Abstract
The acid-sensing ion channel 1a (ASIC1a) is abundantly expressed in the amygdala complex and other brain regions associated with fear. Studies of mice with a disrupted ASIC1 gene suggested that ASIC1a may contribute to learned fear. To test this hypothesis, we generated mice overexpressing human ASIC1a by using the pan-neuronal synapsin 1 promoter. Transgenic ASIC1a interacted with endogenous mouse ASIC1a and was distributed to the synaptosomal fraction of brain. Transgenic expression of ASIC1a also doubled neuronal acid-evoked cation currents. The amygdala showed prominent expression, and overexpressing ASIC1a enhanced fear conditioning, an animal model of acquired anxiety. These data raise the possibility that ASIC1a and H+-gated currents may contribute to the development of abnormal fear and to anxiety disorders in humans.
Anxiety disorders such as posttraumatic stress disorder and panic disorder can cause significant distress and are frequently disabling (1). Pavlovian fear conditioning is an important animal model of anxiety with both anatomical and physiological parallels to anxiety disorders in humans (2-5). Thus, understanding the mechanisms that underlie fear conditioning might offer the opportunity for new treatment and prevention strategies for these debilitating illnesses. Our recent data suggest that acid-sensing ion channels (ASICs) may play a role in fear conditioning (6).
ASICs are neuronally expressed members of the degenerin/epithelial Na+ channel family (for review, see refs. 7-10). ASIC subunits form homomultimeric and heteromultimeric channel complexes that are activated by a fall in extracellular pH. Central neurons express three ASIC subunits (ASIC1a, ASIC2a, and ASIC2b, where a and b refer to splice variants) (11-15). ASIC1a appears to play a prominent role in determining current amplitude and also affects the kinetics of H+-gated current (14, 16-18). ASIC2a modulates desensitization, recovery from desensitization, pH-sensitivity, and the response to modulatory agents such as Zn2+ and FMRFamide (19-23). The role of ASIC2b is uncertain, although in the absence of ASIC1a it inhibits ASIC2a-mediated current (12, 17).
Earlier work showed ASIC1a is expressed throughout the brain, with prominent expression in areas that receive rich synaptic input (6, 11, 14, 24). Endogenous ASIC1a was enriched in synaptosome-containing brain fractions, and ASIC1a transfected into neurons appeared in the cell body and colocalized with the postsynaptic density 95 protein (PSD-95) at synapses (16). Consistent with a role in synaptic physiology, targeted disruption of the ASIC1 gene in mice impaired long-term potentiation and temporal summation of excitatory postsynaptic potentials in hippocampal slices (16). Loss of ASIC1a also eliminated hippocampal neuron currents evoked by pH 5 stimuli and markedly reduced currents generated by more extreme acidosis (6, 16, 17).
In previous studies, ASIC1a expression was abundant in the amygdala complex (6, 14, 25), which is required for fear conditioning and the expression of fear (4, 5, 26). Moreover, extracellular acidosis elicited a greater H+-gated current density in amygdala neurons than in hippocampal neurons (6). ASIC1 null mice displayed deficits in cue and context fear conditioning, while baseline fear on the elevated plus maze remained intact. These studies suggested that ASIC1a and H+-gated currents contribute to the neural mechanisms underlying fear conditioning. To test this hypothesis, we asked whether overexpressing ASIC1a would increase H+-gated currents and enhance fear conditioning.
Materials and Methods
Transgenic (Tg) Mice. The plasmid vector was constructed by using a strategy described by Stec et al. (27). Briefly, the human ASIC1a cDNA, with the FLAG epitope sequence inserted immediately after the first ATG (16), was subcloned into pSTEC-2 (27). A PCR fragment containing the rat synapsin I promoter (a generous gift of Manfred Killiman, Ruhr-Universität, Bochum, Germany) was inserted upstream of the FLAG-hASIC1a sequence (28). A chimeric intron, composed of the 5′ splice site from the β-globin intron and the 3′ splice site from an IgG intron, was inserted between the synapsin I promoter and the first ATG of FLAG-hASIC1a, as described (27). An EcoRV restriction enzyme site was engineered by QuikChange mutagenesis (Stratagene) into the 5′ flanking sequence of the synapsin I promoter so that an EcoRV restriction enzyme digestion could be used to excise the entire transgene from the prokaryotic vector. It was then microinjected into one-cell fertilized mouse embryos obtained from superovulated C57BL/6J × SJL/J (B6SJL F2) mice by using standard procedures (29). Genotype of offspring was determined from tail DNA by using PCR and the following primers: 5′-TTC CCA TAC CGC GTG AAG ACC AC-3′ and 5′-GTC ATC GTC GTC CTT GTA GTC-3′. Tg lines were maintained by backcross breeding to C57BL/6.
The rate of transgene inheritance in lines 1 and 2 was 43% (n = 49) and 45% (n = 97), respectively, and Tg mice survived to adulthood. The overall size and appearance of Tg mice was normal, and there were no obvious signs of toxicity from overexpressing ASIC1a. All mice received standard mouse chow (LM-485, Teklad, Madison, WI) and water ad libitum. Care of the mice used in the experiments met the standard set forth by the National Institutes of Health in their guidelines for the care and use of experimental animals, and all procedures were approved by the University Animal Care and Use Committee at the University of Iowa.
