Abstract
Four dioxin-inducible enzymes—NAD(P)H:quinone oxidoreductase-1 (NQO1) and three cytochromes P450 (CYP1A1, CYP1A2 & CYP1B1)—are implicated in both detoxication and metabolic activation of various endobiotics and xenobiotics. NQO1 is generally regarded as a cytosolic enzyme; whereas CYP1 proteins are located primarily in endoplasmic reticulum (ER), CYP1A1 and CYP1A2 proteins are also targeted to mitochondria. This lab has generated Cyp1a1(mc/mc) and Cyp1a1(mtt/mtt) knock-in mouse lines in which CYP1A1 protein is targeted exclusively to ER (microsomes) and mitochondria, respectively. Comparing dioxin-treated Cyp1(+/+) wild-type, Cyp1a1(mc/mc), Cyp1a1(mtt/mtt), and Cyp1a1(−/−), Cyp1b1(−/−) and Nqo1(−/−) knockout mice, in the present study we show that [a] NQO1 protein locates to cytosol, ER and mitochondria, [b] CYP1B1 protein (similar to CYP1A1 and CYP1A2 proteins) traffics to mitochondria as well as ER, and [c] NQO1 and CYP1B1 targeting to mitochondrial or ER membranes is independent of CYP1A1 presence in that membrane.
Keywords: NAD(P)H:quinone oxidoreductase-1 (NQO1); Cytochrome P450 1B1 (CYP1B1); CYP1A1; subcellular localization; mitochondrial targeting; endoplasmic reticulum targeting; dioxin-inducible enzymes; mouse tissues (lung, spleen, uterus, small intestine, liver, kidney)
Membrane-bound cytochrome P450 (CYP) monooxygenases catalyzing the oxygenation of innumerable endogenous compounds and foreign chemicals; there are 103 functional protein-coding Cyp genes in the mouse genome, and 57 CYP genes in the human genome [23;24]. Members of the CYP1, CYP2, CYP3 and CYP4 families are largely involved in metabolism of drugs and environmental pollutants [20;21;28], although lipid mediators including eicosanoids [22] and many other endogenous compounds [23] are also substrates.
The mammalian CYP1 family has three members—CYP1A1, CYP1A2 and CYP1B1—which are highly conserved between mouse and human. CYP1A1 and CYP1B1 are best known for polycyclic aryl hydrocarbon (PAH) metabolism, whereas CYP1A2 preferentially metabolizes arylamines [21]. All three monooxygenases are known to transform numerous environmental chemicals into reactive intermediates that can cause genotoxicity, mutagenesis, and oxidative stress—associated in laboratory animals with increased risk of toxicity, birth defects, mutagenesis and cancer.
Cyp1 knockout mouse studies have confirmed the relevance of CYP1-mediated metabolic activation of PAHs such as benzo[a]pyrene and 7,12-dimethylbenzo[a]anthracene and arylamines such as 4-aminobiphenyl and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP, a food mutagen) in causing toxicity or tumorigenesis. Toxic or carcinogenic effects depend on the CYP1 enzyme present, route-of-administration, dose and rate of exposure, and the target organ being studied [7;13;17;18;35;36;38;40]. Similarly, these knockout mouse lines have helped us understand the importance of CYP1-mediated detoxication of topical 4-aminobiphenyl [38–40] and oral benzo[a]pyrene [38–40].
NAD(P)H:quinone oxidoreductase-1 (NQO1) is historically regarded as a cytosolic flavoenzyme. NQO1 catalyzes the obligatory 2-electron reduction of toxic quinones to hydroquinones, thereby detoxifying reactive intermediates which can be generated via 1-electron reduction “recycling” pathways {19, 8}. Quinones can be toxic as electrophiles, and may also undergo 1-electron reduction via redox cycling to generate semiquinones that cause oxidative stress due to formation of reactive oxygen species (ROS) [16].
CYP1 proteins were historically regarded as located only in the endoplasmic reticulum (ER). Over the past two decades, however, studies by the Avadhani lab have convincingly demonstrated that CYP1A1 protein is partially targeted to mitochondrial (MT) inner membrane; MT- vs ER-targeting is determined by the NH2-terminal protein sequence [2;5]. Using Cyp1a1(−/−) and Cyp1a2(−/−) knockout mouse lines, we confirmed the unequivocal presence of not only CYP1A1 but also CYP1A2 protein in MT [33]. Recently, we generated Cyp1a1 knock-in mouse lines in which the CYP1A1 protein is exclusively targeted to either ER or MT [10].
Examining NH2-terminal sequences of CYP1B1 and NQO1, we posited that these two redox enzymes might also be trafficked to MT. Comparing Cyp1b1(−/−) and Nqo1(−/−) knockout mice with our previously generated ER-specific- and MT-specific CYP1A1 lines, plus Cyp1a1(−/−) knockout and Cyp1(+/+) wild-type (WT) mice as controls, we set out to prove unequivocally the existence of mitochondrial CYP1B1 and NQO1 protein.
