ABSTRACT
The sexually transmitted infection gonorrhea is caused exclusively by the human-specific pathogen Neisseria gonorrhoeae. Type IV pili are an essential virulence factor uniformly expressed on clinical gonococcal isolates and are required for several aspects of gonococcal pathogenesis, including adherence to host tissues, autoagglutination, twitching motility, and the uptake of DNA during transformation. Symptomatic gonococcal infection is characterized by the influx of neutrophils or polymorphonuclear leukocytes (PMNs) to the site of infection. PMNs are a key component of gonococcal pathogenesis, mediating the innate immune response through the use of oxidative and nonoxidative killing mechanisms. The M23B family zinc metallopeptidase NGO1686 is required for gonococci to survive oxidative killing by H2O2- and PMN-mediated killing through unknown mechanisms, but the only known target of NGO1686 is peptidoglycan. We report that the effect of NGO1686 on survival after exposure to H2O2 and PMNs is mediated through its role in elaborating pili and that nonpiliated mutants of N. gonorrhoeae are less resistant to killing by H2O2, LL-37, and PMNs than the corresponding piliated strains. These findings add to the various virulence-associated functions attributable to gonococcal pili and may explain the selection basis for piliation in clinical isolates of N. gonorrhoeae.
IMPORTANCE
Successful infectious agents need to overcome host defense systems to establish infection. We show that the Neisseria pilus, a major virulence factor of this organism, which causes gonorrhea, helps protect the bacterium from two major killing mechanisms used by the host to combat infections. We also show that to express the pilus, an enzyme needs to partially degrade the cell wall of the bacterium.
Introduction
The human-specific pathogen Neisseria gonorrhoeae is the sole causative agent of the sexually transmitted infection gonorrhea. Type IV pili are an essential virulence factor of gonococci (Gc), as only piliated bacteria are isolated from patients with gonorrhea (1). Type IV pili are thin, hair-like appendages that have been implicated in the virulence of both Gram-negative and Gram-positive bacteria, including the closely related species Neisseria meningitidis, as well as a variety of other pathogens, including Legionella pneumophila, Vibrio cholerae, Pseudomonas aeruginosa (reviewed in reference 2), and Clostridium perfringens (3). Several proteins are required for type IV pilus biogenesis in pathogenic Neisseria species; however, only a few of these proteins are absolutely required for pilus assembly (4). For a subset of these pilus biogenesis proteins, type IV pili can be restored when they are absent by inactivation of the twitching motility ATPase PilT (4, 5).
Pilus expression is essential for Gc pathogenesis. Whereas all clinical isolates of Gc are piliated, both nonpiliated and underpiliated variants frequently arise when Gc are grown in vitro, suggesting that there is strong selective pressure for pilus expression in the human host (1). Gc pili have a number of established functions that are important for pathogenesis, including adherence to the host’s epithelium, autoagglutination, twitching motility, and uptake of DNA during transformation (6). Since Gc do not express exotoxins or a type II or type III secretion system, and the type IV secretion system is expressed in only some isolates, the pilus can be considered both a fibril organelle and the major secretion system for this pathogen. However, the only known secreted substrate of the type IV secretion system is the pilus itself.
A second important aspect of gonococcal pathogenesis is the interaction of Gc with polymorphonuclear leukocytes (PMNs). Symptomatic gonorrhea is characterized by the influx of PMNs to the site of infection. The clinical manifestation of this infection is the production of a purulent exudate that consists almost entirely of PMNs with attached and internalized gonococcal cells. PMNs possess both oxidative and nonoxidative mechanisms of killing, yet some Gc found in the purulent exudate remain viable, suggesting that Gc have evolved mechanisms to circumvent killing by PMNs (7, 8). Gc express a plethora of gonococcal proteins that protect against oxidative damage caused by reactive oxygen species (ROS). Some act by detoxifying ROS (9–11), while others repair damage caused by ROS (12, 13). Although many of these antioxidant defenses have been shown to aid in gonococcal survival within primary human cervical epithelial cells and the mouse genital tract (14, 15), evidence suggests that ROS do not participate in PMN-mediated killing of Gc (16, 17). The nonoxidative components of PMN-mediated killing include degradative enzymes and cationic antimicrobial peptides (18). Gc possess two efflux pump systems which confer resistance to cationic antimicrobial peptides and long-chain fatty acids (19, 20) and aid in colonization of the murine genital tract (21). It is likely that in becoming a successful pathogen, Gc have evolved diverse ways to subvert the killing mechanisms of PMNs to promote infection and transmission (7).
The gonococcal NGO1686 locus, encoding the mpg gene (for M23B metalloprotease active against peptidoglycan), was identified by microarray analysis as being highly upregulated by sublethal levels of H2O2, and mpg mutants are sensitive to H2O2 and nonoxidative PMN-mediated killing (16, 22). Thus far, RecN and NGO1686 are the only gonococcal proteins that have been shown to aid in survival to nonoxidative PMN-mediated killing (16). NGO1686 was characterized as a bifunctional, M23B family, zinc-dependent carboxy- and endopeptidase that hydrolyzes peptidoglycan (PG) side chains (23). Other M23B family metalloproteases have been shown to affect PG cross-linking, contributing to the cellular morphology of Helicobacter pylori (24, 25). Several M23B family proteins have also been shown to cleave septal PG to allow for efficient cell separation (26–29). In contrast, the N. gonorrhoeae mpg mutant (also termed the Δ1686 mutant) shows no defects in cellular morphology but does exhibit an altered colony morphology. Here we provide evidence that NGO1686 is involved in pilus biogenesis and that the mpg mutant colony morphology as well as its corresponding phenotypes of resistance to H2O2 and PMNs are directly mediated through NGO1686’s effect on piliation. This work establishes new roles for the gonococcal pilus in pathogenesis.
RESULTS
The mpg mutant is underpiliated.
The mpg mutant strain has a colony morphology different from that of the parent strain, and it can be complemented by supplying mpg at an ectopic locus in the chromosome (22, 23) (Fig. 1 and data not shown). We noted that the colony morphology of the mpg mutant was intermediate between that of the fully piliated (P+) parent strain and spontaneous nonpiliated (P−) pilin variants (Fig. 1). To directly examine the piliation state of the mpg mutant, the mpg mutation was transformed into the FA1090 (RM11.2nv, where “nv” indicates “nonvarying”) genetic background, which expresses a pilin variant that can be detected using immunoelectron microscopy (immuno-EM) (30) and cannot undergo further antigenic variation due to a transposon mutation affecting the guanine quartet sequence required for pilin variation (31, 32). The RM11.2nv mpg mutant also exhibited an altered colony morphology similar to that of the 1-81-S2 mpg strain (data not shown). Enumeration of pilin bundles on each strain revealed that the parent strain FA1090 (RM11.2nv) expressed an average of 3.5 bundles per gonococcal cell (Fig. 2A to C), whereas the corresponding mpg mutant expressed an average of 0.7 bundle per gonococcal cell (Fig. 2D to F). These data demonstrate that the mpg mutant shows decreased pilus expression, which is likely the basis of the altered colony morphology.
