ABSTRACT
Bacteria that express contact-dependent growth inhibition (CDI) systems outcompete siblings that lack immunity, suggesting that CDI mediates intercellular competition. To further explore the role of CDI in competition, we determined the target cell range of the CDIEC93 system from Escherichia coli EC93. The CdiAEC93 effector protein recognizes the widely conserved BamA protein as a receptor, yet E. coli EC93 does not inhibit other enterobacterial species. The predicted membrane topology of BamA indicates that three of its extracellular loops vary considerably between species, suggesting that loop heterogeneity may control CDI specificity. Consistent with this hypothesis, other enterobacteria are sensitized to CDIEC93 upon the expression of E. coli bamA and E. coli cells become CDIEC93 resistant when bamA is replaced with alleles from other species. Our data indicate that BamA loops 6 and 7 form the CdiAEC93-binding epitope and their variation between species restricts CDIEC93 target cell selection. Although BamA loops 6 and 7 vary dramatically between species, these regions are identical in hundreds of E. coli strains, suggesting that BamAEcoli and CdiAEC93 play a role in self-nonself discrimination.
IMPORTANCE
Contact-dependent growth inhibition (CDI) systems are widespread among Gram-negative bacteria, enabling them to bind to neighboring bacterial cells and deliver protein toxins that inhibit cell growth. In this study, we tested the role of CDI in interspecies competition using intestinal isolate Escherichia coli EC93 as an inhibitor cell model. Although E. coli EC93 inhibits different E. coli strains, other bacterial species from the intestine are completely resistant to CDI. We show that resistance is due to small variations in the CDI receptor that prevent other species from being recognized as target cells. CDI receptor interactions thus provide a mechanism by which bacteria can distinguish siblings and other close relatives (self) from more distant relatives or other species of bacteria (nonself). Our results provide a possible means by which antimicrobials could be directed to one or only a few related bacterial pathogens by using a specific receptor “zip code.”
Introduction
Bacterial contact-dependent growth inhibition (CDI) was discovered and characterized in Escherichia coli strain EC93. This enteric isolate uses the CdiB/CdiA two-partner secretion system to inhibit the growth of other E. coli strains upon direct cell-to-cell contact (1). Based on other two-partner systems, CdiBEC93 is localized to the outer membrane and mediates the export of CdiAEC93 (1, 2). CdiAEC93 is a hemagglutinin (HA) repeat protein that is predicted to form a long β-helical filament extending from the surface of E. coli EC93 cells (1, 3). CDIEC93 toxin activity is contained within the C-terminal 224 residues of CdiAEC93 (CdiA-CTEC93), and this domain inhibits growth when expressed inside E. coli cells (4, 5). CdiA-CTEC93-mediated inhibition is associated with dissipation of the proton motive force and low ATP levels (5), suggesting that the toxin forms a pore in the inner membrane of target bacteria. E. coli EC93 protects itself from this activity by producing a small CdiIEC93 immunity protein that is encoded immediately downstream of cdiAEC93 (1, 5). The precise mechanism of immunity is unknown, but CdiIEC93 contains two predicted transmembrane regions, suggesting that it localizes to the inner membrane, where it blocks toxin activity. CDI systems are also found in other E. coli isolates and a variety of alpha-, beta-, and gammaproteobacteria (4, 6). Within a given genus, CdiA proteins are typically conserved throughout much of their length but the CdiA carboxy-terminal toxin regions (CdiA-CTs) are highly variable (4, 6). Additionally, the predicted CdiI immunity proteins are also diverse, suggesting that cdi loci constitute a family of polymorphic toxin/immunity pairs (6, 7). This hypothesis is supported by studies showing that many CdiA-CTs have distinct nuclease activities that are specifically neutralized by their cognate CdiI proteins (4, 8, 9).
CDI toxin delivery has been studied most extensively in the CDIEC93 system. Genetic selections for CDIEC93-resistant mutants revealed two target cell proteins that are required for growth inhibition. A transposon insertion in the bamA promoter region renders E. coli partially resistant to CDIEC93 because of decreased BamA expression (10). BamA is an essential outer membrane protein (OMP) that forms the core of the β-barrel assembly machine (BAM) complex (11–16). The BAM complex is required for assembly of β-barrel proteins into the outer membrane, but notably, the biogenesis function of BamA is not required for susceptibility to CDIEC93 (10). BamA plays a critical role in cell-cell adhesion during CDIEC93, and binding interactions between inhibitor and target cells are blocked by anti-BamA antibodies (10). Anti-BamA antibodies also protect target cells from CDIEC93-mediated growth inhibition. These observations suggest that BamA is the receptor for CdiAEC93. Additionally, acrB null mutations confer resistance to CDIEC93 (10). AcrB is a trimeric inner membrane protein that functions together with AcrA and TolC as a multidrug efflux pump (17, 18). However, acrA and tolC mutants have no resistance phenotype (10), indicating that the role of AcrB in CDIEC93 is distinct from its efflux function. The localization of AcrB suggests that this protein could facilitate CdiA-CTEC93 insertion into the membrane or perhaps stabilize the pore once assembled. Together, these studies have led to a model postulating that the CdiAEC93 effector exploits specific cell envelope proteins to deliver and activate its toxin domain.
E. coli EC93 inhibits a variety of E. coli strains (1, 5, 10), but its activity against other bacterial species has not been examined. BamA and AcrB are both well conserved among enterobacteria, raising the possibility that E. coli EC93 could inhibit other species. However, we find that the growth of Salmonella enterica serovar Typhimurium, Citrobacter freundii, Enterobacter aerogenes, Enterobacter cloacae, and Proteus mirabilis cells is not inhibited during coculture with E. coli EC93. BamA from these enterobacteria has 73% to 93% sequence identity with E. coli BamA, but sequence differences are concentrated within three of the predicted extracellular loops. These observations suggest that CdiAEC93 is unable to bind BamA from other species because of surface residue variability. In accord with this hypothesis, expression of E. coli bamA sensitizes other species to CDIEC93. Furthermore, replacement of the E. coli bamA gene with alleles from other species renders cells resistant to inhibition. Using chimeric receptors, we localized the CdiAEC93-binding region to extracellular loops 6 and 7 of E. coli BamA. These findings demonstrate that CDIEC93 is restricted at the level of target cell selection and suggest that CDI may function more broadly in kin selection.
RESULTS
Diverse enterobacteria are resistant to CDIEC93.
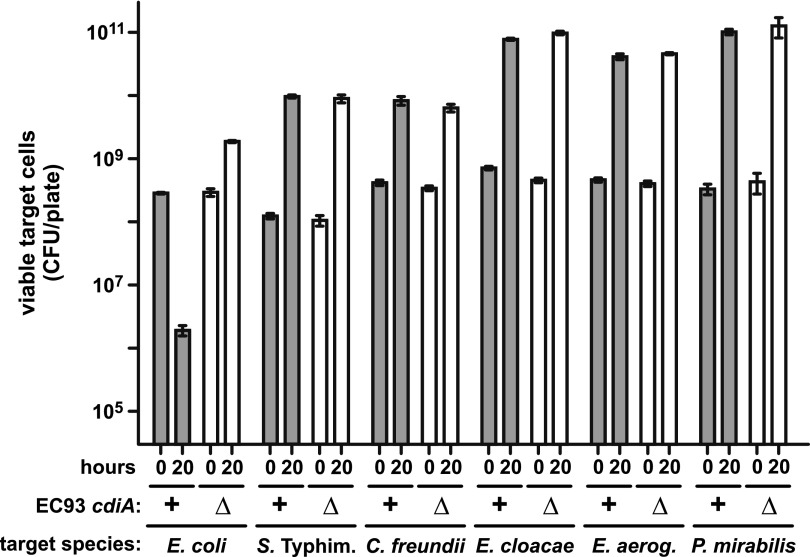
To test whether CDIEC93 exhibits cross-species inhibition activity, we examined the growth of Enterobacter aerogenes (ATCC 13048), Enterobacter cloacae (ATCC 13047), Citrobacter freundii (ATCC 8090), Proteus mirabilis (ATCC 7002), and Salmonella Typhimurium LT2 cells in cocultures with E. coli EC93. E. coli EC93 cells were suspended with target bacteria at a 10:1 ratio, and the suspension was plated onto LB agar at high density to facilitate cell-cell contact. Under these conditions, viable E. coli MC4100 target cell counts decreased ~150-fold after 20 h of coculture with E. coli EC93 (Fig. 1). This inhibition is attributable to CDIEC93 because E. coli MC4100 cell counts increased 6-fold in mock competitions with E. coli EC93 ∆cdiA mutant cells (Fig. 1). In contrast, all other target species grew to about the same cell density when cocultured with wild-type E. coli EC93 (CDI+) or the E. coli EC93 ∆cdiA mutant (CDI−) (Fig. 1). Thus, other species are resistant to CDIEC93, suggesting that E. coli EC93 only inhibits related E. coli strains. We also measured E. coli EC93 growth during interspecies competition and found that cell counts increased 9-fold to 50-fold in all cocultures except those with E. cloacae, in which E. coli EC93 cells did not increase significantly (see Fig. S1 in the supplemental material). Because the cultures were seeded with 10-fold more E. coli EC93 cells, the latter data suggest that E. cloacae ATCC 13047 inhibits E. coli EC93.
