Abstract
Placebo treatment may affect multiple components of pain, including inhibition of nociceptive input, automatic or deliberative appraisal of pain, or cognitive judgments involved in pain reporting. If placebo analgesia is due in part to an attenuation of early nociceptive processing, then pain-evoked event-related potentials (ERPs) should be reduced with placebo. In this study, we tested for placebo effects in P2 laser-evoked potentials at midline scalp electrodes. We found that placebo treatment produced significant decreases in P2 amplitude, and that P2 placebo responses were large enough to reflect a meaningful difference in nociceptive processing. However, we also found evidence that the very robust placebo-induced decreases in reported pain are not solely explained by early reductions in P2. N2 amplitude was affected by neither placebo nor reduction of laser intensity. These results suggest that placebo treatment affects early nociceptive processing, but that another component of placebo effects in reported pain occurs later, either in evaluation of pain or cognitive judgments about pain reports.
Keywords: Placebo, Laser, Pain, EEG, ERP, LEP, P2, Evoked potentials, Placebo effect
1. Introduction
A major theme in contemporary neuroscientific research is that subjective experience is not a direct reflection of events in the world, but rather is constructed within the brain. According to this view, sensory signals are only one component of an experience, whether that experience is the perception of an object or the feeling of pain (Bruner et al., 1951). These “bottom-up” signals are integrated with “top-down” information about the context of the experience (Miller and Cohen, 2001), including memories of relevant past experiences, expectations for the future, and the significance of the experience for the self. Recent research suggests that placebo effects emerge from such interactions, as cognitive expectations interact with ongoing processes in the brain and body. Placebo effects and other context effects are particularly powerful in pain, a multifaceted experience that is closely tied to physical and mental well-being (Koyama et al., 2005; Lorenz et al., 2005; Melzack and Casey, 1968; Price, 2000; Sawamoto et al., 2000; Wager et al., 2004).
A question with major implications for understanding the neurobiology of expectation and brain–body interactions is the question of how deep into the body placebo effects reach. Though a number of studies have reported reliable placebo effects in reported pain (e.g., Benedetti et al., 1999; De Pascalis et al., 2002; Pollo et al., 2001; Price and Barrell, 2000; Vase et al., 2002), reports are based on cognitively constructed representations of experience. Judgment of pain is an active neurobiological process that appears to engage affective and decision circuits in the brain (Moulton et al., 2005). Like other forms of judgment (e.g., Ericsson and Simon, 1980; Manis et al., 1991), pain reports may be highly susceptible to expectancy-induced biases in a variety of settings (Kirsch, 1985; Moerman, 2000).
Two primary issues that bear on the physiological ‘depth’ of placebo effects are whether placebo treatments have active psychobiological effects (Wager, 2005a,b), as opposed to resulting from demand characteristics or statistical artifacts (Hrobjartsson and Gotzsche, 2001, 2004; Kienle and Kiene, 1997); and if placebo effects are active, whether they affect biological processes related to physical health and mental well-being. A recent fMRI study provided evidence that placebo treatment involves active recruitment of cortical regions involved in the regulation of attention and pain (Wager et al., 2004), suggesting that the placebo response is an active psychobiological process. The study also found that placebo treatment suppressed pain-induced activity in the insula, thalamus, and anterior cingulate cortex, suggesting that it alters ongoing processing of pain. Zubieta et al. (2005), using PET, found evidence that placebo treatment both reduces pain and elicits increases in endogenous opioid activity (cf. Benedetti et al., 1999).
However, these neuroimaging studies are limited in their ability to address a critical question about the physiological ‘depth’ of placebo: whether placebo treatments can alter nociceptive processing, rather than or in addition to pain affect, evaluation, and judgments about pain. The fMRI study of Wager et al. found decreases in pain regions only late during pain, after the stimulus had been turned off, though strong responders also showed evidence for greater decreases in anterior cingulate activity during the first several seconds of painful stimulation. Either effect could be related to the evaluation of pain, rather than to the suppression of nociceptive processing, particularly since a key area showing decreases—the insula—is also involved in cognitive judgments of pain (Moulton et al., 2005). The Zubieta et al. study provides converging, but also indirect, evidence: opioid systems are involved in pain, but also in affect, reward, and motivation, and so the evidence that placebo effects inhibit nociceptive processing remains indirect.
In the present study, we recorded brain potentials evoked by painful laser stimuli to test for placebo effects on early nociceptive responses. Laser-evoked potentials (LEPs) are a reliable, objective marker of pain processing (Bromm and Treede, 1984), and they are considered by many to be the best tool for probing the function of nociceptive pathways (Cruccu et al., 2004). Laser stimuli selectively activate A-delta and C nociceptive fibers, and so activate the nociceptive system without activating touch and vibration pathways (Bromm and Treede, 1984). LEPs are influenced by arousal and attention (Kakigi et al., 2000), as is pain-induced fMRI activity (Petrovic and Ingvar, 2002), consistent with the idea that pain processing is sensitive to behavioral context. However, unlike measures of fMRI or PET activity, which may reflect the process of making subjective cognitive judgments about pain, LEPs arise from nociceptive processes that occur before most evaluation and decision processes begin. Some studies have suggested that strategic response processes do not affect stimulus processing until relatively late (at least 450 ms; Ratcliff and McKoon, 1981), and that strategic control is unlikely to affect responses faster than 700 ms (Seymour et al., 2000). Thus, the cognitive biases known to affect decisions about sensory experience and other types of self-report are unlikely to affect LEPs.
A demonstration that placebo treatment affects LEPs would provide converging evidence that placebo treatment can affect early (pre-evaluative) nociceptive processing, with implications for the relationship between cognitive expectations and the function of one of the body’s most basic systems for avoiding harm. There are both theoretical and empirical reasons to expect such effects. The theory is that cognitive expectations maintained in prefrontal cortex may activate the PAG, which has the capability to inhibit pain signals at the level of the spinal cord (e.g., Fields, 2004). The evidence comes from two recent studies, in addition to the fMRI and opioid placebo studies discussed above. Matre et al. (2006) induced secondary hyperalgesia by heating the skin to 46 °C for 5 min. Sensitization of the skin area surrounding the stimulation site is thought to result from sensitization in the spinal dorsal horn. Expectation of pain relief reduced the size of the secondary hyperalgesic area, compared to a control session where pain relief was not expected, implicating a spinal mechanism in the placebo effect. Converging electrophysiological evidence comes from a study by Lorenz et al. (2005), who found that expectations about the intensity of a laser stimulus produced systematic changes in laser-evoked magneto-encephalogram (MEG) potentials. They delivered laser stimuli of high and low intensities, and crossed intensity with a manipulation of whether the expected intensity was high or low. They found that MEG potentials localized approximately to SII—a cortical area critical for nociceptive processing—were reduced in the low-expectation condition and increased in the high-expectation condition.
There are several components of LEPs that may be affected by placebo expectancies, with different implications for the cognitive control of nociception. The major components of LEPs are a lateralized mid-latency negativity (N160) likely to be localized in the parietal operculum (SII) and the late N2/P2 complex (200–300 ms; Lorenz and Garcia-Larrea, 2003). The N2/P2 complex arises from the activation of Aδ fibers and is sometimes followed by an ultralate component (400–600 ms) thought to arise from C-fiber activation (Bromm and Treede, 1984; Bromm et al., 1984). The P2 increases as a function of both laser intensity and reported pain (Iannetti et al., 2004). It is likely to be separable from the P3, but it may overlap with the P3a and reflect cognitive appraisal or attention to pain (Lorenz and Garcia-Larrea, 2003). This is consistent with a view of P2 LEPs as markers of early brain processing of pain, which may involve attention and appraisal of behavioral context as integral components (Garcia-Larrea et al., 1997, 2003; Legrain et al., 2005).
A likely source of the P2 is the anterior cingulate gyrus (Garcia-Larrea et al., 2003; Lenz et al., 1998), which plays a central role in both attention and pain, and also appears to be modulated by placebo in studies of pain and emotion (Petrovic et al., 2002, 2005; Wager et al., 2004; Zubieta et al., 2005). Though the cingulate may show both increases and decreases in different subregions during different phases of pain anticipation and regulation (Porro et al., 2003), the analyses in this study are sensitive to changes occurring within several hundred milliseconds of laser stimulus onset. Slower changes in anterior cingulate activity (i.e., sustained changes beginning in anticipation) will neither influence nor be detected by the measures employed here. The N160 is also of interest because it is a marker for early nociceptive processing, but the midline electrode configuration used in this study was not suitable for examining that component.
