Abstract
Fragile X syndrome (fraX) is the most common known cause of inherited developmental disability. fraX is associated with a CGG expansion in the FMR1 gene on the long arm of the X chromosome. Behavioral deficits, including problems with impulse control and distractibility, are common in fraX. We used functional brain imaging with a Go/NoGo task to examine the neural substrates of response inhibition in females with fraX (ages 10-22) and age- and gender-matched typically developing subjects. Although subjects with fraX had significantly lower IQ scores, as a group their performance on the Go/NoGo task was equivalent to that of the typically developing group. However, females with fraX showed abnormal activation patterns in several cortical and subcortical regions, with significantly reduced activation in the supplementary motor area, anterior cingulate and midcingulate cortex, basal ganglia, and hippocampus. An important finding of our study is that neural responses in the right ventrolateral prefrontal cortex (PFC) and the left and right striatum were correlated with the level of FMR1 gene expression. Our findings support the hypothesis that frontostriatal regions typically associated with response inhibition are dysfunctional in females with fraX. In addition to task-related activation deficits, reduced levels of “deactivation” were observed in the ventromedial PFC, and, furthermore, these reductions were correlated with the level of FMR1 gene expression. The ventromedial PFC is a key node in a “default mode” network that monitors mental and physiological states; we suggest that self-monitoring processes may be aberrant in fraX.
Keywords: genetic, Go/NoGo, response inhibition, prefrontal cortex, basal ganglia
The effect of genetic factors on human brain function is a topic of increasing interest within the field of cognitive neuroscience. In particular, the study of individuals with homogeneous genetic abnormalities and neurocognitive dysfunction can potentially serve as a prototype for advancing our knowledge of specific associations among genetic, neurobiological, and behavioral variables (1, 2). In this study, we used functional MRI (fMRI) to study deficits in executive function among females with fragile X syndrome (fraX).
fraX occurs in 1 of every 2,000-5,000 live births and is the most common known cause of inherited developmental disability. fraX affects both males and females. However, because the mutation occurs in an X chromosome gene, males are more uniformly and severely affected as opposed to females, who show a considerably wider spectrum of severity. Thus we chose to study females to more accurately elucidate the spectrum of neurobiological effects associated with fraX and reduced fragile X mental retardation protein (FMRP). In individuals with fraX, the cytogenetic fragile site on the long arm of the X chromosome, from which the syndrome derives its name, is typically caused by a mutation in which >200 (up to 2,000) cytosine-guanine-guanine (CGG) triplet repeats occur within the promoter region of the FMR1 gene. In females with the full mutation, the number of CGG triplet repeats is generally >200, and the gene is hypermethylated, leading to transcriptional silencing. This transcriptional suppression of the FMR1 gene and the subsequent diminished or absent production of the FMR1 protein results in aberrant brain development and function (3, 4).
Immunohistochemical studies have revealed that the hippocampus, cerebellum (Purkinje cells), and the nucleus basalis contain the highest levels of FMRP in the mammalian brain (3, 4). Structural neuroimaging studies have shown specific volumetric differences between normal controls and females with fraX in the hippocampus, cerebellar vermis, fourth ventricle, caudate nucleus, lateral ventricles, and thalamus (5-9). fraX in females is associated with a well characterized neurocognitive and neurobehavioral profile of deficits in executive functioning (10), visuospatial processing (11, 12), and mathematical reasoning (13). These and other problems significantly disrupt academic, vocational, and social functioning in females with fraX.
To date, functional brain imaging studies of cognitive function in fraX have been limited. Two recent studies in females with fraX have examined brain function during working memory (14) and mental arithmetic (15) tasks. These studies showed that in parallel with performance deficits, females with fraX also demonstrate deficits in frontoparietal networks associated with these tasks. As a result, it was not clear whether deficits in brain activation primarily reflect performance deficits or aberrant neural processing. To address this important issue, we used a response-inhibition task that incorporates a key component of executive brain function and yet is simple enough to be performed at relatively high levels of accuracy by females with fraX.
The Go/NoGo task provides a simple paradigm to study brain function during response inhibition. A number of brain imaging studies in adults and typically developing (TD) children have shown that the ventrolateral prefrontal cortex (VLPFC), anterior cingulate cortex (ACC), and basal ganglia play a critical role in this task (14, 16, 17). Based on previously reported neuroanatomical abnormalities in fraX, we expected the fraX group to show activation patterns different from those of TD individuals, particularly in brain networks underlying response inhibition and in some of the brain regions previously shown to be anatomically aberrant in this condition. Using a correlational approach, we were able to take advantage of the larger variability in FMRP levels in females with fraX. Because FMR1 gene expression of FMRP plays a key role in brain development, we hypothesized that activation deficits would be correlated with measures of FMR1 gene expression.
