Abstract
The hippocampus is critical for formation of spatial memories. Hippocampal pyramidal neurons in freely behaving animals exhibit spatially selective firing patterns, which taken together form an internal representation of the environment. This representation is thought to contribute to the hippocampal spatial memory system. Behavioral long-term memories differ from short-term memories in requiring the synthesis of new proteins. Does the development of the internal hippocampal representation also require the synthesis of new proteins? We found that blocking protein synthesis in the brain of mice by 95% does not affect short-term stability of newly formed hippocampal place fields but abolishes stability in the long term. By contrast, inhibiting protein synthesis does not affect the retention and recall of previously established fields in a familiar environment, indicating that protein synthesis-dependent reconsolidation is not required for recall. Our results indicate that place fields parallel both behavioral memories and the late phase of long-term potentiation in requiring the synthesis of new proteins for consolidation.
Keywords: hippocampus, place cells, anisomycin, long-term memory, consolidation
In humans, explicit memory storage is concerned with memories about places, objects, and people and requires selective attention and conscious effort (1). This type of memory requires the hippocampus and associated structures in the medial temporal lobe (2). A major focus in the study of explicit memory has been on the storage of spatial information, or memory about place (3).
In freely moving rodents, pyramidal cells of the CA1 region of the hippocampus exhibit a spatially selective activity pattern, firing only when the animal is in certain cell-specific locations (place fields) in the environment (4). Place fields are environmentally specific; the firing field of a place cell in one environment does not predict its field in another (5). When the animal enters a new environment, new place fields form within a matter of minutes (6, 7) and can be stable for months (8). The long-term stability of place fields implies that the representation is recalled and not created de novo each time the animal enters a familiar environment, as would be expected of elements of a spatial memory system in the hippocampus.
Behavioral memories depend on the synthesis of new proteins for long-term stability. Specifically, it has been found that the initial acquisition and early retention of memory are independent of synthesis of new proteins, whereas protein synthesis during or shortly after training is required for the formation of long-term memories (9). Synaptic models of memory such as long-term potentiation (LTP) also share a similar requirement of protein synthesis (10). The most studied locus of LTP has been that of synapses onto hippocampal pyramidal CA1 cells, the same neurons acting as place cells in behaving animals (11). This makes place cells an ideal system to relate the molecular studies of memory storage to those of behavioral memory.
LTP in the CA1 region requires N-methyl-d-aspartate (NMDA) receptors and has two phases: an early phase that does not require new protein synthesis and a late phase (L-LTP) that does (12-14). We have previously shown that injecting animals with an NMDA receptor antagonist blocks the long-term stability of place fields, whereas the formation and short-term maintenance of the maps are not affected (15). This suggests that the mechanism underlying place-field stability may overlap with those underlying L-LTP. Because the synthesis of new proteins is required both for L-LTP and behavioral memories, we wondered whether protein synthesis is also required for the stabilization of place fields in the hippocampus. Thus, we injected animals with the protein synthesis blocker anisomycin immediately after they had seen a novel environment and compared the stability of the newly formed place field map with that of a previously formed map.
Materials and Methods
Surgery. Young male C57BL6/J mice (10 weeks old, The Jackson Laboratory) were anesthetized with ketamine/xylazine (100 and 7 mg/kg, in saline) and implanted stereotactically (anterior, -1.8 mm; lateral, 1.8 mm; dorsal, 0.9 mm) with a drivable four-tetrode (each wire 25-μm nichrome, California Fine Wire, Grover Beach, CA) microelectrode array ≈0.3 mm dorsal to the CA1 hippocampal pyramidal cell layer. The animals were allowed to recover for 1 week before the start of screening for units.