One account of placebo effects is that they induce an affective/motivational state that permits reduced attention to pain. The motivational state that regulates the allocation of attention appears to be only partly under voluntary control (e.g., it is very difficult to willfully ignore a rattlesnake next to one’s foot), and the effects of placebo serve as a safety signal and permit attention to be directed away from pain. Placebo effects on the mid-frontal P2 would be consistent with this view. Notably, behavioral context (i.e., factors that affect motivated attention) also affects sensory pathways in the dorsal spinal horn (Duncan et al., 1987), which suggests that attentional set can have far-reaching physiological effects.
Understanding the psychobiological mechanisms of placebo will likely be an enduring research question. The immediate goal of assessing whether placebo treatment affects early nociceptive processing is a preliminary step towards this understanding. Thus, in this study, we sought to test three specific hypotheses: (a) that placebo treatment would reduce P2 LEP amplitude; (b) that placebo reductions in LEP would correlate with reductions in reported pain; and (c) that the placebo P2 reduction would be comparable in magnitude to an equivalent reduction in the intensity of the laser.
2. Methods
2.1. Subjects
Thirty-nine subjects participated in the study (age: 23.2 ± 5.0 years old; four females). Ten additional subjects participated in a preliminary experiment to measure the relationship between evoked potentials, laser intensity, and reported pain. Of the 39 subjects, four were excluded because LEPs could not be identified reliably, and 11 were additionally excluded because they did not report that the laser stimulus was sufficiently painful (more detail is provided in Section 3). All subjects were free of medication and gave informed consent before testing. The experimental protocol was conducted in accordance with the Declaration of Helsinki and was approved by the Internal Review Boards of the Veterans Affairs Medical Center and the University of Michigan, Ann Arbor, MI, USA.
2.2. Laser stimulation
Laser stimulation was delivered by a Thulium YAG infrared laser (Neurolaser, BAASEL Lasertech, Starnberg Germany), activating heat-sensitive A-delta and C nociceptors. Spot diameter was 5 mm and pulse duration 1 ms. The output energy was kept below 700 mJ to avoid skin damage. The subject and the experimenter wore protective eye goggles.
2.3. Preliminary experiment
N2 and P2 LEP amplitudes were the primary physiological measures collected. Although N2 and P2 amplitudes are sensitive to changes in laser intensity and perceived pain intensity (Kakigi et al., 2000), we conducted a preliminary experiment (n = 10) to assess the relationship of these parameters to N2 and P2 amplitudes specifically. By varying the intensity of the laser stimulus within subjects, we hoped to determine roughly what sort of intensity decrease corresponds to a decrease in reported pain of the magnitude observed in placebo studies.
2.4. Experimental protocol
Participants were told that they were taking part in a study that compared brain responses to an analgesic cream (Lidocaine) with a control cream (ineffective). In reality both creams were ineffective (Vaseline skin cream).
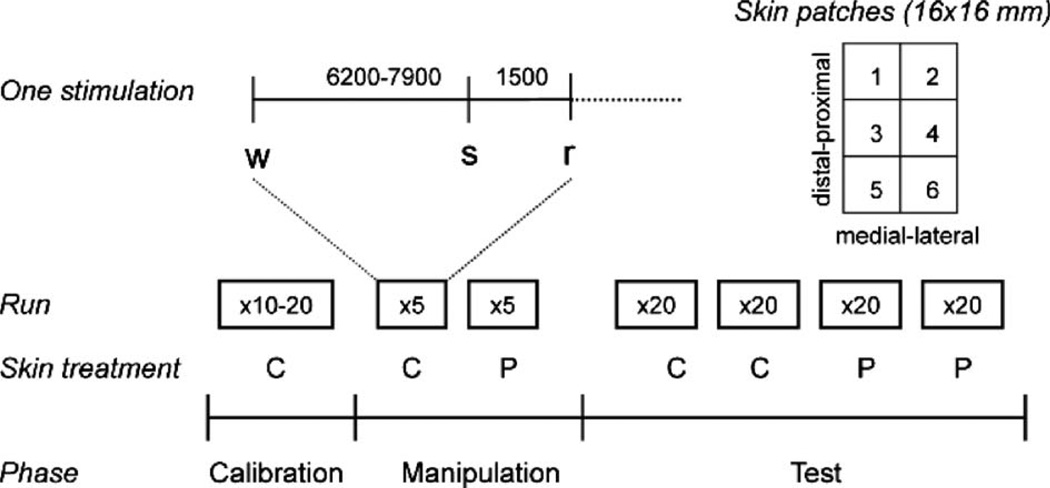
The subjects were first tested for warmth-insensitive fields (WIFs) by touching different spots on the volar forearm for 3 s with a thermode heated to 41 °C (Green and Cruz, 1998). Any WIFs were marked on the skin. Six 16-by-16 mm patches were then marked on the skin of the volar forearm, avoiding WIFs (Fig. 1). ‘Analgesic’ (placebo) cream was applied to patches 1 and 2 and control cream to patches 5 and 6 (Fig. 1; location counterbalanced across subjects). Control cream was also applied to patches 3 and 4, which served as a calibration area. In the first section of the experiment, contact-heat stimulations were applied to the treated areas. These data were not analyzed in the present study.
Fig. 1.
Experimental protocol. Six 16-by-16 mm patches were marked on the skin of the volar forearm and treated with an placebo cream or a control cream. The experiment involved three phases: calibration, manipulation, and test. Each laser stimulus was cued by an auditory warning cue (a beep) presented 6200–7900 ms before the stimulus, and followed by another auditory cue 1500 ms post-stimulus that signaled the participant to make a rating of reported pain. After a calibration phase to determine each individual’s pain intensity vs. applied laser intensity, a manipulation phase followed in which participants received laser stimuli to the control- and placebo-treated areas at ‘high’ and ‘low’ intensities, respectively. During the test phase, subjects received 2 runs of 20 stimuli each on the control (C)- and placebo (P)-treated areas at medium intensity. The figure shows the order of testing for a C-first subject; order was counterbalanced across participants. The manipulation and test phases were performed on different skin patches.
Subjects were then prepared for the evoked-potentials section, which involved three phases (Calibration, Manipulation, and Test), as shown in Fig. 1. In the calibration phase, 10–20 laser stimuli of various intensities (300–700 mJ) were delivered to patch 3 and 4 in Fig. 1 to identify stimulus intensities corresponding to low- (level 1), medium- (level 2), and high-intensity (level 3 or above) pain. Many participants reported low or medium pain at the maximum intensity of 700 mJ, in which case this value was used in subsequent testing.
A manipulation phase followed to enhance participants’ expectations of pain relief and thereby increase placebo responding. In this phase, pain was surreptitiously reduced in the placebo condition (Price et al., 1999). Five stimuli were applied to the placebo-treated (‘analgesic’ cream) patches of skin, and subsequently five stimuli were applied to the control-treated patches (order counterbalanced across participants). Participants were told that all stimuli were at level ‘high.’ However, they were administered at level ‘low’ in the placebo-treated patch and at level ‘high’ in the control-treated patch.
Finally, during the critical test phase, two runs of stimuli were administered to placebo- and control-treated patches of skin (80 stimulations in total: 2 patches × 2 runs × 20 stimuli). Locations of placebo and control patches and testing order were counterbalanced across subjects. A short warning beep (1 kHz tone) alerted the subject at pseudorandom intervals 6200–7900 ms before each laser stimulus. As before, participants were told these were at level ‘high,’ but all were delivered at level ‘medium,’ in keeping with the paradigm used in Price et al. (1999). Because the stimuli on placebo and control patches were identical, any differences in reported pain and evoked potential amplitude (control–placebo) during this phase are attributable to placebo effects.
2.5. Psychophysics
After a 1.5 s inter-stimulus interval following each laser stimulation, a beep prompted the subject to rate the perceived intensity. The subjective rating was done verbally on a 13-point numerical ratings scale ranging from −2 to 10, with anchor points described by the following verbal instructions: −2 was ‘not perceived,’ −1 was ‘non-painful warmth,’ 0 was ‘non-painful pinprick,’ 1 was a painful pinprick, and 10 was ‘worst pain imaginable.’ The verbal ratings were recorded by the experimenter.
