Abstract
The principal inhibitory neurotransmitter in the mammalian brain, γ-aminobutyric acid (GABA), is thought to regulate memory processes by activating transient inhibitory postsynaptic currents. Here we describe a nonsynaptic, tonic form of inhibition in mouse CA1 pyramidal neurons that is generated by a distinct subpopulation of GABA type A receptors (GABAARs). This tonic inhibitory conductance is predominantly mediated by α5 subunit-containing GABAARs (α5GABAARs) that have different pharmacological and kinetic properties compared to postsynaptic receptors. GABAARs that mediate the tonic conductance are well suited to detect low, persistent, ambient concentrations of GABA in the extracellular space because they are highly sensitive to GABA and desensitize slowly. Moreover, the tonic current is highly sensitive to enhancement by amnestic drugs. Given the restricted expression of α5GABAARs to the hippocampus and the association between reduced α5GABAAR function and improved memory performance in behavioral studies, our results suggest that tonic inhibition mediated by α5GABAARs in hippocampal pyramidal neurons plays a key role in cognitive processes.
The γ-aminobutyric acid (GABA) subtype A receptor (GABAAR) is a pentameric anion-selective ion channel that assembles from different classes of subunits (α1-6, β1-3, γ1-3, δ, π, θ, and ε) (1). The combination of various GABAAR subunits confers different biophysical and pharmacological properties and regulates regional and subcellular patterns of distribution (2, 3). The subunit composition critically determines agonist affinity, receptor kinetics, and sensitivity to a variety of clinically important drugs, including benzodiazepines and general anesthetics.
Studies of gene-targeted mice have implicated specific GABAAR subunit isoforms in critical aspects of information processing in the brain. Notably, α5-null mutant mice (α5-/-) exhibit improved performance in the water maze model of spatial learning, a hippocampus-dependent learning task (4). Further, mice carrying a point mutation at position 105 of the α5 subunit (H105R) experience an unexpected selective reduction of α5GABAARs in hippocampal pyramidal neurons and improved performance for learning tasks (5). Pharmacological studies further support the involvement of α5GABAARs in learning processes; for example, α5 subunit-selective inverse agonists such as L-655,708 enhance learning performance in rats in the Morris water maze test (6, 7). Moreover, α5 subunit-selective inverse agonists exhibit desirable nootropic effects without causing adverse convulsant activities associated with nonselective GABAAR inverse agonists. Thus, inhibition of α5GABAARs presents an attractive strategy for developing memory-enhancing drugs.
The neuronal substrates underlying improved cognitive performance associated with reduced α5GABAAR function remain unknown. The α5 subunit has a unique and limited pattern of distribution in the mammalian brain. Although α5-containing receptors constitute <5% of the total GABAAR population, they are abundantly expressed in the hippocampus, where they account for >20% of all GABAARs (8-12). The α5 subunit is present at considerably lower levels in the cerebral cortex and is virtually absent from most brain regions (3, 10, 13). The relatively restricted expression of the α5 subunit isoform in the hippocampus provides an unprecedented opportunity to establish links between specific populations of GABAARs and the mechanisms of learning and memory. Thus, it is of great interest to determine the neuronal correlate(s) underlying the improved behavioral performance in α5-/- mice (4, 5).
Immunocytochemistry and in situ hybridization studies indicate that α5GABAARs are localized primarily to extrasynaptic regions of pyramidal neurons in the CA1 and CA3 regions of the hippocampus (5, 11, 14, 15). In cerebellar granule neurons, α6δGABAARs that are localized primarily to extrasynaptic regions (2, 16, 17) generate a persistent tonic inhibitory conductance that is distinct from inhibitory synaptic currents (18). This tonic conductance regulates neuronal excitability and possibly information processing in the cerebellar cortex (18-20). In dentate gyrus granule cells, GABAARs containing the δ subunit, and likely α4 subunits, also generate a tonic current (21). The subunit composition of GABAARs that underlie a tonic current in the CA1 region of the hippocampus has not been determined.
Whole-cell recordings from rat hippocampal CA1 pyramidal cells and cultured hippocampal neurons show two distinct forms of GABAergic inhibition: a persistent low-amplitude tonic current and a transient synaptic current (22, 23). Low, ambient concentrations of γ-aminobutyric acid (GABA) activate a tonic current rather than spontaneous GABAAR openings (22). We previously showed that the benzodiazepine midazolam enhances the tonic current in cultured hippocampal neurons, indicating that the underlying GABAAR complexes lack α4 and δ subunits but contain γ subunits in combination with α1, α2, α3, or α5 subunits (22). The GABAARs that generate tonic and synaptic currents in hippocampal neurons have different pharmacological and biophysical properties (22, 24, 25). This differential sensitivity to GABAergic compounds could result from different molecular compositions of the underlying receptors, conditions of receptor activation, or a combination of these factors. Here, we test the hypothesis that GABAARs composed of a unique complement of subunits generate a tonic current in cultured hippocampal neurons and CA1 pyramidal cells; specifically, α5GABAARs underlie tonic, but not synaptic, inhibitory currents.
Materials and Methods
Cell Culture and Electrophysiological Techniques. Experiments were approved by the Animal Care Committee of the University of Toronto. Cultures of hippocampal and cortical neurons were prepared as described (26) from α5-/- mice and WT littermates on postnatal day 1. The generation of the α5-/- mice has been described (4). Briefly, mice were kept in a mixed genetic background (≈50% C57BL/6 and ≈50% 129SvEv), and α5-/- and WT mice were generated by crossing α5+/- mice. For several experiments, cell cultures were prepared from embryonic Swiss White mice because the number of WT mice available from the heterozygous α5+/- breeding pairs was limited. We have previously used Swiss White mice extensively to characterize the properties of the tonic current in hippocampal neurons (22, 25). To increase the amplitude of the tonic current and facilitate its pharmacological characterization, cultures were treated with the GABA-transaminase inhibitor vigabatrin (100 μM) for 24 h before recording (23), although a low-amplitude tonic current is detected in cultured hippocampal neurons without treatment (22).
