Abstract
Post-translational degradation of protein plays an important role in cell life. We employed chimeric molecules (dihydrotestosterone-based proteolysis-targeting chimeric molecule [DHT-PROTAC]) to facilitate androgen receptor (AR) degradation via the ubiquitin–proteasome pathway (UPP) and to investigate the role of AR in cell proliferation and viability in androgen-sensitive prostate cancer cells. Western blot analysis and immunohistochemistry were applied to analyse AR levels in LNCaP cells after DHT-PROTAC treatment. Cell counting and the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) cell viability assay were used to evaluate cell proliferation and viability after AR elimination in both LNCaP and PC-3 cells. AR was tagged for elimination via the UPP by DHT-PROTAC, and this could be blocked by proteasome inhibitors. Degradation of AR depended on DHT-PROTAC concentration, and either DHT or an ALAPYIP-(arg)8 peptide could compete with DHT-PROTAC. Inhibition of cell proliferation and decreased viability were observed in LNCaP cells, but not in PC-3 or 786-O cells after DHT-PROTAC treatment. These data indicate that AR elimination is facilitated via the UPP by DHT-PROTAC, and that the growth of LNCaP cells is repressed after AR degradation.
Keywords: androgen receptor, LNCaP, prostate cancer, proteolysis, ubiquitin
Introduction
The androgen receptor (AR) and its ligands play an important role in the development and maintenance of the male genital system and are involved in the pathogenesis of benign prostate hyperplasia and prostate cancer, which show an increasing morbidity in elderly males 1, 2. AR expression is maintained throughout the progression of prostate cancer, and the majority of androgen-independent or hormone-refractory prostate cancers express AR. Although AR plays an important role in prostate cancer, the mechanisms underlying the development and progression of this disease are not well understood 3.
AR ligands include testosterone and dihydrotestosterone (DHT). Although both androgens can bind to AR, DHT has a 10-fold higher binding affinity than testosterone and is consequently the primary androgen bound by AR 4. After a conformational change induced by DHT binding in the cytoplasm that allows dissociation of a heat shock protein, AR forms a homo-dimer and is phosphorylated at several sites. Subsequently, the ligand-receptor complex translocates to the nucleus, where it initiates gene transcription by binding to specific androgen-response elements in the promoter regions of its target genes. After DNA binding, the RNA polymerase machinery is recruited to the initiation site and transcription of AR-regulated genes begins 5. AR migrates rapidly back to the cytoplasm upon androgen withdrawal, where it maintains its ability to re-enter the nucleus for at least four rounds of AR recycling 6.
AR proteins undergo systematic protein degradation via the ubiquitin–proteasome pathway (UPP). Degradation via the UPP involves two discrete and successive steps: (i) covalent attachment of multiple ubiquitin molecules to the AR protein to form a polyubiquitin chain and (ii) degradation of the tagged protein by the 26S proteasome or, in certain cases, by the lysosomes/vacuole 7. Ubiquitin, a highly conserved 76-amino acid protein 8, is conjugated to the target protein by a three-step mechanism. Initially, the C-terminal carboxyl group of ubiquitin is activated by a ubiquitin-activating enzyme (E1). The thioester formed by attachment of ubiquitin to the E1 enzyme is then transferred through a transacylation reaction to a ubiquitin-conjugating enzyme (E2). Finally, the E2 enzyme transfers ubiquitin from E1 to a member of the ubiquitin–protein ligase family, E3, to which the substrate protein is specifically bound. E3 catalyses the last step in the conjugation process, which is the covalent attachment of ubiquitin to the substrate 9. Successive conjugations of ubiquitin to the internal lysines of previously added ubiquitin molecules lead to the formation of polyubiquitin chains 10. The polyubiquitinated target protein is then recognized by the 26S proteasome and eliminated.
The regulation of protein expression can be described as occurring at three basic levels. First, at the genetic level, genetic knockouts disrupt protein function at the DNA level by directly inactivating the gene responsible for a protein product. Second, at the post-transcriptional level, removal of a protein of interest may be accomplished by RNA interference (RNAi) 11. Finally, at the post-translational level, modifications such as glycosylation, phosphorylation or degradation significantly affect both the intracellular levels and the activity of a protein 12. Methods based on each of these three levels of regulation have been developed to disrupt protein expression 13. A number of studies have focused on the regulation of AR expression in the development and progression of prostate cancer, and various methods were devised to downregulate AR protein levels. AR gene knockout in mice 14 and AR expression suppressed by RNAi 15, 16 were accomplished in earlier studies. In contrast, interference with proteins at the post-translational level has been relatively less explored. Several biochemical or chemical genetic approaches, such as the chimeric F-box approach 17, the use of small molecules as biological probes to induce protein ubiquitination and degradation 18 and the use of proteolysis-targeting chimeric molecules (PROTACs) 19, have emerged and gained the attention of researchers.
In this study, PROTAC was used to eliminate the AR protein post-translationally. PROTAC is a heterobifunctional molecule composed of a ligand for the target protein, a linker moiety and a ligand for an E3 ubiquitin ligase 20. PROTAC induces interaction between the target protein and the E3 ubiquitin ligase, leading to artificial polyubiquitination of the target protein and subsequently degradation via the UPP. According to the different target–ligand pairs and recognition of different E3 ubiquitin ligases, five different PROTACs targeting methionine aminopeptidase (MetAP-2), human oestrogen receptor (hER), mutant FK506 binding protein and AR have shown their effectiveness in post-translational protein degradation. We synthesized DHT-PROTAC as described earlier 12, 21. This molecule consists of DHT, an ALAPYIP sequence coupled to a poly-D-arginine tag on its carboxy terminus, and a linker moiety. DHT, the natural ligand of AR, was designed in this molecule to target AR. The seven-residue polypeptide fragment from the hypoxia-inducible factor 1α (HIF1α) is the minimal recognition domain for the von Hippel–Lindau tumour suppressor protein (VHL), which is part of the VBC-Cul2 E3 ubiquitin ligase complex 22, 23. When DHT-PROTAC is presented to a cell, the AR and VBC-Cul2 E3 ubiquitin ligase complex are believed to be conjugated and the exposed lysine of the AR facilitated for ubiquitination by E3. The poly-D-arginine tag ensures the membrane permeability of the molecule through a mechanism similar to that of the Antennapedia 24 and HIV Tat proteins 25. This tag also protects the molecule from nonspecific proteolysis 26.