Materials and Methods
Subjects and Assessments. Participants included 18 females with the fraX full mutation (mean age 15.95 years, SD = 4.02 years) and 16 age-matched TD healthy females (mean age 15.5 years, SD = 3.85 years). The groups were roughly equated for race and ethnicity. The distribution for the fraX group was white = 16, Hispanic = 1, and Pacific Islander = 1. The distribution for the TD group was white = 13, Asian = 1, and unknown = 2. Intellectual functioning was assessed with the Wechsler Intelligence Scale for Children, Third Edition or the Wechsler Adult Intelligence Scale, Third Edition. TD subjects were screened for significant psychiatric and behavioral problems by using the Child Behavior Checklist (CBCL; age 4-18 years) (18) and the Symptom Checklist-90-R (SCL-90-R; age 13 years through adulthood) (19). All TD subjects had CBCL or SCL-90-R T scores within 1 SD of the mean of a normative standardized sample. Diagnosis for fraX was confirmed by standard DNA testing (Southern blot), and procedures for calculating FMRP were based on methods described by Willemson et al. (20). All protocols used in this study were approved by the Stanford University School of Medicine, and written informed consent was obtained from subjects and their parents.
Experimental Task. The experimental task consisted of a 30-s rest epoch and 12 alternating 26-s epochs of Go and Go/NoGo conditions, followed by a 30-s rest epoch. During both conditions, letters were presented every 2 s. In the Go/NoGo condition, subjects responded with a key press to every letter except “X” (presented on 50% of the trials), to which they were instructed to withhold response. In the Go condition, subjects responded with a key press to every letter (no X's were presented). At the beginning of each epoch, a 2-s instruction alerted the subject to the new task condition. Errors of omission, commission, and reaction time (RT) to correct trials during the experimental condition were recorded. Other details are described elsewhere (21).
Image Acquisition. Images were acquired on a 1.5-T General Electric Signa scanner with Echospeed gradients by using a custom-built whole head coil that provides a 50% advantage in signal-to-noise ratio over that of the standard General Electric coil. A custom-built head holder was used to prevent head movement. Eighteen axial slices (6 mm thick, 1 mm skip) parallel to the anterior and posterior commissure covering the whole brain were imaged with a temporal resolution of 2 s by using a T2*-weighted gradient echo spiral pulse sequence [repetition time (TR) = 2000 ms, echo time (TE) = 40 ms, flip angle = 89° and 1 interleave]. The field of view was 240 mm, and the effective in-plane spatial resolution was 4.35 mm. To aid in localization of functional data, high-resolution T1-weighted spoiled grass gradient recalled 3D MRI sequence with the following parameters was used: TR = 24 ms, TE = 5 ms, flip angle = 40°, 24-cm field of view, 124 slices in sagittal plane, 256 × 192 matrix, acquired resolution = 1.5 × 0.9 × 1.2 mm. The images were reconstructed as a 124 × 256 × 256 matrix with a 1.5 × 0.9 × 0.9-mm spatial resolution.
Stimulus Presentation. The experimental task was programmed with psyscope (http://psyscope.psy.cmu.edu). Initiation of the scan and task was synchronized by using a transitor-transistor logic (TTL) pulse delivered to the scanner timing microprocessor board. Stimuli were presented visually at the center of a screen by using a custom-built magnet compatible projection system.
Image Preprocessing. Images were reconstructed, by inverse Fourier transform, for each of the 186 time points into 64 × 64 × 18 image matrices (voxel size, 3.75 × 3.75 × 7 mm). fMRI data were preprocessed with spm99 (www.fil.ion.ucl.ac.uk/spm). Images were corrected for movement by using least-square minimization without higher-order corrections for spin history and were normalized to stereotaxic Talairach coordinates (22). Images were then resampled every 2 mm by using sinc interpolation and smoothed with a 4-mm Gaussian kernel to decrease spatial noise.
Statistical Analysis. Statistical analysis was performed on individual and group fMRI data by using the general linear model and the theory of Gaussian random fields as implemented in spm99 (23). Activation maps were superimposed on high-resolution T1-weighted images, and their locations were interpreted by using known neuroanatomical landmarks (24). Activation coordinates were transformed to Tailairach space by using a nonlinear transformation (25).
A within-subject procedure was first used to model all of the effects of interest for each subject. Confounding effects of fluctuations in global mean were removed by proportional scaling where, for each time point, each voxel was scaled by the global mean at that time point. Low-frequency noise was removed with a high-pass filter (0.5 cycles per min) applied to the fMRI time series at each voxel. A temporal smoothing function (Gaussian kernel corresponding to dispersion of 8 s) was applied to the fMRI time series to enhance the temporal signal-to-noise ratio. We defined the effects of interest for each subject with the relevant contrasts of the parameter estimates. For each of these contrasts, a corresponding contrast image was also created.
Group analysis was performed by using a random-effects model in a two-stage hierarchical procedure. This model estimates the error of variance for each condition of interest across subjects rather than across scans (26) and therefore provides a stronger generalization to the population. The aim of this analysis was to determine which brain regions showed significant activation for each main effect and interaction of interest. Initially, we computed a contrast image corresponding to the Go/NoGo versus Go condition for each subject. We used these contrast images to compute (i) task-related activation within each group, (ii) between-group differences in activation, and (iii) the correlation between FMRP and brain activation in the fraX group. For between-group comparisons, an exclusive mask was used to rule out effects that might arise from task-related decreases in activation (“deactivation”). The t statistics were then normalized to Z scores, and significant clusters of activation were determined by using height (P < 0.05) and extent thresholds (P < 0.05).