MATERIALS AND METHODS
Chemicals
TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin; also called “dioxin”) was purchased from Accustandard, Inc. (New Haven, CT). Anti-CYP1A1/1A2 polyclonal antibody (α-1A1/1A2) was bought from BD Gentest (Woburn, MA). Anti-CYP1B1 polyclonal antibody (α-1B1) was generated in chicken against the mouse protein [25]. Anti-NQO1 polyclonal antibody (α-NQO1) was made in rabbits against the rat protein [29]. Anti-P450 oxidoreductase (α-POR) and anti-prohibitin (α-PHB) were purchased from Abcam (Cambridge, MA). Antibody to mouse γ-glutamylcysteine ligase modifier subunit (α-GCLM) was generated in chicken [8].
Animals
The Cyp1a1(mc/mc) mouse line, hereafter referred to as mc1A1, contains CYP1A1 protein exclusively in ER and not MT; the Cyp1a1(mtt/mtt) mouse line, hereafter referred to as mtt1A1, contains CYP1A1 protein exclusively in mitochondria and not ER —due to truncation and thus loss of the ER-trafficking signal [10]. Both genotypes have been backcrossed into C57BL/6J (B6) for at least eight generations—to ensure that the knock-in genotype resides in >99.8% B6 genetic background. Age-matched B6 mice, purchased from The Jackson Laboratory (Bar Harbor, ME), could therefore be used as Cyp1(+/+) WT controls. Cyp1a1(−/−) [9], Cyp1b1(−/−) [7], and Nqo1(−/−) [26] knockout mouse lines, hereafter referred to as 1A1 KO, 1B1 KO and NQO1 KO, have previously been described. All animal experiments were approved by, and conducted in accordance with, the National Institutes of Health standards for care and use of experimental animals and the University Cincinnati Medical Center Institutional Animal Care and Use Committee.
Treatment
TCDD (5 µg/ml) was dissolved in corn oil; untreated controls received corn oil only; this inducer was used to amplify the amount of NQO1 and CYP1 proteins located in various subcellular fractions. Female mice (age 6–8 weeks) were administered TCDD (15 µg/kg i.p. for 3 consecutive days), which is known to accumulate maximal amounts of CYP1A1 protein in MT [14]. On the 11th day after initiating TCDD treatment, we euthanized the mice with CO2 asphyxiation and collected lung, spleen, uterus, proximal small intestine (PSI), liver and kidney. PSI includes 5 cm of intestine beyond the pyloric sphincter, thus containing duodenum and a portion of jejunum.
Reverse transcription, Quantitative real-time PCR, Western immunoblotting analysis, and treating TCDD with precaution as a Biohazard
These sections are included in our Supplemental Data file online.
Statistical analyses