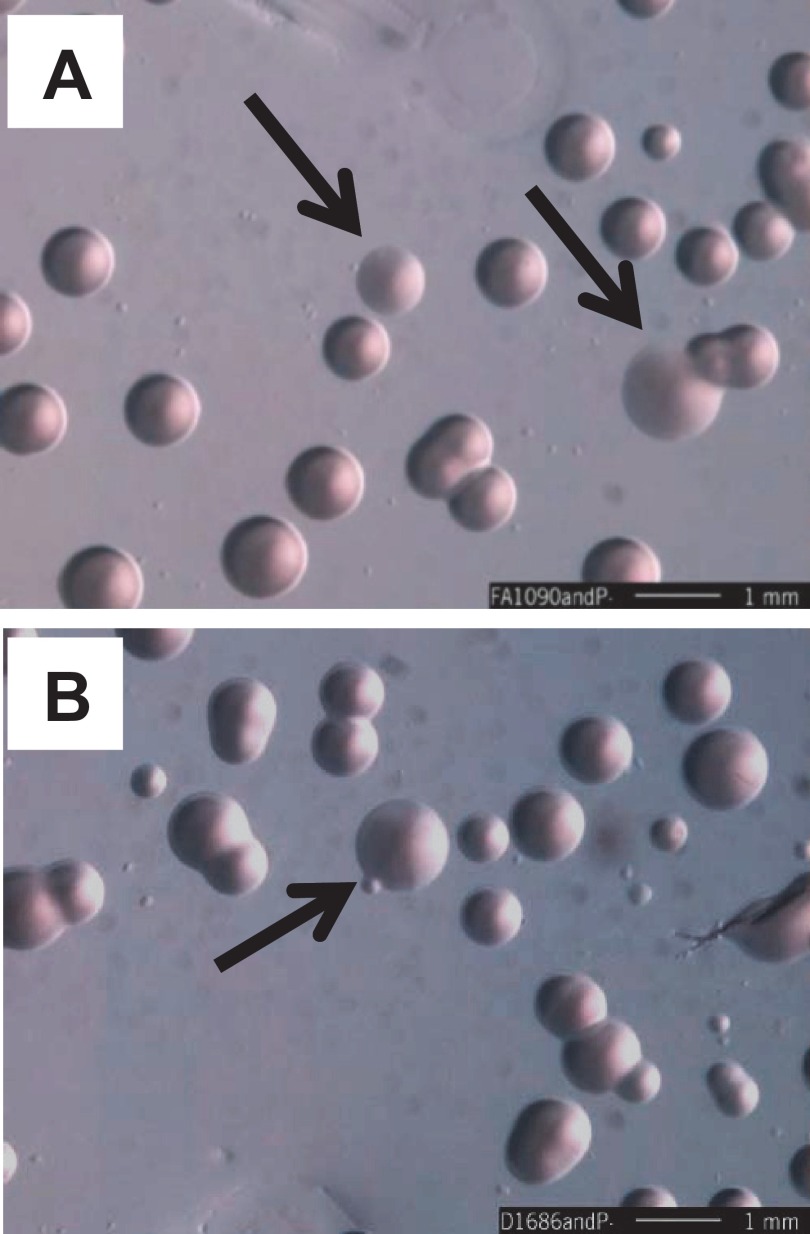
FIG 1 .
Colony morphologies of strains FA1090, the mpg (Δ1686) mutant, and nonpiliated (P−) derivatives. (A) FA1090; (B) mpg mutant. Arrows indicate colonies with P− morphologies among P+ colonies. The more P+ colonies of the mpg strain show a colony morphology intermediate between those of FA1090 P+ and FA1090 P−. Representative stereomicroscope images of strains after 24 h of growth on solid media (bars, 1 mm). Smaller colonies can appear with both strains for unknown reasons.
FIG 2 .
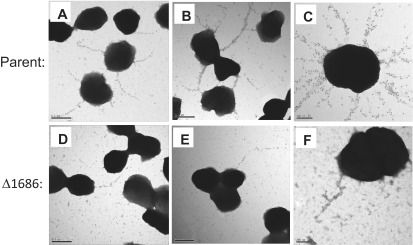
Electron micrographs of immunogold-labeled bundled pili on strains RM11.2nv and RM11.2nv mpg (Δ1686). Representative electron micrographs are shown for strain RM11.2nv (parent) and RM11.2nv mpg (Δ1686). Bars, 0.5 µm (A, B, D, and E) and 200 nm (C and F). Pili were detected using a rabbit antipeptide polyclonal antiserum directed against the RM11.2 hypervariable pilin sequence and a gold-labeled secondary antibody.
The mpg strain is deficient in natural transformation.
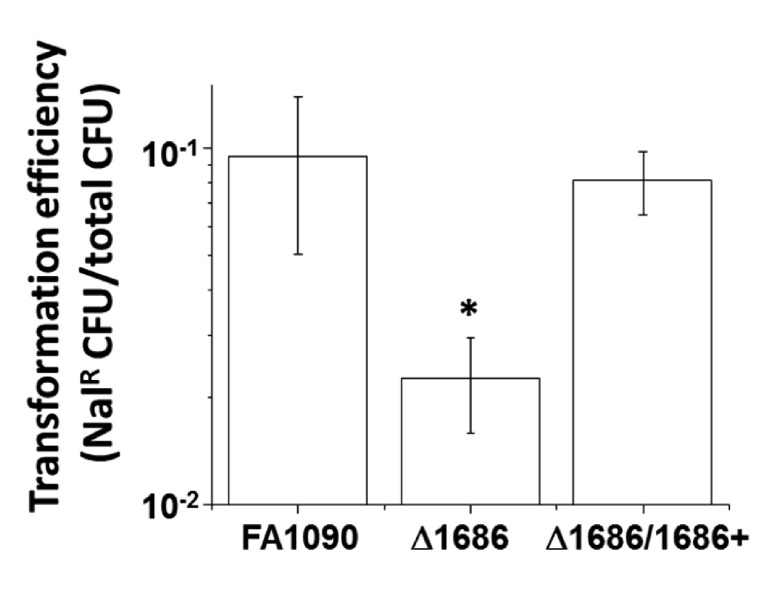
Gc are naturally competent for DNA transformation at all stages of growth, and the pilus is essential for efficient DNA transformation (33). The number of pili elaborated on the gonococcal cell surface correlates with the transformation efficiency of a given strain (30, 34). To further test whether piliation is affected in the mpg mutant, we compared the transformation efficiencies of the parental strain, the previously described mpg loss-of-function mutant (D1686), and the mutant complemented with the gene expressed from its own promoter at an ectopic locus (Δ1686/1686+ mutant). The mpg mutant showed a statistically significant 3- to 4-fold decrease in transformation efficiency relative to that of the parent strain, and this deficiency was restored in the complemented strain (Fig. 3). These results show that the mpg mutant has decreased transformation competence and suggest that the additional mpg phenotypes of decreased resistance to H2O2- and PMN-mediated killing may also be attributable to decreased piliation.