Immunoblotting. Mouse brain protein lysate was prepared as described (6). Briefly, tissue was homogenized in lysis buffer, which contained PBS, aprotinin (40 μg/ml), leupeptin (40 μg/ml), pepstatin A (20 μg/ml), phenylmethanesulfonyl fluoride (40 μg/ml), and ethylenediamine tetraacetate (2 mM), by using a Dounce homogenizer (Wheaton Scientific). The homogenate was cleared of large particles with a 10-min centrifugation at 700 × g. Membrane proteins were precipitated at 170,000 × g for 30 min (Beckman TL-100, TLA-100 rotor). The pellet was resuspended in PBS with protease inhibitors. All steps in sample preparation were performed on ice or at 4°C. Protein concentration was determined by Lowry assay (30), and 50 μg was run on 8% acrylamide gel and western blotted. Signals were detected by enhanced chemiluminescence (Pierce). For deglycosylation, N-glycanase (Prozyme, San Leandro, CA) was used to deglycosylate 50 μg of mouse brain protein lysate. The sample was denatured at 100°C for 5 min and cooled to room temperature, and Nonidet P-40 was added to a final concentration of 0.75%. The reaction mixture was incubated overnight with 2 μl of N-glycanase (37°C) and western blotted. For immunoprecipitation, Triton X-100 (Pierce) was added to a final concentration of 1% to the cleared homogenate (described above). The sample was then further purified with a 1-min spin at 2,800 × g. The supernatant was incubated overnight at 4°C with 1 μl of anti-Flag antibody (Sigma) with shaking. It was then bound to 50 μl of protein A Sepharose for 30 min at 4°C. After three washes with cold PBS, the pellet was resuspended in sample buffer with 2% SDS, run on 8% acrylamide gels, and western blotted. The signal was detected by enhanced chemiluminescence (Pierce). The primary antibodies used in immunoblotting were anti-Flag (Sigma) at 1:2,000, anti-PSD-95 (Upstate, Charlottesville, VA) at 1:100,000, anti-actin (Santa Cruz Biotechnology) at 1:10,000, and anti-ASIC (MTY) antiserum at 1:15,000 (6). The MTY antibody is directed against an epitope common to the C-termini of both human and mouse ASIC1. Secondary antibodies were anti-rabbit IgG HRP (Amersham Pharmacia), anti-mouse IgG HRP (Amersham Pharmacia), and anti-goat IgG HRP (Jackson ImmunoResearch), all at 1:10,000.
Synaptosome Preparation. Sucrose gradients were prepared by layering 10 ml of 1.2, 1.0, and 0.85 M sucrose from bottom to top in a centrifuge tube (326823, Beckman) and allowing 2 h for settling. Mouse brain lysates (three per gradient) were prepared as described above. After the 170,000 × g centrifugation, the membrane pellet was resuspended in 2.5 ml of 0.32 M sucrose, in 1 mM NaHCO3 with protease inhibitors. The resuspended membrane fraction was layered on the gradient, centrifuged at 82,000 × g for 120 min (Beckman L8-70M, SW28 rotor), and allowed to come to a stop without braking. The synaptosome-containing band (between 1.0 and 1.2 M sucrose) was collected (31), and the relative amount of ASIC1a protein in this fraction was compared to the amount in whole brain and total membrane fractions by Western blotting at 50 μg per lane.
Immunocytochemistry. Mouse hippocampal neurons were prepared as described (6) and plated on CC2 eight-well slides (Nalge Nunc). After 10-14 days, cultures were fixed in PBS with 4% formaldehyde (Electron Microscopy Sciences, Fort Washington, PA) and 0.25% Triton X-100 (Pierce) for 5 min. Slides were rinsed twice and blocked with 10% BSA in PBS for 30 min at room temperature. Affinity-purified MTY antibody (1:25) (6) and secondary antibody (Cy3-conjugated anti-rabbit IgG, 1:300, Jackson ImmunoResearch) were prepared in 3% BSA in PBS and applied for 2 h at room temperature and 1 h at 37°C, respectively. Slides were washed with PBS followed by distilled H2O, mounted with VECTASHIELD (Vector Laboratories), and visualized by using a Bio-Rad MRC 1024 confocal microscope.
Whole-Cell Voltage-Clamp Experiments. Mouse hippocampal cultures were generated from postnatal day 1-2 pups as described (6). Whole-cell patch-clamp recordings were performed 7-14 days after seeding on neurons from at least two different litters as described (6, 17).