Recording Environment. At least 7 days after surgery, animals were food-deprived and trained to chase randomly dropped food pellets (Bioserv, Frenchtown, NJ) in a 49-cm-diameter 34-cm-high cylindrical chamber. The familiar environment cylinder was white with several asymmetric orienting cues on its walls. The novel environment cylinder was gray, with one white cue card providing an asymmetric cue. Care was taken to minimize the salience of any cues outside of the cylinders. The arena was surrounded by black curtains, and the only light source was eight symmetrical lights on the ceiling. Animals were always introduced to the environment in the same orientation and always returned to their home cage between recording sessions. To minimize olfactory cues, the floor paper was changed between every reintroduction to the environment, and if urine soaked through the paper, the floor was washed with ethanol.
Screening Procedure. After five training sessions, we started screening the animals for cells while they chased pellets in the chamber. If no complex spike cells were found on the wires, the electrode was advanced no more than 40 μm, and the animal was returned to its home cage until the next day. If complex spikes above 200 μV were found [based on the definition by Fox and Ranck (11)], a test session was done to detect whether units were sufficiently discriminable. When complex spike units that had clear and distinct cluster boundaries had been isolated, a second session was done 1 h after the first session. If the cluster boundaries of the units (see procedure below) were stable at the 1-h time point, an experiment was started. Every animal was screened at least five times before recording, so that it had seen the familiar environment at least 10 times before the first recording.
Experimental Protocol. The animal was recorded in the familiar environment, followed by a recording session in the novel environment. Immediately after the novel environment session, the animal was injected with either drug or saline. One hour after the injection, the animal was recorded in the novel and then the familiar environment. The procedure was repeated 6 and 24 h later (see Fig. 1). Every animal was used only once during this study.
Fig. 1.
Experimental protocol. Recordings were made on 2 consecutive days. On the first day, at least two cells were identified and recorded in the familiar environment, shown here as a white cylinder with a black cue. This was followed by a session in a novel environment: a cylinder of the same size but of a different color, and with different cues on its walls. Immediately after removal from the novel environment, the animal was injected with either drug or saline. The gray box indicates the duration of protein synthesis inhibition. We recorded from the animals in both the novel and familiar environments at 1, 6, and 24 h postinjection.
Injection. Animals were injected with 150 mg/kg anisomycin (in 0.1 ml of PBS) or saline s.c. Protein synthesis inhibitors (PSIs) are known to cause sickness in mice, in particular piloerection, decreased food and water intake, and decreased locomotor drive (9). At our dosage of anisomycin, the animals had significantly decreased locomotor drive, as is evident in the rate maps for the 1-h-postinjection sessions (see Fig. 2 and Results). However, place cell activity was still present, and correlations between before and during PSI were no different from before-before or after-after correlations.
Fig. 2.
Firing fields in familiar and novel environments. Examples of the firing fields of four cells from a saline-injected mouse and four cells from an anisomycin-injected mouse. (A and B) Place fields of saline-injected mouse in familiar (A) and novel (B) environments. Firing-rate maps for each pyramidal cell are shown as a row. The rate maps are shown in the time order they were performed. (C and D) Place fields of anisomycin-injected mouse in familiar (C) and novel (D) environments. Each square pixel in a rate map represents a 2 × 2-cm area in the apparatus. Yellow encodes regions where the animal visited and the cell never fired. Orange, red, green, blue, and purple pixels encode progressively higher firing rates and are autoscaled in each session. (Peak firing rates for all cells are in Table 1.) White pixels were not visited. (E) Waveforms of the four cells from the saline-injected mouse shown in A and B, during the first and last session of the experiment. (F) Waveforms of the four cells from the anisomycin-injected mouse shown in C and D, during the first and last session of the experiment.
Recording and Cluster Isolation. Units were amplified ≈10,000 times by using an eight-channel amplifier (Neuralynx, Tucson, AZ) and bandpass filtered at 300-10,000 Hz. The amplifier output was digitized at 40 KHz and acquired into the computer by using discovery ver. 6.1 software package (Datawave, Longmont, CO). Data were recorded in the tetrode or occasionally stereotrode configuration; no single-wire recordings are included in the data set. To be recorded, cells had to be at least 200 μV, and the noise was typically 50 μV. The number of cells per animal simultaneously recorded during an experiment ranged from 4 to 12. All recording sessions were 16 min long. The position of the animal in the chamber was recorded simultaneously with the recording of the neuronal firing, as described (15). In this way, we could measure the firing rate of each cell as a function of the animal's head position within the cylinder.