FIG 3 .
Transformation efficiencies of the indicated strains to nalidixic acid resistance (Nalr) by plasmid pSY6. Error bars represent the standard errors of the means of results from at least 4 experiments. *, P < 0.02, relative to values for FA1090 and the Δ1686 1686+ mutant (D1686/1686+), by Student’s two-tailed t test.
The decreased resistance of the mpg mutant to H2O2- and PMN-mediated killing is due to decreased piliation.
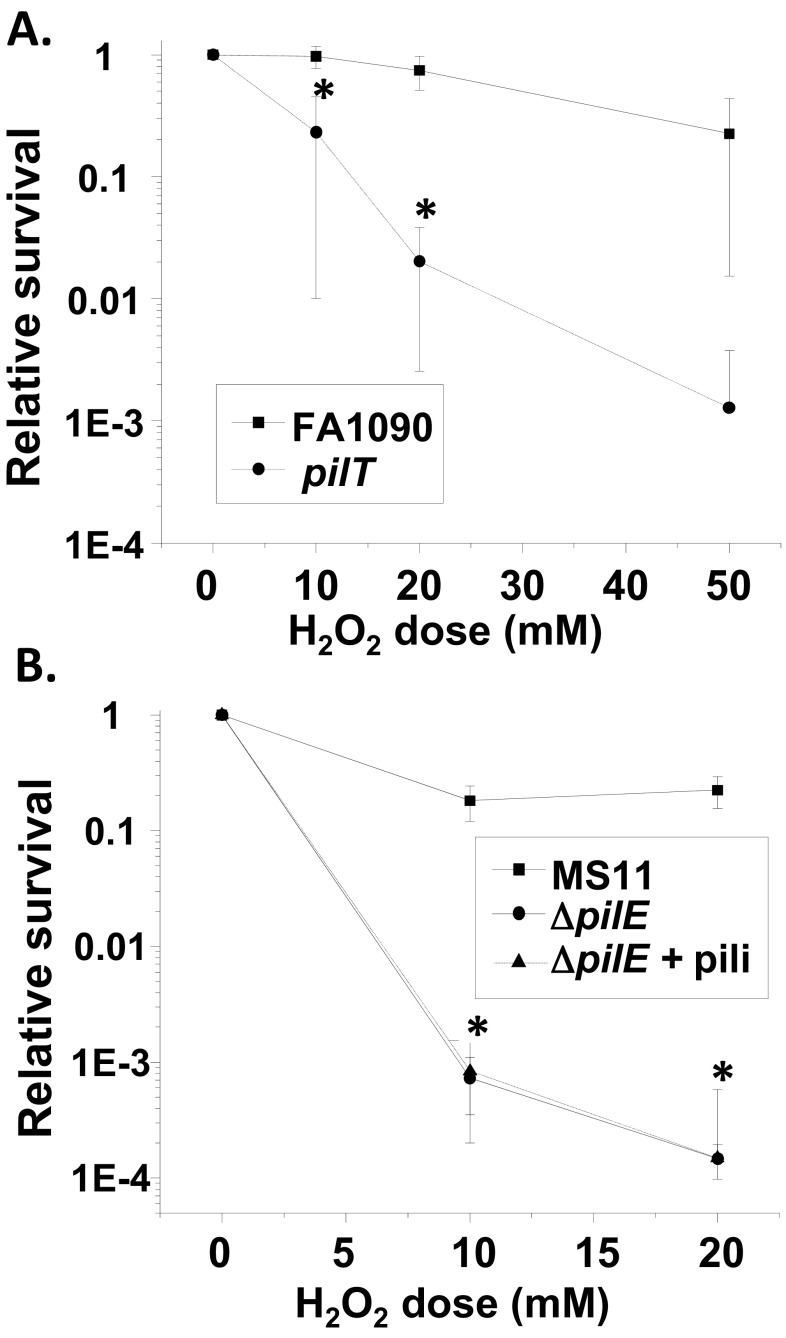
To genetically test whether the mpg strain’s H2O2 sensitivity phenotype was mediated through pili, we compared the levels of H2O2 resistance conferred by the ΔpilE, mpg, and ΔpilE mpg mutations in strain FA1090 (variant 1-81-S2) (Fig. 4A). The ΔpilE strain was less resistant to H2O2 than the mpg strain, but both the mpg and ΔpilE strains were less resistant than the parental strain, consistent with the complete lack of piliation in the ΔpilE strain and the underpiliated phenotype of the mpg strain. There was no further decrease in H2O2 resistance from that shown by the ΔpilE strain when the two mutations were combined, indicating that the two mutations are in the same epistasis group and are in the same pathway for H2O2 resistance.
FIG 4 .
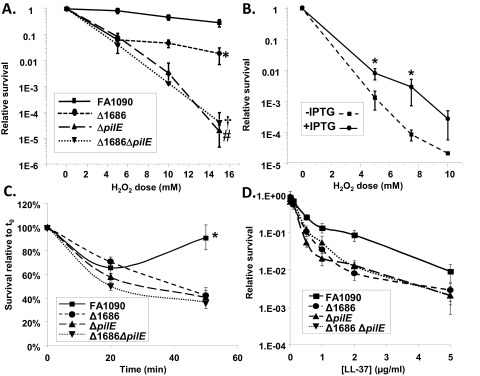
Effect of piliation on H2O2 and PMN-mediated resistance. (A and B) Dose-response curves of the H2O2 resistance of the indicated gonococcal strains after 15 min of treatment (A) and of regulatable N. gonorrhoeae strain RM11.2 either expressing pili (+IPTG) or not expressing pili (−IPTG) (B). (A) Error bars represent the standard errors of the means from at least 4 experiments. *, P < 0.02, in comparisons with all strains at 5, 10, and 15 mM H2O2; #, P < 7.8E−4, in comparisons with the mpg (Δ1686) mutant and FA1090 at 10 and 15 mM; †, P > 0.37, in a comparison with the ΔpilE strain at all doses by Student’s two-tailed t test. (B) Error bars represent the standard errors of the means of results from at least 3 experiments. *, P < 0.04, relative to values for regulatable strain RM11.2 (without IPTG) at the indicated doses by Student’s two-tailed t test. (C) PMN-mediated survival of the indicated strains over time relative to that at time zero (t0). At 50 min, P was <0.005 for the Δ1686 mutant versus the wild type, the pilE strain versus the wild type, and the double mutant versus the wild type. (D) LL-37 resistance. The indicated strains were exposed to increasing concentrations of LL-37. Bacterial survival after 45 min is expressed as survival relative to that of the inoculum. Statistically significant survival differences were obtained for FA1090 versus the Δ1686 mutant at 0.5, 1, and 2 µg/ml LL-37 and for FA1090 versus the ΔpilE mutant at 0.5 and 1 µg/ml LL-37 (P < 0.05, Student’s two-tailed t test).