Statistics were performed using SigmaStat Statistical Analysis software (SPSS Inc.; Chicago, IL). Group means were compared by one-way analysis of variance, followed by Student’s t test for pair-wise comparison of means. All data were normally distributed and reported as the means ± S.E.M.; for qRT-PCR we used confidence intervals and population means to calculate fold-induction of TCDD-induced vs untreated WT mRNA. P-values of <0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Continuity of ER with the outer membrane of mitochondria [12;32] makes it extremely difficult by differential centrifugation to separate microsomal and MT fractions without some degree of contamination of one organelle by the other. This is especially true with ER-rich liver tissue; hence, we preferred to use nonhepatic tissues for better separations of microsomal and MT fractions [10]. Another reason for not studying liver when characterizing the subcellular localization of CYP1B1 protein is that TCDD-inducible CYP1B1 is negligible in liver, compared with ~100-times-greater levels of TCDD-inducible CYP1A1 [19].
Subcellular localization of CYP1A1 protein
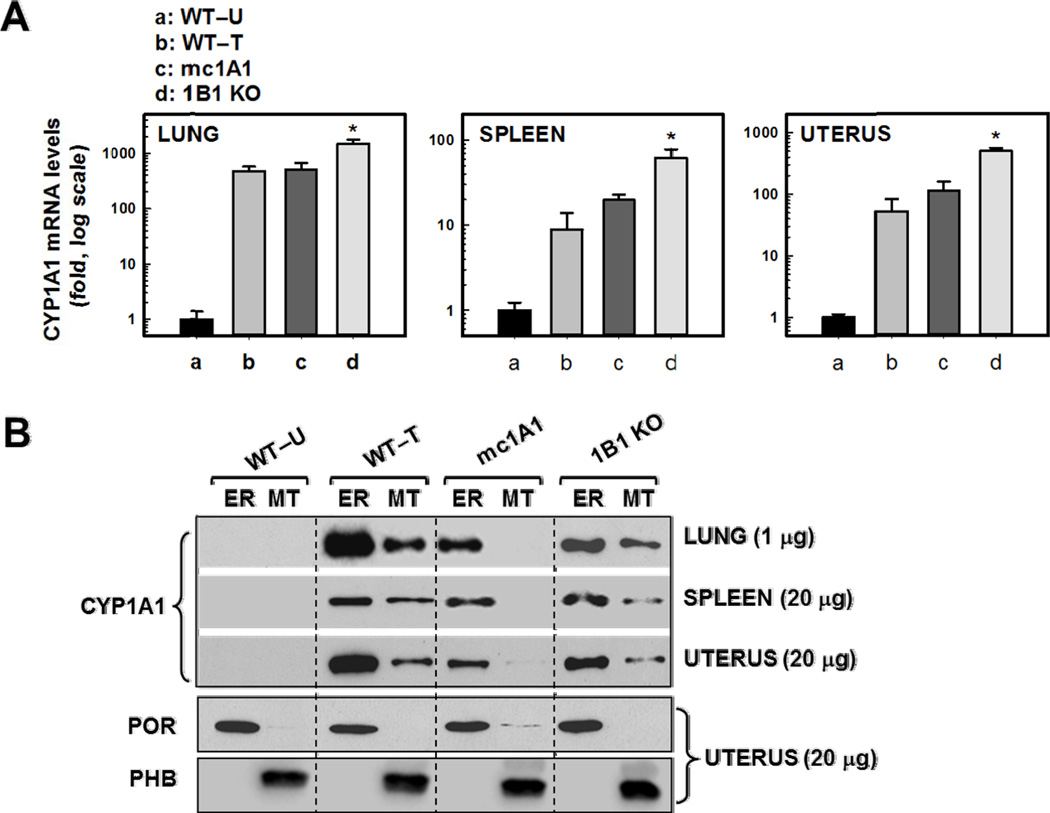
Compared with untreated Cyp1(+/+) WT mice (Fig. 1A), CYP1A1 mRNA levels were highly induced in lung, spleen and uterus of TCDD-treated Cyp1(+/+) wild-type, mc1A1 and 1B1 KO mice; CYP1A1 mRNA was absent in 1A1 KO mice. Whereas no CYP1A1 protein was detectable in ER or MT fractions of untreated wild-type mice (Fig. 1B), CYP1A1 protein (59,230 Da) was more abundant in ER than MT of lung, spleen and uterus from TCDD-treated WT and 1B1 KO mice but undetectable in 1A1 KO mice. Presence of TCDD-inducible CYP1A1 in ER, but none in MT in mc1A1 mice, was previously demonstrated in lung, kidney and PSI [10]; here we show the same results for lung, spleen and uterus. Inclusion of the microsomal marker POR and mitochondrial marker PHB (Fig. 1B, bottom) illustrates the lack of significant cross-contamination of ER with MT.
FIG. 1.
CYP1A1 mRNA and protein levels in lung, spleen and uterus from WT, mc1A1 and CYP1B1 KO mice. A, For qRT-PCR, untreated wild-type values were set at 1.0 (100), and TCDD-induced (fold increases in mRNA) are shown on a log scale. Data are reported as means ± S.E.M. (N = 4 mice per group). *P <0.05, when compared with mRNA levels in TCDD-treated wild-type mice. ND, non-detectable. B, Western immunoblots of microsomal (endoplasmic reticulum; ER) and mitochondrial (MT) fractions from lung, spleen and uterus; α-1A1/1A2 was the antibody used and lanes were loaded with 1 µg protein for lung and 20 µg protein for spleen and uterus. NADPH-P450-oxidoreductase (POR) is marker for ER fraction, and prohibitin (PHB) a marker for MT fraction (20 µg per lane); a representative blotting image of uterus is shown.
Subcellular localization of CYP1B1 protein
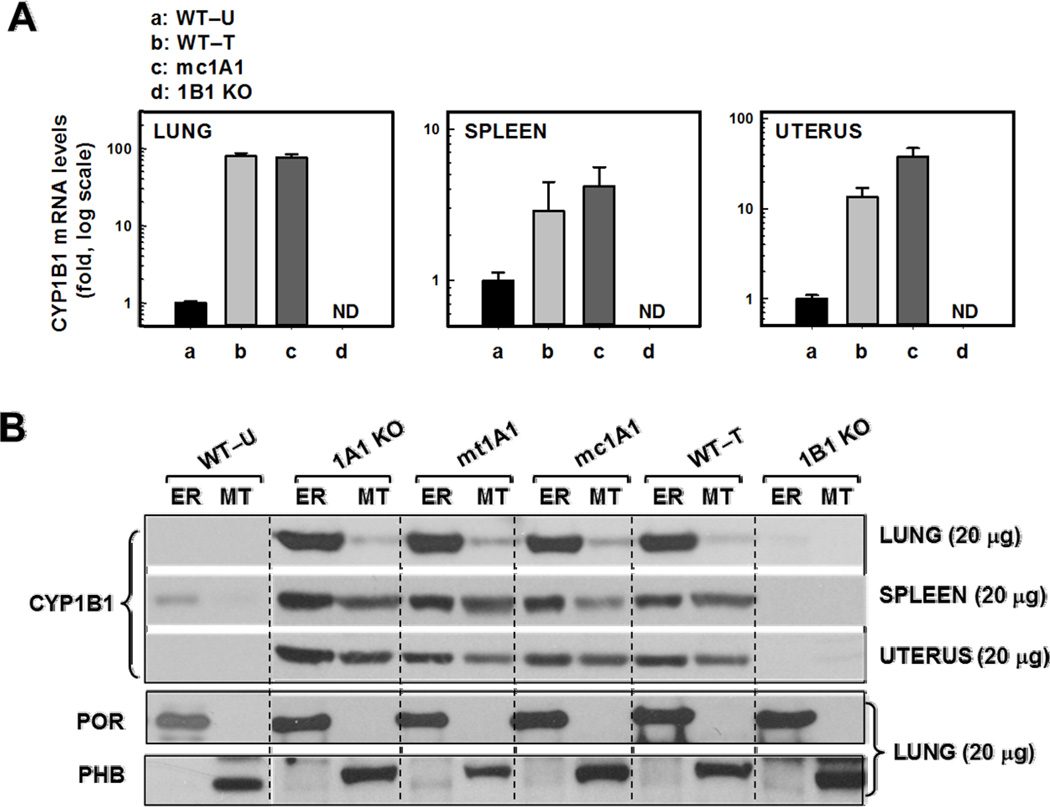
Compared with untreated WT (Fig. 2A), CYP1B1 mRNA levels were highly induced in lung (~70-fold), spleen (~3-fold) and uterus (~30-fold) of TCDD-treated WT and mc1A1 mice. CYP1B1 mRNA levels were even 2- to 3-fold greater in 1A1 KO than in WT mice; this (presumptive compensatory) increase has previously been noted [40;41]. CYP1B1 mRNA was absent in Cyp1b1(−/−) mice.