Results
Neuropsychological Assessment. Subjects <17 years of age received the Wechsler Intelligence Scale for Children, Third Edition, and subjects 17 years or older received the Wechsler Adult Intelligence Scale, Third Edition. The TD group (mean = 117, SD = 11.8) had significantly higher full-scale IQ scores (P < 0.0001) than the fraX group (mean = 84, SD = 15.16). In the fraX group, there was no significant correlation between FMRP levels and IQ (Spearman's correlation r = 0.18, P = 0.48).
Behavioral Performance. RTs and number of correct and incorrect responses to stimuli in the experimental and control conditions were computed separately. Behavioral data were examined by using parametric or nonparametric tests as appropriate. Behavioral data were unavailable for two subjects from each group because of technical difficulties with recording hardware. Table 1 shows accuracy and RT during the Go and Go/NoGo conditions during fMRI task performance. TD subjects performed better than subjects with fraX during both the Go and Go/NoGo conditions; however, these differences were not significant for either the Go [t(28) = 1.420, P < 0.17] or Go/NoGo conditions [t(28) = 0.893, P < 0.38]. There was no significant difference in RT for the Go trials in either the Go [Mann-Whitney U test Z (28) = -0.13, P < 0.89] or the Go/NoGo [Mann-Whitney U test Z (28) = -0.46, P < 0.64] conditions.
Table 1. Behavioral performance during the Go/NoGo and Go conditions in the TD and fraX groups.
| Condition | Subject group | Accuracy, % | RTs to Go trials, ms | RTs to NoGo errors, ms |
|---|---|---|---|---|
| Go | TD | 98.5 ± 2.4 | 396.6 ± 109.4 | — |
| fraX | 92.2 ± 16 | 410.2 ± 98.9 | — | |
| Go/NoGo | TD | 90.4 ± 10 | 509.9 ± 120.6 | 382.1 ± 79.0 |
| fraX | 86.3 ± 14.4 | 515.2 ± 78.3 | 450.0 ± 134.8 |
Note that RTs in the Go/NoGo condition refer to Go trials within the Go/NoGo condition.
We also examined the relationship between task performance and FMRP within the fraX group. There was no significant relationship between FMRP and accuracy (r = 0.26, P = 0.34) in the Go condition; RTs in this condition were not significantly correlated with FMRP (Spearman's r = -0.32, P = 0.22). For the Go/NoGo condition, there was no significant relationship between FMRP and accuracy (r = 0.37, P = 0.15). RTs to correct Go trials in the Go/NoGo condition were not correlated with FMRP (Spearman's r = -0.37, P = 0.15).
Brain Activation. We examined within- and between-group activation during the Go/NoGo, compared with the Go, condition.
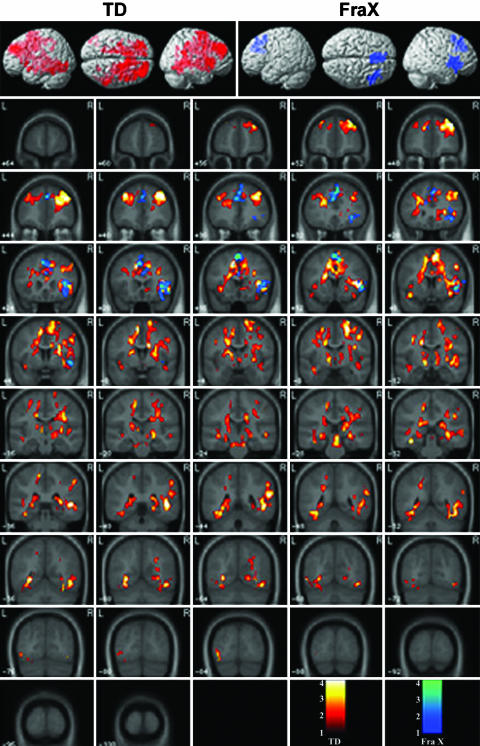
TD group. TD subjects showed significant activation in the right (R) dorsolateral PFC [DLPFC; and Brodmann's area (BA) 46/9], R VLPFC (BA 44, 45, 47), left (L) and R motor and premotor cortex (BA 4/6), supplementary motor area (SMA; BA 6), L and R caudate, putamen, and thalamus, L and R superior and middle temporal gyrus (BA 21), L and R fusiform and lingual gyri (BA 37), and L and R hippocampus and parahippocampal gyrus, as shown in Fig. 1.
Fig. 1.
Surface rendering and coronal cross sections of brain regions that showed significant activation during the Go/NoGo, compared with the Go, condition in TD females (red scale) and females with fraX (blue scale).
fraX group. fraX subjects showed significant activation in the L SMA and pre-SMA (BA 6/8), L cingulate cortex (BA 24), and R VLPFC, as shown in Fig. 1.