Extracellular solutions contained 140 mM NaCl, 1.3 mM CaCl2, 5.4 mM KCl, 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Hepes), and 28 mM glucose, pH 7.4. Tetrodotoxin (300 nM) was added to the extracellular solution to block voltage-sensitive Na+ channels, and 6-cyano-7-nitroquinoxaline-2,3-dione (10 μM) and 2-amino-4-phosphonovaleric acid (40 μM) were added to inhibit ionotropic glutamate receptors. The extracellular solution was applied to neurons by a computer-controlled multibarreled perfusion system (SF-77B, Warner Instruments, Hamden, CT) with an exchange time of ≈3 ms. Whole-cell currents were recorded under voltage-clamp (-60 mV) conditions using an Axopatch 200 amplifier (Axon Instruments, Union City, CA) that was interfaced to a Digidata 1200 (Instrutech, Elmont, NY). To monitor series resistance, a hyperpolarizing voltage step of 10 mV was applied. Only cells that demonstrated a stable access resistance were used for data analysis. Patch electrodes were filled with a solution containing 140 mM CsCl, 10 mM Hepes, 11 mM EGTA, and 2 mM MgCl2, 1 mM CaCl2, 4 mM MgATP, and 2 mM tetraethylammonium, pH 7.3. Experiments were performed at room temperature.
Drugs were dissolved in water with the exception of L-655,708, etomidate (ETMD), loreclezole, allotetrahydrodeoxycorticosterone, and furosemide, which were prepared in DMSO. Concentrations of DMSO that were equivalent to those present in drug solutions did not affect tonic or synaptic currents.
Data Analysis for Cell Cultures. The amplitude of the tonic current under control conditions was measured as the difference in the holding current (Ihold) before and during the application of bicuculline methiodide (BIC; 100 μM) (22, 27). Drug efficacy was reported as percent control, where control was equal to the amplitude of the tonic current revealed by the application of BIC. The rms of the noise was calculated from recording segments that lacked miniature inhibitory postsynaptic currents (mIPSCs) by using mini analysis software (Synaptosoft, Decatur, GA). The total charge transfer was measured as previously described (22). GABA-evoked currents were activated by applying GABA (600 μM) for 2 s. The time course of current desensitization and deactivation was calculated by fitting a single- or biexponential function. The weighted time constant (τweighted) was used to compare differences between groups (25).
Hippocampal Slice Recordings. Hippocampal slices were prepared from mice (18-23 days after birth) according to standard protocols (28). Animals were killed in accordance with Schedule 1 of the U.K. Government Animals (Scientific Procedures Act 1986). The brain was placed in artificial cerebrospinal fluid solution containing 225 mM sucrose, 2.95 mM KCl, 1.25 mM NaH2PO4, 26 mM NaHCO3, 0.5 mM CaCl2, 10 mM MgSO4, 10 mM glucose, 1 mM ascorbic acid, and 3 mM pyruvic acid bubbled with 95% O2/5% CO2 to give a pH of 7.4 (330-340 milliosmolar). The tissue was cut into horizontal 300-μm slices by using a Vibratome (Intracel, Hertsfordshire, U.K.). The extracellular solution contained 126 mM NaCl, 2.95 mM KCl, 26 mM NaHCO3, 1.25 mM NaH2PO4, 2 mM CaCl2, 10 mM d-glucose, and 2 mM MgCl2 (pH 7.4 when bubbled with 95% O2/5% CO2; 300-310 milliosmolar). Slices were incubated at 32°C for 1 h before being used for the recordings.
Whole-cell patch clamp recordings were made at 35°C from hippocampal CA1 pyramidal neurons that were visually identified by using a microscope that was equipped with differential interference contrast/IR optics. Patch pipettes had open tip resistances of 3-5 MΩ when filled with an intracellular solution that contained 140 mM CsCl, 10 mM Hepes, 10 mM EGTA, 2 mM MgATP, 1 mM CaCl2, 5 mM lidocaine derivative QX-314 (pH 7.3 with CsOH, 295-305 milliosmolar). The extracellular recording solution contained 2 mM kynurenic acid (Sigma-Aldrich) to block ionotropic glutamate receptors. Experiments were performed on slices previously incubated in the presence of vigabatrin (50 μM; Sigma-Aldrich) for 2-4 h. Currents were sampled at 10 kHz and filtered at 2 kHz by using an eight-pole low pass Bessel filter.
Hippocampal Slice Data Analysis. Data from slice recordings were recorded onto a digital audio tape by using a Biologic DTR 1200 recorder and analyzed offline by using the Strathclyde Electrophysiology Software, winedr (courtesy of J. Dempster, University of Strathclyde, Glasgow, U.K.). The amplitude of the tonic current and rms were calculated as described above.
Heterologous Expression of Recombinant GABAARs. Human embryonic kidney 293 cells were transiently transfected with cDNAs encoding human GABAAR α5 or α1, β2 or β3, and γ2L subunit isoforms (1:1:1 ratio) by using Lipofectamine 2000 (Invitrogen). All cDNAs were subcloned into the mammalian expression vectors pCDM8 or pcDNA3.1 for heterologous expression. Human embryonic kidney 293 cells were grown on culture dishes and incubated for 24-48 h before investigation.
Statistics. All results are reported as the mean ± SEM. Normalized data are expressed as percent of control. Statistical significance was assessed by using the Student's t test or one-way ANOVA, as appropriate.