Units were analyzed offline manually (cluster cutting) with the autocut software package (Datawave). Clusters isolated were clear Gaussian ellipses generally based on peak-to-peak projections of different tetrode wires with minimal overlap with neighboring clusters or noise. They were also inspected to ensure that the complex spike interval (4-7 msec) was the largest bin in an autocorrelogram, to ensure analysis of only complex spike units.
Only units with the exact same cluster boundaries throughout the experiment (and that had essentially no overlap with either other clusters or noise) are included in the analysis. Cluster stability was the sole criterion used for deciding that cells were acceptable for inclusion in the final dataset. If the cluster boundaries of a unit drifted during the course of an experiment, it was excluded from the experiment; the other units from the same animal that had maintained their cluster boundaries were included.
Our screening procedure is specifically designed to look for long-term stable recordings from a few animals rather than recordings from more animals that may not stay stable. Of a large number of surgeries performed, 28 animals yielded complex spike cells >200 μV. Of those 28, 16 were either never started on an experiment or were abandoned midway due to recording instability. From the rest of the 12 experiments, one was excluded because of recording instability at the 6-h time point. There are 11 experiments in the study, of which data from two are included only through the 6-h time point because of instability at the 24-h time point. Data from nine experiments are included through the 24-h time point. Although, as always with unit recording studies, it is possible that in some cases a unit was really two neurons, this would work against our main findings by increasing the variability of the data.
Data Analysis. Correlation coefficients (Pearson's linear correlation) were calculated by treating the rate maps produced by a given cell in two sessions as two lists of numbers. Only those pixels that the animal visited during both sessions were considered for calculating correlations (each square pixel in a rate map represents a 2 × 2-cm area in the apparatus). Cells that had a peak firing rate of less than one spike per second or a coherence <0.25 in both sessions were excluded from quantitative analysis.
We calculated a similarity score for a session pair by computing the correlation between the firing rates on a pixel-by-pixel basis. Because the correlation scores of cells within an animal tended to be more similar than between animals, treating cells as independent observations would lead to overestimates of the significance levels. We corrected for this data clustering by performing ANOVAs with cells as a random effect nested within animals, by using the statistical software sas (SAS Institute, Cary, NC) as described in Singer (16). The figures show the means and standard errors estimated in the ANOVA.
Coherence was calculated as the correlation coefficient between the rate of a pixel and the average rate of its eight nearest neighbors (17). Information content was calculated as ΣPi(Ri/ R)log2(Ri/R), where i is the bin number, Pi is the probability for occupancy of bin i, Ri is the mean firing rate for bin i, and R is the overall mean firing rate (18). All statistical analysis was done on the basis of number of animals.
For calculating the similarity slope of two rate maps, we calculated a slope for each pixel across those two rate maps (y = rate difference, x = time difference) and averaged the slopes of all pixels with rates in the top fifth percentile of each rate map.
For rotational analysis, every cell was correlated with its counterpart in a second session after successive 5° rotations around the center. Similarity scores >0.1 were treated as high enough to consider that the field may have rotated. For comparisons to random cells, every cell in our experiments was compared to a different randomly selected cell out of a database of 1,144 mouse place-cell firing-rate maps (none of which were used in this experiment).
Histological Procedures. After completion of experiments, the mice were anesthetized by using Isoflurane (Abbott) and decapitated. Iron was deposited from the electrode wire, which had given cells by passing 10 μA of current. The craniotomized brain was fixed overnight for Prussian blue staining in a 10% formalin solution containing 3% potassium ferrocyanide. The brain was then dehydrated through a graded alcohol series and lightly stained with cresyl violet. Brains were sectioned by a Vibratome (Vibratome, St. Louis) at 50 μm in the coronal plane. The sections were then dehydrated, mounted on slides, and coverslipped.