FIG. 2.
CYP1B1 mRNA (A) and protein (B) levels in lung, spleen and uterus from the same genotypes shown in Figure 1, plus CYP1A1 KO and mtt1A1 mice. All parameters are same as in Figure 1. The α-1B1 antibody was used; lanes were loaded with 20 µg protein for each of the three tissues. Representative blotting image of the lung (also 20 µg protein), probed for ER and MT marker proteins, is shown
No CYP1B1 protein was detectable in microsomal or mitochondrial fractions of untreated WT (Fig. 2B). However, inducible CYP1B1 protein (60,581 Da) was more abundant in ER than MT in lung, spleen and uterus of TCDD-treated 1A1 KO, mt1A1, mc1A1, and WT mice. CYP1B1 protein was undetectable in 1B1 KO mice.
Because the absence of CYP1A1 causes compensatory increases in CYP1B1 mRNA and protein levels [40;41], we wondered if lack of CYP1A1 in either ER or MT would affect CYP1B1 trafficking. Fig. 2B confirms this is not the case. CYP1B1 protein targeting to ER or MT is independent of presence of CYP1A1 in either membrane.
Subcellular localization of NQO1 protein
The NQO1 enzyme (originally called DPN/TPN [DT] diaphorase IV [34]) has generally been regarded as cytosolic [11]. In human cancer cells NQO1 was reported to be largely cytosolic and also present in the nuclear fraction—but not in MT, Golgi or ER [43]. The unequivocal experiment to prove where NQO1 might be localized in subcellular organelles would be to compare WT with NQO1 KO mice.
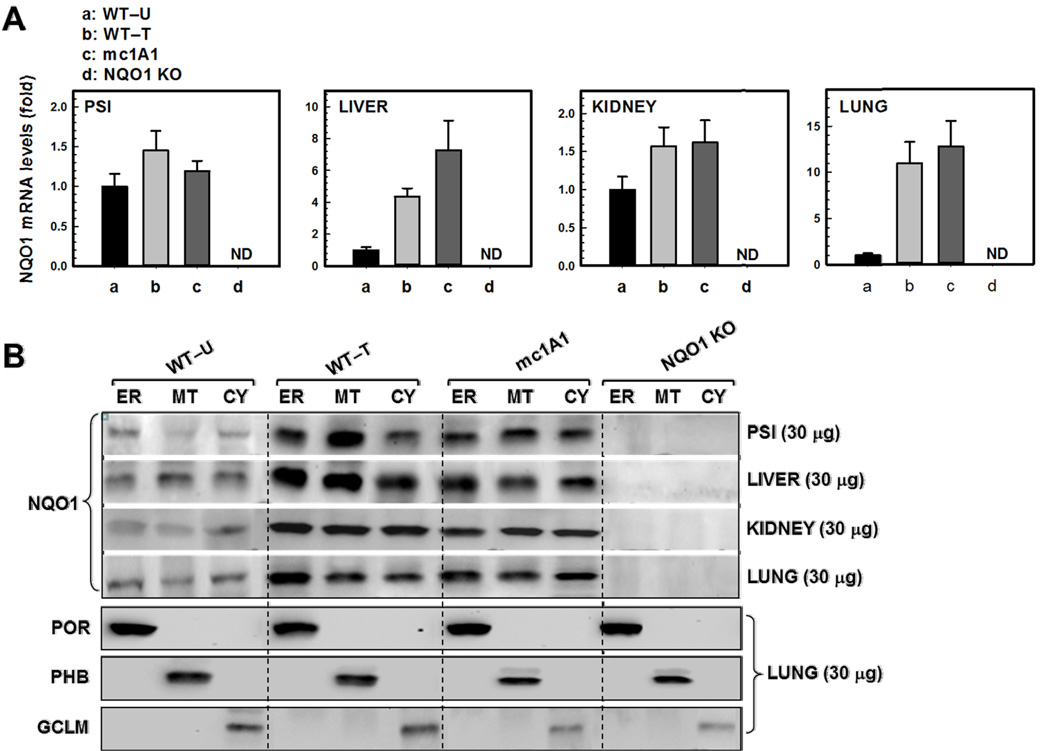
Compared with untreated WT (Fig. 3A), NQO1 mRNA levels were induced 4- to 8-fold in liver and lung but <2-fold in PSI and kidney of TCDD-treated WT and mc1A1 mice. NQO1 mRNA was absent in all four tissues of NQO1 KO mice.