Results
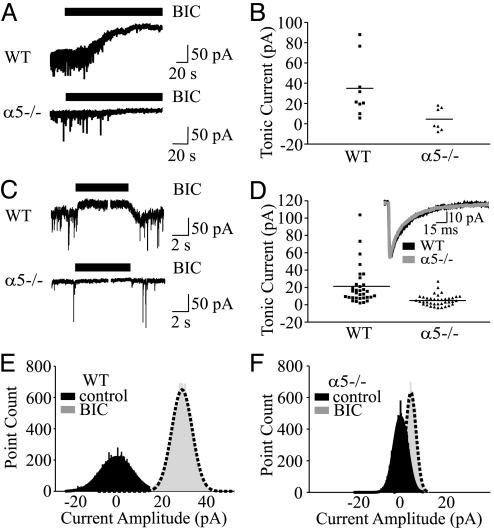
If GABAARs that contain α5 subunits generate the tonic current, then hippocampal neurons that do not express the α5 subunit should exhibit no tonic current. To address this postulate, we recorded whole-cell currents from pyramidal neurons in the CA1 region of hippocampal slices from WT and α5-/- mice. Application of BIC (100 μM) to WT neurons inhibited the tonic current and reduced Ihold by 34.5 ± 9.6 pA (n = 9; Fig. 1A). A reduced tonic current was exhibited by α5-/- neurons (4.3 ± 4.3 pA, n = 7, P < 0.05, Fig 1 A and B). The baseline noise was also reduced in α5-/- compared to WT neurons as indicated by a decrease in the rms value (σ: 2.8 ± 0.2 pA, n = 9 vs. 3.6 ± 0.3 pA, n = 11, P < 0.05, respectively). BIC also inhibited the sIPSCs in both WT and α5-/- neurons, as evidenced by the blockade of transient inward currents (Fig. 1A).
Fig. 1.
Reduced tonic current in α5-/- hippocampal slice and cultured neurons. (A) BIC (100 μM; solid bar) inhibited a tonic current recorded from CA1 pyramidal neurons in hippocampal slices from WT but not α5-/- mice. (B) Scatterplots illustrate the amplitude of the tonic current in hippocampal slices. The horizontal lines in B and D represent the mean amplitude of the tonic current. (C) Tonic current is reduced in α5-/- neurons grown in primary cultures. (D) Scatterplots illustrate the amplitude of the tonic current in cultured neurons. Inset shows that mIPSCs in α5-/- and WT neurons are similar. (E and F) The all-point histograms illustrate the amplitude of the current in the absence (black) and presence (gray) of BIC for WT and α5-/- cultured neurons. The dashed line represents the line of best fit.
We next investigated whether dissociated cultures of hippocampal neurons from WT and α5-/- mice also generated an α5 subunit-mediated tonic current. Although the network architecture of the hippocampus is disrupted during cell isolation, the sorting and targeting of GABAAR subunits that occurs in vivo also occurs in dissociated cultures, especially in regards to the distribution of extrasynaptic receptors (11). Microscopy revealed no differences in the size or morphology of the cells, and cell capacitance values were similar for neurons from WT and α5-/- mice (18.7 ± 1.8 pF, n = 24 vs. 20.2 ± 1.9 pF, n = 25, respectively). As was observed in recordings from hippocampal slices, the application of BIC revealed a larger tonic current in WT compared to α5-/- neurons (20.6 ± 3.8 pA, n = 33 vs. 5.2 ± 1.0 pA, n = 38, P < 0.05; Fig. 1 C-F). The larger tonic current in WT neurons was accompanied by a larger baseline noise (WT: 3.5 ± 0.2 pA, n = 33 vs. α5-/-: 2.6 ± 0.1 pA, n = 38, P < 0.01). Despite a reduced tonic current in α5-/- neurons, no differences were detected in the frequency, peak amplitude, rise time, charge transfer (Q), or decay of mIPSCs between WT and α5-/- neurons (Table 1 and Fig. 1D Inset). As a negative control, we investigated whether cortical neurons that express low levels of α5GABAARs also generate a tonic current. Application of BIC revealed minimal tonic current in α5-/- and WT cortical neurons (6.7 ± 1.6 pA, n = 32 and 5.1 ± 1.6 pA, n = 13, respectively). Baseline noise values were similar for α5-/- and WT neurons (2.4 ± 0.1 pA, n = 32 vs. 2.4 ± 0.2 pA, n = 13, respectively) as were the properties of mIPSCs (Table 1).
Table 1. Spontaneous mIPSCs from WT and α5−/− cultured neurons.
| Group | n | Frequency, Hz | Amplitude, pA | Rise, ms | Q, pA·ms | Decay, ms |
|---|---|---|---|---|---|---|
| Hippocampus | ||||||
| WT | 14 | 1.6 ± 0.4 | 55.0 ± 2.5 | 2.3 ± 0.2 | 1620.1 ± 121.9 | 25.5 ± 2.1 |
| α5−/− | 15 | 1.8 ± 0.6 | 49.9 ± 3.3 | 2.0 ± 0.1 | 1350.9 ± 86.0 | 26.5 ± 1.6 |
| Cortex | ||||||
| WT | 7 | 1.1 ± 0.3 | 30.0 ± 3.9 | 2.4 ± 0.2 | 848.9 ± 96.9 | 24.8 ± 2.5 |
| α5−/− | 9 | 0.6 ± 0.2 | 32.6 ± 5.3 | 2.7 ± 0.1 | 1070.4 ± 165.6 | 30.3 ± 2.6 |
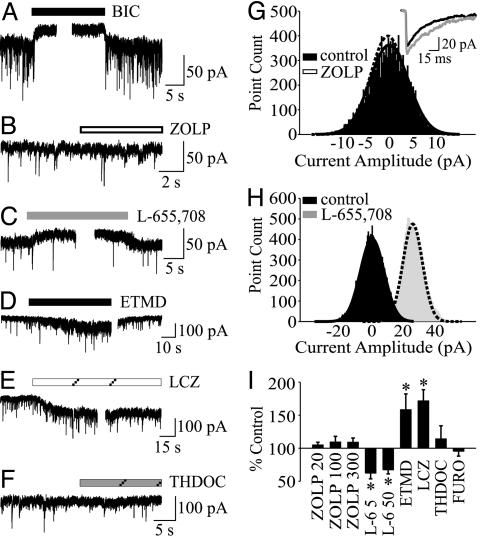
To support the hypothesis that α5 subunit-containing GABAARs generate the tonic current but not mIPSCs in hippocampal pyramidal neurons, we tested compounds that interact with GABAARs in a subunit-selective manner. BIC inhibited a tonic current in cultured hippocampal neurons (34.5 ± 6.9 pA, n = 19, Fig. 2A) as previously shown (20, 22). The α5 subunit confers a high affinity for positive modulation by classical benzodiazepine agonists, including midazolam, but a low affinity for the imidazopyridine zolpidem (ZOLP) (29, 30). We previously showed that the tonic current in hippocampal neurons was sensitive to enhancement by midazolam (22). ZOLP (20, 100, and 300 nM) failed to enhance the tonic current (104.8 ± 4.17 pA, n = 13; 109.3 ± 8.7% n = 11, and 109.0 ± 6.5, n = 8 respectively; Fig. 2 B, G, and I). Although ZOLP (100 nM) caused no change in the tonic current, it nevertheless slowed the decay of mIPSCs (Table 2 and Fig. 2G Inset) and increased the charge transfer of the mIPSCs to 132.7 ± 6.2% of control (n = 15, P < 0.05). Binding studies showed that L-655,708 exhibits at least a 50-fold selectivity for α5β2γ2 GABAARs compared with heteromeric complexes containing α1, α2, α3, or α6 subunits (31, 32). Application of L-655,708 (5 μM and 50 μM; Fig. 2 C and I) reduced the tonic current to 62.4 ± 8.7% (n = 10) and 67.3 ± 6.2% of control (n = 15, P < 0.05), respectively, but failed to change the mIPSCs (Table 2).