2.6. Evoked potential acquisition
Electroencephalographic (EEG) registrations were made from four midline electrodes (FCz, Cz, CPz, and Pz) according to the international 10–20 system, using a standard EEG cap and Neuroscan software (Scan 4.2, Compumedics, El Paso, Texas). The recordings were referenced to linked bilateral earlobes (A1 + A2). Electrooculogram (EOG) was recorded from supra- and infraorbital electrodes for offline artifact rejection. The impedance was maintained below 5 kΩ. The signals were amplified 100,000 times (Synamp, Compumedics), sampled at 500 Hz, and bandpass filtered at 0.1–50 Hz. Room temperature was 22–23 °C and skin temperature was always above 30 °C. The subjects were instructed to keep their eyes open, to focus on a fixed point on the wall and to avoid blinking, particularly in the interval between the warning and rating beeps.
2.7. Data analysis
Individual pain intensity ratings were averaged for each run of 20 stimuli. Individual behavioral effect scores were then calculated for each run by subtracting placebo ratings from control ratings (C–P). Furthermore, an overall pain score was calculated for each subject as the average across all 80 laser stimuli to determine each participant’s pain sensitivity. Because the effect scores and average pain scores were positively skewed, nonparametric analyses were conducted on ranks of placebo effect scores and ranks of average pain scores.
EEG data were preprocessed using Neuroscan’s built-in functions. First, ocular artifact rejection was done on the continuous recording. Manually counting the number of eye blinks or saccades in ten randomly selected runs (each lasting −5 min) showed a variation between 16 and 65 ocular artifacts. Blinking was most pronounced in the intervals between stimuli, as we instructed subjects to avoid blinking between the warning and rating beeps. To avoid sampling bias resulting from the manual rejection of some sweeps (e.g., those with the highest subjective pain, which may also lead to increases in ocular activity), we employed a computational correction method that corrects for artifacts rather than deleting sweeps.
The Neuroscan EOG correction procedure involved the following steps. First, the EOG channel was scanned for the maximum eye movement potential. EOG deviations of more than 10% from the maximum were used as indicators of blinks, and these were used to estimate an average blink artifact response for each channel. The procedure discarded artifacts starting <400 ms before a previous artifact, to avoid double detection. If less than 20 blinks were detected, no correction was made. Otherwise, EEG data were corrected using a regression approach. From the average EOG ocular artifact, transmission coefficients (b) were computed for each EEG channel by estimating the covariance of the averaged potentials of the EOG channel with the EEG channels according to this equation: b = cov(EOG,EEG)/var(EOG). A fraction of the average EOG artifact (b Æ EOG) was subtracted from each EEG channel on a sweep-by-sweep, point-by-point basis. After ocular artifact correction, the continuous EEG signal was split into epochs (−500 to 2000 ms relative to stimulus onset). Finally, each epoch was baseline corrected by subtracting 100-ms pre-stimulus EEG from each point in the epoch. This procedure prevents slow changes that may begin during anticipation of pain from influencing P2 amplitude estimates.
To extract peak LEPs, the 20 sweeps in each run were averaged and the latency and amplitude of the first major negative (N2) and positive (P2) component was extracted for each subject and each electrode using a peak-detection function. The search interval was limited a priori to 150–350 ms (N2) and 250–500 ms (P2) relative to stimulus onset. For each run, each detected peak was confirmed by visual inspection before the estimates were accepted. In six subjects, no peaks were identified by the automatic procedure. In two of these subjects the N2/P2 complex was identified by visual inspection of the averaged sweeps. In the remaining four subjects, no valid N2/P2 complex could be identified in the search interval, although an ultralate positivity was identified with peak amplitude between 600 and 1000 ms. Data from these four subjects are not included in the remaining analysis, leaving 35 subjects. The visual inspection resulted in minor corrections to the automatic procedure in 4 of the remaining 140 runs (35 subjects × 4 runs each). These were typically cases where the EEG signal was distorted by relatively large α-band (−10 Hz) components.
2.8. Statistics
Statistical analysis was done in the General Linear Model (GLM) framework using SPSS (SPSS, Chicago, Illinois). Placebo effects in reported pain (RP) were analyzed separately for Run 1 and Run 2 with a 1 within (Placebo, C vs. P), 1 between (Order, C first or P first) GLM. Due to habituation of pain reports and LEPs, we expected the strongest placebo effects in Run 1. Follow-up analysis included Run as a factor in a 2 × 2 within, 1 between ANOVA.
LEP amplitudes were analyzed in two ways. First, we analyzed amplitudes in a 4 × 2 within, 2 between GLM for each run. Within-subjects factors were Electrode (FCz, Cz, CPz, and Pz) and Placebo (C vs. P). Between-subjects factors were Order (C first or P first) and ranked Reported Placebo.1 Runs were analyzed separately because reported pain and P2 amplitudes both showed strong habituation effects, and we expected the test to be sensitive to placebo effects only when subjects were experiencing pain (i.e., primarily in Run 1). Supplementary analyses added Run as a within-subjects factor. Nonsphericity is not an issue for the effects of Placebo and Order, and Huynh-Feldt correction for nonsphericity was used for effects of Electrode (Keselman, 1998); thus, fractional degrees of freedom are reported for these effects. MANOVA analyses (Keselman, 1998) revealed a qualitatively identical pattern of results (data not shown).
In the second analysis we divided the sample into tertiles based on RP, and looked for differences in P2 amplitudes between RP placebo Responders (those in the highest third, n = 8) and Nonresponders (the lowest third, n = 8). The GLM model for each run was the same as in the previous analysis, that is a 4 × 2 within (Electrode and Placebo), 2-between (Order and Responder Status) design.
We used one-tailed tests for one-sided hypotheses about which we had a priori expectations, including that placebo would decrease P2 amplitude (main effect of Placebo, particularly in Run 1) and that the placebo effect would be weaker in Run 2 (Placebo × Run interaction) due to habituation in both pain and P2 amplitude. T-contrasts are presented for these comparisons to test the one-sided alternative (the F statistic gives equivalent results, but for a 2-sided alternative). Two-tailed tests were conducted on other effects. An α level of 0.05 was used throughout.
2.8.1. Test of intensity-reduction hypothesis of placebo
Another analysis was performed only at electrode Cz, where P2 effects are maximal, to test whether P2 placebo effects were significantly smaller than would be expected if placebo worked only by reducing nociceptive input. We refer to this hypothesis as the “intensity-reduction” hypothesis. We assessed this by testing whether placebo effects in P2 amplitude were significantly smaller than P2 reductions in laser intensity that produce equivalent decreases in reported pain. For this analysis, we began with the placebo effect in reported pain (n = 24), and used the estimated curves from the preliminary experiment (n = 10) to calculate the reduction in both P2 and laser intensity required to produce an equivalent decrease in reported pain. Performing these calculations for each of the 10 participants allowed us to estimate the variance of predicted P2 reductions. We then calculated the standard error of the difference between observed and expected P2 reductions, as given by the equation
where σ2 is the variance, n is the sample size, and O and E refer to the observed P2 effects in the placebo experiment and the expected placebo effects in the intensity mapping experiment, respectively. Degrees of freedom are given by the Sattherwaite approximation. This allowed us to perform a t test of the difference between observed and expected P2 reductions, where the null hypothesis is a placebo effect produced only by intensity reduction.
3. Results
3.1. Placebo effects in pain ratings
There was a strong effect of Placebo on pain intensity ratings. For Run 1, C–P = 0.64, t (38) = 4.26, p = .0001, indicating that pain ratings were decreased with the placebo treatment. For Run 2, effects were also significant, C–P = 0.65, t (38) = 4.75, p < .0001. Adding Run as a factor, the effect of Run was significant, effect = 0.19, t (37) = 2.07, p = .04, indicating that participants habituated to the laser stimulus. The effect of placebo remained significant, C–P = 0.64, t (37) = 4.30, p < .0001. There was a trend towards a Placebo × Order interaction, t (37) = 1.90, p = .066. Mean pain ratings for the P first group were 2.40 and 1.57 for C and P, respectively, and means for the C first group were 1.47 and 1.15. Placebo effects were significant for both order groups: for P first, C–P = 0.82, t (19.0) = 4.00, p = .0008, and for C first, C–P = 0.46, t (18.0) = 2.90, p = .009. No other interactions were significant.
Examining bivariate correlations revealed that placebo scores (C–P) in reported pain were positively correlated with overall pain (r = .41, p < .05), but not with other variables (stepwise regression confirmed these results). After controlling for this variable, placebo effects in reported pain remained significant, t (22) = 2.25, p = .035. The relationship between placebo and reported pain appeared to be due to the fact that a number of participants (n = 11) rated the laser stimuli as non-painful, i.e., an average score below 1, where 1 was defined as a “painful pinprick” and 0 was a “non-painful pinprick.” These participants still reported a placebo effect [C–P] = 0.33, t (10) = 2.29, p = .04 (the non-parametric Wilcoxon sign-rank test was also significant, Z = −2.05, p = .04). However, the placebo effect was much reduced compared with that of the remaining 24 participants, [C–P] = 0.79, t (23) = 3.97, p = .001; Wilcoxon Z = −2.83, p = .005. Because we were interested in placebo effects in pain, participants who did not find the stimulation painful were excluded from further analyses.