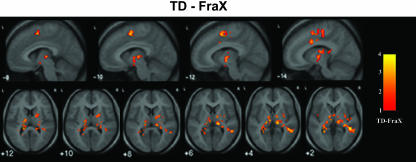
TD group minus fraX group. Compared with fraX subjects, TD subjects had significantly greater activation in the L and R SMA (BA 6), R anterior cingulate gyrus and midcingulate gyrus (BA 23), R putamen, L and R thalamus, R middle and inferior temporal gyri (BA 21), L fusiform gyrus (BA 37), and the L and R hippocampus. Fig. 2 highlights differences observed in the SMA, cingulate cortex, basal ganglia, and thalamus; a more complete rendering is provided in Fig. 5, which is published as supporting information on the PNAS web site.
Fig. 2.
Brain regions in which females with fraX showed significantly reduced activation, compared with TD subjects, during the Go/NoGo task.
fraX group minus TD group. Compared with TD subjects, subjects with fraX did not demonstrate significantly greater activation in any brain region.
Correlation Between FMRP and Brain Activation. Within the fraX group, we examined the relation between FMRP and brain activation during the Go/NoGo, compared with the Go, condition.
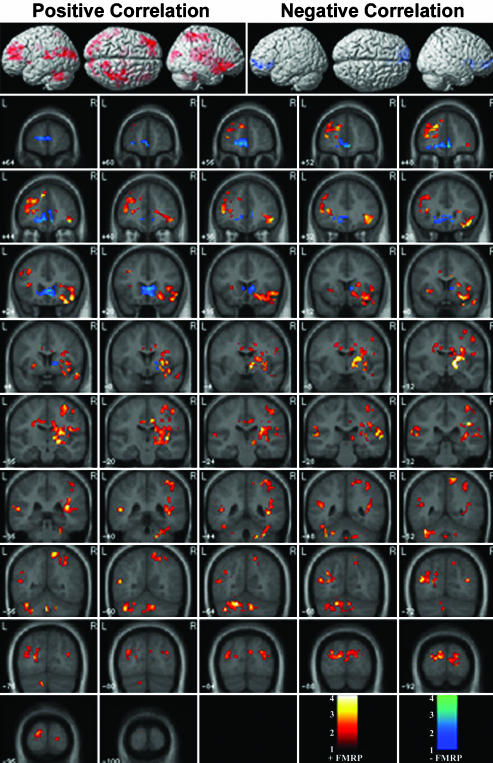
Positive correlation with FMRP. A significant positive correlation between FMRP and activation during the Go/NoGo, compared with the Go, condition was observed in the L cerebellum (regions VI, VIII, and IX), R cerebellum (regions VIII, IX, and IV/V), vermis (regions VI, VII, and VIII), R thalamus, putamen, and caudate, R ventral striatum, L putamen, R insula, L and R midcingulate gyrus (BA 23), R hippocampus, L DLPFC (BA 9/46), L VLPFC (BA 45/47), L and R superior and middle occipital gyri (BA 18 and 19), L angular gyrus (BA 39), R precuneus, superior parietal lobule (BA 7), L and R middle temporal gyrus, and superior temporal sulcus (BA 21), as shown in Fig. 3 and Table 2. Fig. 4 A-C further clarifies the relation between FMRP and neural responses in brain regions known to be involved in response inhibition.
Fig. 3.
Brain regions that showed significant positive and negative correlation with FMRP during the Go/NoGo task.
Table 2. Brain regions that showed significant positive and negative correlation with FMRP in females with fraX during the Go/NoGo task.
| Brain region | P value, corrected | No. of voxels | Peak Z score | Peak coordinates | ||
|---|---|---|---|---|---|---|
| Increase with FMRP | ||||||
| L cerebellum (VI, VIII, IX), R cerebellum (VIII, IX, IV/V), vermis (6, 7, 8) | <0.000 | 1,339 | 3.89 | −4 | −56 | −27 |
| R thalamus, L and R putamen, R caudate, R ventral striatum, R insula, L and R midcingulate gyrus (BA 23), R hippocampus, R VLPFC (BA 45/47) | <0.000 | 6,046 | 3.74 | 6 | −4 | −1 |
| L DLPFC (BA 9/46), L VLPFC (BA 45/47) | <0.000 | 1,433 | 3.71 | −12 | 42 | 33 |
| L and R superior and middle occipital gyri (BA 18 and 19), L angular gyrus (BA 39) R precuneus/superior parietal lobule (BA 7), L and R middle temporal gyrus/superior temporal sulcus (BA 21) | <0.000 | 1,758 | 3.53 | 38 | −69 | 20 |
| Decrease with FMRP | ||||||
| L and R VMPFC (BA 11, 10, 32, and 25) | <0.000 | 1,413 | 3.32 | 4 | 48 | −11 |
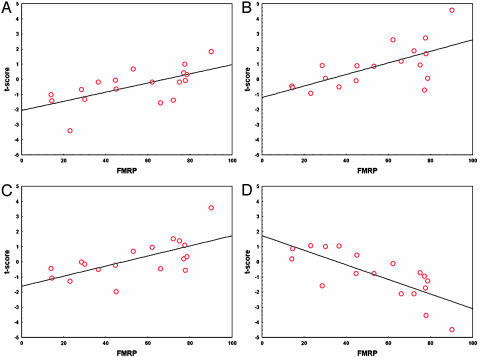
Fig. 4.