Fig. 2.
Pharmacological profile of the tonic current in hippocampal neurons from Swiss White mice. Representative traces of Ihold before and during the application of BIC (100 μM) (A), ZOLP (100 nM) (B), L-655,708 (50 μM) (C), ETMD (100 nM) (D), loreclezole (LCZ) (10 μM) (E), and allotetrahydrodeoxycorticosterone (THDOC) (10 nM) (F). (G) The corresponding all-point histograms for ZOLP treatment are shown on the right. Inset shows the averaged traces of mIPSCs recorded in the absence (black) and presence (gray) of ZOLP (100 nM). ZOLP increased the decay and charge transfer of mIPSCs. (H) The shift in the all-point histogram by L-655,708 is consistent with a reduced tonic current. (I) The bar graph summarizes the effects of drugs, including furosemide (FURO, 600 μM), on the amplitude of the tonic current. Data are presented as percent control relative to the BIC-mediated shift in current amplitude (*, P < 0.05).
Table 2. Effects of ZOLP and L-655,708 on mIPSCs from Swiss White mice.
| Group | n | Frequency, Hz | Amplitude,.pA | Rise, ms | Q, pA·ms | Decay, ms |
|---|---|---|---|---|---|---|
| Control | 9 | 4.3 ± 1.0 | 43.2 ± 1.5 | 2.0 ± 0.1 | 1004.6 ± 71.8 | 21.6 ± 5.8 |
| ZOLP (20 nM) | 9 | 5.4 ± 1.5 | 44.3 ± 3.2 | 2.2 ± 0.2 | 1116.2 ± 115.2 | 23.1 ± 1.9 |
| ZOLP (100 nM) | 8 | 4.7 ± 1.6 | 44.6 ± 3.7 | 2.1 ± 0.1 | 1324.8 ± 140.8* | 29.6 ± 2.7* |
| Control | 7 | 1.9 ± 0.6 | 44.5 ± 6.7 | 2.2 ± 0.2 | 902.3 ± 76.1 | 18.5 ± 1.7 |
| L-655,708 (50 μM) | 7 | 2.6 ± 0.6 | 43.8 ± 6.7 | 2.4 ± 0.4 | 944.2 ± 111.1 | 18.6 ± 1.7 |
*, P < 0.05 vs. control.
We next sought to identify the subunits that coassemble with α5 subunits by comparing the properties of native GABAARs with those of recombinant receptors composed of known subunit isoforms. Several lines of evidence indicate that most α5GABAARs contain β3 subunits in pyramidal neurons (9, 29, 33). Further, β3-containing GABAARs are present in extrasynaptic regions of hippocampal neurons (8), and binding studies indicate that native α5GABAARs display the pharmacological properties of α5β3γ2 receptors (9). Further, the GABA sensitivity and kinetic properties of GABA-evoked currents recorded from hippocampal neurons suggest that α5β3γ2L GABAARs represent a native receptor subtype (34). We tested whether α5 subunits coassemble with β3 subunits by examining the sensitivity of the tonic current to compounds selective for β2/3 subunits.
ETMD is a positive allosteric modulator selective for β2 or β3 subunit-containing GABAARs (35, 36). ETMD (100 nM) enhanced the tonic current to 158.3 ± 23.9% of control (n = 8, P < 0.05; Fig. 2 D and I). To ensure that the increase in tonic current by ETMD did not result from an undetected enhancement of mIPSCs, the GABAAR antagonist penicillin (5 mM) was added to the bath solution. We previously showed that penicillin inhibits synaptic but not tonic current in hippocampal neurons by an unidentified mechanism (25). Penicillin blocked the mIPSCs but not the ETMD-enhanced tonic current (not shown). Loreclezole (10 μM), a β2/3 subunit-selective anticonvulsant (37), increased the tonic current to 171.6 ± 17.0% of control (n = 6, P < 0.05; Fig. 2 E and I). The α4, α6, and ε subunit-selective antagonist furosemide (600 μM) failed to inhibit the tonic current (95.5 ± 7.4% of control, Fig. 2I), indicating that these subunits do not mediate the tonic current in hippocampal pyramidal neurons. The δ subunit-preferring neuroactive steroid 3α,21-dihydroxy-5α-pregnan-20-one (allotetrahydrodeoxycorticosterone; 10 nM), failed to enhance the tonic current (114.0 ± 19.9% of control, n = 5, P < 0.05; Fig. 2 F and I). Insensitivity to allotetrahydrodeoxycorticosterone (10 nM) and furosemide indicates that the tonic current in pyramidal neurons is distinct from the highly steroid-sensitive tonic current in dentate gyrus, thalamic relay neurons, and cerebellar granule cells that is generated by δ-containing GABAARs (38-40).