Results
To investigate the role of protein synthesis in the stability of place fields, we studied the effect of protein synthesis inhibition under two conditions: (i) when the representation of the environment is newly formed, and (ii) when the representation has been stably established earlier. Our experimental strategy is summarized in Fig. 1. After their first time in the novel environment, animals were randomly injected with either saline or 150 mg/kg anisomycin s.c. At this dosage, anisomycin inhibits cerebral protein synthesis by ≈95% and lasts 4-5 h (19).
Examples of firing patterns in the two environments of four pyramidal cells simultaneously recorded from a saline-injected mouse are shown in Fig. 2 A and B, and four pyramidal cells simultaneously recorded from an anisomycin-injected mouse are shown in Fig. 2 C and D. Each row shows firing-rate maps for a single cell during eight recording sessions over 2 days. The maps are first grouped according to the environment (familiar environment, Fig. 2 A-D Left; novel environment, Fig. 2 A-D Right) and then by the time order of the session. Fig. 2 E and F show the waveforms of the cells shown in Fig. 2 A-D during the first and last sessions of the experiment, and Table 1 shows the peak firing rates of the cells shown in Fig. 2 A-D.
Table 1.
Peak firing rates (spikes per second) for the cells shown in Fig. 2
| Environment
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Familiar | Novel | |||||||
| Saline | 3.02 | 2.89 | 2.36 | 2.09 | 4.04 | 3.56 | 3.46 | 1.62 |
| 6.85 | 5.14 | 5.33 | 5.17 | 11.57 | 10.58 | 9.85 | 8.86 | |
| 13.71 | 10.00 | 9.04 | 13.71 | 8.06 | 6.79 | 15.01 | 9.47 | |
| 11.31 | 8.69 | 9.48 | 8.48 | 4.55 | 11.10 | 7.53 | 7.93 | |
| Anisomycin | 15.36 | 13.11 | 12.41 | 9.03 | 10.42 | 22.00 | 5.16 | 4.28 |
| 15.84 | 9.30 | 18.70 | 12.38 | 18.50 | 25.71 | 7.27 | 5.19 | |
| 13.36 | 17.14 | 18.46 | 11.41 | 9.90 | 13.02 | 10.91 | 7.80 | |
| 7.50 | 8.02 | 13.33 | 13.54 | 12.95 | 12.78 | 12.07 | 14.24 | |
Protein Synthesis Inhibition Abolishes the Long-Term Stability of Newly Formed Place Fields. The results show that under protein synthesis inhibition, place cells in the novel environment were stable in the short term (1 h) but not for a long period (6 and 24 h). The representation of the novel environment on the second day bore no resemblance to that of the first day, as if the animal had never seen the environment before. The same cells had stable fields in the familiar environment, indicating that the representation of the familiar environment was not affected by protein synthesis inhibition.
To quantify these results, we compared positional firing patterns in pairs of sessions to look for stability of new fields before, during, and after protein synthesis inhibition. We calculated a similarity score for a session pair by computing the correlation between the firing rates on a pixel-by-pixel basis (see Materials and Methods).
The high similarity for the novel environment 1 h after injection (see Fig. 3A) indicates that new protein synthesis is not required for the acquisition and short-term stability of place fields. This is in agreement with behavioral memory (9) as well as LTP (12-14, 20), both of which do not require protein synthesis for short-term maintenance. The stability of place-cell firing fields for the novel environment is significantly impaired 6 h after anisomycin injection (n = 34 cells from six animals, P = 0.0009). The new place fields formed in the novel environment 6 h later were then stable 24 h later (Fig. 3), indicating that anisomycin did not destroy the ability to form stable place fields; it just abolished the stability of the initial place fields. This is similar to our earlier study of place fields with the N-methyl-d-aspartate receptor antagonist (±)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphoric acid (15).
Fig. 3.