To directly test the hypothesis that piliated Gc are more resistant to H2O2 killing than nonpiliated Gc, we utilized a gonococcal strain containing a regulatable pilE gene. The regulatable RM11.2 strain contains the RM11.2 variant pilE sequence under the control of the lac regulatory system, thereby allowing the control of pilE expression and piliation by addition or removal of IPTG (isopropyl-β-d-thiogalactopyranoside) (30). The regulatable RM11.2 strain was ~10-fold more resistant to H2O2 in the presence of IPTG (when wild-type levels of pili were expressed) than in the absence of IPTG (when few or no pili were expressed) (Fig. 4B).
To determine whether the decreased resistance of the mpg mutant to PMN-mediated killing was also mediated through pili, ex vivo PMN survival measurements were performed using freshly isolated human PMNs incubated with the parent strain, the mpg and ΔpilE single mutants, and the ΔpilE mpg double mutant. The mpg strain showed the previously reported decreased resistance to PMN-mediated killing (Fig. 4C, and see reference 22). The ΔpilE strain was less resistant to PMN-mediated killing than the parent or the mpg strain, and the double mutant showed levels of resistance similar to those of the ΔpilE mutant (Fig. 4C). These results indicate that the basis for decreased resistance to PMN-mediated killing in the mpg strain is also due to decreased levels of piliation and reveal a role for piliation in gonococcal resistance to PMN killing.
Since we previously found that the mpg strain was more sensitive to nonoxidative killing by PMNs (22), we began to explore what nonoxidative-killing mechanisms of PMNs might be responsible for the increased killing of non- and underpiliated Gc. The parent strain, the mpg and ΔpilE single mutants, and the ΔpilE mpg double mutant were exposed to the cationic antimicrobial peptide LL-37, which is produced by PMNs. All of the mutant strains showed decreased resistance to human LL-37 (Fig. 4D), implying that at least some of the PMN sensitivity was dependent on the action of this cathelicidin and confirming that pilE and mpg were epistatic and likely in the same pathway.
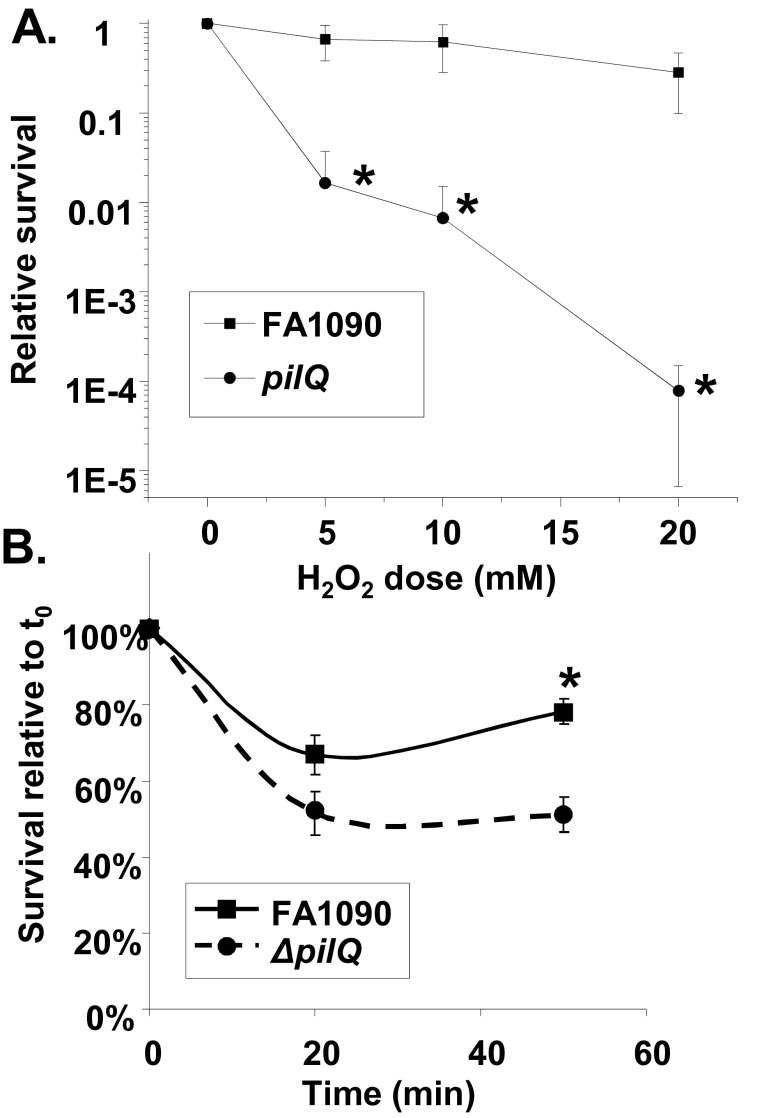
To confirm a role for piliation in gonococcal resistance to H2O2 and PMN-mediated killing, we examined the effect of a pilQ mutation on resistance to H2O2 and PMN-mediated killing. PilQ encodes the pore through which the pilus is extruded, and a pilQ mutant has been shown to not elaborate pili (35). The pilQ mutant showed decreased resistance to both H2O2 and PMN killing (Fig. 5). A pilC mutant, which is also P−, was also less resistant to H2O2 than the parent strain (data not shown). Thus far, all the gonococcal mutants which decrease or abolish piliation that we have tested also confer decreased resistance to H2O2 (data not shown).
FIG 5 .
Resistance of pilQ mutant strains to H2O2 and PMN-mediated killing. (A) Dose-response curve of H2O2 resistance after 15 min of treatment of the pilQ mutant strain. *, P < 0.02. (B) Resistance of the pilQ mutant strain to PMN-mediated killing. *, P < 0.0005 (Student’s two-tailed t test).
Pseudomonas aeruginosa type IV pili do not confer resistance to H2O2 killing.
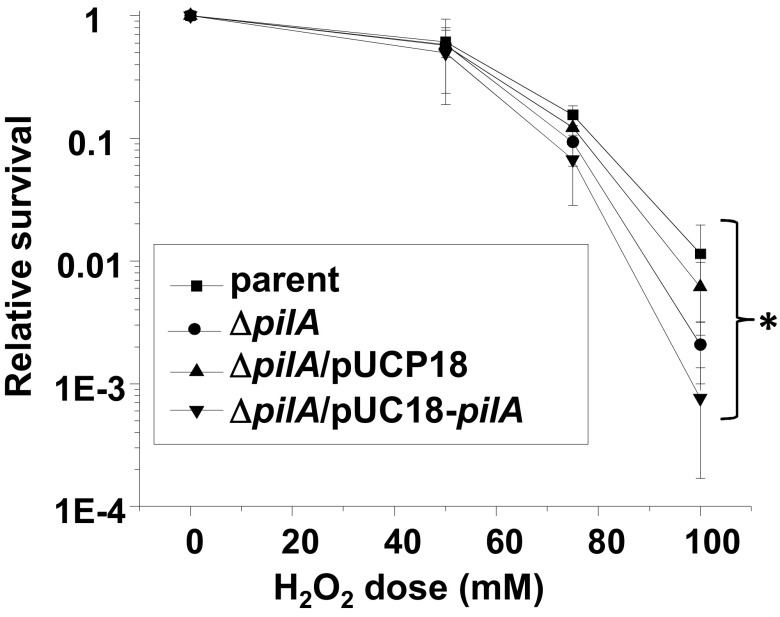
We next considered whether H2O2 resistance is a trait conferred by all type IV pili. We measured the H2O2 resistance of P. aeruginosa strain 15692, an isogenic pilA mutant (pilA encodes the pilin subunit), the complemented strain containing the pilA gene in vector pUCP18, and the empty vector (36). The observed small differences are not consistent with pili conferring H2O2 resistance on P. aeruginosa (Fig. 6). These results indicate that H2O2 resistance is not a phenotype shared among type IV pili from all bacterial species, although it is still possible that other type IV-pilus-expressing species share this property with Gc.