FIG. 3.
NQO1 mRNA (A) and protein (B) levels in proximal small intestine (PSI), liver, kidney and lung from the same genotypes shown in Figure 1, plus NQO1 KO mice. All parameters are same as in Figure 1. The α-NQO1 antibody was used and all lanes were loaded with 30 µg protein for each of the four tissues. A representative blotting image of lung (30 µg protein)—probed for ER (POR), MT (PHB), and cytosol (GCLM) marker proteins—is shown. CY, cytosol.
Constitutive as well as TCDD-inducible NQO1 protein (30,960 Da) was detectable in microsomal, mitochondrial and cytosolic fractions of all mouse lines except NQO1 KO mice (Fig. 3B). Interestingly, NQO1 protein appeared to be at least as abundant in the ER and MT fractions, as in cytosol of PSI, liver, kidney and lung. NQO1 protein levels in all three subcellular fractions appeared to be slightly greater in PSI and liver, compared with that in kidney and lung. The degree to which each antibody (α-NQO1, α-POR, α-PHB, & α-GCLM) recognizes its matching mouse protein, plus possible subtle differences in Western blot lane-loading, makes it difficult to be any more quantitative than what we have stated; in fact, liver and lung NQO1 mRNA levels are considerably higher than PSI or kidney NQO1 mRNA levels. However, our findings (Fig. 3B) show that—similar to the CYP1B1 protein—NQO1 protein is targeted to MT regardless of the presence or absence of mitochondrial CYP1A1 protein.
Posttranslational modification and targeting of proteins
In eukaryotic cells, proteins are transported to their designated subcellular compartments via various targeting mechanisms—usually involving specific amino-acid stretches. ER-targeted proteins in general contain a hydrophobic domain at the NH2-terminus, which is involved in a signal-recognition-particle (SRP)-mediated co-translational mechanism [27], whereas nuclear localization sequences function critically in delivering proteins to the nucleus [37]. MT-targeting sequences also participate in translocation of nuclear genome-encoded proteins onto or across the mitochondrial membrane [15].
More recently it has become appreciated that certain proteins can be imported into MT from their “historical” subcellular locations—such as ER, plasma membrane or cytosol—and these MT-targeted proteins exhibit distinctly different functions from their historical counterparts. For example, plasma membrane-bound amyloid precursor protein can undergo MT-targeting via its NH2-terminal chimeric signal sequence, leading to mitochondrial dysfunction and impaired energy metabolism [4]. Also, phosphorylation of a COOH-terminus Ser and/or Thr can cause cytosolic glutathione S-transferase A4-4 to undergo MT-targeting, and the mitochondrial enzyme shows different substrate specificities [31]. In addition, multiple CYP monooxygenases that historically had been regarded as microsomal proteins have now been shown to undergo MT-targeting; these include CYP1A1 [5;33], CYP1A2 [33], CYP2B1 and/or CYP2B2 [1;3], CYP2E1 [1;30], and CYP3A1 and/or CYP3A2 [1]. CYP1A1, CYP2B1, and CYP2E1 have specifically been shown to carry cryptic NH2-terminal MT-targeting signal sequences that are activated by a cytosolic protease or phosphorylation [1;1;3;5;30;33]. The MT-targeting mechanisms of the other above-mentioned proteins remain to be determined. To this list we can unequivocally now add CYP1B1 and NQO1.
NH2-terminal analysis
For ER- vs MT-targeting of mouse CYP1A1 protein (Fig. 4), the amino-acid stretch from 1 to 30 supplies hydrophobic signals for ER membrane insertion and stop transfer; amino acids 33 to 43 provide MT-targeting signals [5]. Leu-7 and Leu-17 in mouse CYP1A1 are critical for SRP-binding (and thus ER-targeting), whereas positively-charged Arg-34 and Lys-39 residues (blue R, K in boxed region of Fig. 4) function critically for MT-targeting. It is likely that Leu-9 and Leu-14 in mouse CYP1A2 (not shown), and Leu-13 and Leu-24 in mouse CYP1B1, participate in ER-targeting. Between the CYP1B1 NH2-terminal hydrophobic stretches and the proline-rich region, the interspersed positively-charged residues Arg-41, Arg-45 and Lys-46 (blue R, K in boxed region of Fig. 4) perhaps participate in MT-targeting of CYP1B1 (Fig. 4).
FIG. 4.

NH2-terminal sixty amino-acid sequences for CYP1A1, CYP1B1 and NQO1 proteins. The boxed areas denote the putative mitochondrial-targeting sequence (MTS)—which in CYP1 but not NQO1 protein immediately precedes the Pro-Pro residues and start of a proline-rich domain (underlined), as discussed in the text. Blue and red stand for positive- and negatively-charged amino acids, respectively.