Similarity scores for the familiar and novel environments. Comparisons of firing pattern similarity in pairs of sessions at the 1-, 6-, and 24-h time points, in the novel (A) and familiar (B) environments. Firing patterns at each time point were compared with the previous session in that environment. Each comparison is shown as two bars indicating the mean similarity score (±SEM) for saline-injected mice (gray, n = 28 cells at the 6-h time point from five animals) and anisomycin-injected mice (black, n = 34 cells at the 6-h time point from six animals). Asterisk indicates statistical significance by a two-tailed Student's t test (P = 0.0009).
The instability in place fields 6 h later is not a result of recording instability. We know this not only because of our stringent unit assignment criteria but also because we recorded the same cells in the familiar environment during the experiment, where the fields were stably maintained during the entire 2-day period (Figs. 2 and 3B and see below).
The Stability and Recall of Existing Place Fields Is Not Affected by Protein Synthesis Inhibition. Interspersed between recordings from an animal in the novel environment, we also recorded from the same neurons in a familiar environment (animals had seen the familiar environment at least 10 times before the experiment started). Our results indicate that protein synthesis inhibition had no effect on either the short-term or long-term stability of the previously formed fields of the familiar environment (Fig. 3). Even as the fields of the novel environment were abolished, those of the familiar environment were maintained.
Behavioral memories are susceptible to blockade by protein synthesis inhibitors either during or immediately after the initial storage of the memory (21, 22); later exposure does not affect the memory (9, 23) (although see below). This is also true for the long-lasting L-LTP, which can be blocked by inhibitors of protein synthesis but only if delivered during or immediately after the LTP-inducing tetanus. Consistent with these results, we find that the hippocampal representation of an already familiar environment is unaffected by blocking protein synthesis in the brain.
We found a few instances of place-field instability in saline-injected animals, even though the recording was stable, as has been previously reported for mice (24). All cells were included in the statistics; this instability is randomly distributed among the various sessions and so works against our results.
To test the possibility of anisomycin blocking reconsolidation of the familiar environment (25), we calculated a similarity slope of the initial rate map to the corresponding rate maps at the 6- and 24-h time points (see Materials and Methods). We found no difference in the slopes at the two time points (P = 0.46), making it unlikely that anisomycin blocks reconsolidation of the familiar environment.
The Effects of Anisomycin Are Not Due to Field Rotations. To investigate whether the new fields in the novel environment could result from rotations of the previous fields around the center, we correlated the firing patterns of each cell in the first novel environment session to the 6-h-postinjection novel environment session after repeated rotations. We found that 8 of 35 cells could have had rotated fields (see Materials and Methods). When the fields did seem to rotate, they did not do so in tandem: the angle for best correlation varied from 110° to 345°, with no apparent clustering of simultaneously recorded units. Moreover, in a circular environment, fields can appear to rotate purely by chance because the number of field shapes is limited. To find out how likely it is that fields could appear to rotate just by chance, we compared every cell in our experiments to a different randomly selected cell out of a database of 1,144 mouse place-cell firing-rate maps (none of which were used in this experiment). We found that of 469 individual rate maps used in our study, 98 could be considered to be rotations of randomly selected maps. So our observation that 8 of 35 cells appear to be rotations could occur purely by chance (Student's t test, P > 0.5). Therefore our results remain consistent with protein synthesis abolishing the long-term stability of place fields.
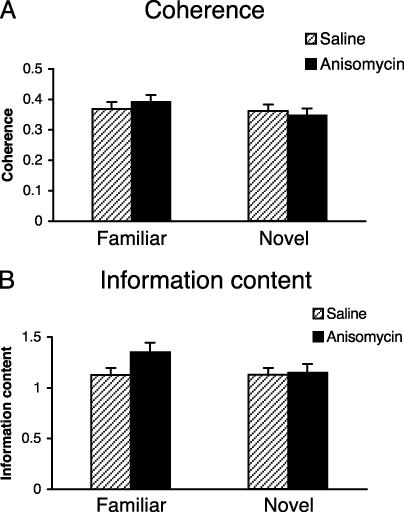
Our findings support a direct correlation between place fields and spatial memory, both of which are similarly susceptible to blockers of protein synthesis. The difference between the anisomycin and saline groups cannot be explained by the fact that cells in the novel environment are less spatial in the animals injected with anisomycin, because information content as well as coherence values of cells recorded in the novel environment in the anisomycin and saline conditions were statistically identical (two-tailed Student's t test, P > 0.5). Fig. 4 shows the coherence and information content values of the cells under all four experimental conditions.