Positive correlation between FMRP and Go/NoGo-related activation in the L DLPFC (A) (Talairach coordinates: -40, 40, and 10 mm), R VLPFC (B) (34, 32, and -2 mm), and basal ganglia (C) (20, 10, and 10 mm). Negative correlation between FMRP and activation in the VMPFC (D) (4, 50, and -10 mm). Note that the negative correlation arises from deactivation, i.e., greater activation during the Go, compared with the Go/NoGo, condition.
Negative correlation with FMRP. A significant negative correlation between FMRP and activation during the Go/NoGo, compared with the Go, condition was observed in the ventromedial PFC (VMPFC; BA 11, 10, 32, and 25), as shown in Fig. 3 and Table 2. Fig. 4D shows that the observed negative correlation arises from increased deactivation (greater activation in the Go compared with the Go/NoGo condition) with FMRP. The VMPFC showed significant deactivation in the TD group, but not in the fraX group, as shown in Fig. 6, which is published as supporting information on the PNAS web site.
Discussion
Despite having significantly lower IQ scores, the fraX group performed the Go/NoGo task at a level similar to the TD group. However, compared with the TD group, the fraX group showed abnormal patterns of activation in several brain regions, with statistically significant deficits in the SMA, cingulate cortex, basal ganglia, thalamus, and fusiform gyrus. Compared with the TD group, the fraX group did not demonstrate significantly greater activation in any brain region. Taken together, these results suggest that in females with fraX, response inhibition engages networks that are both quantitatively and qualitatively different from brain networks engaged by TD individuals. Because accuracy and RTs on the Go/NoGo condition were similar in both groups, the anomalous pattern of brain activation observed in our study may reflect aberrant neuronal organization in fraX. Our results also suggest that females with fraX may have the capacity to overcome executive functioning weaknesses (10, 27), particularly in simple tasks like the one used in our study.
The correlations between FMRP, accuracy, and RT (for both experimental and control conditions) are in the correct direction, lower performance with lower FMRP. Correlations between FMRP levels and task performance measures were moderate (r ≈ 0.3-0.4); these relations may become significant with larger sample sizes or with inclusion of females with lower IQs than those used in our study. Correlation between FMRP levels and IQ were weaker (r < 0.2). Although there is no disagreement that IQ is affected by reduced FMRP levels in fraX (typically 15-25 points lower in females and 40-50 points lower in males, compared with unaffected siblings), IQ is sufficiently polygenic and multifactorial that a simple correlation is often not sufficient to characterize the association (5). The important point here is that within a fraX group in which task performance and IQ are not confounding factors the effects of FMRP on neurofunctional organization can be assessed in a more direct way.
FMRP is required for normal dendritic pruning, and its absence can lead to immature synapses (28), aplastic and nonspecific connections, and presumed aberrant activity within affected structures. An important finding of our study is that deficits in brain activation during response inhibition were correlated with reduced FMRP levels. In particular, reduced FMRP levels were associated with reduced activation in the PFC, basal ganglia, and hippocampus, all regions that showed significant task-related activation in TD subjects. As discussed in detail below, these findings support our hypothesis of abnormal neuronal organization in fraX within brain regions associated with response inhibition.
Lesion, neurophysiological, and brain imaging studies have consistently implicated specific regions of the PFC including the DLPFC (29, 30), VLPFC (31, 32), and ACC (33, 34) in response inhibition. Positive correlations with FMRP were observed in all of these brain regions except the ACC, although the fraX group did show task-related deficits in the caudal aspects of the ACC. Activation in the DLPFC and VLPFC regions identified in Fig. 3 has been consistently reported in prior functional imaging studies of response inhibition (14, 16, 17). In particular, we observed FMRP correlations in the R VLPFC, consistent with findings from event-related studies emphasizing the greater involvement of the R, compared with the L, VLPFC in response inhibition (32). More broadly, these PFC regions also play critical roles in inhibition of perseverative behavior (35), inhibition of distracting sensory information (36), and inhibition of inappropriate prepotent response tendencies in motor (31, 37) and cognitive (38) operations. Our results strongly suggest that FMR1 gene expression can alter the ability to engage PFC control processes. The deficits observed here also suggest that inhibitory control, an important function of the PFC and ACC (39-41), may be dysfunctional in individuals (e.g., fraX males) with low FMRP levels.
