bignotto M, Machado RB, Tufik S. Different stress modalities result in distinct steroid hormone responses by male rats. Braz J Med Biol Res. 2004;37:791–7. doi: 10.1590/s0100-879x2004000600003. [DOI] [PubMed] [Google Scholar]
- Farooqui SM, Brock JW, Zhou J. Changes in monoamines and their metabolite concentrations in REM sleep-deprived rat forebrain nuclei. Pharmacol Biochem Behav. 1996;54:385–91. doi: 10.1016/0091-3057(95)02072-1. [DOI] [PubMed] [Google Scholar]
- Martins PJ, D'Almeida V, Pedrazzoli M, Lin L, Mignot E, et al. Increased hypocretin-1 (orexin-a) levels in cerebrospinal fluid of rats after short-term forced activity. Regul Pept. 2004;117:155–8. doi: 10.1016/j.regpep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Baumgartner A, Riemann D, Berger M. Neuroendocrinological investigations during sleep deprivation in depression. II. Longitudinal measurement of thyrotropin, TH, cortisol, prolactin, GH, and LH during sleep and sleep deprivation. Biol Psychiatry. 1990;28:569–87. doi: 10.1016/0006-3223(90)90395-i. [DOI] [PubMed] [Google Scholar]
- Cortes-Gallegos V, Castaneda G, Alonso R, Sojo I, Carranco A, et al. Sleep deprivation reduces circulating androgens in healthy men. Arch Androl. 1983;10:33–7. doi: 10.3109/01485018308990167. [DOI] [PubMed] [Google Scholar]
- Singer F, Zumoff B. Subnormal serum testosterone levels in male internal medicine residents. Steroids. 1992;57:86–9. doi: 10.1016/0039-128x(92)90035-8. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Bignotto M, Tufik S. Influence of paradoxical sleep deprivation and cocaine on development of spontaneous penile reflexes in rats of different ages. Brain Res. 2003;968:130–8. doi: 10.1016/s0006-8993(03)02228-5. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Tiba PA, Tufik S. Hormonal and behavioural responses of paradoxical sleep-deprived rats to the elevated plus maze. J Neuroendocrinol. 2002;14:549–54. doi: 10.1046/j.1365-2826.2002.00812.x. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Tufik S. Social stability attenuates the stress in the modified multiple platform method for paradoxical sleep deprivation in the rat. Physiol Behav. 2000;68:309–16. doi: 10.1016/s0031-9384(99)00181-x. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Lobo LL, Hipolide DC, Tufik S. Increased ACTH and corticosterone secretion induced by different methods of paradoxical sleep deprivation. J Sleep Res. 1998;7:276–81. doi: 10.1046/j.1365-2869.1998.00122.x. [DOI] [PubMed] [Google Scholar]
- Opstad PK, Aakvaag A. The effect of sleep deprivation on the plasma levels of hormones during prolonged physical strain and calorie deficiency. Eur J Appl Physiol Occup Physiol. 1983;51:97–107. doi: 10.1007/BF00952542. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Bignotto M, Machado RB, Tufik S. Does paradoxical sleep deprivation and cocaine induce penile erection and ejaculation in old rats. Addict Biol. 2002;7:285–90. doi: 10.1080/13556210220139497. [DOI] [PubMed] [Google Scholar]
- Palma BD, Suchecki D, Tufik S. Differential effects of acute cold and footshock on the sleep of rats. Brain Res. 2000;861:97–104. doi: 10.1016/s0006-8993(00)02024-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Takeyasu K, Mizutani S, Hamanaka Y, Uozumi T. Plasma testosterone levels following surgical stress in male patients. Acta Endocrinol (Copenh) 1970;65:11–7. doi: 10.1530/acta.0.0650011. [DOI] [PubMed] [Google Scholar]
- Gao HB, Tong MH, Hu YQ, Guo QS, Ge R, et al. Glucocorticoid induces apoptosis in rat leydig cells. Endocrinology. 2002;143:130–8. doi: 10.1210/endo.143.1.8604. [DOI] [PubMed] [Google Scholar]
- Ringstrom SJ, Schwartz NB. Differential effect of glucocorticoids on synthesis and secretion of luteinizing hormone (LH) and follicle stimulating hormone (FSH) J Steroid Biochem. 1987;27:625–30. doi: 10.1016/0022-4731(87)90362-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress-induced suppression of testicular function in the wild baboon: role of glucocorticoids. Endocrinology. 1985;116:2273–8. doi: 10.1210/endo-116-6-2273. [DOI] [PubMed] [Google Scholar]
- Demura R, Suzuki T, Nakamura S, Komatsu H, Odagiri E, et al. Effect of immobilization stress on testosterone and inhibin in male rats. J Androl. 1989;10:210–3. doi: 10.1002/j.1939-4640.1989.tb00089.x. [DOI] [PubMed] [Google Scholar]
- Frungieri MB, Zitta K, Pignataro OP, Gonzalez-Calvar SI, Calandra RS. Interactions between testicular serotoninergic, catecholaminergic, and corticotropin-releasing hormone systems modulating cAMP and testosterone production in the golden hamster. Neuroendocrinology. 2002;76:35–46. doi: 10.1159/000063682. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Park SP, Lopez-Rodriguez F, Wilson CL, Maidment N, Matsumoto Y, et al. In vivo microdialysis measures of extracellular serotonin in the rat hippocampus during sleep–wakefulness. Brain Res. 1999;833:291–6. doi: 10.1016/s0006-8993(99)01511-5. [DOI] [PubMed] [Google Scholar]
- Wu JL, Wu RS, Yang JG, Huang CC, Chen KB, et al. Effects of sleep deprivation on serum testosterone concentrations in the rat. Neurosci Lett. 2011;494:124–9. doi: 10.1016/j.neulet.2011.02.073. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neurosci Biobehav Rev. 1992;16:115–30. doi: 10.1016/s0149-7634(05)80175-7. [DOI] [PubMed] [Google Scholar]
- Schussler P, Uhr M, Ising M, Weikel JC, Schmid DA, et al. Nocturnal ghrelin, ACTH, GH and cortisol secretion after sleep deprivation in humans. Psychoneuroendocrinology. 2006;31:915–23. doi: 10.1016/j.psyneuen.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Mastorakos G, Bixler EO, Kales A, Gold PW, et al. Sleep deprivation effects on the activity of the hypothalamic–pituitary–adrenal and growth axes: potential clinical implications. Clin Endocrinol (Oxf) 1999;51:205–15. doi: 10.1046/j.1365-2265.1999.00763.x. [DOI] [PubMed] [Google Scholar]
- Mdzinarishvili AL, Molodavkin GA, Voronina TA. The effect of valproic acid on sleep structure and ethanol consumption in rats with various types of individual reactivity before and after stress exposure. Biull Eksp Biol Med. 1989;108:294–6. [PubMed] [Google Scholar]
- Morita K, Kuwada A, Fujihara H, Morita Y, Sei H. Influence of sleep disturbance on steroid 5alpha-reductase mRNA levels in rat brain. Neuroscience. 2002;115:341–8. doi: 10.1016/s0306-4522(02)00456-6. [DOI] [PubMed] [Google Scholar]