FIG 6 .
Relative survival of P. aeruginosa strain 15692, the isogenic pilA mutant, an empty vector control, and the pilA complemented strain. Error bars represent the standard errors of the means of results from at least 5 experiments. *, P > 0.21, between all strains at all doses.
Resistance to H2O2 requires active pili.
To determine whether functional pili or merely the presence of pili affects resistance to H2O2 killing, we measured the effect of pilT mutation on H2O2 resistance. pilT encodes the ATPase that mediates the retraction of the pilus. Although pilT mutants are piliated, they no longer exhibit twitching motility and are defective in DNA uptake (37). An FA1090 pilT mutant was less resistant to H2O2 than the parent strain (Fig. 7A), showing that active pili are necessary to mediate resistance to H2O2 killing. We also purified pili from a distinct gonococcal strain, strain MS11, and added them to MS11 ΔpilE bacteria to determine whether the pili were able to confer protection on the ΔpilE strain. The addition of purified pili did not confer H2O2 resistance to the MS11 ΔpilE strain (Fig. 7B). These data indicate that active pili are required to mediate resistance to H2O2 and that the absence of pili in both strain FA1090 and strain MS11 leads to decreased H2O2 resistance.
FIG 7 .
Effect of active pili on H2O2 resistance. (A) Dose-response curve of H2O2 resistance after 15 min of treatment of strains FA1090 and FA1090 pilT. Error bars represent the standard errors of the means of results of 4 to 8 experiments. *, P < 0.05, between strains at the indicated doses. (B) H2O2 resistance of N. gonorrhoeae strain MS11 and the isogenic ΔpilE mutant with and without purified pili added back. Error bars represent the standard errors of the means of results from 2 experiments. *, P > 0.89, between the ΔpilE strain and the ΔpilE strain with pili added at both doses.
NGO1686 is involved in pilus biogenesis.
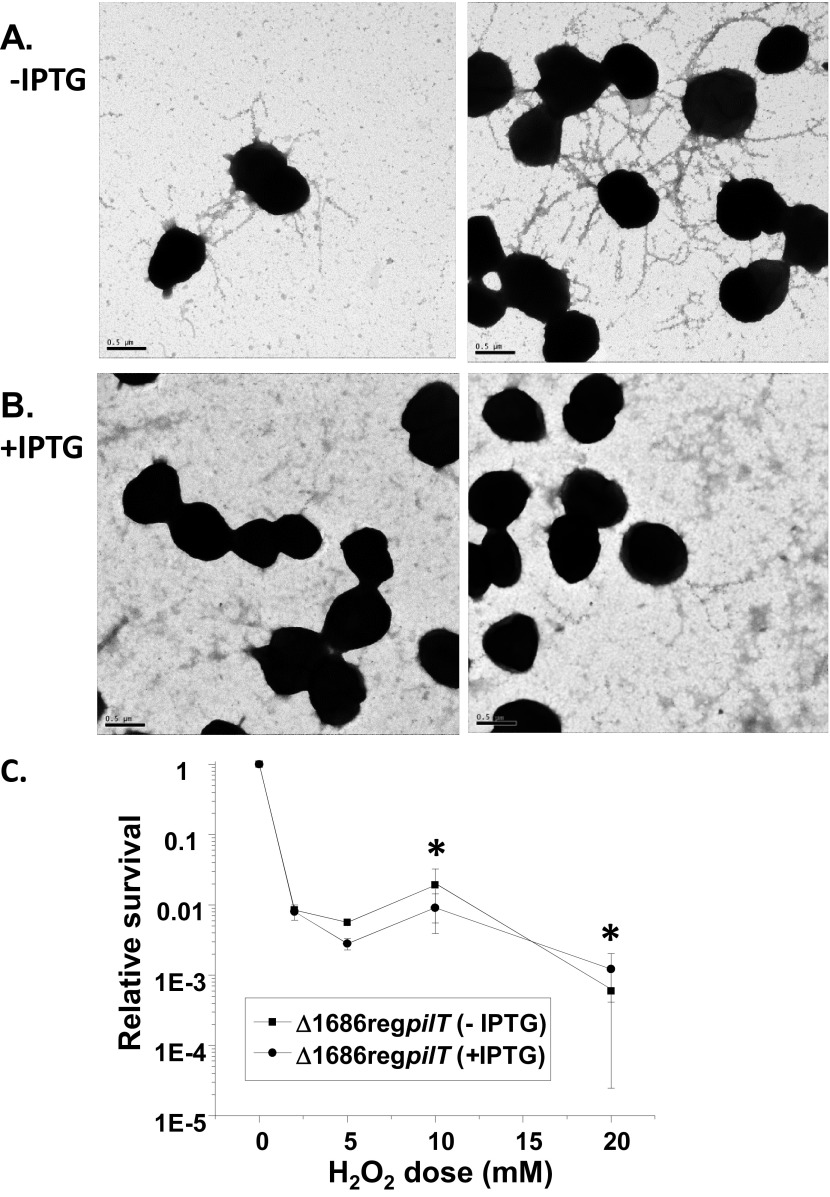
The underpiliated phenotype of the mpg mutant was similar to phenotypes described for pathogenic Neisseria strains with mutations in pilus assembly genes. For a subset of these mutants, concomitant mutation of the retraction ATPase gene, pilT, restores piliation (4, 5, 38). We therefore introduced an IPTG-regulatable pilT (reg pilT) construct (37) into the FA1090 (RM11.2nv) mpg mutant background and used immunogold transmission EM (immunogold-TEM) to analyze piliation. In the presence of IPTG, strain RM11.2nv mpg reg pilT expressed an average of 0.6 pilus bundle per gonococcal cell (Fig. 8B), a number of bundles similar to that of strain RM11.2nv mpg (Fig. 2D to F). However, in the absence of IPTG, the strain expressed an average of 3.3 pilus bundles per gonococcal cell (Fig. 8A), a number of bundles nearly equivalent to that of strain RM11.2nv (Fig. 2A to C). By analogy with Neisseria pilus mutants having similar pilT-dependent phenotypes, this suggested a role for NGO1686 in pilus biogenesis.
FIG 8 .