Immunohistochemistry. Fresh frozen coronal slices (7.5 μm) through the forebrain were prepared as described (6). Slices were postfixed in PBS with 4% formaldehyde/4% sucrose for 15 min, followed by 0.25% Triton X-100 in PBS for 5 min at room temperature. They were immunolabeled by using the TSA Fluorescence Systems (Perkin-Elmer) as described (6). Primary antibody was anti-Flag (Sigma) at 1:1,000, and secondary was anti-mouse IgG HRP (Amersham Pharmacia) at 1:200. Slices were mounted with VECTASHIELD and visualized with an Olympus (Melville, NY) BX-51 epifluorescence microscope equipped with Spot RT Slater (Diagnostic Instruments, Sterling Heights, MI.)
Context Fear Conditioning. On day 1, naíve mice were placed in a conditioning chamber (MED Associates, St. Albans, VT) (6). After 3 min, animals received a foot-shock (1 sec, 0.5 mA) through an electric floor grid. Three foot-shocks were given, separated by 1-min intervals. Mice were then returned to their home cages. On day 2, mice were placed in the conditioning chamber for 15 min. Freezing, defined as the absence of movement, was scored from videotape during 1-min intervals by trained observers, blinded to genotype. To determine the sensory stimulus response threshold, naíve mice were placed in the test chamber, and sets of 10 foot-shocks were delivered starting at 0.01 mA. For each subsequent set, the foot-shock amplitude was increased by 0.005 mA. The number of responses per set of 10 shocks was defined as foot lifting or head twitching that coincided with shock delivery. To determine vocalization threshold, foot-shocks were delivered beginning at 0.08 mA. Subsequent foot-shocks were delivered with increasing current steps of 0.01 mA until vocalization occurred. For behavioral experiments the results did not differ between the two lines of Tg mice, therefore the data were combined. Mice used for these experiments were 15-20 weeks of age at the time of data collection. Non-Tg age and sex-matched littermates were used as controls.
Elevated Plus Maze. Naíve mice were placed in the center of the plus maze and allowed to roam freely for 5 min as described (6). Behavior was scored from videotapes by observers blinded to genotype.
Results
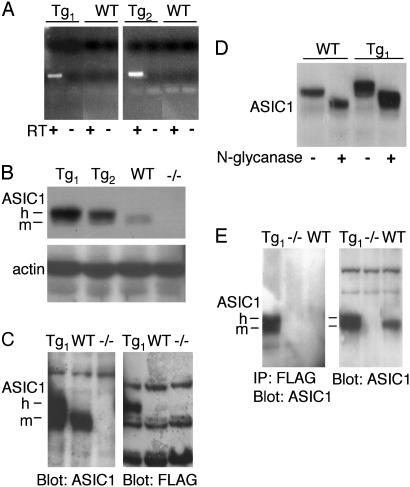
ASIC1a Was Overexpressed in Tg Mouse Brain. We generated Tg mice by using the pan-neuronal synapsin I promoter (28) cloned upstream of the human ASIC1a cDNA. We included a FLAG-epitope engineered into the 5′ coding sequence just after the first ATG. Two independent lines of Tg mice (Tg1 and Tg2) were generated. Allele-specific RT-PCR detected transgene expression in total brain RNA from both lines (Fig. 1A). Western blots of whole brain lysates with an antibody that detects mouse and human ASIC1 proteins revealed increased ASIC1 levels in both Tg lines (Fig. 1B). In Tg mice, the antibody detected the endogenous mASIC1a, plus a more slowly migrating species (Fig. 1 B, C, and E; m and h, respectively). Blotting with an antibody to the FLAG epitope identified the higher molecular mass species as the FLAG-tagged human ASIC1a protein (Fig. 1C).
Fig. 1.
FLAG-hASIC1 expression in Tg mouse brain. (A) RT-PCR analysis of transgene expression in total brain RNA from Tg1, Tg2, and WT mice. Experiments were performed with (+) or without (-) reverse transcriptase (RT). (B) Western blot analysis of ASIC1 expression in whole brain protein lysates from mice of indicated genotype. Blot was probed with antibody (MTY) directed against an epitope common to the C-termini of both mouse and human ASIC1. Actin immunoblotting was used to confirm equivalent protein loading. -/-, ASIC1 null animals. h and m, human and mouse ASIC1a, respectively. (C) Whole brain lysates were blotted with anti-ASIC1 antibody (MTY) or anti-FLAG antibody. Both blots were obtained from the same gel. Compared to Western blot in B, the electrophoresis time was extended for better resolution of the two ASIC1a species. (D) Whole brain lysates were treated with (+) or without (-) N-glycanase and blotted with MTY. (E) Whole brain lysates were immunoprecipitated with anti-FLAG antibody followed by immunoblotting with MTY (Left) or blotted with MTY without immunoprecipitation (Right).
We also tested the processing of hASIC1a and its interaction with endogenous mASIC1a. ASIC1 protein from both WT and Tg mice was sensitive to N-glycanase digestion (Fig. 1D), suggesting that the endogenous and transgenic proteins were similarly glycosylated and processed. To test whether transgenic and endogenous ASIC1a formed multimers, we used the anti-FLAG antibody to immunoprecipitate FLAG-tagged hASIC1 from whole brain lysates and then blotted with the MTY antibody. The anti-FLAG antibody immunoprecipitated both transgenic and endogenous ASIC1a from Tg but not WT or ASIC1 -/- brain (Fig. 1E). These results indicate that transgenic hASIC1a formed a multimeric complex with mASIC1.