Our findings that FMRP is significantly correlated with activation in the basal ganglia are also noteworthy. Specific areas in the basal ganglia that showed such a relation included the dorsal caudate, putamen, and ventral striatum. Several brain imaging studies have established an association between reduced FMRP and morphological abnormalities in the basal ganglia (2, 6-8, 42-44). Here, our prior finding that caudate volumes are increased in individuals with fraX is of particular interest. Correlation of FMR1 gene inactivation with caudate volume further supports the validity and relevance of aberrant volumes of these neuroanatomical regions in individuals with fraX. For TD subjects, larger caudate size is associated with higher IQ, whereas in subjects with fraX, larger caudate size is correlated with lower IQ (2). This finding suggests that the developmental increases in caudate volume in individuals with fraX reflect aberrant neuronal organization. Although well known for involvement in movement, the caudate nucleus is reported to play an important role in cognition via connections with the DLPFC and ACC (45, 46). Disturbances of these frontal subcortical circuits are known to produce problems in executive function, motor programming, regulation of affect, social behavior, impulse control, and flexibility in response to environmental cues (45, 46). Accordingly, dysfunction of neuroanatomical circuits involving prefrontal striatal regions would be consistent with some of the cognitive and behavioral abnormalities observed in fraX including attention deficit hyperactivity, stereotypic and perseverative language and motor behavior, and problems with impulse control (2, 47).
In addition to these frontostriatal regions, the cerebellum and hippocampus showed activation that was significantly correlated with FMRP levels, regions that have reported structural abnormalities in subjects with fraX (7, 8). Also, immunohistochemical analyses have shown that FMR1 concentrations are among the highest in the mammalian hippocampus and cerebellum (3). Furthermore, Castren et al. (48) have provided evidence that FMRP play a role in brain-derived neurotrophic factor-induced synaptic plasticity in the hippocampus. Abnormal hippocampus functioning associated with decreased FMRP may therefore directly affect memory functions needed to sustain performance on even simple behavioral tasks. The cerebellar vermis also showed activation that was positively correlated with FMRP; this region has been implicated in a wide range of functions, including movement, sensory acquisition, and attention (7, 49-53), all operations that are needed in our Go/NoGo task.
Only one brain region, the VMPFC, showed task-related decreases in activation that were correlated with increased FMRP. A closer examination revealed that the decrease arose from an increase in deactivation, reflecting greater activation in the Go minus Go/NoGo conditions, rather than decrease in Go/NoGo minus Go-related activation. Functional brain imaging has traditionally focused on brain regions showing task-related increases in neural activity, i.e., greater activity during an experimental task than during a baseline state, typically rest or a sensory motor control task with reduced cognitive demand. However, there are several brain regions in which neural activity is greater during the baseline state than during an experimental task. Such decreases in activation are referred to as deactivation, and some brain regions consistently demonstrate such task-related decreases in activity across a broad range of cognitive tasks (54). Raichle et al. (55) have suggested that some of these brain regions constitute an organized “default model network” whose activity is ongoing during rest or simple tasks and suspended during performance of externally cued tasks. In our study, the VMPFC showed significant levels of deactivation in the Go minus Go/NoGo, compared with the TD, group. In fraX subjects taken as a whole, this region did not show significant deactivation. Our data suggest that individuals with fraX may not be able to modulate this default mode network in the same way as TD subjects and that the ability to engage this network depends on FMRP levels. The VMPFC is one of the most consistent regions to show such deactivation (56). This region is associated with affective and autonomic regulatory processes via links to paralimbic and subcortical regions including the orbitofrontal cortex, ventral striatum, and hypothalamus/midbrain (57, 58). Taken together, our data suggest that monitoring internal mental and physiological states may be aberrant in fraX.
The inability of individuals with fraX to appropriately engage brain networks typically invoked during response inhibition may significantly affect their performance when task difficulty is increased beyond the level used in this study. Further studies are needed to examine brain responses related to parametric variations in task difficulty by using more appropriate IQ-matched controls. Neuroimaging studies are also needed to investigate hippocampus and cerebellar function in individuals with fraX by using cognitive tasks that are more commonly associated with these regions in TD individuals.
Supplementary Material
Acknowledgments
We thank two anonymous reviewers for their helpful comments. This research was supported by National Institutes of Health Grants HD40761 and MH50047.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ACC, anterior cingulate cortex; PFC, prefrontal cortex; DLPFC, dorsolateral PFC; VLPFC, ventrolateral PFC; VMPFC, ventromedial PFC; fMRI, functional MRI; FMRP, fragile X mental retardation protein; fraX, fragile X syndrome; RT, reaction time; SMA, supplementary motor area; TD, typically developing; BA, Brodmann's area.