3.2. Placebo effects in P2 amplitude
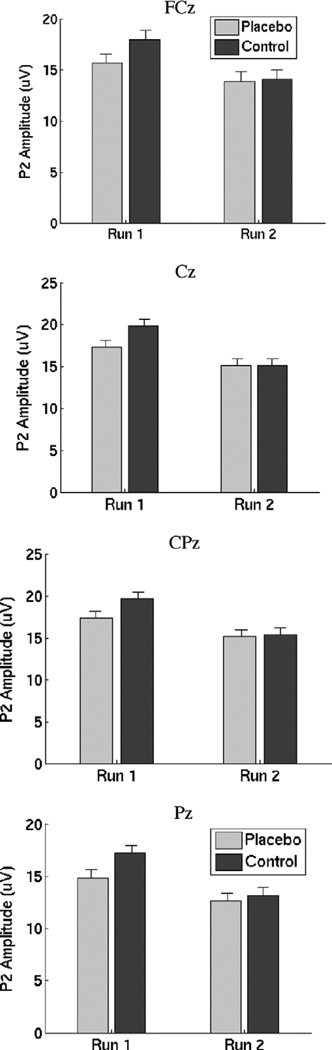
A planned test on Run 1 showed that Placebo was significant, t (21) = 2.37, p = .013 one-tailed (equivalent ANOVA F(1, 21) = 5.63). The result, shown in Fig. 2, provides evidence that P2 amplitude was reduced in the placebo condition (a 16.7% reduction on average at Electrode Cz). However, placebo effects were negligible in Run 2. Across runs, Placebo showed a trend towards significance, t (21) = 1.59 (F = 2.53), p = .063 one-tailed, an 8.8% reduction at electrode Cz. The Placebo × Run interaction was significant, t (21) = 1.75 (F = 3.06), p = .047 (one-tailed), demonstrating that as expected, placebo effects were reduced in Run 2. Placebo did not interact with Electrode, F(2.0, 42.0) = 0.13, p > .8.
Fig. 2.
P2 amplitudes by placebo condition and run for each electrode, controlling for order of testing. Notably, placebo reductions in P2 amplitude are apparent in all electrodes for Run 1, but not Run 2.
A strong effect of Run, F(1, 21) = 16.37, p < .0001 indicated that P2 amplitudes decreased substantially with repeated testing. A Placebo × Order interaction, F(1, 21) = 6.37, p = .02, indicated that placebo effects were stronger in the C first group (3.72 µV for C first vs. −1.16 µV for P first at Cz). A significant Electrode × Placebo × Order effect F(2.0, 42.0) = 3.91, p = .03 suggested that the order effects were strongest at the anterior sites.
Electrode did not interact with other effects, consistent with our expectations that the midline electrodes recorded provided largely redundant information (max. F = 2.28, p > .11). Other effects were not significant, including between-subjects effects of RP (all p’s > .2). Controlling for Order, partial correlations between C–P P2 effects and RP ranged from r = 0.01 to 0.18 across electrodes, and from r = −.16 to .02 for run 1 only (all p > .10). Additional multiple regressions showed no effects of laser intensity or reported pain on placebo responses, suggesting that these were not confounding or masking variables.2
In a second GLM model, we considered only Responder and Nonresponder subgroups. A planned test on Run 1 showed that Placebo was significant, t (13) = 2.53, p = .013 one-tailed (equivalent two-tailed ANOVA F(1, 13) = 6.39, p = .025), indicating that P2 amplitudes were reduced with placebo. Again, placebo effects were negligible in Run 2. Across runs, Placebo was significant, t (13) = 2.11 (F = 4.48), p = .027 one-tailed. Placebo × Responder Status, which would indicate a difference in P2 placebo effects between RP responder groups, was not significant. Neither was Run × Responder Status. However, the Placebo × Run × Responder Status interaction was significant, F(1, 13) = 6.58, p = .02. Examination of the means suggested that Responders showed placebo reductions in P2 amplitude in both runs, whereas Nonresponders showed it only in Run 1. The Placebo × Responder Status relationship did not interact with Electrode. Other effects were qualitatively similar to those reported above and do not change the interpretation of results.
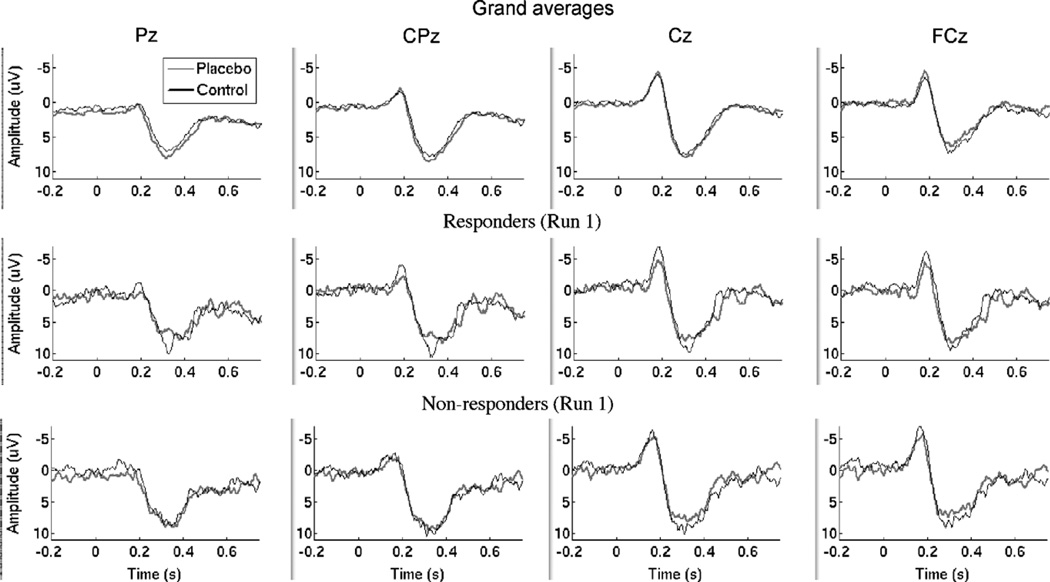
Grand averages, illustrated in the top panels of Fig. 3, show the N2 and P2 responses across the 24 subjects included in our analyses. These generally did not show apparent differences between C and P (thin black line and thicker gray line, respectively), which might be expected given the individual variability in the amplitude and latency of reported peaks. The middle and bottom panels of Fig. 3 show grand averages for Responders (n = 8) and Nonresponders (n = 8), respectively. Responders show an apparent decrease in P2 with placebo, though the effect of Responder Status was not significant in P2 peak amplitude, as described above.
Fig. 3.
Top row: grand averages by placebo condition across all runs and participants. For display, data were not adjusted for baseline activity. Responses in the control condition are shown by the thin black lines, and in the placebo condition in thicker gray lines. Middle row: grand averages by placebo condition for the placebo Responder group (n = 8). Bottom row: grand averages for Nonresponders (n = 8).