FIG 1 .
Enterobacteria are resistant to CDIEC93. Wild-type E. coli EC93 (cdiA+) or E. coli EC93 ∆cdiA mutant cells were mixed with the indicated target species at a 10:1 ratio, and the suspension were incubated on LB agar for 20 h. Cells were harvested, washed, and replated on LB agar supplemented with streptomycin to enumerate viable target cells as CFU. All competitions were conducted at least twice, and the reported values are the mean ± the standard error of the mean.
E. coli bamA sensitizes other enterobacterial species to CDIEC93.
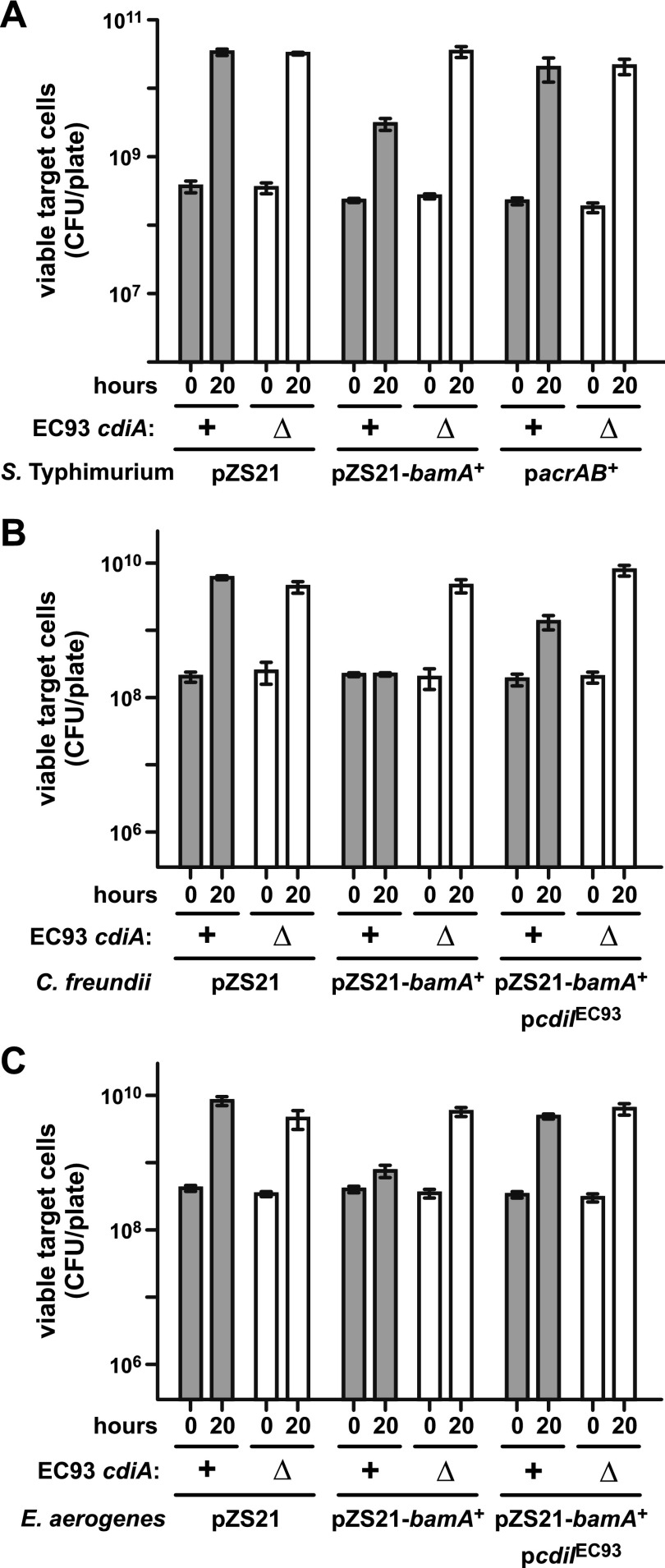
Mutations that decrease bamA or acrB expression confer CDIEC93 resistance on E. coli cells (10). Each of the tested target species contains bamA and acrB genes that are homologous to but distinct from those of E. coli (see Fig. S2 and S3 in the supplemental material). Therefore, we tested whether E. coli alleles of bamA (bamAEcoli) and acrB (acrBEcoli) are required for susceptibility to CDIEC93. We introduced plasmids (see Text S1 in the supplemental material) carrying either bamAEcoli (pZS21-bamA+) or acrBEcoli (pacrAB+) into S. Typhimurium and cultured the resulting strains with E. coli EC93 on LB agar. S. Typhimurium targets harboring pZS21-bamA+ grew to a 5-fold lower level than cells with the empty plasmid vector (Fig. 2A). In principle, this growth inhibition could reflect toxicity from heterologous bamAEcoli expression. However, S. Typhimurium cells carrying pZS21-bamA+ were not inhibited in cocultures with E. coli EC93 ∆cdiA mutant cells (Fig. 2A), indicating that inhibition is due to CDIEC93. In contrast, S. Typhimurium cells carrying plasmid pacrAB+ were not inhibited during coculture with E. coli EC93 (Fig. 2A). These results suggest that other species are resistant to CDIEC93 because they lack the BamA receptor allele from E. coli. We extended this analysis to E. aerogenes and C. freundii and found that plasmid pZS21-bamA+ also sensitizes these bacteria to CDIEC93 (Fig. 2B and C). In each instance, growth inhibition was dependent upon a functional CDIEC93 system and the sensitized bacteria were protected by the expression of a plasmid-borne cdiIEC93 immunity gene (Fig. 2B and C). Thus, expression of BamAEcoli in normally resistant enterobacterial species renders them susceptible to growth inhibition by E. coli EC93.
FIG 2 .
Expression of bamAEcoli sensitizes enterobacteria to CDIEC93. (A) Competitions between E. coli EC93 and S. Typhimurium target cells carrying plasmid pZS21 or plasmids that express E. coli bamA or acrAB. (B) Competitions between E. coli EC93 and C. freundii cells carrying plasmid pZS21 or pZS21-bamA+. Plasmid pcdiIEC93 corresponds to pDAL741 and constitutively expresses the cdiI immunity gene from E. coli EC93. (C) Competitions between E. coli EC93 and E. aerogenes cells carrying plasmid pZS21, pZS21-bamA+, and/or pcdiIEC93, as indicated. For all competitions, wild-type E. coli EC93 (cdiA+) or E. coli EC93 ∆cdiA mutant cells were cocultured with target bacteria at a 10:1 ratio on LB agar for 20 h. Cells were harvested, washed, and replated on LB agar supplemented with streptomycin for enumeration of viable target cells as CFU. All competitions were conducted at least twice, and the reported values are the mean ± the standard error of the mean.
Heterologous bamA confers CDIEC93 resistance on E. coli.
A previous study proposed that the extracellular regions of BamA vary between different species (19), but the structure of the BamA β-barrel domain is unknown. Therefore, we generated a topological model of BamA based on the crystal structure of the homologous FhaC protein from Bordetella pertussis (2). We then aligned 41 enterobacterial BamA sequences and projected the results onto the topology model as a conservation heat map (Fig. 3A). Strikingly, the regions of lowest conservation (≤30% identity) lie within loops 4, 6, and 7, which are the longest of the predicted extracellular loops (Fig. 3A). In accord with these observations, BamA proteins from E. coli, S. Typhimurium, C. freundii, E. aerogenes, E. cloacae, and P. mirabilis have very diverse loop 4, 6, and 7 regions (see Fig. S2 in the supplemental material). In general, each species has a unique set of BamA loop sequences, but Shigella species and an isolate of Salmonella arizonae have the same loops as E. coli strains.
FIG 3 .