Fig. 4.
Coherence and information content values in the familiar and novel environments. Coherence (A) and information content (B) of place cell rate maps in the familiar and novel environments. Each bar represents the pooled data from all sessions recorded in that environment. The two bars indicate the mean similarity score (±SEM) for saline-injected (gray) and anisomycin-injected (black) mice.
Anisomycin and State-Dependent Learning. A drug such as anisomycin could act as a discriminative stimulus for state-dependent learning (26). The combination of drug and initial exposure to the novel environment could effectively provide a different environment than the novel environment alone 6 h later, when the drug has ceased to act. We specifically designed our experiment to circumvent this problem by injecting anisomycin after the first exposure to the novel environment. Thus, as described, there cannot be any difference in state between the time when the animal is first exposed to the novel environment and the time when the animal is exposed to the environment after the effect of anisomycin has worn off. If the place-cell firing fields are different between those two sessions (as they are in our case), it can result only from abolition of the newly formed place fields by anisomycin.
Discussion
Declarative memories require the hippocampus for their storage and protein synthesis for their consolidation. This requirement for protein synthesis is also shared by synaptic models of memory-like LTP. In particular, the L-LTP in the CA1 region of the hippocampus is disrupted by inhibitors of protein synthesis when delivered during and immediately after the induction protocol but not 30-45 min later (13, 20). It is assumed that protein synthesis is required for long-term synaptic changes that maintain the newly formed memory.
Because place cells represent the in vivo activity of the primary neurons of the hippocampus, they form a bridge between behavioral memories and in vitro studies of plasticity-like LTP. Our results provide previously undescribed evidence that there is a protein synthesis-dependent consolidation step in the formation of a stable hippocampal representation of an environment. Retrieval and recall of already existing fields were not affected by protein synthesis inhibition, a phenomenon also observed for behavioral memory storage. This suggests that the reason for the amnesic effect of anisomycin is an unstable hippocampal representation, implicating this representation in the storage of the memory itself.
Sensory Predetermination of Place Fields. Most maps in the central nervous system are determined almost exclusively by their sensory inputs. For example, a receptive field map in the primary visual cortex depends on the exact shape and position of a stimulus on the retina. Place fields have been thought not to be directly determined by sensory input alone, reflecting the complex and processed nature of the input into the hippocampus. Our experiments with protein synthesis inhibition have allowed us an interesting perspective into the sensory predetermination of place fields.
Animals exposed to anisomycin have a different map (set of fields) during the first exposure to the novel environment and exposure to the novel environment after the effect of anisomycin has worn off. As stated above, there is no difference in state between these two conditions. Thus, exactly the same sensory stimuli gave rise to more than one map. The first map of the environment, formed during initial exposure, was disrupted by anisomycin, and the next time the animal saw the environment after drug exposure, it generated another map de novo, quite different from the first. Together with a previous report with novel environments (27) and as reported for aging animals (28), this argues against any hard-wiring of place fields, unlike what has previously been reported for task-related neurons in the hippocampus (29).
Place Cells and General Sickness. Although it is possible that the abolition of place-field stability may be the result of general sickness, it is unlikely, because place fields in the familiar environment remained stable throughout the experiment. Moreover, under the influence of anisomycin, when the animals were exhibiting sickness and thigmotaxis (see Materials and Methods and Fig. 2), place fields remained intact and stable in both the familiar and novel environments. This strongly suggests that place perception of the animal remains intact during the duration of protein synthesis inhibition.