3.3. Relationship of N2 and P2 amplitude to intensity and reported pain
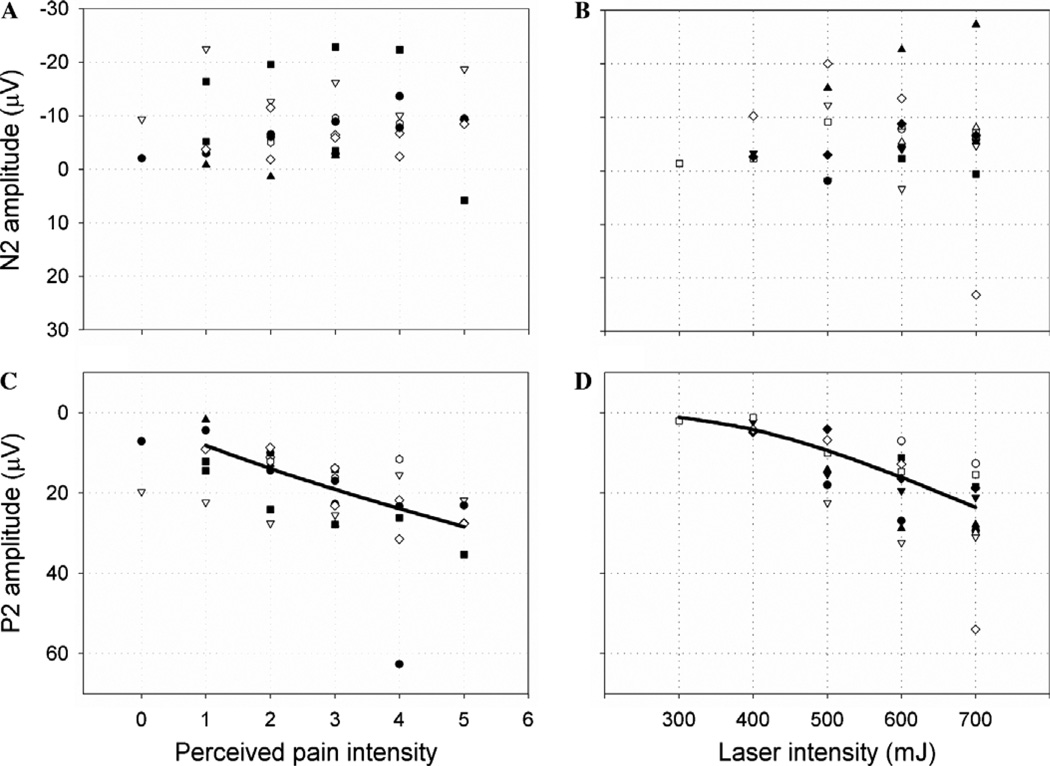
We conducted a preliminary experiment to assure that LEP responses were sensitive to laser intensity variations and test the intensity reduction hypothesis. Results of the preliminary experiment are shown in Fig. 4. The scatterplots show how N2 and P2 vary with reported pain intensity. Different subjects are plotted with different symbols. N2 did not vary in a systematic way with laser intensity (R2 = 0.0, F = 0.0, p = .99) or pain (R2 = 0.002, F = 0.085, p = .77). These relationships are shown in Figs. 4A and B. Because N2 amplitude was not sensitive to changes in laser intensity or pain, we did not analyze it further.
Fig. 4.
Scatterplots of N2/P2 amplitude vs. intensity. The relationship between LEP amplitude and perceived and applied intensity was determined in a separate experiment on ten subjects who each received 50 stimuli at five different laser intensities. This enabled us to test whether P2 placebo effects were lower than would be expected if placebo worked only by reducing nociceptive input. N2 amplitude did not change with either perceived intensity (A) or laser intensity (B). P2 amplitude changed with both perceived intensity (C) and laser intensity (D). A power equation best described the overall relationship in (C), though the response was not essentially linear in the range of reported pain placebo effects in the main study, whereas a S function best described the relationship in (D). To estimate expected P2 amplitude for a given change in perceived intensity, linear regressions were computed within each participant and the variance in predicted values corresponding to reported pain and placebo effects was assessed across participants.
P2 amplitude, however, increased significantly with increasing laser intensity (R2 = 0.658, F = 57.77, p < .0001) and pain (R2 = 0.42, F = 24.7, p < .0001). We found that the relationship between P2 amplitude and pain ratings was fit best by a power function (though other functions provided similar fits). The best-fitting function was P2 = 8.1X0.78 (where X = pain rating), which was roughly linear in the range of reported intensities observed in our experiment (1–5; see Fig. 4C).
We applied the same procedure to the relationship between P2 amplitude and laser intensity to estimate the effective reduction in laser intensity for the observed placebo effects. The best-fitting function was a sigmoid, P2 = e(5.5−1622/X), where X = laser intensity. While the relationship between P2 amplitude and reported pain was roughly linear, the relationship with laser intensity was nonlinear, as shown in Fig. 2D. In particular, much larger decreases in laser intensity are required to achieve a unit change in P2 amplitude if intensity and reported pain are low. The expected P2 placebo effect, a reduction from 18 to 14 µV, is in a sensitive part of the curve in Fig. 2D, as the response is roughly linear between 10 and 20 µV (corresponding approximately to 400–700 mJ). The observed P2 amplitudes of 18.6 µV for Run 1 and 15.1 µV for Run 2 are also in the sensitive portion of the curve, indicating that P2 amplitude was expected to be responsive to placebo suppression of nociceptive input.
3.4. Testing the intensity reduction hypothesis
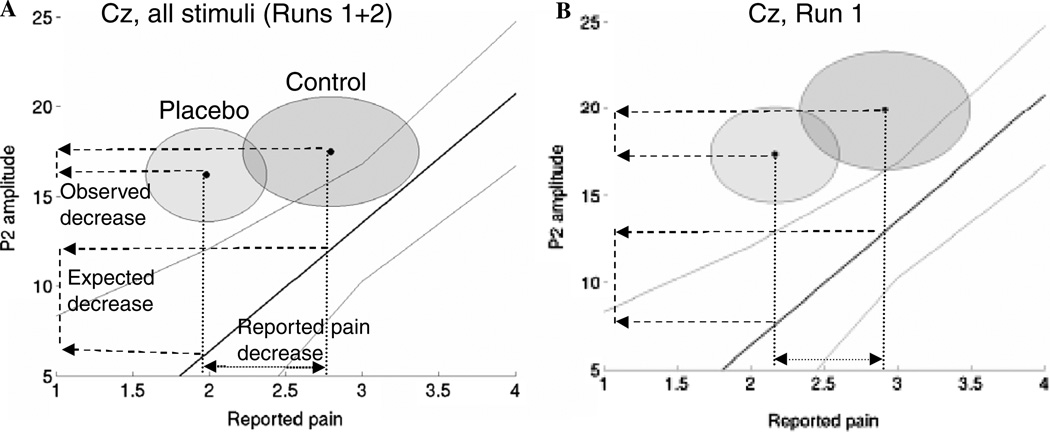
We next compared observed P2 placebo effects with those expected under the intensity-reduction hypothesis, as shown in Fig. 5. The x-axis shows reported pain for both placebo (n = 24) and intensity-mapping (n = 10) groups. The y-axis shows P2 amplitude in both experiments, for both runs together (left panel) and for Run 1 only (right panel). The solid line is the regression line for P2 amplitude regressed on reported pain (n = 10, with 90% confidence bands), and the expected placebo reduction is shown as the lower of two dashed lines on the y-axis. The darker and lighter circles in Fig. 5 show the 90% confidence intervals for C and P effects (n = 24; though placebo effects were calculated as within-subjects contrasts), and the uppermost dashed line on the y-axis shows the observed placebo effect.
Fig. 5.
Test of the intensity reduction hypothesis of placebo. The solid line is the regression slope for the relationship between reported pain and P2 amplitude in the preliminary experiment (n = 10). Light gray lines show 90% confidence intervals, corresponding to the one-sided hypothesis of expected decreases in P2 with decreasing pain. The circles show 90% confidence regions in the control and placebo conditions (n = 24). Dashed lines on the y-axis show the observed placebo decrease and the expected decrease if the same reported pain decrease were produced by an intensity reduction. Confidence regions shown are between subjects, though tests of control–placebo and expected decreases were calculated within subjects. The observed decrease is significantly smaller than the expected decrease across both runs (A), but is not significantly different in the first run alone (B).
Notably, P2 amplitudes were lower (and/or reported pain was higher) for the intensity-mapping group overall than for the placebo group. This effect may have multiple causes, including differences between subjects, the placebo context, and the length of the test. However, what is critical is the within-subject differences between higher and lower reported pain. If the relationship between P2 and reported pain is nonlinear, then the difference in offset between groups could make interpretation of the critical reductions in P2 between groups problematic; it would be difficult to tell a whether the difference is due to differences in the magnitude or the scale of the response. However, Fig. 2 shows that the relationship is roughly linear across the range of intensities tested, so the intensity-reduction hypothesis predicts equal reductions in P2 per unit reduction in reported pain for the placebo and intensity-mapping groups.
The observed placebo reduction in P2 was 1.28 µV, with a variance of 20.47 µV2 (n = 24; left panel, top dashed line on y-axis). The expected placebo reduction due to an intensity decrease was 5.65 µV, with a variance of 29.36 µV2 (left panel in Fig. 5, bottom dashed line on y-axis). The difference between observed and expected P2 reductions of 4.37 µV was significant, t (14.5) = −2.25, SE = 1.95, p = .02. The overall difference between P2 amplitudes for the placebo and intensity-mapping groups suggests that P2 amplitudes were higher in the placebo experiment, but the critical effect of interest is the relative drop in P2 amplitude with a decrease in reported pain.