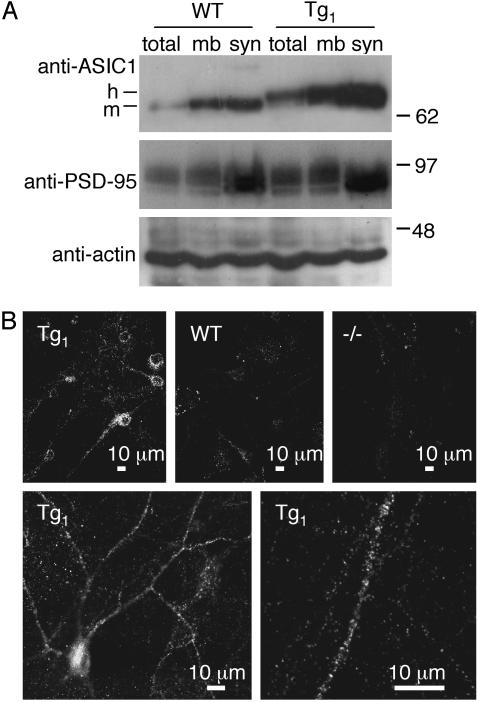
Transgenic ASIC1a Was Expressed in Neurons and Enriched in Synaptosomes. We used the Tg animals to test the hypothesis that ASIC1a protein is distributed in a pattern consistent with synaptic localization. Synaptosome-containing brain fractions revealed that ASIC1a protein coenriched with PSD-95 in both WT and Tg mice, although the abundance was greater in Tg brain (Fig. 2A). In hippocampal neurons from Tg mice, we found ASIC1a at the soma and distributed along dendrites in a punctate pattern (Fig. 2B). In contrast, we found little or no immunostaining of glial cells, which in these cultures form a dense bed on which the neurons settle. This result suggests that transgene expression was neuron-specific. There was little ASIC1a staining in WT neurons, consistent with our previous observation that endogenous ASIC1a has low levels of expression in the hippocampus (6). Together, the biochemical fractionation and immunolocalization data suggest that ASIC1a exists in the soma and neurites, where it may have a synaptic distribution.
Fig. 2.
Subcellular distribution of ASIC1 in WT and Tg mice. (A) Whole brain lysates (total), membranes (mb), and the synaptosome-containing brain fraction (syn) were western blotted with the indicated antibodies. (B) Cultured hippocampal neurons were prepared from mice with indicated genotypes and immunolabeled with affinity-purified anti-ASIC MTY antibody. (Lower) Immunolabeling of Tg1 neurons at higher magnification. The bed of glial cells underlying the neurons showed no immunofluorescence.
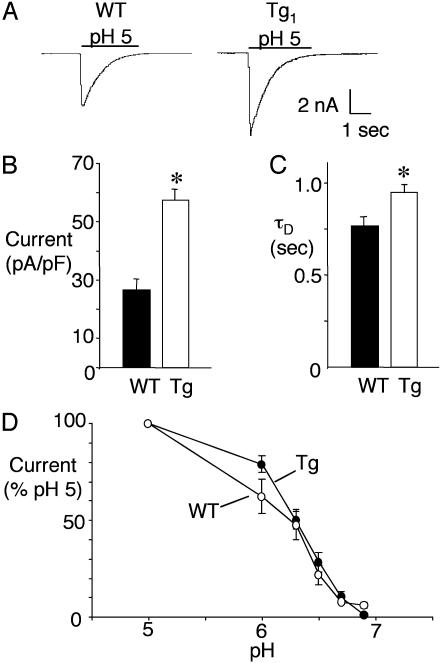
ASIC1a Overexpression Augmented Central H+-Evoked Currents. Previous studies proposed that ASIC1a was the subunit most important for determining the amplitude of H+-gated current, whereas ASIC2a influenced kinetics, including the desensitization rate of the current (τD) (16-18). Supporting this hypothesis, we found that overexpressing ASIC1a doubled acid-evoked current density in Tg neurons (Fig. 3 A and B). This increase could result from more ASIC1a homomultimers or more heteromultimeric channels. τD might distinguish between these alternatives because currents from ASIC1a homomultimers desensitize more slowly than ASIC1a/2a heteromultimers (17, 32, 33). In ASIC1a transgenic neurons, we found a slowing of τD compared to WT neurons (Fig. 3 A and C). Although the proportion of homomultimeric and heteromultimeric channels remains uncertain, these data suggest that overexpressing ASIC1a increases current at least in part by producing more ASIC1a homomultimers. It is possible that the increase in current amplitude might be caused by a shift in the pH dose-response; however, this did not appear to be the case because the pH-sensitivity of H+-evoked currents was similar for WT and Tg neurons (Fig. 3D).
Fig. 3.