References
- 1.Bellugi, U., Lichtenberger, L., Jones, W., Lai, Z. & St. George, M. (2000) J. Cognit. Neurosci. 12, Suppl. 1, 7-29. [DOI] [PubMed] [Google Scholar]
- 2.Reiss, A. L., Abrams, M. T., Greenlaw, R., Freund, L. & Denckla, M. B. (1995) Nat. Med. 1, 159-167. [DOI] [PubMed] [Google Scholar]
- 3.Tamanini, F., Willemsen, R., van Unen, L., Bontekoe, C., Galjaard, H., Oostra, B. A. & Hoogeveen, A. T. (1997) Hum. Mol. Genet. 6, 1315-1322. [DOI] [PubMed] [Google Scholar]
- 4.Devys, D., Lutz, Y., Rouyer, N., Bellocq, J. P. & Mandel, J. L. (1993) Nat. Genet. 4, 335-340. [DOI] [PubMed] [Google Scholar]
- 5.Reiss, A. L., Freund, L. S., Baumgardner, T. L., Abrams, M. T. & Denckla, M. B. (1995) Nat. Genet. 11, 331-334. [DOI] [PubMed] [Google Scholar]
- 6.Reiss, A. L., Lee, J. & Freund, L. (1994) Neurology 44, 1317-1324. [DOI] [PubMed] [Google Scholar]
- 7.Mostofsky, S. H., Mazzocco, M. M., Aakalu, G., Warsofsky, I. S., Denckla, M. B. & Reiss, A. L. (1998) Neurology 50, 121-130. [DOI] [PubMed] [Google Scholar]
- 8.Kates, W. R., Abrams, M. T., Kaufmann, W. E., Breiter, S. N. & Reiss, A. L. (1997) Psychiatry Res. 75, 31-48. [DOI] [PubMed] [Google Scholar]
- 9.Jakala, P., Hanninen, T., Ryynanen, M., Laakso, M., Partanen, K., Mannermaa, A. & Soininen, H. (1997) J. Clin. Invest. 100, 331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franke, P., Leboyer, M., Hardt, J., Sohne, E., Weiffenbach, O., Biancalana, V., Cornillet-Lefebre, P., Delobel, B., Froster, U., Schwab, S. G., et al. (1999) Psychiatry Res. 87, 223-231. [DOI] [PubMed] [Google Scholar]
- 11.Crowe, S. F. & Hay, D. A. (1990) Neuropsychologia 28, 9-16. [DOI] [PubMed] [Google Scholar]
- 12.Freund, L. S., Reiss, A. L. & Abrams, M. T. (1993) Pediatrics 91, 321-329. [PubMed] [Google Scholar]
- 13.Riddle, J. E., Cheema, A., Sobesky, W. E., Gardner, S. C., Taylor, A. K., Pennington, B. F. & Hagerman, R. J. (1998) Am. J. Ment. Retard. 102, 590-601. [DOI] [PubMed] [Google Scholar]
- 14.Menon, V., Kwon, H., Eliez, S., Taylor, A. K. & Reiss, A. L. (2000) Brain Res. 877, 367-370. [DOI] [PubMed] [Google Scholar]
- 15.Rivera, S. M., Menon, V., White, C. D., Glaser, B. & Reiss, A. L. (2002) Hum. Brain Mapp. 16, 206-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casey, B. J., Trainor, R. J., Orendi, J. L., Schubert, A. B., Nystrom, L. E., Giedd, J. N., Castellanos, F. X., Haxby, J. V., Noll, D. C., Cohen, J. D., et al. (1997) J. Cognit. Neurol. 9, 835-847. [DOI] [PubMed] [Google Scholar]
- 17.Vaidya, C. J., Austin, G., Kirkorian, G., Ridlehuber, H. W., Desmond, J. E., Glover, G. H. & Gabrieli, J. D. (1998) Proc. Natl. Acad. Sci. USA 95, 14494-14499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Achenbach, T. M. (1991) Manual for the Child Behavior Checklist/4-18 and 1991 Profile (Univ. of Vermont Press, Burlington).
- 19.Derogatis, L. R. (1977) SCL-90: Administration, Scoring, and Procedure Manual (Johns Hopkins Univ. Press, Baltimore).
- 20.Willemsen, R., Smits, A., Mohkamsing, S., van Beerendonk, H., de Haan, A., de Vries, B., van den Ouweland, A., Sistermans, E., Galjaard, H. & Oostra, B. A. (1997) Hum. Genet. 99, 308-311. [DOI] [PubMed] [Google Scholar]
- 21.Menon, V., Adleman, N., White, C. D., Glover, G. H. & Reiss, A. L. (2001) Hum. Brain Mapp. 12, 131-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talairach, J. & Tournoux, P. (1988) Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System: An Approach to Cerebral Imaging (Thieme, New York).
- 23.Friston, K. J., Holmes, A. P., Poline, J. B., Grasby, P. J., Williams, S. C., Frackowiak, R. S. & Turner, R. (1995) NeuroImage 2, 45-53. [DOI] [PubMed] [Google Scholar]
- 24.Duvernoy, H. M., Bourgouin, P., Cabanis, E. A. & Cattin, F. (1999) The Human Brain: Functional Anatomy, Vascularization and Serial Sections with MRI (Springer, New York).
- 25.Brett, M., Johnsrude, I. S. & Owen, A. M. (2002) Nat. Rev. Neurosci. 3, 243-249. [DOI] [PubMed] [Google Scholar]
- 26.Holmes, A. P. & Friston, K. J. (1998) NeuroImage 7, S754. [DOI] [PubMed] [Google Scholar]
- 27.Munir, F., Cornish, K. M. & Wilding, J. (2000) Neuropsychologia 38, 1261-1270. [DOI] [PubMed] [Google Scholar]
- 28.Weiler, I. J. & Greenough, W. T. (1999) Am. J. Med. Genet. 83, 248-252. [DOI] [PubMed] [Google Scholar]
- 29.Shimamura, A. P. (1995) Memory and Frontal Lobe Function (MIT Press, Cambridge, MA).