For Run 1 only (right panel of Fig. 5), the observed decrease was 2.80 µV, with a variance of 41.42 µV. The RP effect of 2.90 µV for C vs. 2.187 µV for P led to an expected P2 decrease of 5.11 µV, with a variance of 24.14 µV. The difference between observed and expected P2 placebo effects of 2.31 µV was not significant, t (22.1) = −1.14, SE = 2.03, p = .13. Adjusting for order did not change the results. Thus, reduction of input (i.e., early pain inhibition) is a possible cause of reported placebo effects in Run 1, but is unlikely to be an adequate explanation for reported placebo effects overall.
4. Discussion
In this study, we recorded evoked brain potentials from midline electrodes in response to painful laser stimuli (LEPs) to test for placebo effects on early nociceptive processing. We compared stimulation of placebo-treated skin (P) with stimulation of control-treated skin (C). The ointments applied to each skin area were identical; the only difference was the induction of expectations of pain relief in the placebo condition. We observed robust placebo effects on reported pain, consistent with previous studies (Price et al., 1999; Vase et al., 2005; Voudouris et al., 1989; Wager et al., 2004). In addition, our results show that placebo treatment reduced P2 LEP amplitude, particularly in the first blocks of stimulation (Run 1). These effects were observed to some degree at all electrodes (FCz, Cz, Cpz, and Pz). Adjusting for Run and Order, placebo effects were maximal at Cz (Table 1), although stronger effects of testing order, habituation, and overall pain made simple C–P effects most reliable at Pz. The N2 component was insensitive to changes in applied pain intensity and to placebo.
Table 1.
Amplitudes and latencies for evoked potential components N2 and P2 across experimental condition (Placebo or Control), Order of stimulation (P first or C first) and Run
| Placebo | Control | |||||||
|---|---|---|---|---|---|---|---|---|
| P first | C first | P first | C first | |||||
| Run 1 | Run 2 | Run 1 | Run 2 | Run 1 | Run 2 | Run 1 | Run 2 | |
| N2 amplitude (µV) | ||||||||
| FCz | −10.35 (2.32) | −10.69 (2.71) | −9.59 (2.61) | −6.62 (2.18) | −11.36 (2.59) | −10.67 (2.62) | −11.11 (3.16) | −8.76 (2.44) |
| Cz | −12.16 (2.24) | −12.43 (2.45) | −8.13 (2.29) | −5.59 (1.93) | −12.69 (2.21) | −11.67 (2.27) | −9.65 (3.06) | −8.28 (2.34) |
| CPz | −9.19 (1.94) | −10.00 (2.06) | −5.05 (1.56) | −3.11 (1.35) | −8.83 (1.74) | −8.34 (1.95) | −5.91 (2.18) | −5.52 (1.69) |
| Pz | −5.96 (1.41) | −7.12 (1.48) | −3.14 (0.87) | −2.21 (0.99) | −5.66 (1.17) | −5.90 (1.49) | −3.57 (1.48) | −3.79 (1.22) |
| P2 amplitude (µV) | ||||||||
| FCz | 16.26 (1.60) | 13.72 (1.09) | 14.76 (2.49) | 14.05 (2.50) | 15.45 (1.40) | 12.50 (1.40) | 20.85 (3.29) | 15.84 (3.29) |
| Cz | 18.13 (2.00) | 15.43 (1.75) | 16.21 (2.60) | 14.68 (2.61) | 17.76 (2.25) | 13.84 (1.49) | 22.21 (3.36) | 16.58 (3.36) |
| CPz | 17.86 (1.65) | 15.49 (1.75) | 16.64 (2.66) | 14.69 (2.56) | 18.04 (2.12) | 14.44 (1.39) | 21.40 (3.46) | 16.29 (3.25) |
| Pz | 15.03 (1.43) | 12.89 (1.39) | 14.56 (2.38) | 12.34 (2.16) | 15.69 (1.69) | 12.05 (1.04) | 18.86 (3.01) | 14.41 (2.69) |
| N2 latency (ms) | ||||||||
| FCz | 201.8 (15.3) | 187.2 (4.2) | 183.7 (7.4) | 201.8 (12.5) | 190.7 (6.6) | 204.3 (14.8) | 206.7 (15.4) | 209.7 (15.1) |
| Cz | 183.3 (8.2) | 180.3 (4.4) | 182.8 (7.3) | 187.2 (9.9) | 186.5 (7.5) | 200.3 (7.9) | 191.0 (11.5) | 202.2 (15.6) |
| CPz | 186.8 (6.8) | 191.7 (8.7) | 178.3 (8.1) | 193.0 (9.8) | 196.7 (8.4) | 197.5 (7.2) | 204.5 (13.5) | 184.8 (8.1) |
| Pz | 188.3 (7.4) | 193.3 (9.3) | 196.3 (12.5) | 211.8 (11.4) | 203.2 (8.7) | 197.7 (8.7) | 206.0 (14.2) | 196.3 (13.0) |
| P2 latency (ms) | ||||||||
| FCz | 321.5 (18.3) | 319.0 (12.8) | 327.5 (18.5) | 340.3 (15.7) | 322.0 (14.1) | 333.7 (17.5) | 328.3 (13.9) | 350.2 (22.2) |
| Cz | 314.5 (16.5) | 319.7 (11.8) | 338.7 (14.7) | 344.2 (14.3) | 320.7 (12.3) | 321.0 (13.4) | 320.8 (11.5) | 344.3 (13.1) |
| CPz | 321.0 (16.9) | 339.0 (15.5) | 334.7 (12.9) | 353.7 (10.8) | 330.7 (14.6) | 331.0 (12.7) | 334.5 (12.5) | 346.0 (13.0) |
| Pz | 322.3 (16.5) | 340.0 (12.9) | 343.7 (14.0) | 363.2 (13.9) | 333.3 (14.5) | 339.5 (11.8) | 338.5 (14.3) | 367.5 (16.6) |
Values are mean and standard error (n = 24)
A reduction in P2 with placebo is consistent with the modulation of pain affect in anterior cingulate suggested by other studies (Rainville et al., 1999; Wager et al., 2004), as cingulate is a likely generator of the laser-evoked P2 (Garcia-Larrea et al., 2003; Iannetti et al., 2004; Lenz et al., 1998). However, we recorded only midline electrodes in the current study and cannot do source localization, which is a limitation of the current study. Because the P2 often has a latency around 300 ms, there is some debate about whether it reflects a P300, but previous work suggests that the P2 and P300 are dissociable (Lorenz et al., 1997a,b; Lorenz and Garcia-Larrea, 2003), though the P2 may overlap with or be augmented by the P3a. In our study, the P2 was pain responsive and the stimulus did not vary across trials, making it unlikely that the effect is a classic P3, though a P3a could have produced the responses, particularly as placebo effects were present only in Run 1. The idea that the P2 modulation we observed reflects changes in cognitive appraisal of the pain stimulus is consistent with theories about expectancy-based placebo effects (Wager, 2005a,b).
4.1. Factors influencing brain placebo effects
The analyses revealed three factors that are important to consider when studying placebo effects in LEPs. The first is overall pain intensity. Consistent with our previous work, if stimulation is less painful (or not painful), then placebo effects are reduced. Thus, care must be taken to ensure that the stimulus is painful enough. One reason for this is that placebo may work by reducing anxiety (Vase et al., 2005), and larger placebo effects may be elicited if the situation is anxiogenic (Staats et al., 2001). A second explanation could be that pain and placebo both induce endogenous opioid release, and these two effects are non-additive (see Zubieta et al., 2005, 2006, for an example of placebo opioid release specific to pain), implying that both pain and expectation are required for a placebo opioid response (Fields, 2004). Finally, it could simply be placebo effects are lower with reduced pain due to floor effects. In this study, LEP analyses were conducted on participants who reported that the stimulus was painful.
A second factor to consider is habituation over time during testing, which influenced the placebo effect size in P2 LEPs, but not in reported pain. As found in other studies of LEPs (Garcia-Larrea et al., 2003), P2 effects habituate more than reported pain does. The habituation of P2 but not reported placebo effects constitutes a striking dissociation between brain and self-report measures of pain processing. We anticipated the habituation of placebo effects due to the decrease in reported pain over time, but overall intensity decreases may not be sufficient to explain the effect.