Proton-gated currents in cultured hippocampal neurons from WT and Tg mice. (A) Representative traces from mice of indicated genotype. (B) Peak current density during pH 5 application (n = 52 for Tg; n = 33 for WT). *, P ≤ 0.001 compared to WT by Student's t test. (C) Desensitization rate (τD) of currents evoked by pH 5 solution (n = 41 for Tg; n = 22 for WT). *, P ≤ 0.05 compared to WT. (D) pH dose-response curve. Error bars represent SEM.
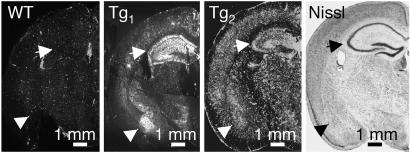
Transgenic ASIC1a Protein Was Abundant in the Amygdala and Hippocampus. To determine the distribution of hASIC1a expression, we immunolabeled coronal sections of forebrain with the anti-FLAG antibody. Although there were variations in the intensity, the staining pattern for the two Tg lines was similar, with the amygdala and hippocampus both showing abundant ASIC1a (Fig. 4). In the hippocampus, transgenic protein was distributed to the dendritic fields in both stratum radiatum and stratum oriens, consistent with the dendritic distribution observed in cultured neurons. In the amygdala, Tg1 brain showed preferential ASIC1a expression in the basolateral nucleus, and Tg2 expressed ASIC1a throughout the amygdala complex.
Fig. 4.
Immunohistochemistry of coronal sections of forebrain from WT, Tg1, and Tg2 mice. Labeling with the anti-FLAG antibody is in white. The arrows point to hippocampal formation, and the arrowheads point to the amygdala complex. A Nissl-stained section from a WT mouse is included for orientation.
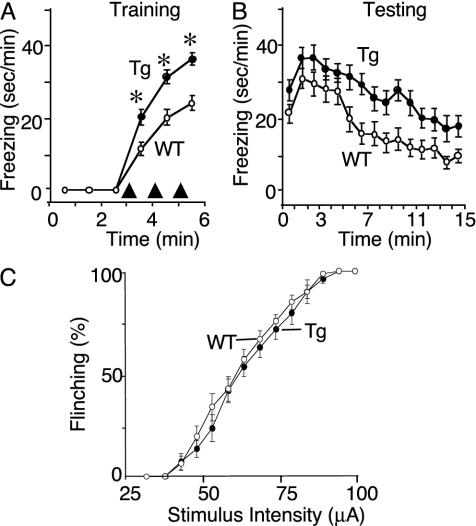
ASIC1a Overexpression Enhanced Context Fear Conditioning. We previously showed rich expression of mASIC1a in the amygdala and found that eliminating ASIC1 markedly reduced H+-gated currents and impaired context and cued fear conditioning (6). Our present finding of abundant hASIC1a in the amygdala and increased acid-evoked currents in Tg animals raised the hypothesis that ASIC1a overexpression might have the opposite effect of ASIC1 disruption and enhance acquired fear. Under baseline conditions, neither WT nor Tg mice showed significant freezing behavior after the introduction into the test cage, suggesting that they were not afraid of the novel environment (Fig. 5A). After foot-shocks, both genotypes froze, but the duration of the behavior increased in Tg mice. This pattern persisted throughout the testing period (Fig. 5B). These results indicate that ASIC1a overexpression enhanced fear-related freezing behavior.
Fig. 5.
Behavioral analysis of context fear conditioning. (A) Amount of time mice froze during 1-min intervals (sec/min) during training. The delivery of foot-shocks is indicated by arrowheads. Data from Tg1 (n = 13) and Tg2 (n = 11) mice were not statistically different (P = 0.98), and so were pooled. Data are mean ± SEM. *, P < 0.0001 compared to WT (n = 25 for WT; n = 24 for Tg). (B) Amount of time mice froze during 1-min intervals (sec/min) during testing. Tg mice spent more time freezing than WT animals (P < 0.0001 by linear mixed model analysis for repeated measures). (C) Percentage of foot-shocks that evoked a flinching response determined over a range of stimulus intensities. At each intensity, 10 foot-shocks were delivered (n = 15 for WT; n = 17 for Tg). P = 0.49 by logistic regression with the procedure genmod (sas/stat v.8.1, SAS Institute, Cary, NC).
Because members of the ASIC family have been implicated in sensory function (10, 34-38), we asked whether the increased freezing in Tg mice might be due to increased ability to feel the shock. To test this, we did several studies. We counted the number of trials in which naíve mice flinched in response to foot-shock; the two genotypes showed similar flinching behavior at all stimulus intensities (Fig. 5C). We also assessed the vocalization threshold by gradually increasing the stimulus intensity until a vocalization was evoked; the vocalization threshold was similar for WT (0.23 ± 0.01 mA; n = 15) and Tg (0.21 ± 0.01 mA; n = 13; mean ± SEM; P = 0.8) animals and was below the 0.5-mA stimulus used during conditioning. In addition, the average duration of hyperactivity after the 0.5-mA foot-shock delivered during fear conditioning did not differ in WT (1.7 ± 0.1 sec; n = 25) and Tg (1.8 ± 0.1 sec; n = 25; P = 0.32). Taken together, these data suggest that the increased freezing response in the Tg mice was likely not due to increased sensory function.