That place fields of the familiar environment remained stable despite protein synthesis inhibition implies that anisomycin did not affect the stability of well established place fields. But there is also a body of literature showing that amnesia can be obtained for a well established memory if the memory trace is reactivated just before amnesic treatment.
Does Anisomycin Disrupt Memories When They Are Retrieved? Nader et al. (25) found that consolidated auditory fear memories, when reactivated during retrieval, become susceptible to blockade by the protein synthesis inhibitor anisomycin. The same treatment with anisomycin, in the absence of memory reactivation, left the memory intact. Debiec et al.¶ have extended this result to contextual fear memories, which depend on the hippocampus for their storage. In our experiments, we find that reactivation of place fields of the familiar environment in the presence of (or followed by) anisomycin did not affect the stability of the hippocampal place fields of that environment. In this sense, our results seem to be at odds with those of Nader et al. (25) and Debiec et al.¶ However, there are several factors that may explain this apparent discrepancy. Aside from using different species, there are two key differences between our experimental designs.
First, our mode of administration of drug is different: we used i.p. injections, as compared to direct brain injections in those studies. We used a dosage of anisomycin that is known to block cerebral protein synthesis by ≈95% (19) and is known to cause long-term deficits in spatial task performance (30). At this dosage, the familiar environment fields remained intact. Second, and perhaps more important whereas our animals had seen the familiar environment at least 10 times, the animals in the studies by Nader et al. (25) and Debiec et al.¶ had been exposed to the stimulus only once before the session that was followed by injection.
There is extensive documentation that memories are labile for a short period after acquisition and are subsequently resistant to amnesic treatments (31, 32). It has been proposed that exposing an already established memory to reconsolidation increases its resistance to future disruption by creating a new memory trace; a multiplicity of traces accounts for the increased resistance of older memories for disruption (33). Recently, Milekic and Alberini (34) found a temporally graded requirement for protein synthesis after memory reactivation. Our results support this notion that there is a gradation of lability for newly stored memories, with those that have been retrieved the fewest number of times being the most labile.
Acknowledgments
This work was supported by Howard Hughes Medical Institute, the National Institutes of Health, and National Institute of Mental Health.
Abbreviations: LTP, long-term potentiation; L-LTP, late-phase LTP.
Footnotes
Debiec, J., LeDoux, J. E. & Nader, K. (2001) Soc. Neurosci. Abstr. 27, 187.10.
References
- 1.Squire, L. R. & Kandel, E. R. (1999) Memory: From Mind To Molecules (Scientific American Library, New York).
- 2.Scoville, W. B. & Milner, B. (1957) J. Neurol. Neurosurg. Psychiatry 20, 11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris, R. G. M. (2001) Philos. Trans. R. Soc. London B 356, 1453-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Keefe, J & Nadel, L. (1978) The Hippocampus as a Cognitive Map (Clarendon Press, Oxford, U.K.).