Predicted membrane topology and conservation of the BamA β-barrel domain. (A) Predicted topology of the BamA β-barrel based on the crystal structure of B. pertussis FhaC (Protein Data Bank code 2QDZ). Residues are numbered according to E. coli BamA. The percent identity for each residue is indicated as a heat map. Predicted extracellular loops 4, 6, and 7 are indicated. (B) Description of the BamAEcoli loop variants used in this study. The mutated residues are indicated, and the HA peptide epitope sequence is Val-Asp-Tyr-Pro-Tyr-Asp-Val-Pro-Asp-Tyr-Ala.
The predicted BamA topology suggests that extracellular loop polymorphism restricts the E. coli EC93 target cell range. If so, then exchange of bamAEcoli with bamA genes from other species should protect E. coli cells from CDIEC93. BamA function is essential for viability (11, 14); therefore, we first asked whether such chimeric E. coli strains could be generated. We deleted bamAEcoli in cells that carry plasmid pZS21-bamA+ and then used plasmid exchange to test whether bamA genes from other enterobacteria support E. coli cell viability. E. coli ∆bamA::cat mutant cells harboring pZS21-bamA+ were transformed with plasmid pZS21amp derivatives that contain the same replication origin as pZS21-bamA+ but confer resistance to ampicillin. Ampicillin-resistant (Ampr) transformants were selected and tested for kanamycin resistance (Kanr) to determine whether plasmid pZS21-bamA+ was retained. Retention of the Kanr phenotype indicates that the introduced Ampr plasmid is unable to complement the chromosomal ∆bamAEcoli mutation. As a proof of principle, we tested plasmid pZS21amp-bamA+ and found that all Ampr transformants were sensitive to kanamycin (see Table S1 in the supplemental material), indicating that plasmid pZS21-bamA+ is readily displaced if the incoming plasmid provides BamA function. In contrast, cells transformed with either the empty pZS21amp vector or a construct encoding inactive BamAEcoli (lacking the POTRA-3 domain) retained plasmid pZS21-bamA+ (see Table S1). We then tested pZS21amp constructs carrying bamA from other enterobacteria and found that the genes from S. Typhimurium LT2, E. cloacae ATCC 13047, and Dickeya dadantii 3937 all support E. coli ∆bamA cell viability (see Table S1). However, bamA from Yersinia pseudotuberculosis YPIII is unable to complement the E. coli ∆bamA mutant (see Table S1). From these results, we conclude that many, but not all, BamA proteins from other enterobacteria are functional for the biogenesis of E. coli OMPs.
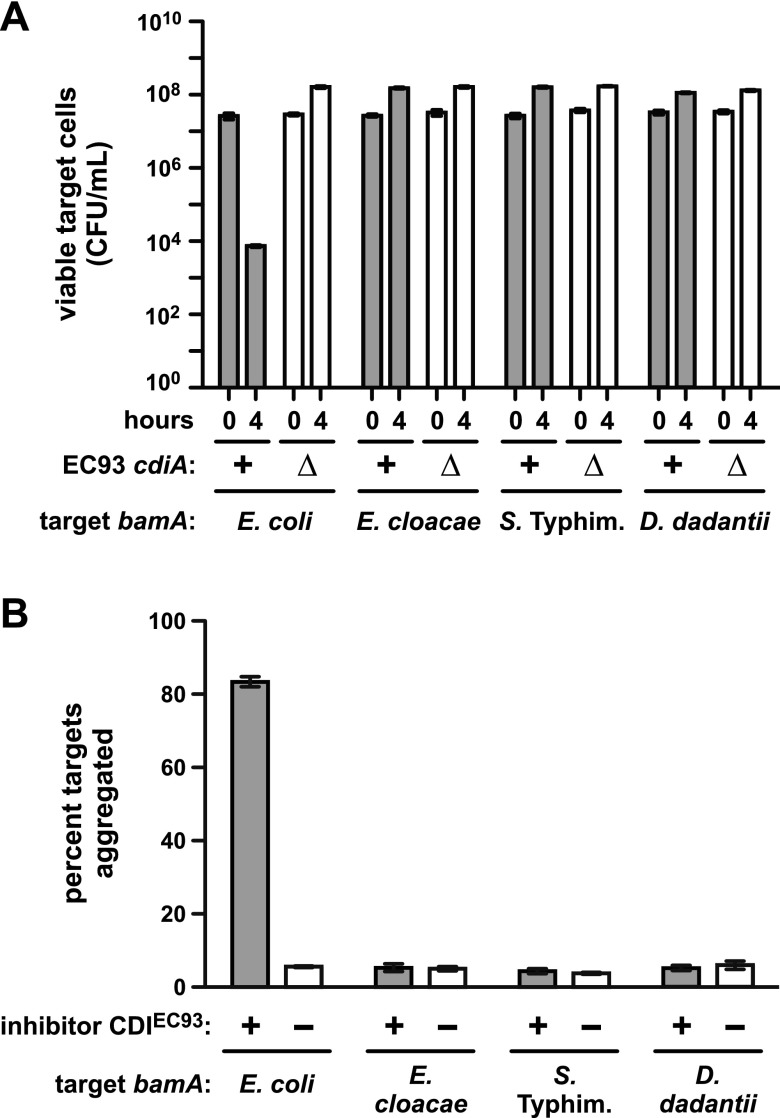
We next tested whether E. coli cells that express heterologous bamA are resistant to CDIEC93. Target cells complemented with pZS21amp-bamA+ were sensitive, sustaining a 3,600-fold decrease in viable cells after 4 h of coculture with E. coli EC93 (Fig. 4A). In contrast, targets expressing bamA from S. Typhimurium, E. cloacae, or D. dadantii 3937 were resistant to CDIEC93 (Fig. 4A). However, the latter target strains were inhibited by CdiA-CTEC93 when the toxin was expressed inside the cells from an inducible promoter (see Fig. S4 in the supplemental material). These results argue that CdiA-CTEC93 is not delivered into cells that express heterologous bamA, presumably because CDIEC93 is disrupted at the level of receptor binding. Our previous work has shown that target cells form stable aggregates with inhibitor cells, provided the inhibitors overexpress the CDIEC93 system from a cosmid (1). Furthermore, this aggregation is proportional to BamAEcoli surface expression on target cells (1), suggesting that cell-cell adhesion reflects the binding interaction between BamAEcoli and CdiAEC93. Therefore, we examined adhesion between targets that express the various bamA alleles and inhibitor cells that overexpress CDIEC93 from cosmid pDAL660∆1-39 (1). Inhibitor cells were labeled with green fluorescent protein (GFP), mixed with DsRed-labeled targets at a 5:1 ratio, and then analyzed by flow cytometry to detect aggregates with both green and red fluorescence. Control experiments showed that 83 ± 1.4% of the targets expressing bamAEcoli formed aggregates with inhibitor cells (Fig. 4B). Moreover, this cell-cell adhesion is dependent upon CDIEC93 because mock inhibitors carrying an empty cosmid vector did not aggregate with target cells (Fig. 4B). Conversely, cells expressing bamA from S. Typhimurium, E. cloacae, or D. dadantii 3937 showed only background levels of aggregation (4 to 6%) with inhibitor cells (Fig. 4B). Together, these results strongly suggest that CdiAEC93 binds specifically to BamAEcoli.
FIG 4 .
Heterologous bamA confers CDIEC93 resistance on E. coli. (A) Competitions between E. coli EC93 and E. coli ∆bamA::cat mutant cells complemented with plasmid-borne copies of bamA from the indicated species. For all competitions, wild-type E. coli EC93 (cdiA+) or E. coli EC93 ∆cdiA mutant cells were cultured with target bacteria at a 1:1 ratio in LB broth for 4 h. Cultures were sampled at 0 and 4 h and plated onto LB agar supplemented with ampicillin to enumerate viable target cells as CFU per milliliter. (B) Adhesion between CDIEC93 inhibitor and target cells. GFP-labeled E. coli MC4100 cells carrying cosmid pDAL660∆1-39 (CDIEC93 +) or pWEB::TNC (CDIEC93 −) were used as inhibitors and mixed in 5-fold excess with DsRed-labeled target bacteria expressing bamA genes from the indicated species. Cell suspensions were analyzed by flow cytometry, and the percentage of red-fluorescent target cells aggregated with green-fluorescent inhibitors was calculated. The reported values are the mean ± the standard error of the mean from two independent experiments. Typhim., Typhimurium.
Disruption of BamAEcoli extracellular loops confers resistance to CDIEC93.