We also asked whether Tg mice have a difference in baseline anxiety that might explain the enhanced behavioral effect. To test this possibility, we used the elevated plus maze, which provides a measure of baseline fear, and scored several anxiety-related behaviors. The average time spent in the closed arms of the maze was similar for the two groups (WT, 172 ± 13 sec, n = 9; Tg, 180 ± 10 sec, n = 8; P = 0.62). The number of open arm entries was the same for both genotypes (WT, 14.4 ± 1.0; Tg, 13.6 ± 1.0; P = 0.66). And the amount of time spent inactive huddled in the closed arms was not significantly different (WT, 63.4 ± 11.3 sec; Tg, 64.3 ± 5.9 sec; P = 0.95). The normal activity of the Tg mice on the elevated plus maze suggests that ASIC1 overexpression did not affect baseline anxiety.
Discussion
The data described here, together with our previous studies of ASIC1 -/- mice indicate that ASIC1a plays an important role in fear-related freezing behavior. Endogenous mASIC1a was abundant in the amygdala (6, 14, 25), a key component of the circuitry for learned fear (4, 5, 26). It was also expressed in the cingulate cortex, nucleus accumbens, and other structures that may contribute to the emotional importance of external stimuli and/or the expression of fear (6, 39). Our studies of mouse behavior indicate that ASIC1a plays a role in the fear response; overexpressing ASIC1a enhanced fear conditioning, whereas eliminating ASIC1a reduced fear in this animal model of anxiety.
Our electrophysiological data support earlier suggestions that ASIC1a plays a key role in determining the amplitude of acid-evoked currents. Compared to WT, increasing ASIC1a expression doubled the current response to a pH 5 challenge, disrupting one of the two ASIC1 alleles cut the current in half (17), and eliminating ASIC1a abolished the response (6, 16, 17). However, central neurons also express ASIC2a and ASIC2b (11-13, 15, 17), which combine with ASIC1a to generate heteromultimeric channels (12, 17, 18, 32, 40). The pH-sensitivity, kinetics, and response to modulatory agents of heteromultimers differs from that of ASIC1a homomultimers (12, 17, 20, 23, 32, 40). At present, we do not know which properties of ASIC-mediated currents are most important in the fear response. However, the results presented here suggest that changing ASIC1a levels or activity could alter H+-gated currents and consequently alter acquired fear. For example, transcriptional regulation might play an important role. In sensory neurons, inflammatory mediators such as serotonin, nerve growth factor (NGF), bradykinin, and interleukin 1 may increase ASIC expression and increase H+-evoked currents (41). Interestingly, serotonin, NGF, and interleukin 1 have been implicated in fear conditioning in rodents (42-44) and may play an important role in anxiety in humans (45). ASIC1 activity might also be influenced by FMRFamide-like peptides (19, 21, 22, 46), which have been associated with fear-related avoidance learning in rodents (47). If polymorphisms or mutations in the ASIC1 gene exist in humans, they might also affect H+-gated currents and fear.
We cannot exclude the possibility that overexpressing ASIC1a could cause some toxicity, but many aspects of behavior were normal in the Tg mice. In fact, if overexpression were toxic to amygdala neurons, we would have expected to reduce freezing rather than enhance it (3, 4, 26). These data suggest that ASIC1a channels are predominantly closed until activated by ligand. However, it is possible that an increase in acid-activated currents could be toxic under pathological conditions such as ischemia and seizures, which are accompanied by extracellular acidosis (48-50). In addition, because the transgenic ASIC1a protein was widely expressed in the brain, it would not be surprising if behaviors other than the fear response were also affected.
We speculate that an increase in H+-gated currents could represent a predisposition for the development of anxiety disorders such as posttraumatic stress disorder and panic disorder. For many years it has been known that inhaling CO2 can trigger panic attacks in patients with panic disorder (51-53). In the brain, the reaction between CO2 and water catalyzed by carbonic anhydrase rapidly generates H+ and causes central acidosis. Perhaps acidosis triggers central H+-gated currents and potentiates feelings of panic. The observation that neither ASIC1 disruption nor ASIC1a overexpression led to gross toxicity suggests that ASIC1a antagonists might represent a safe approach for reducing anxiety in the clinical setting.
Acknowledgments
We thank Samuel Hartman and Joe Orlando for excellent technical assistance, Tom Moninger and the University of Iowa Central Microscopy Research Facility for assistance with microscopy and image analysis, Dr. Jim Hynes and the University of Iowa Transgenic Mouse Facility for assistance with mice, Bridget Zimmerman for assistance with statistical analysis, and the University of Iowa DNA Core Facility (National Institutes of Health Grant DK25295) for assistance. This work was supported in part by a Veteran's Administration Research Career Development Award and the Howard Hughes Medical Institute Biomedical Research Support Program (to J.A.W.). C.C.A. and A.S.L. were Associates and M.J.W. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations: ASIC, acid-sensing ion channel; Tg, transgenic.