The NQO1 protein is not evolutionarily related to CYP1 proteins (Fig. 4), and posttranslational mechanisms by which NQO1 protein undergoes cytosol vs ER-targeting vs MT-targeting remain to be determined. Interestingly, considerable quantities of NQO1 protein were found not only in cytosol and MT, but also in microsomal fractions (Fig. 3B). NQO1 does not contain any recognizable ER-targeting signal in its NH2-terminus, whereas the NH2-terminal sequence of hydrophobic residues interspersed with positively charged residues Arg-4 & -5; Lys-15, 23, 31 & 32; Arg-33 (blue R, K in boxed region of Fig. 4) likely participates in MT-targeting. It had been reported in rat [6] that predominantly cytosolic NQO1 protein was detected in small amounts in mitochondria (13%), microsomes (2%), and Golgi (1%). In the present study, however, NQO1 protein was detected at comparable levels in ER, MT and cytosolic fractions (Fig. 3B).
Mitochondrial-mediated ROS formation and redox cycling
If ample amounts of NQO1 are present in all subcellular organelles including the nucleus, what benefit to the cell would this be? NQO1 catalyzes the 2-electron reduction of quinones to hydroquinones, thus preventing ROS production generated from redox cycling of semiquinones formed via 1-electron reduction. Quinones comprise a large class of aromatic compounds—found endogenously in all organisms as flavonoids, electron-carrying coenzymes, and metabolic end-products of oxidation—but also found exogenously as plant metabolites and in the dye industry, hide-tanning, and photography {8}. Because ROS formation causes oxidative stress that can be very destructive to any subcellular organelle, presence of NQO1 would help prevent that; further, no organelle is more important to protect from oxidative disruption than MT [16].
If ample amounts of all three CYP1 enzymes also exist in mitochondria as well as ER, what might be their benefits to the cell? Substrate-specificity differences between the mc1A1 and mt1A1 enzymes have been reported [3]. Endogenous functions of MT vs ER CYP1 enzymes are not yet known and will require further study.
Supplementary Material
HIGHLIGHTS.
Dioxin-treated Cyp1(+/+) wild-type, Cyp1a1(mc/mc), Cyp1a1(mtt/mtt), and Cyp1a1(−/−), Cyp1b1(−/−) and Nqo1(−/−) knockout mice were compared
Analysis included both mRNA and protein quantification of CYP1A1, CYP1B1 and NQO1
NQO1 protein locates to cytosol, ER and mitochondria
CYP1B1 protein (similar to CYP1A1 and CYP1A2 proteins) traffics to mitochondria as well as ER
NQO1 and CYP1B1 targeting to mitochondrial or ER membranes is independent of CYP1A1 presence in that membrane
Acknowledgments
We thank our colleagues, especially Narayan Avadhani for fruitful discussions, and careful readings of this manuscript. We thank Bin Wang for excellent technical assistance, and Lucia F Jorge-Nebert for precise genotyping of all mouse lines. Supported, in part, by NIH Grants R01 ES08147 (D.W.N.), R01 ES014403 (D.W.N.), and Center for Environmental Genetics P30 ES06096 (H.G.S., M.B.G., D.W.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All coauthors declare no conflicts of interest.
REFERENCES
- 1.Anandatheerthavarada HK, Addya S, Dwivedi RS, Biswas G, Mullick J, Avadhani NG. Localization of multiple forms of inducible cytochromes P450 in rat liver mitochondria: immunological characteristics and patterns of xenobiotic substrate metabolism. Arch Biochem Biophys. 1997;339:136–150. doi: 10.1006/abbi.1996.9855. [DOI] [PubMed] [Google Scholar]
- 2.Anandatheerthavarada HK, Addya S, Mullick J, Avadhani NG. Interaction of adrenodoxin with P450 1A1 and its truncated form P450MT2 through different domains: differential modulation of enzyme activities. Biochemistry. 1998;37:1150–1160. doi: 10.1021/bi972046j. [DOI] [PubMed] [Google Scholar]
- 3.Anandatheerthavarada HK, Biswas G, Mullick J, Sepuri NB, Otvos L, Pain D, Avadhani NG. Dual targeting of cytochrome P450 2B1 to endoplasmic reticulum and mitochondria involves a novel signal activation by cyclic AMP-dependent phosphorylation at Ser-128. EMBO J. 1999;18:5494–5504. doi: 10.1093/emboj/18.20.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161:41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhagwat SV, Biswas G, Anandatheerthavarada HK, Addya S, Pandak W, Avadhani NG. Dual targeting property of the N-terminal signal sequence of P450 1A1. Targeting of heterologous proteins to endoplasmic reticulum and mitochondria. J Biol Chem. 1999;274:24014–24022. doi: 10.1074/jbc.274.34.24014. [DOI] [PubMed] [Google Scholar]