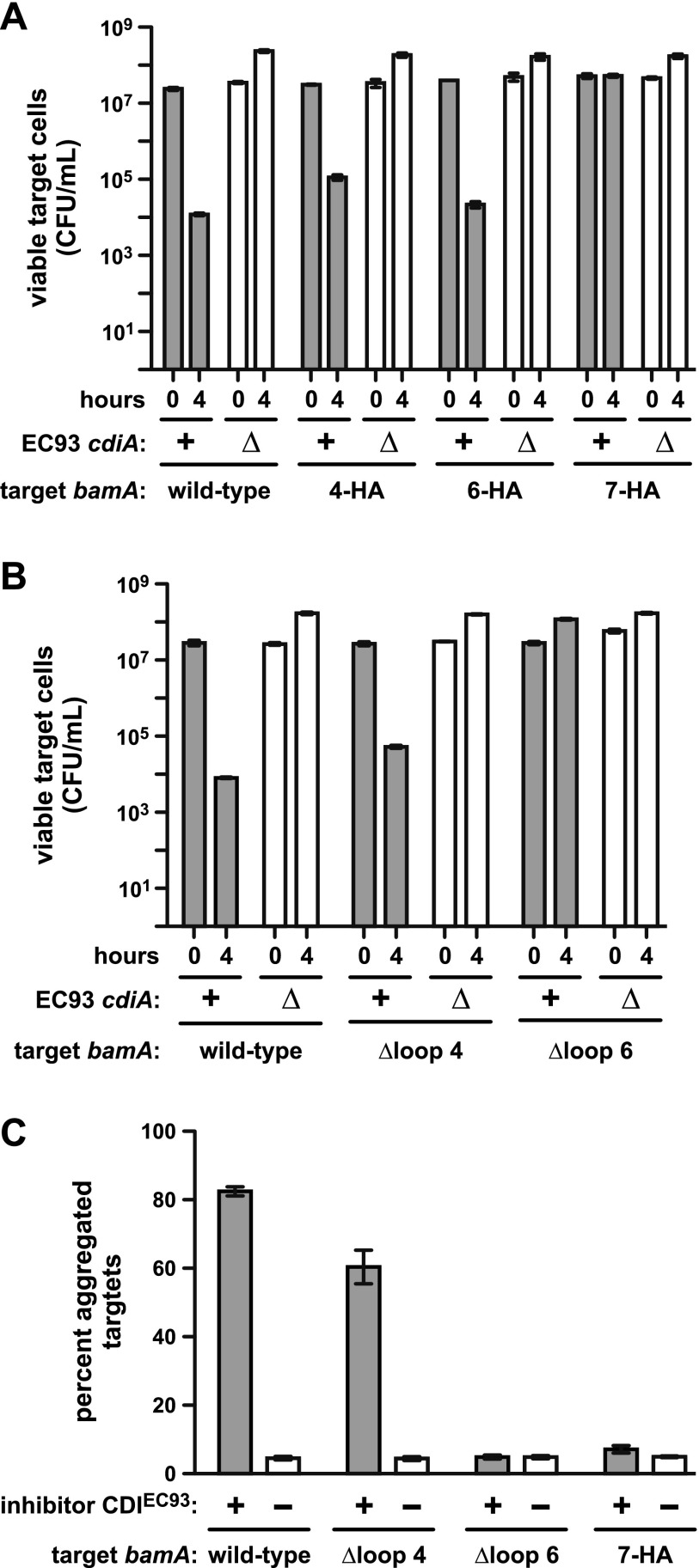
To determine whether the predicted extracellular loops are exposed on the cell surface, we examined BamA topology. Three bamA constructs were generated in which nine residues within loops 4, 6, and 7 were replaced with the HA peptide epitope (Fig. 3B). Each of the HA epitope constructs could displace plasmid pZS21-bamA+ from E. coli ∆bamA::cat cells (see Table S1 in the supplemental material), indicating that the modified BamA proteins are functional and presumably assembled correctly into the outer membrane. We then used immunofluorescence microscopy to examine the surface exposure of HA epitopes. Cells were fixed without permeabilizing the outer membrane, which allows the detection of cell surface antigens but prevents antibody access to periplasmic and cytoplasmic antigens (20). Immunostaining with anti-HA antibody detected the epitopes within loops 4 and 6 (see Fig. S5 in the supplemental material), indicating that these regions are accessible to antibody and therefore could potentially interact with CdiAEC93. However, no signal from the loop 7 epitope was detected (see Fig. S5). The latter result could indicate that loop 7 is not extracellular, but it is also possible that this region is occluded by another extracellular loop. We next tested whether HA mutations provide resistance to CDIEC93. Cells expressing bamA4-HAEcoli and bamA6-HAEcoli were still sensitive to CDIEC93, but bamA7-HAEcoli provided nearly full protection against growth inhibition (Fig. 5A). This resistance presumably results from a defect in CdiA-CTEC93 delivery, because the strain expressing bamA7-HAEcoli is sensitive to CdiA-CTEC93 toxin produced internally (see Fig. S4).
FIG 5 .
BamAEcoli loops 6 and 7 are required for CDIEC93. (A and B) Competitions between E. coli EC93 and E. coli ∆bamA::cat targets that express the indicated bamAEcoli alleles. For all competitions, wild-type E. coli EC93 (cdiA+) or E. coli EC93 ∆cdiA mutant cells were cultured with target bacteria at a 1:1 ratio in LB broth for 4 h. Cultures were sampled at 0 and 4 h and plated on LB agar supplemented with ampicillin to enumerate viable target cells as CFU per milliliter. (C) Adhesion between CDIEC93 inhibitor and target cells. GFP-labeled E. coli MC4100 cells carrying cosmid pDAL660∆1-39 (CDIEC93 +) or pWEB::TNC (CDIEC93 −) were used as inhibitors and mixed in 5-fold excess with DsRed-labeled target bacteria expressing the indicated bamAEcoli alleles. Cell suspensions were analyzed by flow cytometry, and the percentage of red-fluorescent target cells aggregated with green-fluorescent inhibitors was calculated. The reported values are the mean ± the standard error of the mean from two independent experiments.
We next generated in-frame deletions to remove loop regions that are unique to the BamAEcoli receptor (Fig. 3B). Notably, we were unable to generate the loop 7 deletion, suggesting that this loop may be required for function of the BAM complex. The bamA∆4Ecoli and bamA∆6Ecoli alleles each supported E. coli ∆bamA::cat cell viability (see Table S1 in the supplemental material), indicating that these loop regions are not required for OMP biogenesis. In competitions with E. coli EC93 cells, targets expressing bamA∆4Ecoli were slightly more resistant than cells expressing wild-type bamAEcoli, but bamA∆6Ecoli conferred complete resistance (Fig. 5B). The 7-HA and ∆loop 6 mutations also disrupted adhesion to inhibitors that overproduce the CDIEC93 system, whereas target cells that express bamA∆4Ecoli still formed aggregates with inhibitors (Fig. 5C). The latter results correlate with CDIEC93 resistance and implicate BamAEcoli loops 6 and 7 as important determinants of target cell recognition.
BamAEcoli extracellular loops 6 and 7 are sufficient for CDIEC93.