References
- 1.Lecrubier, Y. (2001) J. Clin. Psychiatry 62, 4-9. [PubMed] [Google Scholar]
- 2.Gorman, J. M., Kent, J. M., Sullivan, G. M. & Coplan, J. D. (2000) Am. J. Psychiatry 157, 493-505. [DOI] [PubMed] [Google Scholar]
- 3.Kim, J. J., Rison, R. A. & Fanselow, M. S. (1993) Behav. Neurosci. 107, 1093-1098. [DOI] [PubMed] [Google Scholar]
- 4.LeDoux, J. E. (2000) Annu. Rev. Neurosci. 23, 155-184. [DOI] [PubMed] [Google Scholar]
- 5.Maren, S. (2001) Annu. Rev. Neurosci. 24, 897-931. [DOI] [PubMed] [Google Scholar]
- 6.Wemmie, J. A., Askwith, C. C., Lamani, E., Cassell, M. D., Freeman, J. H. J. & Welsh, M. J. (2003) J. Neurosci. 23, 5496-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishtal, O. (2003) Trends Neurosci. 26, 477-483. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi, L. & Driscoll, M. (2002) Neuron 34, 337-340. [DOI] [PubMed] [Google Scholar]
- 9.Welsh, M. J., Price, M. P. & Xie, J. (2002) J. Biol. Chem. 277, 2369-2372. [DOI] [PubMed] [Google Scholar]
- 10.Waldmann, R. & Lazdunski, M. (1998) Curr. Opin. Neurobiol. 8, 418-424. [DOI] [PubMed] [Google Scholar]
- 11.García-Añoveros, J., Derfler, B., Neville-Golden, J., Hyman, B. T. & Corey, D. P. (1997) Proc. Natl. Acad. Sci. USA 94, 1459-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lingueglia, E., de Weille, J. R., Bassilana, F., Heurteaux, C., Sakai, H., Waldmann, R. & Lazdunski, M. (1997) J. Biol. Chem. 272, 29778-29783. [DOI] [PubMed] [Google Scholar]
- 13.Price, M. P., Snyder, P. M. & Welsh, M. J. (1996) J. Biol. Chem. 271, 7879-7882. [DOI] [PubMed] [Google Scholar]
- 14.Waldmann, R., Champigny, G., Bassilana, F., Heurteaux, C. & Lazdunski, M. (1997) Nature 386, 173-177. [DOI] [PubMed] [Google Scholar]
- 15.Waldmann, R., Champigny, G., Voilley, N., Lauritzen, I. & Lazdunski, M. (1996) J. Biol. Chem. 271, 10433-10436. [DOI] [PubMed] [Google Scholar]
- 16.Wemmie, J. A., Chen, J., Askwith, C. C., Hruska-Hageman, A. M., Price, M. P., Nolan, B. C., Yoder, P. G., Lamani, E., Hoshi, T., Freeman, J. H. J. & Welsh, M. J. (2002) Neuron 34, 463-477. [DOI] [PubMed] [Google Scholar]
- 17.Askwith, C. C., Wemmie, J. A., Price, M. P., Rokhlina, T. & Welsh, M. J. (2004) J. Biol. Chem., in press. [DOI] [PubMed]
- 18.Escoubas, P., De Weille, J. R., Lecoq, A., Diochot, S., Waldmann, R., Champigny, G., Moinier, D., Menez, A. & Lazdunski, M. (2000) J. Biol. Chem. 275, 25116-25121. [DOI] [PubMed] [Google Scholar]
- 19.Askwith, C. C., Cheng, C., Ikuma, M., Benson, C. J., Price, M. P. & Welsh, M. J. (2000) Neuron 26, 133-141. [DOI] [PubMed] [Google Scholar]
- 20.Baron, A., Schaefer, L., Lingueglia, E., Champigny, G. & Lazdunski, M. (2001) J. Biol. Chem. 276, 35361-35367. [DOI] [PubMed] [Google Scholar]
- 21.Allen, N. J. & Attwell, D. (2002) J. Physiol. 543, 521-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catarsi, S., Babinski, K. & Seguela, P. (2001) Neuropharmacology 41, 592-600. [DOI] [PubMed] [Google Scholar]
- 23.Baron, A., Waldmann, R. & Lazdunski, M. (2002) J. Physiol. 539, 485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvarez de la Rosa, D., Krueger, S. R., Kolar, A., Shao, D., Fitzsimonds, R. M. & Canessa, C. M. (2003) J. Physiol. 546, 77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olson, T. H., Riedl, M. S., Vulchanova, L., Ortiz-Gonzalez, X. R. & Elde, R. (1998) Neuron 9, 1109-1113. [DOI] [PubMed] [Google Scholar]
- 26.Faneslow, M. S. & LeDoux, J. E. (1999) Neuron 23, 229-232. [DOI] [PubMed] [Google Scholar]
- 27.Stec, D. E., Morimoto, S. & Sigmund, C. D. (2001) BioTechniques 31, 256-260. [DOI] [PubMed] [Google Scholar]
- 28.Hoesche, C., Sauerwald, A., Veh, R. W., Krippl, B. & Kilimann, M. W. (1993) J. Biol. Chem. 268, 26494-26502. [PubMed] [Google Scholar]
- 29.Sigmund, C. D. (1993) Hypertension 22, 599-607. [DOI] [PubMed] [Google Scholar]
- 30.Lowry, O. H. & Passanneau, J. V. (1972) in A Flexible System of Enzymatic Analysis (Academic, New York), pp. 86-92.