Effect of pilT mutation on the RM11.2nv mpg (Δ1686) piliation state and H2O2 resistance. (A and B) Representative immunoelectron micrographs of immunogold-labeled pilus bundles on strain RM11.2nv mpg with regulatable pilT grown on solid medium in the presence (B) or absence (A) of IPTG and lifted onto Formvar-coated grids. (C) H2O2 resistance of the strain in the presence and absence of IPTG. Cells were treated with the indicated doses of H2O2 for 15 min, and the relative survival at each dose was calculated. Error bars represent the standard errors of the means of results from 2 to 8 experiments. *, P > 0.48.
We next measured the H2O2 resistance of the RM11.2nv mpg reg pilT strain in the presence and absence of IPTG. This allowed us to modulate PilT and pilus expression for the purpose of determining whether the pili elaborated in the absence of PilT could mediate resistance to H2O2. The strain showed the same level of H2O2 resistance in the presence and absence of IPTG (Fig. 8C). These results in combination with those obtained with the pilT mutant strongly support the role of active pili in mediating resistance to H2O2.
DISCUSSION
We have determined that the three previously described phenotypes conferred by the PG-targeted metalloprotease NGO1686—colony morphology, resistance to H2O2, and PMN-mediated killing—are all mediated through NGO1686’s effect on piliation. This discovery identifies a novel activity required for gonococcal type IV pilus biogenesis and also reveals two new pathogenesis-related phenotypes for the pilus.
NGO1686 is an M23B metalloprotease that exhibits dual peptidoglycanase activities in vitro. Mutation of several M23B active sites in NGO1686 results in loss of enzymatic activity as well as decreased piliation (23). Although there are many published examples of PG-hydrolyzing enzymes that facilitate the emergence of macromolecular structures on the cell surface (reviewed in reference 39), there is only one PG-hydrolyzing enzyme that has previously been shown to play a role in type IV pilus biogenesis. The PleA protein of Caulobacter crescentus is required for assembly of both the flagellum and pili (40). The enzymatic activity of PleA has not been verified experimentally, but it is predicted to encode a lytic transglycosylase, and mutation of the proposed active site recapitulates the pleA mutant phenotype (40). Whereas lytic transglycosylases cleave the PG sugar backbone, NGO1686 cleaves PG side chains. NGO1686 is the first carboxypeptidase/endopeptidase demonstrated to function in type IV pilus biogenesis. Results from a tBLASTn search of available bacterial entries revealed that all of the genomes containing NGO1686 homologues (defined as having >50% amino acid similarity over >66% of the protein) also contained genes coding for either type IV pili or flagellar-biogenesis proteins. These data suggest that NGO1686 homologues may also have a role in pilus biogenesis in other species.
The exact role that NGO1686 plays in pilus biogenesis is unclear. Piliation of the mpg mutant strain was restored in a pilT mutant background, where PilT-mediated pilus retraction was abolished. Thus, NGO1686 must have a direct or indirect role in preventing pilus retraction. Similar effects have been observed with numerous other Neisseria pilus biogenesis proteins (4, 5, 41, 42). It has been proposed that each of these proteins somehow antagonizes retraction of the pilus, perhaps by forming a prefilament that promotes pilin polymerization (PilC/H-PilL) (4) or by interacting with the pili from neighboring bacteria (PilX) (41), but no mechanism for preventing retraction has been demonstrated. Since NGO1686 has peptidoglycanase activities (23), it is possible that NGO1686 specifically cleaves PG as part of the pilus apparatus assembly process, thus removing a barrier that would otherwise prevent the elaboration of pili on the cell surface. However, if this were the case, we would expect the mpg mutant to express no pili on its cell surface rather than simply to exhibit a large decrease in the numbers of pili. Moreover, if PG were a complete barrier, a Δ1686 mpg pilT mutant would show a growth defect similar to that of a pilQ pilT mutant (43). Therefore, it is unlikely that NGO1686’s role in pilus elaboration is simply through restructuring the cell wall to allow passage of pili. It is feasible that the pili produced in the mpg mutant are using a different pathway through the PG to exit the cell, but we think it is unlikely that this putative alternative pathway for pilus extrusion would allow for full piliation when PilT was inactivated. Therefore, we do not favor the model where the sole role of NGO1686 in piliation is to remove a barrier to assembly; rather, we think that it remodels the PG to allow an antiretraction complex to form.
The most surprising observation made in these studies was that the ability of Gc to elaborate pili is important for conferring resistance to both H2O2- and PMN-mediated killing. This observation is even more surprising given that H2O2 and PMN kill by disparate mechanisms and that PMN-mediated killing is likely to be mediated in part by LL-37. Our data imply that pili do not simply have antioxidant properties that directly detoxify H2O2 associated with them. Our observations that only “active” pili (from pilT-expressing strains) and not exogenously added pili have a role in H2O2 resistance show that inert pili do not function to provide H2O2 resistance. We have confirmed that there is no change in catalase activity or in intracellular iron, magnesium, or manganese levels in either the mpg or ΔpilE mutant (data not shown), suggesting that these molecules are not differentially altered in the mutants. Although it is certainly plausible that there are distinct mechanisms by which piliated Gc maintain resistance to H2O2, PMN, and LL-37, it is possible that there is a common physiological change in piliated N. gonorrhoeae that renders the organism more resistant to killing by both H2O2 and PMNs.
The gonococcal type IV pilus is the linchpin of gonococcal pathogenesis. Several decades of work have catalogued the myriad functions of this organelle, and our current work has revealed two new pilus-dependent phenotypes: resistance to H2O2 and PMN-mediated killing. These findings may alter the conclusion of the role of the pilus in cell interactions if differential survival of the bacteria are not taken into account. The observation that pilus expression is required for resistance may also explain the strong selection for piliated clinical isolates. Most clinical isolates are taken from the purulent exudate that is characteristic of symptomatic gonorrhea. The ability of the pilus to confer resistance to H2O2- and PMN-mediated killing enriches for piliated gonococci in the purulent exudate, where PMNs are in excess. Since pili are required for full colonization and the establishment of disease, the linkage of resistance to piliation provides a unique mechanism for maximizing the infectious potential of this strict human pathogen. This report also raises the possibility that underpiliated or nonpiliated Gc variants have unrealized roles in the pathogenesis of gonorrhea.
MATERIALS AND METHODS
Bacterial strains and media.
All gonococcal strains were derivatives of FA1090 or MS11. Gonococcal strains were grown at 37°C on Gc medium base (GCB; Difco) plus Kellogg supplement I (22.2 mM glucose, 0.68 mM glutamine, 0.45 mM cocarboxylase) and II [1.23 mM Fe(NO3)3] (44) at 37°C in 5% CO2 or in Gc liquid (GCBL) medium (1.5% proteose peptone no. 3 [Difco], 0.4% K2HPO4, 0.1% KH2PO4, 0.1% NaCl). Liquid-grown gonococcal strains additionally contained Kellogg supplement I and II and 0.042% sodium bicarbonate and were grown to exponential phase as previously described (22). Cultures were then grown at 37°C to mid-log phase (optical density at 600 nm [OD600] ≅ 0.5). The following antibiotics (Sigma) were used at the concentrations for Escherichia coli: kanamycin (Kan), 40 µg/ml; erythromycin (Erm), 275 µg/ml; and chloramphenicol (Cam), 25 µg/ml. For Gc, the concentrations were 0.75 µg/ml Erm, 40 µg/ml Kan, and 0.2 µg/ml tetracycline (Tet).