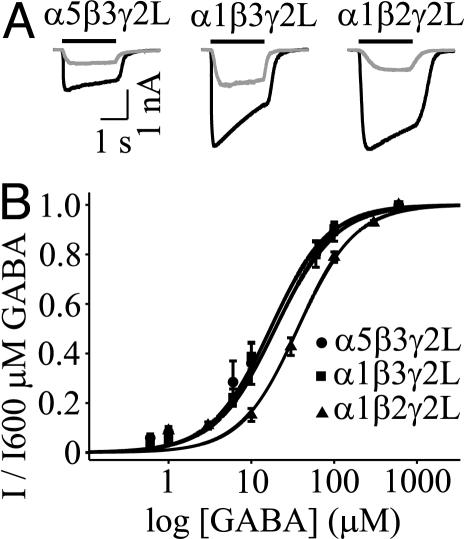
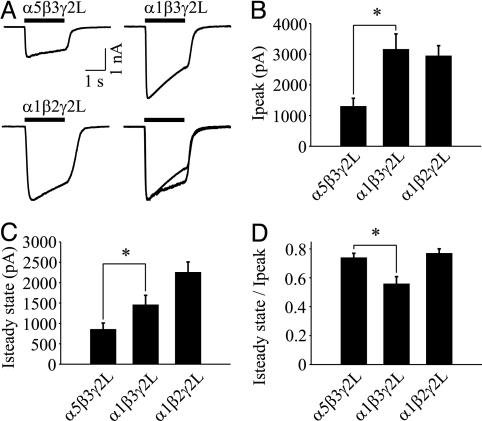
Having determined that α5GABAARs generate a tonic current in hippocampal neurons, we investigated the unique properties conferred by α5 subunits by recording from recombinant GABAAR subunits expressed in human embryonic kidney 293 cells. We compared α5β3γ2L to α1β3γ2L and α1β2γ2L GABAARs, because the α1 subunit isoform is highly abundant in the mammalian brain (1), and α1β2γ2L GABAARs are likely to be the most common synaptic complexes. Applications of GABA (600 μM) to cells expressing α5β3γ2L, α1β3γ2L, and α1β2γ2L activated currents that peaked (Ip) then decayed to a steady state (Iss). The concentration-response curves for α5β3γ2L and α1β3γ2L receptors revealed no difference in the EC50 values (19.4 ± 3.5 μM, n = 7 vs. 17.0 ± 2.3 μM, n = 6, respectively), and the Hill coefficients for both curves were 1.2 ± 0.1 (Fig. 3). Sensitivity of α1β2γ2L GABAARs was reduced 2-fold (EC50 = 36.3 ± 3.3 μM, n = 4, P < 0.05), indicating that replacement of β3 with β2 reduces the potency of GABA. Thus, the potency of GABA is greater for α5β3GABAARs (putatively extrasynaptic) than for α1β2GABAARs (putatively synaptic), as previously suggested from studies of hippocampal neurons (25). We next compared the amplitude of current evoked by a saturating concentration of GABA (600 μM), because our previous reports suggested that the tonic current is generated by low-conductance GABAARs (22, 25) (Fig. 4A). Ip was reduced 2.4-fold for α5β3γ2L compared with α1β3γ2 GABAARs (1.30 ± 0.27 nA, n = 22 vs. 3.16 ± 0.51 nA, n = 18, P < 0.05; Fig. 4B). The steady-state current was also reduced for α5β3γ2L GABAARs (Fig. 4C). These differences are consistent with a lower channel conductance, although reduced open probability or surface expression of GABAARs cannot be excluded.
Fig. 3.
(A) Representative traces for currents evoked by 10 μM GABA (gray) and 600 μM GABA (black) from α5β3γ2L, α1β3γ2L, and α1β2γ2L GABAARs. The solid line denotes the duration of GABA application. (B) GABA concentration-response curves for α5β3γ2L, α1β3γ2L, and α1β2γ2L GABAARs. GABA sensitivity was reduced 2-fold for α1β2γ2L GABAARs compared with α5β3γ2L and α1β3γ2L GABAARs.
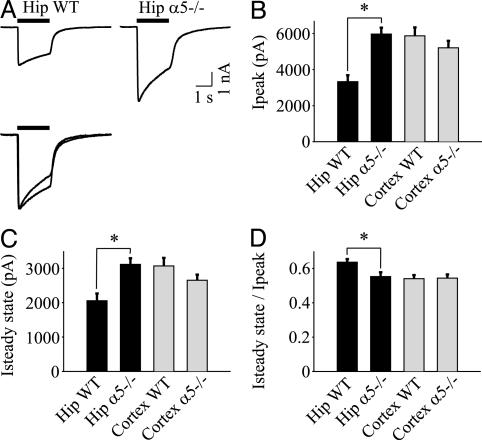
Fig. 4.
α5GABAARs expressed in human embryonic kidney 293 cells generate low-amplitude, slowly desensitizing current. (A) Representative traces of GABA-evoked currents recorded from α5β3γ2L, α1β3γ2L, and α1β2γ2L GABAARs. The solid line denotes the duration of GABA application (600 μM). Currents from α5β3γ2L and α1β3γ2L GABAARs are normalized and superimposed for illustration purposes (Lower Right). (B and C) Ip and Iss are reduced for α5β3γ2L vs. α1β3γ2L. (D) The Iss/Ip ratio is greater for α5β3γ2L than for α1β3γ2L GABAARs (*, P < 0.05).
We next determined whether the α5 subunit regulates desensitization of GABAARs, because our previous report suggested that extrasynaptic receptors desensitize more slowly than synaptic receptors in hippocampal neurons (25). GABAARs display a complex pattern of desensitization characterized by fast and slow components (41). Here, we examined slow desensitization, a process that could influence GABAARs that are continually exposed to ambient GABA. The time course of desensitization was 2.3-fold slower for α5β3γ2L compared with α1β3γ2L GABAARs (τweighted = 3.54 ± 0.44 s, n = 22 vs. 1.54 ± 0.22 s, n = 18, P < 0.05, respectively). The extent of desensitization, estimated by the Iss/Ip ratio, was also reduced for α5β3γ2L (Fig. 4D). Therefore, substitution of α5 for α1 in αxβ3γ2L complexes generates a low-amplitude current that desensitizes slowly (34).