What, then, might cause the habituation of placebo effects in LEPs? One possibility is that the effect of placebo on early pain processing is due to anxiety reduction, and thus has a most pronounced effect on the initial pain stimuli. Participants report that early trials are substantially more painful than later trials, possibly due to anxiety and uncertainty about the stimulus and testing situation. A second possibility is that placebo treatment can elicit an early, pre-stimulation opioid release—but once the opioid system is activated by painful stimulation, the placebo treatment offers little additional benefit. Either of these scenarios involves the additional assumption that reported pain is influenced by judgments about the experimental context (e.g., the stimulus history) in ways that LEPs are not. For instance, pain reports may be subject to hysteresis or self-consistency biases. That is, if pain is initially high, then participants may form an overall impression of the stimulus as painful and continue to report high levels of pain even when the stimulation is reduced.
A third factor is the order of testing (control first or placebo first), which were strongest at Cz. We observed strong placebo effects in the C first group, but no reliable differences in the P first group. This could be because of a real psychological difference between receiving C first or P first, but it could also simply reflect habituation to the stimulus across the first (C or P) and second (C or P) testing blocks, for the following reason: If P2 effects habituate, as we observed, then even if there were no true effect of placebo, we would expect to observe C–P differences in the C first group and P–C differences of equal magnitude in the P first group, creating an apparent Placebo × Order interaction. Another way of saying this is that our design, which was intended to study placebo effects overall but not differences in placebo due to administration order, cannot separate effects of habituation from psychologically caused Placebo × Order interactions. Thus, we cannot say whether psychobiological placebo effects are larger in the C first group. Future studies with additional subject groups that receive only placebo or only control stimulation across the entire experiment may resolve this ambiguity.
Critically for our main hypotheses, however, all our analyses controlled for testing order in the GLM. A significant placebo effect, controlling for order, is statistically equivalent to testing whether C–P effects in the C first group are larger than P–C effects in the P first group, and implies that the placebo treatment had an impact independent of the effects of habituation. We observed strong placebo effects in the C first group, but no reliable differences in the P first group, consistent with the idea that both placebo effects and habituation influence P2 responses. Thus, we can reject the null hypothesis that placebo treatment had no effect, but we cannot assess whether the testing order has a real psychological impact on placebo responses.
4.2. The intensity-reduction hypothesis
The strongest hypothesis for why brain placebo effects may be observed is that placebo treatment blocks nociceptive input to the brain, thus reducing the impact of those signals on brain activity. Recent evidence from a secondary hyperalgesia paradigm suggests that there may be a spinal inhibition component to placebo (Matre et al., 2006). Thus, goal of this research was to test whether the magnitude of placebo effects in LEPs is consistent with spinal pain inhibition. We equated the reported pain decrease in the placebo experiment (n = 24) and a separate laser intensity- mapping experiment (n = 10), and asked whether there were also equivalent reductions in P2 amplitude. We found that P2 placebo effects in the first run were strong enough to be consistent with an intensity reduction, but that P2 placebo effects collapsing across runs were significantly smaller than would be expected under the intensity reduction hypothesis.
This finding is consistent with the finding that placebo effects in P2 were only found in the first run, but equivalent reported placebo effects were found in both runs. Together, the selective habituation of placebo effects in LEPs and the intensity-reduction results suggest that placebo effects on early nociceptive processing (including spinal inhibition and attention- or affect-related effects) are not the only component of placebo analgesia in reported pain. Thus, the model that placebo effects are either completely mediated by spinal inhibition or they are not is probably too simplistic. An alternative is that there are multiple components of a placebo response, including effects on central processing of pain affect (Wager, 2005a,b) and on cognitive judgments about pain (Clark, 1969).
An alternative account is that different regions of the cingulate (the presumed source of the P2) have different phasic responses to the laser stimulus, and that placebo effects in different directions (increases vs. decreases in activity) in these regions offset one another, producing smaller than expected placebo decrements in P2. LEP analysis cannot discriminate the activity of multiple cingulate subregions based on spatial location, but independent components analysis may reveal whether there are multiple superimposed effects hidden within the overall P2 response. Supplementary independent components analysis (ICA) did not show evidence for multiple components that are affected differentially by placebo (data not shown for space reasons). In addition, for this hypothesis to explain the pattern of results, placebo-induced increases in a subcomponent of P2 would have to increase over the course of the session (between Run 1 and Run 2); we are not aware of evidence that might support the existence of such an effect.
4.3. Correspondence between P2 and reported placebo effects
A related question is whether those participants who reported the largest placebo reductions in reported pain also showed the largest P2 placebo reductions. We looked for these effects in two ways: by using ranked reported effects in the GLM analysis and by comparing P2 effects for the highest and lowest thirds of the group on reported effects. None of these tests were significant, although the grand averages (Fig. 4) show effects of Responders and Nonresponders in the appropriate direction. The low brain-behavior correlations are consistent with the notion that placebo effects on reported pain involve multiple components, only one of which is an effect on early nociceptive processing. The existence of both early (LEP-influencing) and late (affect or cognitive judgment) components of reported placebo effects would make relationships between P2 effects and reported effects difficult to detect.
5. Conclusions
In this study, we report that a placebo treatment produced detectable amplitude decreases in the P2 component of laser-evoked pain potentials. Brain placebo responses were large enough to reflect a meaningful difference in nociceptive processing, but the effects were smaller than the very robust decreases in reported pain. Placebo responses in P2 potentials were smaller than those expected if the entire reported response were produced by a decrease in nociceptive input, suggesting that there are both early and late phases of the placebo response.
Acknowledgments
This work was supported by the Mind, Brain, Body, and Health Initiative (T.W.), by the Research Council of Norway (D.M.), and by the Department of Veterans’ Affairs. We are grateful to Alex Sokolik and Raza Zaidi for assisting with the experiments.
Footnotes
Ranked reported placebo was computed by taking the ranks of data (1−n participants, where the lowest score is assigned rank 1, the second lowest rank 2, and so on) and using those as a continuous predictor in the GLM. This is a typical procedure for making a test nonparametric, because using ranks limits the influence of extreme cases on the regression outcome and avoids problems relating to violations of the normality assumption.
We tested for effects of laser intensity and overall pain by using R2 change tests. C–P placebo scores were used as the dependent variable. The basic (reduced) model included only administration Order, Reported Placebo (Placebo), and their interaction. To test effects of intensity, we added intensity and its interactions with Placebo and order. Thus, the full model included Order, laser intensity, Placebo, and all two-way interactions. For Cz, The increase in R2 compared with the reduced model was 0.20, F(3, 17) = 2.29, p = .11, which did not provide convincing evidence for intensity effects. In a second analysis, we added overall pain to the reduced model, so that the full model included Order, Placebo, reported overall pain, and all two-way interactions. This model did not account for significantly more of the variance in P2 placebo effects, R2 change = 0.11, F(3, 17) = 1.09, p = .3817. Analyses showed similar results for other electrodes.