- 6.Bianchet MA, Faig M, Amzel LM. Structure and mechanism of NAD(P)H:quinone acceptor oxidoreductases (NQOs) Methods Enzymol. 2004;382:144–174. doi: 10.1016/S0076-6879(04)82009-3. [DOI] [PubMed] [Google Scholar]
- 7.Buters JT, Sakai S, Richter T, Pineau T, Alexander DL, Savas U, Doehmer J, Ward JM, Jefcoate CR, Gonzalez FJ. Cytochrome P450 CYP1B1 determines susceptibility to 7,12-dimethylbenzo[a]anthracene-induced lymphomas. Proc Natl Acad Sci U S A. 1999;96:1977–1982. doi: 10.1073/pnas.96.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Yang Y, Miller ML, Shen D, Shertzer HG, Stringer KF, Wang B, Schneider SN, Nebert DW, Dalton TP. Hepatocyte-specific Gclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure. Hepatology. 2007;45:1118–1128. doi: 10.1002/hep.21635. [DOI] [PubMed] [Google Scholar]
- 9.Dalton TP, Dieter MZ, Matlib RS, Childs NL, Shertzer HG, Genter MB, Nebert DW. Targeted knockout of Cyp1a1 gene does not alter hepatic constitutive expression of other genes in the mouse [Ah] battery. Biochem Biophys Res Commun. 2000;267:184–189. doi: 10.1006/bbrc.1999.1913. [DOI] [PubMed] [Google Scholar]
- 10.Dong H, Dalton TP, Miller ML, Chen Y, Uno S, Shi Z, Shertzer HG, Bansal S, Avadhani NG, Nebert DW. Knock-in mouse lines expressing either mitochondrial or microsomal CYP1A1: differing responses to dietary benzo[a]pyrene as proof of principle. Mol Pharmacol. 2009;75:555–567. doi: 10.1124/mol.108.051888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernster L, Danielson L, Ljunggren M. DT diaphorase. I. Purification from the soluble fraction of rat-liver cytoplasm, and properties. Biochim Biophys Acta. 1962;58:171–188. doi: 10.1016/0006-3002(62)90997-6. [DOI] [PubMed] [Google Scholar]
- 12.Franke WW, Kartenbeck J. Outer mitochondrial membrane continuous with endoplasmic reticulum. Protoplasma. 1971;73:35–41. doi: 10.1007/BF01286409. [DOI] [PubMed] [Google Scholar]
- 13.Gálvez-Peralta M, Shi Z, Chen J, Miller ML, Nebert DW. Oral benzo[a]pyrene in Cyp1a1/1b1(−/−) double-knockout mice: Microarray analysis during squamous cell carcinoma formation in preputial gland duct. Intl J Cancer. 2013;132:2065–2075. doi: 10.1002/ijc.27897. [DOI] [PubMed] [Google Scholar]
- 14.Genter MB, Clay CD, Dalton TP, Dong H, Nebert DW, Shertzer HG. Comparison of mouse hepatic mitochondrial versus microsomal cytochromes P450 following TCDD treatment. Biochem Biophys Res Commun. 2006;342:1375–1381. doi: 10.1016/j.bbrc.2006.02.121. [DOI] [PubMed] [Google Scholar]
- 15.Hartl FU, Pfanner N, Nicholson DW, Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989;988:1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- 16.Jones DP. Disruption of mitochondrial redox circuitry in oxidative stress. Chem Biol Interact. 2006;163:38–53. doi: 10.1016/j.cbi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Kimura S, Kawabe M, Ward JM, Morishima H, Kadlubar FF, Hammons GJ, Fernandez-Salguero P, Gonzalez FJ. CYP1A2 is not the primary enzyme responsible for 4-aminobiphenyl-induced hepatocarcinogenesis in mice. Carcinogenesis. 1999;20:1825–1830. doi: 10.1093/carcin/20.9.1825. [DOI] [PubMed] [Google Scholar]
- 18.Ma X, Idle JR, Malfatti MA, Krausz KW, Nebert DW, Chen CS, Felton JS, Waxman DJ, Gonzalez FJ. Mouse lung CYP1A1 catalyzes the metabolic activation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Carcinogenesis. 2007;28:732–737. doi: 10.1093/carcin/bgl184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray GI, Melvin WT, Greenlee WF, Burke MD. Regulation, function, and tissue-specific expression of cytochrome P450 CYP1B1. Annu Rev Pharmacol Toxicol. 2001;41:297–316. doi: 10.1146/annurev.pharmtox.41.1.297. [DOI] [PubMed] [Google Scholar]
- 20.Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- 21.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 22.Nebert DW, Karp CL. Endogenous functions of the aryl hydrocarbon receptor (AHR): intersection of cytochrome P450 1 (CYP1)-metabolized eicosanoids and AHR biology. J Biol Chem. 2008;283:36061–36065. doi: 10.1074/jbc.R800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nebert DW, Wikvall K, Miller WL. Human cytochromes P450 in health and disease. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120431. doi: 10.1098/rstb.2012.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 2004;14:1–18. doi: 10.1097/00008571-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Pottenger LH, Christou M, Jefcoate CR. Purification and immunological characterization of a novel cytochrome P450 from C3H/10T1/2 cells. Arch Biochem Biophys. 1991;286:488–497. doi: 10.1016/0003-9861(91)90070-y. [DOI] [PubMed] [Google Scholar]