- 5.Muller, R. U. & Kubie, J. L. (1987) J. Neurosci. 7, 1951-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bostock, E., Muller, R. U. & Kubie, J. L. (1991) Hippocampus 1, 193-206. [DOI] [PubMed] [Google Scholar]
- 7.Wilson, M. A. & McNaughton, B. L. (1993) Science 261, 1055-1058. [DOI] [PubMed] [Google Scholar]
- 8.Thompson, L. T. & Best, P. J. (1989) J. Neurosci. 9, 2382-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, H. P. & Squire, L. R. (1984) Psychol. Bull. 96, 518-559. [PubMed] [Google Scholar]
- 10.Bliss, T. V. P. & Collingridge, G. L. (1993) Nature 361, 31-39. [DOI] [PubMed] [Google Scholar]
- 11.Fox, S. E. & Ranck, J. B., Jr. (1975) Exp. Neurol. 49, 299-313. [DOI] [PubMed] [Google Scholar]
- 12.Stanton, P. K. & Sarvey, J. M. (1984) J. Neurosci. 4, 3080-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frey, U., Krug, M., Reymann, K. G. & Matthies, H. (1988) Brain Res. 452, 57-65. [DOI] [PubMed] [Google Scholar]
- 14.Huang, Y.-Y., Nguyen, P. V., Abel, T. & Kandel, E. R. (1996) Learn. Mem. 3, 74-85. [DOI] [PubMed] [Google Scholar]
- 15.Kentros, C., Hargreaves, E., Hawkins, R. D., Kandel, E. R., Shapiro, M. & Muller, R. U. (1998) Science 280, 2121-2126. [DOI] [PubMed] [Google Scholar]
- 16.Singer, J. D. (1998) J. Educ. Behav. Stat. 23, 323-355. [Google Scholar]
- 17.Rotenberg, A., Mayford, M., Hawkins, R. D., Kandel, E. R. & Muller, R. U. (1996) Cell 87, 1351-1361. [DOI] [PubMed] [Google Scholar]
- 18.Markus, E. J., Barnes, C. A., McNaughton, B. L., Gladden, V. L. & Skaggs, W. E. (1994) Hippocampus 4, 410-421. [DOI] [PubMed] [Google Scholar]
- 19.Flood, J. F., Rosenzweig, M. R., Bennett, E. L. & Orme, A. E. (1973) Physiol. Behav. 10, 555-562. [DOI] [PubMed] [Google Scholar]
- 20.Frey, U., Huang, Y.-Y. & Kandel, E. R. (1993) Science 260, 1661-1664. [DOI] [PubMed] [Google Scholar]
- 21.Meiri, N. & Rosenblum, K. (1998) Brain Res. 789, 48-55. [DOI] [PubMed] [Google Scholar]
- 22.Lattal, K. M. & Abel, T. (2001) J. Neurosci. 21, 5773-5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourtchouladze, R., Abel, T., Berman, N., Gordon, R., Lapidus, K. & Kandel, E. R. (1998) Learn. Mem. 5, 365-374. [PMC free article] [PubMed] [Google Scholar]
- 24.Rotenberg, A., Abel, T., Hawkins, R. D., Kandel, E. R. & Muller, R. U. (2000) J. Neurosci. 20, 8096-8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nader, K., Schafe, G. E. & LeDoux, J. E. (2000) Nature 406, 722-726. [DOI] [PubMed] [Google Scholar]
- 26.Kumar, R., Stolerman, I. P. & Steinberg, H. (1970) Annu. Rev. Psychol. 21, 595-628. [DOI] [PubMed] [Google Scholar]
- 27.McNaughton, B. L., Barnes, C. A., Gerrard, J. L., Gothard, K., Jung, M. W., Knierim, J. J., Kudrimoti, H., Qin, Y., Skaggs, W. E., Suster, M., et al. (1996) J. Exp. Biol. 199, 173-185. [DOI] [PubMed] [Google Scholar]
- 28.Barnes, C. A., Suster, M. S., Shen, J. & McNaughton, B. L. (1997) Nature 388, 272-275. [DOI] [PubMed] [Google Scholar]
- 29.Hampson, R. E., Simeral, J. D. & Deadwyler, S. A. (1999) Nature 402, 610-614. [DOI] [PubMed] [Google Scholar]
- 30.Squire, L. R. & Barondes, S. H. (1974) Brain Res. 66, 301-308. [Google Scholar]
- 31.Müller, G. E. & Pilzecker, A. (1900) Z. Psychol. S1, 1-288. [Google Scholar]
- 32.McGaugh, J. L. (2000) Science 287, 248-251. [DOI] [PubMed] [Google Scholar]
- 33.Nadel, L. & Land, C. (2000) Nat. Rev. Neurosci. 1, 209-212. [DOI] [PubMed] [Google Scholar]
- 34.Milekic, M. H. & Alberini, C. M. (2002) Neuron 36, 521-525. [DOI] [PubMed] [Google Scholar]