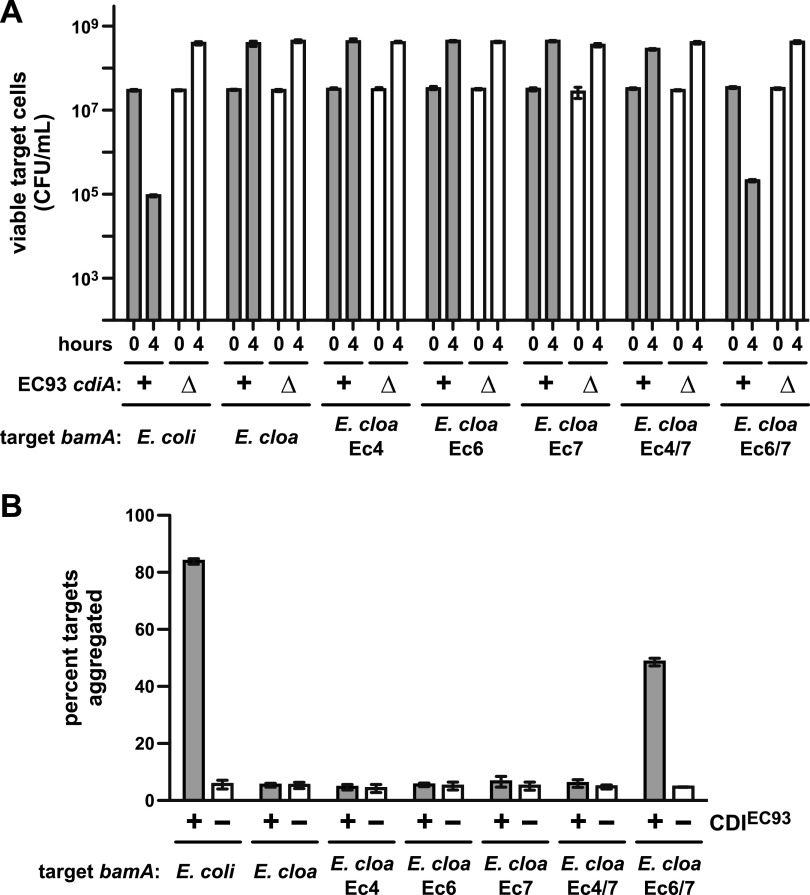
The cell-cell adhesion data suggest that BamAEcoli loops 6 and 7 interact with CdiAEC93. If so, then these loops should restore sensitivity to CDIEC93 when grafted onto BamA from another species. Chimeric bamAEcloacae genes were constructed in which individual loop-coding sequences were replaced with the corresponding region from bamAEcoli (see Fig. S6 in the supplemental material). Each of the single-loop chimeras (bamAEc4Ecloacae, bamAEc6Ecloacae, and bamAEc7Ecloacae) supported E. coli ∆bamA::cat cell viability (see Table S1). Cells expressing these chimeras were as resistant to CDIEC93 as cells that express bamAEcloacae (Fig. 6B), indicating that no single E. coli loop is sufficient for target cell recognition. Similarly, cells expressing bamAEcloacae with E. coli loops 4 and 7 were also CDIEC93 resistant. However, cells expressing bamAEc6/7Ecloacae, which encodes loops 6 and 7 of E. coli, were almost fully sensitive (Fig. 6B). The loop 6/7 chimera also supported CDIEC93-dependent cell-cell adhesion, whereas the other chimeric bamAEcloacae alleles did not (Fig. 6C). Together, these results indicate that BamAEcoli loops 6 and 7 are sufficient for target cell selection during CDIEC93.
FIG 6 .
BamAEcoli loops 6 and 7 are sufficient for CDIEC93. (A) Competitions between E. coli EC93 and E. coli ∆bamA::cat targets that express the indicated bamA alleles. For all competitions, wild-type E. coli EC93 (cdiA+) or E. coli EC93 ∆cdiA mutant cells were cultured with target bacteria at a 1:1 ratio in LB broth for 4 h. Cultures were sampled at 0 and 4 h and plated on LB agar supplemented with ampicillin to enumerate viable target cells as CFU per milliliter. (B) Adhesion between CDIEC93 inhibitor and target cells. GFP-labeled E. coli MC4100 cells carrying cosmid pDAL660∆1-39 (CDIEC93 +) or pWEB::TNC (CDIEC93 −) were used as inhibitors and mixed in 5-fold excess with DsRed-labeled target bacteria expressing the indicated bamA alleles. Cell suspensions were analyzed by flow cytometry, and the percentage of red-fluorescent target cells aggregated with green-fluorescent inhibitors was calculated. The reported values are the mean ± the standard error of the mean from two independent experiments.
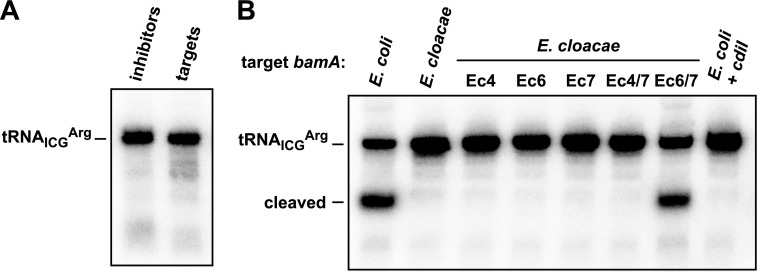
Previous studies have shown that CdiAEC93 is modular and capable of delivering different CdiA-CTs from other systems (8, 20, 21). This heterologous toxin delivery is dependent upon BamAEcoli (20), suggesting that specific CdiA-CT sequences are not required for target cell selection. Therefore, we asked whether the chimeric BamAEc6/7Ecloacae receptor supports the delivery of other CdiA-CTs into target cells. We tested the previously characterized CdiAEC93-CTo1EC93 effector protein, in which the original CdiA-CTEC93 membrane-pore toxin is replaced with a tRNase toxin encoded by the “orphan-1” cdiA-CTo1EC93 gene from E. coli EC93 (21). CdiA-CTo1EC93 is an anticodon nuclease (21, 22); therefore, we assayed toxin activity by Northern blotting with a probe for E. coli tRNAICGArg. We first examined RNA from inhibitor and target cells that had been cultured separately and detected no tRNase activity in either population (Fig. 7A). However, tRNase activity was observed when inhibitors were cocultured with target cells that express bamAEcoli (Fig. 7B). This nuclease activity was not detected when target cells carried a plasmid-borne copy of the cognate cdiIo1EC93 immunity gene (Fig. 7B), verifying that the nuclease activity is due to the CdiA-CTo1EC93 toxin. We next tested targets that express bamAEcloacae and the various bamA chimeras and detected tRNase activity only in cocultures with inhibitor cells that express bamAEc6/7Ecloacae (Fig. 7B). These results demonstrate that chimeric BamAEc6/7Ecloacae supports the delivery of other CDI toxins into E. coli cells.
FIG 7 .
BamAEc6/7Ecloacae allows delivery of another CdiA-CT. (A) Northern blot analysis of tRNAICGArg from E. coli EPI100 carrying cosmid pDAL879 (inhibitors) and E. coli CH9350 (targets). (B) Northern blot analysis of RNA isolated from competition cocultures. Inhibitors were mixed with target cells expressing the indicated bamA alleles at a 1:1 ratio and incubated for 1 h. RNA was isolated from the mixed cultures and analyzed by Northern hybridization using a radiolabeled probe to tRNAICGArg. Target cells from the sample on the far right also expressed the cognate cdiIo1EC93 immunity gene from plasmid pDAL867.
DISCUSSION
The results presented here show that the CDI system from E. coli EC93 is ineffective against other enteric gammaproteobacterial species. Because CDI-mediated inhibition requires the translocation of protein toxins across the target cell envelope, multiple steps could potentially be disrupted to prevent toxin import into other species. However, our results indicate that cross-species inhibition is blocked primarily at the level of target cell recognition because S. Typhimurium, E. aerogenes, and C. freundii cells become susceptible to CDIEC93 upon the expression of the BamAEcoli receptor. BamA is conserved between enterobacteria, but three of the predicted extracellular loops contain regions of sequence variability. Two of these variable loops play an important role in target cell selection by E. coli EC93. Perturbations of BamAEcoli loops 6 and 7 prevent adhesion to inhibitor cells and protect target cells from CDIEC93-mediated growth inhibition. Moreover, these loops are sufficient for cell-cell adhesion and growth inhibition when grafted onto BamA from another species. The latter observation indicates that loops 6 and 7 together form the CdiAEC93-binding epitope on E. coli target cells. These data also demonstrate that, given the appropriate receptor, CdiA-CTEC93 toxin can be delivered into other species. This finding underscores previous work showing that CdiA-CTs from Yersinia pestis CO92 and Dickeya dadantii 3937 can be delivered into E. coli cells provided the toxins are fused onto E. coli CdiA proteins (4, 20). Thus, although the target cell range of a given CDI system may be restricted, CdiA-CTs appear to possess a general import activity sufficient for translocation into a variety of Gram-negative bacteria.