- 31.Carlin, R. K., Grab, D. J., Cohen, R. S. & Siekevitz, P. (1980) J. Cell Biol. 86, 831-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benson, C. J., Xie, J., Wemmie, J. A., Price, M. P., Henss, J. M., Welsh, M. J. & Snyder, P. M. (2002) Proc. Natl. Acad. Sci. USA 99, 2338-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutherland, S. P., Benson, C. J., Adelman, J. P. & McCleskey, E. W. (2001) Proc. Natl. Acad. Sci. USA 98, 711-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benson, C. J., Eckert, S. P. & McCleskey, E. W. (1999) Circ. Res. 84, 921-928. [DOI] [PubMed] [Google Scholar]
- 35.Chen, C. C., England, S., Akopian, A. N. & Wood, J. N. (1998) Proc. Natl. Acad. Sci. USA 95, 10240-10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price, M. P., Lewin, G. B., McIlwrath, S. L., Cheng, C., Xie, J., Heppenstall, P. A., Stucky, C. L., Mannsfeldt, A. G., Brennan, T. J., Drummond, H. A., et al. (2000) Nature 407, 1007-1011. [DOI] [PubMed] [Google Scholar]
- 37.Price, M. P., McIllwrath, S. L., Xie, J., Cheng, C., Qiao, J., Tarr, D. E., Sluka, K. A., Brennan, T. J., Lewin, G. R. & Welsh, M. J. (2001) Neuron 32, 1071-1083. [DOI] [PubMed] [Google Scholar]
- 38.Chen, C. C., Zimmer, A., Sun, W. H., Hall, J. & Brownstein, M. J. (2002) Proc. Natl. Acad. Sci. USA 99, 8992-8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cardinal, R. N., Parkinson, J. A., Hall, J. & Everitt, B. J. (2002) Neurosci. Biobehav. Rev. 26, 321-352. [DOI] [PubMed] [Google Scholar]
- 40.Bassilana, F., Champigny, G., Waldmann, R., de Weille, J. R., Heurteaux, C. & Lazdunski, M. (1997) J. Biol. Chem. 272, 28819-28822. [DOI] [PubMed] [Google Scholar]
- 41.Mamet, J., Baron, A., Lazdunski, M. & Voilley, N. (2002) J. Neurosci. 22, 10662-10670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song, C., Phillips, A. G. & Leonard, B. (2003) Eur. J. Neurosci. 18, 1739-1743. [DOI] [PubMed] [Google Scholar]
- 43.Winkler, J., Ramirez, G. A., Thal, L. J. & Waite, J. J. (2000) J. Neurosci. 20, 834-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell, B. M. & Merchant, K. M. (2003) Brain Res. 993, 1-9. [DOI] [PubMed] [Google Scholar]
- 45.Anderson, I. M. & Mortimore, C. (1999) Adv. Exp. Med. Biol. 467, 43-55. [DOI] [PubMed] [Google Scholar]
- 46.Deval, E., Baron, A., Lingueglia, E., Mazarguil, H., Zajac, J. M. & Lazdunski, M. (2003) Neuropharmacology 44, 662-671. [DOI] [PubMed] [Google Scholar]
- 47.Telegdy, G. & Bollók, I. (1987) Neuropeptides 10, 157-163. [DOI] [PubMed] [Google Scholar]
- 48.Obrenovitch, T. P., Garofalo, O., Harris, R. J., Bordi, L., Ono, M., Momma, F., Bachelard, H. S. & Symon, L. (1988) J. Cereb. Blood Flow Metab. 8, 866-874. [DOI] [PubMed] [Google Scholar]
- 49.Wasterlain, C., Fujikawa, D., Penix, L. & Sankar, R. (1993) Epilepsia 34, S37-S53. [DOI] [PubMed] [Google Scholar]
- 50.Siesjo, B. K. (1988) Neurochem. Pathol. 9, 31-88. [DOI] [PubMed] [Google Scholar]
- 51.Gorman, J. M., Askanazi, J., Liebowitz, M. R., Fyer, A. J., Stein, J., Kinney, J. M. & Klein, D. F. (1984) Am. J. Psychiatry 141, 857-861. [DOI] [PubMed] [Google Scholar]
- 52.Coryell, W., Fyer, A., Pine, D., Martinez, J. & Arndt, S. (2001) Biol. Psychiatry 49, 582-587. [DOI] [PubMed] [Google Scholar]
- 53.Klein, D. F. (1993) Arch. Gen. Psychiatry 50, 306-317. [DOI] [PubMed] [Google Scholar]