Construction of strains.
All RM11.2 (45) strains with the subscript “nv” (indicating “nonvarying”) carry a Tn3 mutation upstream of the pilE gene that abrogates antigenic pilin variation (32). RM11.2nv mpg was created by transforming RM11.2nv with plasmid construct pBLUNT/1686Erm and selecting for colonies on GCB-Erm using spot transformation (46). The altered colony morphology as well as PCR confirmed replacement with the mutated allele. This strain was subsequently transformed with genomic DNA containing the IPTG-regulatable pilT allele (37) and plated on GCB-Tet medium to select for transformants. Tetr transformants were screened for the characteristic pilT colony morphology and were verified to contain the mutant mpg allele by selection on GCB-Ermr and PCR of the mpg mutant. To create strains MS11 ΔpilE and FA1090 mpg ΔpilE, genomic DNA from strain FA1090 ΔpilE, which was created by transforming genomic DNA from strain RM11.2 recA6 ΔpilE (47), was used to transform strains MS11 and FA1090 mpg. Colonies exhibiting a nonpiliated colony morphology were screened for full deletion of the pilE gene by PCR using primers OPAEJ and PILRBS.
Pilus detection.
Immunoelectron microscopy was performed as described previously (30). Grids were viewed using a JEOL JEM-1220 transmission electron microscope.
Pilus purification.
Pili were purified using the method described in reference 37.
Killing assays.
Gc were grown as described above to mid-log phase, and H2O2 killing assays were performed as described previously (22).
Adherent, interleukin 8 (IL-8)-treated, primary human PMNs were collected from consenting healthy human volunteers, according to a protocol approved by the University of Virginia Institutional Review Board for Health Sciences Research. Bacterial survival after exposure to PMNs was determined from cell lysates at the time points indicated in the figures by colony counting as described in reference 22.
Mid-logarithmic-phase Gc were exposed to increasing concentrations of LL-37 (synthesized by Jan Pohl, Centers for Disease Control and Prevention) dissolved in 0.01% acetic acid as described previously (20) and in 0.1× GCBL with 20 mM HEPES, pH 7.4. Bacterial survival at each concentration was calculated as the CFU enumerated after 45 min divided by the number of CFU present in the input. Results are the averages ± standard errors of the means from 3 to 7 replicates per strain.
ACKNOWLEDGMENTS
We thank Mark Anderson for supplying the FA1090 pilT mutant, Lennell Reynolds and the Northwestern University Cell Imaging Facility for assistance with TEM, Bill Shafer (Emory University) and Jan Pohl (Centers for Disease Control and Prevention) for LL-37, and Matt Parsek for P. aeruginosa strains.
These studies were supported by NIH grants R01 AI044239 and R37 AI033493 to H.S.S. and R00 TW008042 and R01 AI097312 to A.K.C.; E.A.S. was partially supported by grant U19 AI01448.
Footnotes
Citation Stohl EA, Dale EM, Criss AK, Seifert HS. 2013. Neisseria gonorrhoeae metalloprotease NGO1686 is required for full piliation, and piliation is required for resistance to H2O2- and neutrophil-mediated killing. mBio 4(4):e00399-13. doi:10.1128/mBio.00399-13.
REFERENCES
- 1. Swanson J, Robbins K, Barrera O, Corwin D, Boslego J, Ciak J, Blake M, Koomey JM. 1987. Gonococcal pilin variants in experimental gonorrhea. J. Exp. Med. 165:1344–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pelicic V. 2008. Type IV pili: e pluribus unum? Mol. Microbiol. 68:827–837 [DOI] [PubMed] [Google Scholar]
- 3. Varga JJ, Therit B, Melville SB. 2008. Type IV pili and the CcpA protein are needed for maximal biofilm formation by the gram-positive anaerobic pathogen Clostridium perfringens. Infect. Immun. 76:4944–4951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Winther-Larsen HC, Wolfgang M, Dunham S, van Putten JP, Dorward D, Løvold C, Aas FE, Koomey M. 2005. A conserved set of pilin-like molecules controls type IV pilus dynamics and organelle-associated functions in Neisseria gonorrhoeae. Mol. Microbiol. 56:903–917 [DOI] [PubMed] [Google Scholar]
- 5. Carbonnelle E, Helaine S, Nassif X, Pelicic V. 2006. A systematic genetic analysis in Neisseria meningitidis defines the Pil proteins required for assembly, functionality, stabilization and export of type IV pili. Mol. Microbiol. 61:1510–1522 [DOI] [PubMed] [Google Scholar]
- 6. Tønjum T, Koomey M. 1997. The pilus colonization factor of pathogenic neisserial species: organelle biogenesis and structure/function relationships—a review. Gene 192:155–163 [DOI] [PubMed] [Google Scholar]
- 7. Criss AK, Seifert HS. 2012. A bacterial siren song: intimate interactions between Neisseria and neutrophils. Nat. Rev. Microbiol. 10:178–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson MB, Criss AK. 2011. Resistance of Neisseria gonorrhoeae to neutrophils. Front. Microbiol. 2:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soler-García AA, Jerse AE. 2004. A Neisseria gonorrhoeae catalase mutant is more sensitive to hydrogen peroxide and paraquat, an inducer of toxic oxygen radicals. Microb. Pathog. 37:55–63 [DOI] [PubMed] [Google Scholar]
- 10. Tseng HJ, Srikhanta Y, McEwan AG, Jennings MP. 2001. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol. Microbiol. 40:1175–1186 [DOI] [PubMed] [Google Scholar]
- 11. Turner S, Reid E, Smith H, Cole J. 2003. A novel cytochrome c peroxidase from Neisseria gonorrhoeae: a lipoprotein from a gram-negative bacterium. Biochem. J. 373:865–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skaar EP, Tobiason DM, Quick J, Judd RC, Weissbach H, Etienne F, Brot N, Seifert HS. 2002. The outer membrane localization of the Neisseria gonorrhoeae MsrA/B is involved in survival against reactive oxygen species. Proc. Natl. Acad. Sci. U. S. A. 99:10108–10113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stohl EA, Seifert HS. 2006. Neisseria gonorrhoeae DNA recombination and repair enzymes protect against oxidative damage caused by hydrogen peroxide. J. Bacteriol. 188:7645–7651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu H, Soler-García AA, Jerse AE. 2009. A strain-specific catalase mutation and mutation of the metal-binding transporter gene mntC attenuate Neisseria gonorrhoeae in vivo but not by increasing susceptibility to oxidative killing by phagocytes. Infect. Immun. 77:1091–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu HJ, Seib KL, Edwards JL, Apicella MA, McEwan AG, Jennings MP. 2005. Azurin of pathogenic Neisseria spp. is involved in defense against hydrogen peroxide and survival within cervical epithelial cells. Infect. Immun. 73:8444–8448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Criss AK, Katz BZ, Seifert HS. 2009. Resistance of Neisseria gonorrhoeae to non-oxidative killing by adherent human polymorphonuclear leucocytes. Cell. Microbiol. 11:1074–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seib KL, Simons MP, Wu HJ, McEwan AG, Nauseef WM, Apicella MA, Jennings MP. 2005. Investigation of oxidative stress defenses of Neisseria gonorrhoeae by using a human polymorphonuclear leukocyte survival assay. Infect. Immun. 73:5269–5272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levy O. 2004. Antimicrobial proteins and peptides: anti-infective molecules of mammalian leukocytes. J. Leukoc. Biol. 76:909–925 [DOI] [PubMed] [Google Scholar]