The clustering of sequence variation within the BamA extracellular loops is striking, but such variability is fairly common with OMPs. These surface-exposed β-barrel proteins interact directly with the environment and, as a consequence, are under selective pressure to diversify (23). OMPs are recognized as antigens by adaptive immune systems and are often exploited as receptors by bacteriophage and bacteriocins. These forces can drive the diversification of a given OMP within an individual bacterial species. For example, extracellular loops 4, 5, and 7 of OmpC vary considerably between E. coli strains, even those isolates that have similar niches and pathogenic lifestyles (24). In contrast, BamA is almost completely conserved across the hundreds of E. coli strains that have been sequenced to date. Although the extracellular loops of BamAEcoli are identical in nearly all strains, these regions are subjected to the same selective forces as other OMPs. Shiga-toxin-producing STX phage use BamA as a receptor to infect E. coli cells (19), and the E. coli EC93 CDI system itself represents a force that could conceivably shape BamAEcoli evolution. Therefore, BamA conservation between E. coli strains implies that the extracellular loops may be critical for function. Indeed, portions of BamA loop 6 are highly conserved between species and these residues are important for OMP biogenesis (25, 26). However, our data show that regions within each loop can be mutated extensively and even deleted without loss of viability. In fact, heterologous BamA proteins with alterations in all three loops still support E. coli growth at nearly wild-type rates under laboratory conditions. Therefore, the E. coli-specific extracellular loops are clearly not required for OMP biogenesis, suggesting that other forces act to prevent BamAEcoli diversification. Though the significance of BamAEcoli conservation is unclear, E. coli EC93 exploits this invariant antigen to target and inhibit closely related competitors.
CDI systems are typically encoded within pathogenicity/genomic islands and are associated with transposable elements. This linkage to mobile genetic elements suggests that cdi genes are transferred horizontally between bacteria. The finding that certain CdiA-CTs from disparate bacterial species have high sequence identities supports this hypothesis (21). If such a transfer occurred between unrelated bacteria, then the recipient bacterium would gain an inhibition system that is probably targeted to the donor species. In principle, the newly acquired system could be deployed immediately, but because the donor bacteria are immune to their own CDI toxin, the recipients would gain no competitive advantage over the donor cells. Furthermore, if all CDI systems are self-targeting, then all horizontally transferred cdiA genes must eventually evolve to recognize receptors on the surface of the recipient species. The selective pressures that drive adaptation to self-receptors are unknown, but perhaps CDI-mediated cell-cell adhesion provides a benefit to bacteria. It is also unclear how cdi genes could be retained during the adaptation process. It is possible that other genes within the transferred genomic island provide a selective advantage and the linked cdi genes could be retained as a result. Alternatively, the toxin/immunity activities encoded by the cdiA-CT/cdiI pair may stabilize the genomic island in a manner similar to that described for some type II toxin/antitoxin (TA) modules (27, 28). In the absence of intercellular CdiA-CT exchange, the latter model would require cryptic translation initiation within cdiA to produce cytoplasmic toxin. If cdi genes do indeed stabilize genomic regions in the absence of selection, then it raises the possibility that CDI systems, like TA modules, are encoded by selfish genetic elements that propagate through horizontal transfer.
The target cell range of E. coli EC93 suggests that CDI is used for self-recognition and could help coordinate multicellular activities. Because CDIEC93 mediates adherence between E. coli cells exclusively, it provides a mechanism for increasing the relatedness of the local population and thus represents a form of “kin selection.” Direct interaction between close kin is a common mechanism to increase the fitness of related cells (29). In accord with this idea, CDI systems enhance biofilm formation in both Burkholderia thailandensis E264 (30) and E. coli EC93 (Z.C.R. and C.S.H., unpublished data). Biofilms require collaboration between individual cells and provide fitness benefits such as protection from predation and antimicrobial agents. These observations indicate that CDI+ siblings recognize and cooperate with one another. But CDI+ cells also harm CDI− kin and therefore also act to exclude related cells that lack cdi genes. These phenomena are evocative of so-called “greenbeard” cooperativity (29, 31). Greenbeard genes give rise to a trait and simultaneously recognize and direct cooperation to other individuals with that same trait (32). Moreover, greenbeard genes are also sometimes capable of harming others that lack the allele. Individuals that have the same greenbeard alleles need not be related at other genetic loci, and therefore, cooperation occurs between those of the same “kind” and not necessarily between kin. However, different strains of a given species typically harbor distinct CDI toxin/immunity pairs (6), so only closely related bacteria that have identical toxin/immunity alleles are likely to associate and cooperate. Thus, CDIEC93 discriminates kin at two levels—potential collaborators are recognized through specific receptor-binding interactions, and favored siblings are selected by virtue of their immunity gene allele.
MATERIALS AND METHODS
Bacteria and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table S2 in the supplemental material. Bacteria were grown in LB broth or LB agar unless otherwise noted. Media were supplemented with antibiotics at the following concentrations: ampicillin, 150 µg ml−1; kanamycin, 50 µg ml−1; chloramphenicol, 66 µg ml−1; spectinomycin, 50 µg ml−1; rifampin, 200 µg ml−1; streptomycin, 100 µg ml−1. The bamA gene was replaced with a chloramphenicol acetyltransferase (cat) resistance cassette in E. coli X90 and EPI100 cells carrying plasmids pZS21-bamA+ (13) and pSIM6 (33). The cat gene was amplified with oligonucleotides bamA-cat-for and bamA-cat-rev (see Table S3 in the supplemental material), and the resulting product was electroporated into cells as previously described (33). Transformants were selected on LB agar supplemented with chloramphenicol at 33 µg ml−1.
For interspecies competitions, inhibitors and targets were first grown to mid-log phase at 37°C in LB broth supplemented with the appropriate antibiotics. Cells were harvested in M9 salts, and cell densities were determined by measuring optical density at 600 nm (OD600). Inhibitors and targets were then mixed at a 10:1 ratio in M9 salts (to a final OD600 of ~33.0), and 100 µl of the cell suspension was plated onto LB agar for incubation at 37°C. After 20 h, cells were harvested into 2 ml of M9 salts. The cell suspensions were serially diluted and plated on LB agar containing the appropriate antibiotics to enumerate viable inhibitor and target cells as colony-forming units (CFU). Intraspecies E. coli competitions were performed in LB broth. The inhibitor and target cells were mixed at a 10:1 ratio (final OD600, ~0.330) in LB without antibiotics and cocultured for 3 h at 37°C with vigorous shaking. Samples of the cocultures were removed, serially diluted, and plated onto selective LB agar to enumerate target cells as CFU ml−1. CdiA-CTEC93 toxicity was assessed by expression inside E. coli cells from an l-arabinose-inducible promoter on plasmid pCH450 (34). Plasmids pCH450, pCH450-cdiA-CTEC93, and pCH450-cdiA-CT/cdiIEC93 were introduced into E. coli X90 ∆bamA::cat strains carrying the various pZS21-bamA constructs, and the resulting cells were cultured at 37°C in LB medium supplemented with tetracycline and 0.2% l-arabinose. Cell growth was monitored by measuring the OD600 as a function of time.
Plasmid exchange.
E. coli X90 ∆bamA::cat carrying plasmid pZS21::bamA+ (Kanr) was transformed with pZS21amp (Ampr) or pZS21amp derivatives carrying the bamA alleles described above (35). Transformed cells were incubated for 1 h at 37°C in LB broth, plated onto LB agar supplemented with ampicillin, and then incubated at 37°C for 20 h. Ampr colonies were picked at random and streaked onto fresh LB agar supplemented with ampicillin. The resulting Ampr colonies were then cross-streaked onto separate LB agar plates supplemented with kanamycin or ampicillin. Cell growth on kanamycin and ampicillin was scored after 20 h at 37°C.
Cell-cell adhesion.
Overnight cultures of E. coli strain DAL4905 carrying cosmid pDAL660∆1-39 (CDI+) or pWEB::TNC (CDI−) were diluted into fresh tryptone broth (TB) and grown to mid-log phase at 30°C. These cells were then mixed at a 5:1 ratio with E. coli EPI100 ∆bamA::cat target cells carrying pZS21-bamA constructs and pDsRedExpress2. The cell suspension was incubated with aeration for 15 min at 30°C, diluted 1:50 into filtered 1× phosphate-buffered saline (PBS), and then analyzed on an Accuri C6 flow cytometer using FL1 (533/30 nm, GFP) and FL2 (585/40 nm, DS-Red) fluorophore filters (Becton Dickinson).
Whole-cell immunofluorescence.
Overnight cultures were inoculated into fresh TB containing the appropriate antibiotics and grown to an OD600 of ~0.8. Cells were harvested and fixed for 15 min in 1× PBS supplemented with 4% formaldehyde. Fixation reactions were quenched with 125 mM glycine (pH 7.4). Cells were washed once with 1× PBS supplemented with 125 mM glycine (pH 7.4) and then washed twice with 1× PBS. Washed cells were resuspended in 1× PBS supplemented with 1% bovine serum albumin (BSA) containing a 1:50 dilution of anti-HA monoclonal antibody (Sigma-Aldrich). Cells were incubated for 30 min at room temperature and washed three times with 1× PBS–1% BSA. Washed cells were resuspended in 1× PBS–1% BSA containing a 1:500 dilution of anti-mouse monoclonal antibody conjugated to Alexa Fluor 488 (Invitrogen). Cells were incubated for 30 min on ice and washed thrice with 1× PBS supplemented with 1% BSA. Cells were resuspended in 1× PBS and allowed to adhere to poly-d-lysine-coated slides for 5 min at room temperature. Nonadhering cells were removed with deionized water. Adhering cells were covered and fixed with FluoroGel solution and imaged by fluorescence microscopy.