- 30.Dias, R., Robbins, T. W. & Roberts, A. C. (1997) J. Neurosci. 17, 9285-9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konishi, S., Nakajima, K., Uchida, I., Sekihara, K. & Miyashita, Y. (1998) Eur. J. Neurosci. 10, 1209-1213. [DOI] [PubMed] [Google Scholar]
- 32.Garavan, H., Ross, T. J. & Stein, E. A. (1999) Proc. Natl. Acad. Sci. USA 96, 8301-8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posner, M. I. (1998) Executive Attention: Conflict, Target Detection, and Cognitive Control (MIT Press, Cambridge, MA).
- 34.Taylor, S. F., Kornblum, S., Lauber, E. J., Minoshima, S. & Koeppe, R. A. (1997) NeuroImage 6, 81-92. [DOI] [PubMed] [Google Scholar]
- 35.Iversen, S. D. & Mishkin, M. (1970) Exp. Brain Res. 11, 376-386. [DOI] [PubMed] [Google Scholar]
- 36.Chao, L. L. & Knight, R. T. (1998) J. Cognit. Neurosci. 10, 167-177. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki, K. & Gemba, H. (1986) Exp. Brain Res. 64, 603-606. [DOI] [PubMed] [Google Scholar]
- 38.Jonides, J., Smith, E. E., Marshuetz, C., Koeppe, R. A. & Reuter-Lorenz, P. A. (1998) Proc. Natl. Acad. Sci. USA 95, 8410-8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuster, J. M. (1997) The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe (Lippincott-Raven, Philadelphia).
- 40.Roberts, A. C., Robins, T. W. & Weiskrantz, L. (1998) The Prefrontal Cortex: Executive and Cognitive Functions (Oxford Univ. Press, New York).
- 41.Paus, T. (2001) Nat. Rev. Neurosci. 2, 417-424. [DOI] [PubMed] [Google Scholar]
- 42.Reiss, A. L., Aylward, E., Freund, L. S., Joshi, P. K. & Bryan, R. N. (1991) Ann. Neurol. 29, 26-32. [DOI] [PubMed] [Google Scholar]
- 43.Reiss, A. L., Freund, L., Tseng, J. E. & Joshi, P. K. (1991) Am. J. Hum. Genet. 49, 279-288. [PMC free article] [PubMed] [Google Scholar]
- 44.Reiss, A. L., Patel, S., Kumar, A. J. & Freund, L. (1988) Am. J. Med. Genet. 31, 407-414. [DOI] [PubMed] [Google Scholar]
- 45.Cummings, J. L. (1993) Arch. Neurol. 50, 873-880. [DOI] [PubMed] [Google Scholar]
- 46.Masterman, D. L. & Cummings, J. L. (1997) J. Psychopharmacol. 11, 107-114. [DOI] [PubMed] [Google Scholar]
- 47.Abrams, M. T. & Reiss, A. L. (1995) Ment. Retard. Dev. Disabilities Res. Rev. 1, 269-275. [Google Scholar]
- 48.Castren, M., Lampinen, K. E., Miettinen, R., Koponen, E., Sipola, I., Bakker, C. E., Oostra, B. A. & Castren, E. (2002) Neurobiol. Dis. 11, 221-229. [DOI] [PubMed] [Google Scholar]
- 49.Critchley, H. D., Corfield, D. R., Chandler, M. P., Mathias, C. J. & Dolan, R. J. (2000) J. Physiol. (London) 523, 259-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levisohn, L., Cronin-Golomb, A. & Schmahmann, J. D. (2000) Brain 123, 1041-1050. [DOI] [PubMed] [Google Scholar]
- 51.Riva, D. & Giorgi, C. (2000) Fiziol. Cheloveka 26, 27-31. [PubMed] [Google Scholar]
- 52.Richter, H. O., Lee, J. T. & Pardo, J. V. (2000) Eur. J. Neurosci. 12, 311-321. [DOI] [PubMed] [Google Scholar]
- 53.Bobee, S., Mariette, E., Tremblay-Leveau, H. & Caston, J. (2000) Behav. Brain Res. 112, 107-117. [DOI] [PubMed] [Google Scholar]
- 54.Shulman, G. L., Fiez, J. A., Corbetta, M., Buckner, R. L., Miezin, F. M., Raichle, M. E. & Petersen, S. E. (1997) J. Cognit. Neurosci. 9, 648-663. [DOI] [PubMed] [Google Scholar]
- 55.Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A. & Shulman, G. L. (2001) Proc. Natl. Acad. Sci. USA 98, 676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greicius, M. D., Krasnow, B., Reiss, A. L. & Menon, V. (2003) Proc. Natl. Acad. Sci. USA 100, 253-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Damasio, A. R., Grabowski, T. J., Bechara, A., Damasio, H., Ponto, L. L., Parvizi, J. & Hichwa, R. D. (2000) Nat. Neurosci. 3, 1049-1056. [DOI] [PubMed] [Google Scholar]
- 58.Aharon, I., Etcoff, N., Ariely, D., Chabris, C. F., O'Connor, E. & Breiter, H. C. (2001) Neuron 32, 537-551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.