- 19. Lee EH, Shafer WM. 1999. The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol. Microbiol. 33:839–845 [DOI] [PubMed] [Google Scholar]
- 20. Shafer WM, Qu X, Waring AJ, Lehrer RI. 1998. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl. Acad. Sci. U. S. A. 95:1829–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jerse AE, Sharma ND, Simms AN, Crow ET, Snyder LA, Shafer WM. 2003. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect. Immun. 71:5576–5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stohl EA, Criss AK, Seifert HS. 2005. The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterized roles in oxidative damage protection. Mol. Microbiol. 58:520–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stohl EA, Chan YA, Hackett KT, Kohler PL, Dillard JP, Seifert HS. 2012. Neisseria gonorrhoeae virulence factor NG1686 is a bifunctional M23B family metallopeptidase that influences resistance to hydrogen peroxide and colony morphology. J. Biol. Chem. 287:11222–11233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boneca IG. 2005. The role of peptidoglycan in pathogenesis. Curr. Opin. Microbiol. 8:46–53 [DOI] [PubMed] [Google Scholar]
- 25. Sycuro LK, Pincus Z, Gutierrez KD, Biboy J, Stern CA, Vollmer W, Salama NR. 2010. Peptidoglycan crosslinking relaxation promotes Helicobacter pylori’s helical shape and stomach colonization. Cell 141:822–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goley ED, Comolli LR, Fero KE, Downing KH, Shapiro L. 2010. DipM links peptidoglycan remodelling to outer membrane organization in Caulobacter. Mol. Microbiol. 77:56–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Möll A, Schlimpert S, Briegel A, Jensen GJ, Thanbichler M. 2010. DipM, a new factor required for peptidoglycan remodelling during cell division in Caulobacter crescentus. Mol. Microbiol. 77:90–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poggio S, Takacs CN, Vollmer W, Jacobs-Wagner C. 2010. A protein critical for cell constriction in the gram-negative bacterium Caulobacter crescentus localizes at the division site through its peptidoglycan-binding LysM domains. Mol. Microbiol. 77:74–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Uehara T, Dinh T, Bernhardt TG. 2009. LytM-domain factors are required for daughter cell separation and rapid ampicillin-induced lysis in Escherichia coli. J. Bacteriol. 191:5094–5107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Long CD, Tobiason DM, Lazio MP, Kline KA, Seifert HS. 2003. Low-level pilin expression allows for substantial DNA transformation competence in Neisseria gonorrhoeae. Infect. Immun. 71:6279–6291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cahoon LA, Seifert HS. 2009. An alternative DNA structure is necessary for pilin antigenic variation in Neisseria gonorrhoeae. Science 325:764–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sechman EV, Rohrer MS, Seifert HS. 2005. A genetic screen identifies genes and sites involved in pilin antigenic variation in Neisseria gonorrhoeae. Mol. Microbiol. 57:468–483 [DOI] [PubMed] [Google Scholar]
- 33. Sparling PF. 1966. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J. Bacteriol. 92:1364–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Long CD, Hayes SF, van Putten JP, Harvey HA, Apicella MA, Seifert HS. 2001. Modulation of gonococcal piliation by regulatable transcription of pilE. J. Bacteriol. 183:1600–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Drake SL, Koomey M. 1995. The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol. Microbiol. 18:975–986 [DOI] [PubMed] [Google Scholar]
- 36. Shrout JD, Chopp DL, Just CL, Hentzer M, Givskov M, Parsek MR. 2006. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 62:1264–1277 [DOI] [PubMed] [Google Scholar]
- 37. Wolfgang M, Lauer P, Park HS, Brossay L, Hébert J, Koomey M. 1998. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol. Microbiol. 29:321–330 [DOI] [PubMed] [Google Scholar]
- 38. Carbonnelle E, Hélaine S, Prouvensier L, Nassif X, Pelicic V. 2005. Type IV pilus biogenesis in Neisseria meningitidis: PilW is involved in a step occurring after pilus assembly, essential for fibre stability and function. Mol. Microbiol. 55:54–64 [DOI] [PubMed] [Google Scholar]
- 39. Scheurwater EM, Burrows LL. 2011. Maintaining network security: how macromolecular structures cross the peptidoglycan layer. FEMS Microbiol. Lett. 318:1–9 [DOI] [PubMed] [Google Scholar]
- 40. Viollier PH, Shapiro L. 2003. A lytic transglycosylase homologue, PleA, is required for the assembly of pili and the flagellum at the Caulobacter crescentus cell pole. Mol. Microbiol. 49:331–345 [DOI] [PubMed] [Google Scholar]
- 41. Helaine S, Dyer DH, Nassif X, Pelicic V, Forest KT. 2007. 3D structure/function analysis of PilX reveals how minor pilins can modulate the virulence properties of type IV pili. Proc. Natl. Acad. Sci. U. S. A. 104:15888–15893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Winther-Larsen HC, Hegge FT, Wolfgang M, Hayes SF, van Putten JP, Koomey M. 2001. Neisseria gonorrhoeae PilV, a type IV pilus-associated protein essential to human epithelial cell adherence. Proc. Natl. Acad. Sci. U. S. A. 98:15276–15281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wolfgang M, van Putten JP, Hayes SF, Dorward D, Koomey M. 2000. Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili. EMBO J. 19:6408–6418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kellogg DS, Jr, Peacock WL, Deacon WE, Brown L, Pirkle CI. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Long CD, Madraswala RN, Seifert HS. 1998. Comparisons between colony phase variation of Neisseria gonorrhoeae FA1090 and pilus, pilin, and S-pilin expression. Infect. Immun. 66:1918–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stein DC. 1991. Transformation of Neisseria gonorrhoeae: physical requirements of the transforming DNA. Can. J. Microbiol. 37:345–349 [DOI] [PubMed] [Google Scholar]
- 47. Chen CJ, Tobiason DM, Thomas CE, Shafer WM, Seifert HS, Sparling PF. 2004. A mutant form of the Neisseria gonorrhoeae pilus secretin protein PilQ allows increased entry of heme and antimicrobial compounds. J. Bacteriol. 186:730–739 [DOI] [PMC free article] [PubMed] [Google Scholar]