SUPPLEMENTAL MATERIAL
Plasmid constructions. Download
Cross-species competitions with E. coli EC93. Wild-type E. coli EC93 (cdiA+) or E. coli EC93 ∆cdiA mutant cells were mixed at a 10:1 ratio with the indicated competitor species, and the suspension was incubated on LB agar plates for 20 h. Cells were harvested, washed, and replated on LB agar supplemented with rifampin to enumerate viable E. coli EC93 cells as CFU. All competitions were conducted at least twice, and the reported values the mean ± the standard error of the mean. Download
Alignment of BamA proteins. BamA sequences from E. coli MG1655 (UniProt code P0A940), Enterobacter cloacae ATCC 13047 (ENTCC; D5CHY0), Enterobacter aerogenes ATCC 13048 (ENTAK; G0E611), Salmonella Typhimurium LT2 (SALTY; Q8ZRP0), Citrobacter freundii ATCC 8090 (CITRI; K8QV34), and Proteus mirabilis strain HI4320 (PROMH; B4F262) were aligned on the UniProt site (http://www.uniprot.org), and the alignment was rendered with Jalview 2.8 (http://www.jalview.org) with 30% sequence identity shading. The numbers over the alignment indicate the predicted extracellular loop regions based on the E. coli BamA protein. Download
Alignment of AcrB proteins. AcrB sequences from E. coli MG1655 (UniProt code P31224), Enterobacter cloacae ATCC 13047 (ENTCC; D5CJE7), Enterobacter aerogenes ATCC 13048 (ENTAK; G0E872), Salmonella enterica serovar Typhimurium strain LT2 (SALTY; Q8ZRA7), Citrobacter freundii ATCC 8090 (CITRI; K8QZQ4), and Proteus mirabilis strain HI4320 (PROMH; B4EU69) were aligned on the UniProt site (http://www.uniprot.org), and the alignment was rendered with Jalview 2.8 (http://www.jalview.org) with 30% sequence identity shading. Download
Expression of CdiA-CTEC93 toxin inside cells. Growth inhibition was assessed by inducing expression of the cdiA-CTEC93 toxin gene (red curves) inside E. coli ∆bamA::cat cells that harbor pZS21 plasmids with the indicated bamA alleles. Coexpression of the cdiIEC93 immunity gene (green curves) served as a control to demonstrate that growth inhibition is due to the cdiA-CTEC93 toxin. All strains were suspended to an OD600 of 0.05 in prewarmed LB medium supplemented with 0.2% l-arabinose to induce toxin or toxin/immunity expression. The black curves show growth profiles for strains that contain the empty vector plasmid pCH450. Download
Anti-HA epitope immunofluorescence microscopy. E. coli MC4100 bamA101::kan cells carrying plasmids that express bamAEcoli, bamAHA-4Ecoli, bamAHA-6Ecoli, and bamAHA-7Ecoli were fixed without permeabilization of the outer membrane and analyzed by immunofluorescence microscopy with monoclonal antibodies to the HA epitope. Light and 4ʹ,6-diamidino-2-phenylindole (DAPI) fluorescence images are shown to indicate the cells in each field. Download
Chimeric BamA sequences. Alignment of the chimeric BamA β-barrel domains is shown. The BamA proteins from E. coli and E. cloacae ATCC 13047 (ENTCC) are depicted along with the corresponding regions from BamAEc4Ecloacae (Ec4), BamAEc6Ecloacae (Ec6), BamAEc7Ecloacae (Ec7), BamAEc4/gEcloacae (Ec4/6), and BamAEc4/7Ecloacae (Ec4/7). The predicted extracellular loops are indicated by numbers over the alignment. Loop regions that vary between E. coli and E. cloacae are depicted in red and green, respectively. Download
Plasmid exchange in E. coli ΔbamA mutant cells.
Bacterial strains and plasmids used in this study.
Oligonucleotides used in this study.
ACKNOWLEDGMENTS
We thank Thomas Silhavy for plasmids.
Z.C.R. was supported in part by the Tri-Counties Blood Bank Postdoctoral Fellowship. This work was supported by grant 0642052 (D.A.L.) from the National Science Foundation and grant GM078634 (C.S.H.) from the National Institutes of Health.
Footnotes
Citation Ruhe ZC, Wallace AB, Low DA, Hayes CS. 2013. Receptor polymorphism restricts contact-dependent growth inhibition to members of the same species. mBio 4(4):e00480-13. doi:10.1128/mBio.00480-13.
REFERENCES
- 1. Aoki SK, Pamma R, Hernday AD, Bickham JE, Braaten BA, Low DA. 2005. Contact-dependent inhibition of growth in Escherichia coli. Science 309:1245–1248 [DOI] [PubMed] [Google Scholar]
- 2. Clantin B, Delattre AS, Rucktooa P, Saint N, Méli AC, Locht C, Jacob-Dubuisson F, Villeret V. 2007. Structure of the membrane protein FhaC: a member of the Omp85-TpsB transporter superfamily. Science 317:957–961 [DOI] [PubMed] [Google Scholar]
- 3. Kajava AV, Cheng N, Cleaver R, Kessel M, Simon MN, Willery E, Jacob-Dubuisson F, Locht C, Steven AC. 2001. Beta-helix model for the filamentous haemagglutinin adhesin of Bordetella pertussis and related bacterial secretory proteins. Mol. Microbiol. 42:279–292 [DOI] [PubMed] [Google Scholar]
- 4. Aoki SK, Diner EJ, de Roodenbeke CT, Burgess BR, Poole SJ, Braaten BA, Jones AM, Webb JS, Hayes CS, Cotter PA, Low DA. 2010. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature 468:439–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aoki SK, Webb JS, Braaten BA, Low DA. 2009. Contact-dependent growth inhibition causes reversible metabolic downregulation in Escherichia coli. J. Bacteriol. 191:1777–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruhe ZC, Low DA, Hayes CS. 2013. Bacterial contact-dependent growth inhibition. Trends Microbiol. 21:230–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aoki SK, Poole SJ, Hayes CS, Low DA. 2011. Toxin on a stick: modular CDI toxin delivery systems play roles in bacterial competition. Virulence 2:356–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morse RP, Nikolakakis KC, Willett JL, Gerrick E, Low DA, Hayes CS, Goulding CW. 2012. Structural basis of toxicity and immunity in contact-dependent growth inhibition (CDI) systems. Proc. Natl. Acad. Sci. U. S. A. 109:21480–21485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nikolakakis K, Amber S, Wilbur JS, Diner EJ, Aoki SK, Poole SJ, Tuanyok A, Keim PS, Peacock S, Hayes CS, Low DA. 2012. The toxin/immunity network of Burkholderia pseudomallei contact-dependent growth inhibition (CDI) systems. Mol. Microbiol. 84:516–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aoki SK, Malinverni JC, Jacoby K, Thomas B, Pamma R, Trinh BN, Remers S, Webb J, Braaten BA, Silhavy TJ, Low DA. 2008. Contact-dependent growth inhibition requires the essential outer membrane protein BamA (YaeT) as the receptor and the inner membrane transport protein AcrB. Mol. Microbiol. 70:323–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J. 2003. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299:262–265 [DOI] [PubMed] [Google Scholar]
- 12. Doerrler WT, Raetz CR. 2005. Loss of outer membrane proteins without inhibition of lipid export in an Escherichia coli YaeT mutant. J. Biol. Chem. 280:27679–27687 [DOI] [PubMed] [Google Scholar]
- 13. Kim S, Malinverni JC, Sliz P, Silhavy TJ, Harrison SC, Kahne D. 2007. Structure and function of an essential component of the outer membrane protein assembly machine. Science 317:961–964 [DOI] [PubMed] [Google Scholar]
- 14. Werner J, Misra R. 2005. YaeT (Omp85) affects the assembly of lipid-dependent and lipid-independent outer membrane proteins of Escherichia coli. Mol. Microbiol. 57:1450–1459 [DOI] [PubMed] [Google Scholar]
- 15. Gentle I, Gabriel K, Beech P, Waller R, Lithgow T. 2004. The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J. Cell Biol. 164:19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gentle IE, Burri L, Lithgow T. 2005. Molecular architecture and function of the Omp85 family of proteins. Mol. Microbiol. 58:1216–1225 [DOI] [PubMed] [Google Scholar]