References
- Benedetti F, Arduino C, Amanzio M. Somatotopic activation of opioid systems by target-directed expectations of analgesia. J. Neurosci. 1999;19(9):3639–3648. doi: 10.1523/JNEUROSCI.19-09-03639.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromm B, Jahnke MT, Treede RD. Responses of human cutaneous afferents to CO2 laser stimuli causing pain. Exp. Brain Res. 1984;55(1):158–166. doi: 10.1007/BF00240510. [DOI] [PubMed] [Google Scholar]
- Bromm B, Treede RD. Nerve fibre discharges, cerebral potentials and sensations induced by CO2 laser stimulation. Hum. Neurobiol. 1984;3(1):33–40. [PubMed] [Google Scholar]
- Bruner JS, Postman L, Rodrigues J. Expectation and the perception of color. Am. J. Psychol. 1951;64(2):216–227. [PubMed] [Google Scholar]
- Clark WC. Sensory-decision theory analysis of the placebo effect on the criterion for pain and thermal sensitivity. J. Abnorm. Psychol. 1969;74(3):363–371. doi: 10.1037/h0027509. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Anand P, Attal N, Garcia-Larrea L, Haanpaa M, Jorum E, et al. EFNS guidelines on neuropathic pain assessment. Eur. J. Neurol. 2004;11(3):153–162. doi: 10.1111/j.1468-1331.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Chiaradia C, Carotenuto E. The contribution of suggestibility and expectation to placebo analgesia phenomenon in an experimental setting. Pain. 2002;96(3):393–402. doi: 10.1016/S0304-3959(01)00485-7. [DOI] [PubMed] [Google Scholar]
- Duncan GH, Bushnell MC, Bates R, Dubner R. Task-related responses of monkey medullary dorsal horn neurons. J. Neurophysiol. 1987;57(1):289–310. doi: 10.1152/jn.1987.57.1.289. [DOI] [PubMed] [Google Scholar]
- Ericsson KA, Simon HA. Verbal reports as data. Psychol. Rev. 1980;87:215–251. [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nat. Rev. Neurosci. 2004;5(7):565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Frot M, Valeriani M. Brain generators of laser-evoked potentials: from dipoles to functional significance. Neurophysiol. Clin. 2003;33(6):279–292. doi: 10.1016/j.neucli.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Peyron R, Laurent B, Mauguiere F. Association and dissociation between laser-evoked potentials and pain perception. Neuroreport. 1997;8(17):3785–3789. doi: 10.1097/00001756-199712010-00026. [DOI] [PubMed] [Google Scholar]
- Green BG, Cruz A. “Warmth-insensitive fields”: evidence of sparse and irregular innervation of human skin by the warmth sense. Somatosens. Mot. Res. 1998;15(4):269–275. doi: 10.1080/08990229870682. [DOI] [PubMed] [Google Scholar]
- Hrobjartsson A, Gotzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N. Engl. J. Med. 2001;344(21):1594–1602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- Hrobjartsson A, Gotzsche PC. Is the placebo powerless? Update of a systematic review with 52 new randomized trials comparing placebo with no treatment. J. Intern. Med. 2004;256(2):91–100. doi: 10.1111/j.1365-2796.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Leandri M, Truini A, Zambreanu L, Cruccu G, Tracey I. Adelta nociceptor response to laser stimuli: selective effect of stimulus duration on skin temperature, brain potentials and pain perception. Clin. Neurophysiol. 2004;115(11):2629–2637. doi: 10.1016/j.clinph.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Kakigi R, Watanabe S, Yamasaki H. Pain-Related somatosensory evoked potentials. J. Clin. Neurophysiol. 2000;17(3):295–308. doi: 10.1097/00004691-200005000-00007. [DOI] [PubMed] [Google Scholar]
- Keselman HJ. Testing treatment effects in repeated measures designs: an update for psychophysiological researchers. Psychophysiology. 1998;35(4):470–478. [PubMed] [Google Scholar]
- Kienle GS, Kiene H. The powerful placebo effect: fact or fiction? J. Clin. Epidemiol. 1997;50(12):1311–1318. doi: 10.1016/s0895-4356(97)00203-5. [DOI] [PubMed] [Google Scholar]
- Kirsch I. Response expectancy as a determinant of experience and behavior. Am. Psychol. 1985;40:1189–1202. [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc. Natl. Acad. Sci. USA. 2005;102(36):12950–12955. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V, Bruyer R, Guerit JM, Plaghki L. Involuntary orientation of attention to unattended deviant nociceptive stimuli is modulated by concomitant visual task difficulty. Evidence from laser evoked potentials. Clin. Neurophysiol. 2005;116(9):2165–2174. doi: 10.1016/j.clinph.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Rios M, Zirh A, Chau D, Krauss G, Lesser RP. Painful stimuli evoke potentials recorded over the human anterior cingulate gyrus. J. Neurophysiol. 1998;79(4):2231–2234. doi: 10.1152/jn.1998.79.4.2231. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Beck H, Bromm B. Cognitive performance, mood and experimental pain before and during morphine-induced analgesia in patients with chronic non-malignant pain. Pain. 1997a;73(3):369–375. doi: 10.1016/S0304-3959(97)00123-1. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Beck H, Bromm B. Differential changes of laser evoked potentials, late auditory evoked potentials and P300 under morphine in chronic pain patients. Electroencephalogr. Clin. Neurophysiol. 1997b;104(6):514–521. doi: 10.1016/s0168-5597(97)00064-6. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Garcia-Larrea L. Contribution of attentional and cognitive factors to laser evoked brain potentials. Neurophysiol. Clin. 2003;33(6):293–301. doi: 10.1016/j.neucli.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Hauck M, Paur RC, Nakamura Y, Zimmermann R, Bromm B, et al. Cortical correlates of false expectations during pain intensity judgments—a possible manifestation of placebo/nocebo cognitions. Brain Behav. Immun. 2005;19(4):283–295. doi: 10.1016/j.bbi.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Manis M, Biernat M, Nelson TF. Comparison and expectancy processes in human judgment. J. Pers. Soc. Psychol. 1991;61(2):203–211. doi: 10.1037//0022-3514.61.2.203. [DOI] [PubMed] [Google Scholar]
- Matre D, Casey KL, Knardahl S. Placebo-induced changes in spinal cord pain processing. J. Neurosci. 2006;26(2):559–563. doi: 10.1523/JNEUROSCI.4218-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, Casey KL. Sensory, motivational, and central control determinants of pain. In: Kenshalo DR, editor. The Skin Senses. C.C Thomas; Springfield: 1968. pp. 423–439. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moerman DE. Cultural variations in the placebo effect: ulcers, anxiety, and blood pressure. Med. Anthropol. Q. 2000;14(1):51–72. doi: 10.1525/maq.2000.14.1.51. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Keaser ML, Gullapalli RP, Greenspan JD. Regional intensive and temporal patterns of functional MRI activation distinguishing noxious and innocuous contact heat. J. Neurophysiol. 2005;93(4):2183–2193. doi: 10.1152/jn.01025.2004. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing—induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46(6):957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Ingvar M. Imaging cognitive modulation of pain processing. Pain. 2002;95((1–2)):1–5. doi: 10.1016/s0304-3959(01)00467-5. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia—imaging a shared neuronal network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, Benedetti F. Response expectancies in placebo analgesia and their clinical relevance. Pain. 2001;93(1):77–84. doi: 10.1016/S0304-3959(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Porro CA, Cettolo V, Francescato MP, Baraldi P. Functional activity mapping of the mesial hemispheric wall during anticipation of pain. Neuroimage. 2003;19(4):1738–1747. doi: 10.1016/s1053-8119(03)00184-8. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288(5472):1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Price DD, Barrell JJ. Mechanisms of analgesia produced by hypnosis and placebo suggestions. Prog. Brain Res. 2000;122:255–271. doi: 10.1016/s0079-6123(08)62144-5. [DOI] [PubMed] [Google Scholar]
- Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83(2):147–156. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- Rainville P, Hofbauer RK, Paus T, Duncan GH, Bushnell MC, Price DD. Cerebral mechanisms of hypnotic induction and suggestion. J. Cogn. Neurosci. 1999;11(1):110–125. doi: 10.1162/089892999563175. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, McKoon G. Automatic and strategic priming in recognition. J. Verbal Learn. Verbal Behav. 1981;20:204–215. [Google Scholar]
- Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, et al. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. J. Neurosci. 2000;20(19):7438–7445. doi: 10.1523/JNEUROSCI.20-19-07438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour TL, Seifert CM, Shafto MG, Mossmann AL. Using response time measures to assess “Guilty Knowledge”. J. Appl. Psychol. 2000;85(1):30–37. doi: 10.1037/0021-9010.85.1.30. [DOI] [PubMed] [Google Scholar]
- Staats PS, Staats A, Hekmat H. The additive impact of anxiety and a placebo on pain. Pain Med. 2001;2(4):267–279. doi: 10.1046/j.1526-4637.2001.01046.x. [DOI] [PubMed] [Google Scholar]
- Vase L, Riley JL, Price DD. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain. 2002;99(3):443–452. doi: 10.1016/S0304-3959(02)00205-1. [DOI] [PubMed] [Google Scholar]
- Vase L, Robinson ME, Verne GN, Price DD. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. 2005;115(3):338–347. doi: 10.1016/j.pain.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Voudouris NJ, Peck CL, Coleman G. Conditioned response models of placebo phenomena: further support. Pain. 1989;38:109–116. doi: 10.1016/0304-3959(89)90080-8. [DOI] [PubMed] [Google Scholar]
- Wager TD. The neural bases of placebo effects in anticipation and pain. Semin. Pain Med. 2005a;3(1):22–30. [Google Scholar]
- Wager TD. The neural bases of placebo effects in pain. Curr. Dir. Psychol. Sci. 2005b;14(4):175–179. [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J. Neurosci. 2005;25(34):7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Yau WY, Scott DJ, Stohler CS. Belief or need? Accounting for individual variations in the neurochemistry of the placebo effect. Brain Behav. Immun. 2006;20(1):15–26. doi: 10.1016/j.bbi.2005.08.006. [DOI] [PubMed] [Google Scholar]