The above results predicted that neurons that express high levels of α5 subunits should generate low-amplitude currents compared with α5-deficient neurons. To test this prediction, GABA (600 μM)-evoked currents were recorded from WT and α5-/- neurons (Fig. 5A). Ip was reduced by 44% for WT compared with α5-/- hippocampal neurons (3.33 ± 0.36 nA, n = 33 vs. 5.97 ± 0.35 nA, n = 36, P < 0.05; Fig. 5B), whereas no difference was detected between WT and α5-/- cortical neurons (Fig. 5B). The steady-state current was also reduced in WT compared with α5-/- hippocampal neurons but not in cortical neurons (Fig. 5C). Consistent with the slow desensitization of α5GABAARs, the extent of desensitization was lower in WT compared with α5-/- neurons (Iss/Ip ratio: WT 0.64 ± 0.02, n = 33 vs. α5-/- 0.55 ± 0.02, n = 36, P < 0.05; Fig. 5D), and the time course of current desensitization was prolonged (τweighted = 2.12 ± 0.32 s, n = 33 vs. 1.31 ± 0.16 s, n = 36, P < 0.05, respectively). No differences were detected in the extent of desensitization of the current recorded in cortical neurons from WT and α5-/- mice. Also, mIPSCs were similar in WT and α5-/- cortical neurons (Table 1). Together, these results suggest that extrasynaptic α5GABAARs generate a lower-amplitude, slower-desensitizing current in hippocampal neurons.
Fig. 5.
Hippocampal α5-/- neurons generate a higher-amplitude, faster-desensitizing current. (A) Representative GABA-evoked currents (600 μM) recorded from α5-/- and WT hippocampal (Hip) neurons. The solid line denotes the duration of GABA application (600 μM). The traces are normalized and superimposed for illustration purposes (Lower). (B and C) WT hippocampal neurons generate smaller Ip and Iss compared with α5-/- neurons. (D) The Iss/Ip ratio is increased in hippocampal WT compared with α5-/- neurons (*, P < 0.05). Note that there are no differences in Ip, Iss, or Iss/Ip for cortical neurons from α5-/- or WT mice.
Discussion
Pharmacological and molecular strategies were used to investigate the GABAAR subunits that generate a tonic inhibitory current in cultured hippocampal neurons and CA1 pyramidal neurons in hippocampal slices. Consistent with our hypothesis, the tonic current but not action potential-independent inhibitory synaptic current was predominantly mediated by α5GABAARs. The α5-/- neurons exhibited a marked reduction in the tonic current and baseline noise. Further, the tonic current was insensitive to enhancement by ZOLP and partially inhibited by the α5 subunit-selective inverse agonist, L-655,708. More importantly, our findings show that tonic and synaptic currents in CA1 pyramidal neurons are generated, at least in part, by populations of GABAARs with differing subunit compositions. Thus, differences in the sensitivity of tonic and synaptic conductances in the CA1 hippocampus to positive allosteric modulators cannot be attributed merely to state-dependent changes in a single GABAAR population (22, 25).
We also examined the properties conferred by the α5 subunit and showed that recombinant α5GABAARs generated smaller macroscopic currents that desensitize more slowly than α1GA BAARs. The low macroscopic conductance of α5GABAARs was consistent with the smaller GABA-evoked currents generated by WT compared with α5-/- neurons. Also, the potency of GABA was greater for α5β3γ2L compared with α1β2γ2L GABAARs, consistent with a greater GABA sensitivity of the tonic compared to synaptic receptors (25). We previously showed that GABA-evoked current recorded from dissociated hippocampal neurons consisted of two components: a relatively fast desensitizing current and a nondesensitizing steady-state current (25). The nondesensitizing component was primarily attributed to extrasynaptic GABAARs because it was not blocked by antagonists that selectively inhibit the synaptic but not tonic currents. Further, consistent with α5 subunits regulating desensitization, a previous report showed that ZOLP-insensitive neurons in CA1 neurons desensitize less than ZOLP-sensitive neurons at depolarizing potentials (34). Slow desensitization and high sensitivity to GABA would allow sustained activation of GABAARs that are continuously exposed to low ambient concentrations of GABA. A similar role was ascribed to α6 subunits that are also expressed in extrasynaptic receptors (20, 42). The α5GABAARs also contribute to a high baseline noise. Noise levels were similar for hippocampal neurons from WT and Swiss White mice, whereas a comparison with our previous reports showed that α5-/- hippocampal neurons, WT cortical neurons, and BIC-treated hippocampal neurons from WT and Swiss White mice (σ = 2.9 pA) (22, 25) exhibit a similar low baseline noise.
We found no differences in the mIPSCs recorded from α5-/- or WT neurons. ZOLP (100 nM) prolonged the mIPSCs, whereas L-655,708 (up to 50 μM) had no effect. Thus, α5GABAARs are unlikely to contribute appreciably to action potential-independent synaptic currents. The lack of change in mIPSCs contrasts with a 33% reduction in amplitude of action potential-dependent spontaneous IPSCs recorded in CA1 hippocampal pyramidal neurons from α5-/- mice (4). The amplitude of stimulus-evoked IPSPs recorded in α5-/- neurons was also reduced to 87% of control. It is generally accepted that evoked IPSCs result from the synchronized release of many vesicles in response to an action potential, whereas individual mIPSCs are the basic quantal units for evoked synaptic responses. The most parsimonious explanation for a reduction in evoked IPSCs with no change in the mIPSCs is that GABA, released during action potential-dependent stimulation, spills out of the synaptic cleft and activates extrasynaptic α5GABAARs that reside in the perisynaptic membrane. Similar actions are attributed to extrasynaptic α6GABAARs in cerebellar granule neurons. The tonic current could also be regulated by the release of GABA from cotransporters. The concentration of GABA present in the extracellular space in vivo (1 μM) is sufficient to activate high-affinity extrasynaptic GABAARs (43).