- 26.Radjendirane V, Joseph P, Lee YH, Kimura S, Klein-Szanto AJ, Gonzalez FJ, Jaiswal AK. Disruption of the DT diaphorase gene (Nqo1) in mice leads to increased menadione toxicity. J Biol Chem. 1998;273:7382–7389. doi: 10.1074/jbc.273.13.7382. [DOI] [PubMed] [Google Scholar]
- 27.Rapoport TA. Protein translocation across and integration into membranes. CRC Crit Rev Biochem. 1986;20:73–137. doi: 10.3109/10409238609115901. [DOI] [PubMed] [Google Scholar]
- 28.Rendic S, Guengerich FP. Summary of information on the effects of diseases and environmental factors on human cytochrome P450 (CYP) enzymes and transporters. Curr Drug Metab. 2010;11:4–84. doi: 10.2174/138920010791110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson JA, Chen HC, Nebert DW. NAD(P)H:menadione oxidoreductase. Novel purification of enzyme, cDNA and complete amino acid sequence, and gene regulation. J Biol Chem. 1986;261:15794–15799. [PubMed] [Google Scholar]
- 30.Robin MA, Anandatheerthavarada HK, Biswas G, Sepuri NB, Gordon DM, Pain D, Avadhani NG. Bimodal targeting of microsomal CYP2E1 to mitochondria through activation of an N-terminal chimeric signal by cAMP-mediated phosphorylation. J Biol Chem. 2002;277:40583–40593. doi: 10.1074/jbc.M203292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robin MA, Prabu SK, Raza H, Anandatheerthavarada HK, Avadhani NG. Phosphorylation enhances mitochondrial targeting of GSTA4-4 through increased affinity for binding to cytoplasmic Hsp70. J Biol Chem. 2003;278:18960–18970. doi: 10.1074/jbc.M301807200. [DOI] [PubMed] [Google Scholar]
- 32.Ruby JR, Dyer RF, Skalko RG. Continuities between mitochondria and endoplasmic reticulum in the mammalian ovary. Z Zellforsch Mikrosk Anat. 1969;97:30–37. doi: 10.1007/BF00331868. [DOI] [PubMed] [Google Scholar]
- 33.Senft AP, Dalton TP, Nebert DW, Genter MB, Puga A, Hutchinson RJ, Kerzee JK, Uno S, Shertzer HG. Mitochondrial reactive oxygen production is dependent on the aromatic hydrocarbon receptor. Free Radic Biol Med. 2002;33:1268–1278. doi: 10.1016/s0891-5849(02)01014-6. [DOI] [PubMed] [Google Scholar]
- 34.Shaw PM, Reiss A, Adesnik M, Nebert DW, Schembri J, Jaiswal AK. The human dioxin-inducible NAD(P)H:quinone oxidoreductase cDNA-encoded protein expressed in COS-1 cells is identical to diaphorase-4. Eur.J Biochem. 1991;195:171–176. doi: 10.1111/j.1432-1033.1991.tb15691.x. [DOI] [PubMed] [Google Scholar]
- 35.Shi Z, Dragin N, Galvez-Peralta M, Jorge-Nebert LF, Miller ML, Wang B, Nebert DW. Organ-specific roles of CYP1A1 during detoxication of dietary benzo[a]pyrene. Mol Pharmacol. 2010;78:46–57. doi: 10.1124/mol.110.063438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Z, Dragin N, Miller ML, Stringer KF, Johansson E, Chen J, Uno S, Gonzalez FJ, Rubio CA, Nebert DW. Oral benzo[a]pyrene-induced cancer: two distinct types in different target organs depend on the mouse Cyp1 genotype. Intl J Cancer. 2010;127:2334–2350. doi: 10.1002/ijc.25222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silver P, Goodson H. Nuclear protein transport. Crit Rev Biochem Mol Biol. 1989;24:419–435. doi: 10.3109/10409238909082557. [DOI] [PubMed] [Google Scholar]
- 38.Tsuneoka Y, Dalton TP, Miller ML, Clay CD, Shertzer HG, Talaska G, Medvedovic M, Nebert DW. 4-Aminobiphenyl-induced liver and urinary bladder DNA adduct formation in Cyp1a2(−/−) and Cyp1a2(+/+) mice. J Natl Cancer Inst. 2003;95:1227–1237. doi: 10.1093/jnci/djg025. [DOI] [PubMed] [Google Scholar]
- 39.Uno S, Dalton TP, Derkenne S, Curran CP, Miller ML, Shertzer HG, Nebert DW. Oral exposure to benzo[a]pyrene in the mouse: detoxication by inducible cytochrome P450 is more important than metabolic activation. Mol Pharmacol. 2004;65:1225–1237. doi: 10.1124/mol.65.5.1225. [DOI] [PubMed] [Google Scholar]
- 40.Uno S, Dalton TP, Dragin N, Curran CP, Derkenne S, Miller ML, Shertzer HG, Gonzalez FJ, Nebert DW. Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Mol Pharmacol. 2006;69:1103–1114. doi: 10.1124/mol.105.021501. [DOI] [PubMed] [Google Scholar]
- 41.Uno S, Dragin N, Miller ML, Dalton TP, Gonzalez FJ, Nebert DW. Basal and inducible CYP1 mRNA quantitation and protein localization throughout the mouse gastrointestinal tract. Free Radic Biol Med. 2008;44:570–583. doi: 10.1016/j.freeradbiomed.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vasiliou V, Ross D, Nebert DW. Update of the NAD(P)H:quinone oxidoreductase (NQO) gene family. Hum Genomics. 2006;2:329–335. doi: 10.1186/1479-7364-2-5-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winski SL, Koutalos Y, Bentley DL, Ross D. Subcellular localization of NAD(P)H:quinone oxidoreductase-1 in human cancer cells. Cancer Res. 2002;62:1420–1424. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.