- 17. Ma D, Cook DN, Alberti M, Pon NG, Nikaido H, Hearst JE. 1995. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol. Microbiol. 16:45–55 [DOI] [PubMed] [Google Scholar]
- 18. Nikaido H, Zgurskaya HI. 2001. AcrAB and related multidrug efflux pumps of Escherichia coli. J. Mol. Microbiol. Biotechnol. 3:215–218 [PubMed] [Google Scholar]
- 19. Smith DL, James CE, Sergeant MJ, Yaxian Y, Saunders JR, McCarthy AJ, Allison HE. 2007. Short-tailed stx phages exploit the conserved YaeT protein to disseminate Shiga toxin genes among enterobacteria. J. Bacteriol. 189:7223–7233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Webb JS, Nikolakakis KC, Willett JL, Aoki SK, Hayes CS, Low DA. 2013. Delivery of CdiA nuclease toxins into target cells during contact-dependent growth inhibition. PLoS One 8:e57609. 10.1371/journal.pone.0057609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poole SJ, Diner EJ, Aoki SK, Braaten BA, t’Kint de Roodenbeke C, Low DA, Hayes CS, t'Kint de Roodenbeke C, Low DA, Hayes CS. 2011. Identification of functional toxin/immunity genes linked to contact-dependent growth inhibition (CDI) and rearrangement hotspot (Rhs) systems. PLoS Genet. 7:e1002217. 10.1371/journal.pgen.1002217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diner EJ, Beck CM, Webb JS, Low DA, Hayes CS. 2012. Identification of a target cell permissive factor required for contact-dependent growth inhibition (CDI). Genes Dev. 26:515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petersen L, Bollback JP, Dimmic M, Hubisz M, Nielsen R. 2007. Genes under positive selection in Escherichia coli. Genome Res. 17:1336–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen SL, Hung CS, Xu J, Reigstad CS, Magrini V, Sabo A, Blasiar D, Bieri T, Meyer RR, Ozersky P, Armstrong JR, Fulton RS, Latreille JP, Spieth J, Hooton TM, Mardis ER, Hultgren SJ, Gordon JI. 2006. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc. Natl. Acad. Sci. U. S. A. 103:5977–5982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leonard-Rivera M, Misra R. 2012. Conserved residues of the putative L6 loop of Escherichia coli BamA play a critical role in the assembly of beta-barrel outer membrane proteins, including that of BamA itself. J. Bacteriol. 194:4662–4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rigel NW, Ricci DP, Silhavy TJ. 2013. Conformation-specific labeling of BamA and suppressor analysis suggest a cyclic mechanism for beta-barrel assembly in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 110:5151–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Szekeres S, Dauti M, Wilde C, Mazel D, Rowe-Magnus DA. 2007. Chromosomal toxin-antitoxin loci can diminish large-scale genome reductions in the absence of selection. Mol. Microbiol. 63:1588–1605 [DOI] [PubMed] [Google Scholar]
- 28. Wozniak RA, Waldor MK. 2009. A toxin-antitoxin system promotes the maintenance of an integrative conjugative element. PLoS Genet. 5:e1000439. 10.1371/journal.pgen.1000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. West SA, Gardner A. 2010. Altruism, spite, and greenbeards. Science 327:1341–1344 [DOI] [PubMed] [Google Scholar]
- 30. Anderson MS, Garcia EC, Cotter PA. 2012. The Burkholderia bcpAIOB genes define unique classes of two-partner secretion and contact dependent growth inhibition systems. PLoS Genet. 8:e1002877. 10.1371/journal.pgen.1002877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strassmann JE, Gilbert OM, Queller DC. 2011. Kin discrimination and cooperation in microbes. Annu. Rev. Microbiol. 65:349–367 [DOI] [PubMed] [Google Scholar]
- 32. Dawkins R. 1976. The selfish gene. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 33. Datta S, Costantino N, Court DL. 2006. A set of recombineering plasmids for gram-negative bacteria. Gene 379:109–115 [DOI] [PubMed] [Google Scholar]
- 34. Hayes CS, Sauer RT. 2003. Cleavage of the A site mRNA codon during ribosome pausing provides a mechanism for translational quality control. Mol. Cell 12:903–911 [DOI] [PubMed] [Google Scholar]
- 35. Chung CT, Niemela SL, Miller RH. 1989. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc. Natl. Acad. Sci. U. S. A. 86:2172–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasmid constructions. Download
Cross-species competitions with E. coli EC93. Wild-type E. coli EC93 (cdiA+) or E. coli EC93 ∆cdiA mutant cells were mixed at a 10:1 ratio with the indicated competitor species, and the suspension was incubated on LB agar plates for 20 h. Cells were harvested, washed, and replated on LB agar supplemented with rifampin to enumerate viable E. coli EC93 cells as CFU. All competitions were conducted at least twice, and the reported values the mean ± the standard error of the mean. Download
Alignment of BamA proteins. BamA sequences from E. coli MG1655 (UniProt code P0A940), Enterobacter cloacae ATCC 13047 (ENTCC; D5CHY0), Enterobacter aerogenes ATCC 13048 (ENTAK; G0E611), Salmonella Typhimurium LT2 (SALTY; Q8ZRP0), Citrobacter freundii ATCC 8090 (CITRI; K8QV34), and Proteus mirabilis strain HI4320 (PROMH; B4F262) were aligned on the UniProt site (http://www.uniprot.org), and the alignment was rendered with Jalview 2.8 (http://www.jalview.org) with 30% sequence identity shading. The numbers over the alignment indicate the predicted extracellular loop regions based on the E. coli BamA protein. Download
Alignment of AcrB proteins. AcrB sequences from E. coli MG1655 (UniProt code P31224), Enterobacter cloacae ATCC 13047 (ENTCC; D5CJE7), Enterobacter aerogenes ATCC 13048 (ENTAK; G0E872), Salmonella enterica serovar Typhimurium strain LT2 (SALTY; Q8ZRA7), Citrobacter freundii ATCC 8090 (CITRI; K8QZQ4), and Proteus mirabilis strain HI4320 (PROMH; B4EU69) were aligned on the UniProt site (http://www.uniprot.org), and the alignment was rendered with Jalview 2.8 (http://www.jalview.org) with 30% sequence identity shading. Download
Expression of CdiA-CTEC93 toxin inside cells. Growth inhibition was assessed by inducing expression of the cdiA-CTEC93 toxin gene (red curves) inside E. coli ∆bamA::cat cells that harbor pZS21 plasmids with the indicated bamA alleles. Coexpression of the cdiIEC93 immunity gene (green curves) served as a control to demonstrate that growth inhibition is due to the cdiA-CTEC93 toxin. All strains were suspended to an OD600 of 0.05 in prewarmed LB medium supplemented with 0.2% l-arabinose to induce toxin or toxin/immunity expression. The black curves show growth profiles for strains that contain the empty vector plasmid pCH450. Download
Anti-HA epitope immunofluorescence microscopy. E. coli MC4100 bamA101::kan cells carrying plasmids that express bamAEcoli, bamAHA-4Ecoli, bamAHA-6Ecoli, and bamAHA-7Ecoli were fixed without permeabilization of the outer membrane and analyzed by immunofluorescence microscopy with monoclonal antibodies to the HA epitope. Light and 4ʹ,6-diamidino-2-phenylindole (DAPI) fluorescence images are shown to indicate the cells in each field. Download
Chimeric BamA sequences. Alignment of the chimeric BamA β-barrel domains is shown. The BamA proteins from E. coli and E. cloacae ATCC 13047 (ENTCC) are depicted along with the corresponding regions from BamAEc4Ecloacae (Ec4), BamAEc6Ecloacae (Ec6), BamAEc7Ecloacae (Ec7), BamAEc4/gEcloacae (Ec4/6), and BamAEc4/7Ecloacae (Ec4/7). The predicted extracellular loops are indicated by numbers over the alignment. Loop regions that vary between E. coli and E. cloacae are depicted in red and green, respectively. Download
Plasmid exchange in E. coli ΔbamA mutant cells.
Bacterial strains and plasmids used in this study.
Oligonucleotides used in this study.