The role of extrasynaptic inhibition may become increasingly important as the number of GABA-releasing synapses declines with increasing postnatal age (44). Although expression of the α5 subunit gene reaches a prominent peak in the early brain (45), the tonic current mediated by α5GABAARs is not restricted to immature neurons. A tonic current is present in CA1 pyramidal neurons from adult rats (21) and guinea pigs (46). Further, GABA-evoked current from CA1 pyramidal neurons obtained from 28- to 35-day-old rats displays voltage-dependent properties consistent with α5GABAARs (34). Nearly one-third of these neurons are insensitive to ZOLP, whereas all of the neurons are sensitive to diazepam. The predominant ZOLP-insensitive GABAAR complex in the brain is thought to be α5β3γ2L (29). A tonic current is also present in interneurons in the stratum radiatum of adult guinea pigs (46); however, α5GABAARs are unlikely to underlie this tonic current because expression of α5 subunits is mostly restricted to pyramidal neurons (11). Certainly, other α subunit isoforms may contribute modestly to the tonic current in hippocampal neurons, including α3 subunits that are expressed extrasynaptically (11). Notably, the tonic current in CA1 neurons is not predominantly mediated by δ subunit-containing GABAARs and is pharmacologically distinct from the tonic current in cerebellar granule cells, dentate gyrus, and thalamic relay neurons (47).
Extrasynaptic α5GABAARs in CA1 pyramidal neurons may play a critical role in learning and memory processes. Reduced α5 subunit expression in CA1 pyramidal neurons is associated with improved performance for hippocampus-dependent learning tasks (4, 5). Long-term potentiation of synaptic efficacy resulting from high-frequency stimulation of afferent pathways is widely accepted as a candidate cellular mechanism of memory in the hippocampus. However, long-term potentiation in CA1 neurons was similar in WT and α5-/- mice (4), whereas the ability of paired-pulse stimuli to enhance excitatory synaptic potential (PPF) was increased in α5-/- slices. The enhanced PPF was specific to the CA1 region and not demonstrated in the dentate gyrus of α5-/- mice. PPF is generally attributed to reduced inhibition by postsynaptic GABAA receptors. Because mIPSCs were unchanged in α5-/- neurons, our results imply that reduced tonic inhibition underlies the enhanced PPF. Pharmacological evidence further supports a role for tonic inhibition in regulating memory processes. Amnestic drugs, including midazolam, propofol (22), and volatile anesthetics (Orser Laboratory, unpublished data) preferentially enhance the tonic current compared with synaptic currents. Consistent with the notion that extrasynaptic GABAARs contribute to behavioral effects of neurodepressive drugs, δGABAARs that generate a tonic inhibition in the dentate gyrus, thalamus, and cerebellum are highly sensitive to low concentrations of ethanol and neuroactive steroids (21, 48). Tonic inhibition in CA1 pyramidal neurons may also contribute to pathological states, because α5 subunit expression is down-regulated in animal models of chronic epilepsy (15). Our results, together with behavioral studies, indicate that the α5GABAAR-mediated tonic inhibitory conductance in hippocampal pyramidal neurons may regulate memory and neuroexcitatory disorders.
Acknowledgments
This research was supported by grants from the Canadian Institutes of Health Research (to B.A.O. and J.F.M.), a Career Scientist Award (to B.A.O.), and Canadian Institutes of Health Research Fellowships (to V.B.C. and J.G.N.). D.B. is a Medical Research Council Senior Fellow. Some experiments were supported by the Commission of the European Communities Research and Technological Development Program “Quality of Life and Management of Living Resources,” QLK1-CT-2000-00179.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BIC, bicuculline methiodide; ETMD, etomidate; GABA, γ-aminobutyric acid; GABAAR, GABA type A receptor; α5GABAAR, α5 subunit-containing GABAAR; mIPSC, miniature inhibitory postsynaptic current; ZOLP, zolpidem.
References
- 1.McKernan, R. M. & Whiting, P. J. (1996) Trends Neurosci. 19, 139-143. [DOI] [PubMed] [Google Scholar]
- 2.Nusser, Z., Sieghart, W. & Somogyi, P. (1998) J. Neurosci. 18, 1693-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fritschy, J. M. & Mohler, H. (1995) J. Comp. Neurol. 359, 154-194. [DOI] [PubMed] [Google Scholar]
- 4.Collinson, N., Kuenzi, F. M., Jarolimek, W., Maubach, K. A., Cothliff, R., Sur, C., Smith, A., Otu, F. M., Howell, O., Atack, J. R., et al. (2002) J. Neurosci. 22, 5572-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crestani, F., Keist, R., Fritschy, J. M., Benke, D., Vogt, K., Prut, L., Bluthmann, H., Mohler, H. & Rudolph, U. (2002) Proc. Natl. Acad. Sci. USA 99, 8980-8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers, M. S., Atack, J. R., Bromidge, F. A., Broughton, H. B., Cook, S., Dawson, G. R., Hobbs, S. C., Maubach, K. A., Reeve, A. J., Seabrook, G. R., et al. (2002) J. Med. Chem. 45, 1176-1179. [DOI] [PubMed] [Google Scholar]
- 7.Chambers, M. S., Atack, J. R., Broughton, H. B., Collinson, N., Cook, S., Dawson, G. R., Hobbs, S. C., Marshall, G., Maubach, K. A., Pillai, G. V., et al. (2003) J. Med. Chem. 46, 2227-2240. [DOI] [PubMed] [Google Scholar]
- 8.Pirker, S., Schwarzer, C., Wieselthaler, A., Sieghart, W. & Sperk, G. (2000) Neuroscience 101, 815-850. [DOI] [PubMed] [Google Scholar]
- 9.Sur, C., Quirk, K., Dewar, D., Atack, J. & McKernan, R. (1998) Mol. Pharmacol. 54, 928-933. [DOI] [PubMed] [Google Scholar]
- 10.Sur, C., Fresu, L., Howell, O., McKernan, R. M. & Atack, J. R. (1999) Brain Res. 822, 265-270. [DOI] [PubMed] [Google Scholar]
- 11.Brunig, I., Scotti, E., Sidler, C. & Fritschy, J. M. (2002) J. Comp. Neurol. 443, 43-55. [DOI] [PubMed] [Google Scholar]
- 12.Sieghart, W. (1995) Pharmacol. Rev. 47, 181-234. [PubMed] [Google Scholar]
- 13.Wisden, W., Laurie, D. J., Monyer, H. & Seeburg, P. H. (1992) J. Neurosci. 12, 1040-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritschy, J. M., Johnson, D. K., Mohler, H. & Rudolph, U. (1998) Neurosci. Lett. 249, 99-102. [DOI] [PubMed] [Google Scholar]
- 15.Houser, C. R. & Esclapez, M. (2003) Hippocampus 13, 633-645. [DOI] [PubMed] [Google Scholar]
- 16.Jones, A., Korpi, E. R., McKernan, R. M., Pelz, R., Nusser, Z., Makela, R., Mellor, J. R., Pollard, S., Bahn, S., Stephenson, F. A., et al. (1997) J. Neurosci. 17, 1350-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nusser, Z., Ahmad, Z., Tretter, V., Fuchs, K., Wisden, W., Sieghart, W. & Somogyi, P. (1999) Eur. J. Neurosci. 11, 1685-1697. [DOI] [PubMed] [Google Scholar]
- 18.Brickley, S. G., Cull-Candy, S. G. & Farrant, M. (1996) J. Physiol. (London) 497, 753-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tia, S., Wang, J. F., Kotchabhakdi, N. & Vicini, S. (1996) J. Neurosci. 16, 3630-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamann, M., Rossi, D. J. & Attwell, D. (2002) Neuron 33, 625-633. [DOI] [PubMed] [Google Scholar]
- 21.Stell, B. M., Brickley, S. G., Tang, C. Y., Farrant, M. & Mody, I. (2003) Proc. Natl. Acad. Sci. USA 100, 14439-14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai, D., Zhu, G., Pennefather, P., Jackson, M. F., MacDonald, J. F. & Orser, B. A. (2001) Mol. Pharmacol. 59, 814-824. [DOI] [PubMed] [Google Scholar]
- 23.Wu, Y., Wang, W. & Richerson, G. B. (2003) J. Neurophysiol. 89, 2021-2034. [DOI] [PubMed] [Google Scholar]
- 24.Stell, B. M. & Mody, I. (2002) J Neurosci. 22, RC223: 1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeung, J. Y., Canning, K. J., Zhu, G., Pennefather, P., MacDonald, J. F. & Orser, B. A. (2003) Mol. Pharmacol. 63, 2-8. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald, J. F., Mody, I., Salter, M. W., Pennefather, P. & Schneiderman, J. H. (1989) Prog. Neuropsychopharmacol. Biol. Psychiatry 13, 481-488. [DOI] [PubMed] [Google Scholar]
- 27.Wall, M. J. & Usowicz, M. M. (1997) Eur. J. Neurosci. 9, 533-548. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds, D. S., Rosahl, T. W., Cirone, J., O'Meara, G. F., Haythornthwaite, A., Newman, R. J., Myers, J., Sur, C., Howell, O., Rutter, A. R., et al. (2003) J. Neurosci. 23, 8608-8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luddens, H., Seeburg, P. H. & Korpi, E. R. (1994) Mol. Pharmacol. 45, 810-814. [PubMed] [Google Scholar]
- 30.Pritchett, D. B. & Seeburg, P. H. (1990) J. Neurochem. 54, 1802-1804. [DOI] [PubMed] [Google Scholar]
- 31.Casula, M. A., Bromidge, F. A., Pillai, G. V., Wingrove, P. B., Martin, K., Maubach, K., Seabrook, G. R., Whiting, P. J. & Hadingham, K. L. (2001) J. Neurochem. 77, 445-451. [DOI] [PubMed] [Google Scholar]
- 32.Quirk, K., Blurton, P., Fletcher, S., Leeson, P., Tang, F., Mellilo, D., Ragan, C. I. & McKernan, R. M. (1996) Neuropharmacology 35, 1331-1335. [DOI] [PubMed] [Google Scholar]
- 33.Merten, S., Benke, D. & Mohler, H. (1993) J. Biol. Chem. 268, 5965-5973. [PubMed] [Google Scholar]
- 34.Burgard, E. C., Tietz, E. I., Neelands, T. R. & Macdonald, R. L. (1996) Mol. Pharmacol. 50, 119-127. [PubMed] [Google Scholar]
- 35.Sanna, E., Murgia, A., Casula, A. & Biggio, G. (1997) Mol. Pharmacol. 51, 484-490. [PubMed] [Google Scholar]
- 36.Hill-Venning, C., Belelli, D., Peters, J. A. & Lambert, J. J. (1997) Br. J. Pharmacol. 120, 749-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wingrove, P. B., Wafford, K. A., Bain, C. & Whiting, P. J. (1994) Proc. Natl. Acad. Sci. USA 91, 4569-4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wall, M. J. (2002) Neuropharmacology 43, 737-749. [DOI] [PubMed] [Google Scholar]
- 39.Korpi, E. R. & Luddens, H. (1997) Br. J. Pharmacol. 120, 741-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korpi, E. R., Grunder, G. & Luddens, H. (2002) Prog. Neurobiol. 67, 113-159. [DOI] [PubMed] [Google Scholar]
- 41.Bai, D., Pennefather, P. S., MacDonald, J. F. & Orser, B. A. (1999) J. Neurosci. 19, 10635-10646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi, D. J., Hamann, M. & Attwell, D. (2003) J. Physiol. 548, 97-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lerma, J., Herranz, A. S., Herreras, O., Abraira, V. & Martin, D. R. (1986) Brain Res. 384, 145-155. [DOI] [PubMed] [Google Scholar]
- 44.de Felipe, J., Marco, P., Fairen, A. & Jones, E. G. (1997) Cereb. Cortex 7, 619-634. [DOI] [PubMed] [Google Scholar]
- 45.Laurie, D. J., Wisden, W. & Seeburg, P. H. (1992) J. Neurosci. 12, 4151-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semyanov, A., Walker, M. C. & Kullmann, D. M. (2003) Nat. Neurosci. 6, 484-490. [DOI] [PubMed] [Google Scholar]
- 47.Porcello, D. M., Huntsman, M. M., Mihalek, R. M., Homanics, G. E. & Huguenard, J. R. (2003) J. Neurophysiol. 89, 1378-1386. [DOI] [PubMed] [Google Scholar]
- 48.Wallner, M., Hanchar, H. J. & Olsen, R. W. (2003) Proc. Natl. Acad. Sci. USA 100, 15218-15223. [DOI] [PMC free article] [PubMed] [Google Scholar]