Schneekloth and colleagues 12 have shown that DHT-PROTAC induces the degradation of a green fluorescent protein (GFP)-AR fusion protein in human embryonic kidney (HEK) 293GFP–AR cells. We applied this approach to prostate cancer LNCaP cells, which have strong AR expression in the nucleus, to see whether DHT-PROTAC can still recruit the E3 ubiquitin ligase complex to the AR protein and facilitate AR degradation by the UPP. In addition, we aimed to determine whether the growth of LNCaP cells would be repressed after AR elimination, in comparison with AR-null cells.
Materials and methods
Antibodies and reagents
For western blot analysis, monoclonal primary antibodies against AR and β-actin and horseradish peroxidase (HRP)-linked secondary antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). For immunohistochemistry (IHC), monoclonal primary antibodies against AR, Supervision Anti-Mouse Detection Reagent (HRP, ready-to-use) and the 3,3′-diaminobenzidine (DAB) kit were from Changdao Biotech (Shanghai, China). DHT was purchased from Sigma-Aldrich (St. Louis, MO, USA). The ALAPYIP sequence coupled to the poly-D-arginine tag peptide was synthesized by Gill Biochem (Shanghai, China). LNCaP, PC-3 and 786-O cells were purchased from the cell bank of the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). Tissue culture media and associated reagents were purchased from GIBCO-Invitrogen (Carlsbad, CA, USA). MG-132 (N-carbobenzoxyl-Leu-Leu-Leucinal), epoxomicin and E-64[trans-epoxysuccinyl-L-leucylamido(4-guanidino)-butane] were purchased from Calbiochem (La Jolla, CA, USA).
Tissue culture
LNCaP and 786-O cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U mL−1 penicillin, 100 mg mL−1 streptomycin and 2 mmol L−1 L-glutamine. PC-3 cells were cultured in F-12 medium with 10% fetal bovine serum, 100 U mL−1 penicillin, 100 mg mL−1 streptomycin and 2 mmol L−1 L-glutamine. The cell lines were maintained at a temperature of 37°C in a humidified atmosphere of 5% CO2.
Detection of AR degradation by IHC
With an adjusted suspension concentration of 105 mL−1, LNCaP cells were incubated in a 24-well plate (coverslip coated with 0.5% poly-L-lysine), 1 mL per well. After the cells were grown to 70% confluency, 4% polyoxymethylene was added and the cells were rinsed with phosphate-buffered saline (PBS) after 30 min. 0.75% H2O2 was used to block the endogenous peroxidase for 30 min. Coverslips were incubated with the AR primary antibody (1:100 dilution) overnight and the HRP-linked secondary antibody (ready-to-use) for 45 min. DAB was added as a chromogen.
Detection of AR degradation by western blot analysis
Cells were washed with cold PBS twice and solubilized in lysis buffer containing 10 mmol L−1 Tris–HCl (pH 7.4), 150 mmol L−1 NaCl, 0.1% sodium dodecyl sulphate (SDS), 0.8% Triton X-100 and a protease inhibitor cocktail (BioVision, CA, USA) at 4°C for 30 min. The cell lysates were subjected to centrifugation at 16 100 × g at 4°C for 15 min. The supernatants were aspirated, and their protein contents were measured. In all, 60 μg of each protein lysate was loaded onto a 10% SDS polyacrylamide gel. After the proteins were separated, they were transferred to polyvinylidene difluoride membranes. Membranes were blocked by SuperBlock blocking buffer (Pierce, Rockford, IL, USA) for 45 min and incubated with the anti-AR antibody (1:500 dilution) by gentle shaking overnight. The antibody was diluted with 5% bovine serum albumin in 1 × TBST (Tris-buffered saline Tween-20) (10 mmol L−1 Tris–HCl, pH 7.5, 100 mmol L−1 NaCl and 0.1% Tween 20). After washing with 1 × TBST, the blots were incubated with the HRP-linked secondary antibody (1:2 000 dilution) in 5% nonfat dry milk in 1 × TBST for 60 min. Immunoreactive bands were detected by the Immobilon Western Chemiluminescent HRP Substrate Kit (Millipore, Billerica, MA, USA).
Cell preliferation assay
A total of 5 000 cells were seeded in each well of a 24-well plate and treated with PBS, dimethyl sulphoxide (DMSO) or DHT-PROTAC every 4 h, for a total of 7 days. Live cells negatively stained by trypan blue were counted at 24-h intervals.
Cell viability assay
A total of 5 000 cells growing in each well of a 96-well microplate were treated with PBS, DMSO or DHT-PROTAC for 24 h and then incubated with 5 mg mL−1 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) for 4 h. The optical density at 590 nm relative to the blank well control was measured for each sample using a microplate spectrophotometer.
Results
DHT-PROTAC induces AR degradation
To demonstrate that the DHT-PROTAC molecule targets the AR protein and induces its degradation, LNCaP cells were treated with 100 μmol L−1 PROTAC, 100 μmol L−1 DHT, 100 μmol L−1 ALAPYIP-(arg)8 peptide or 100 μmol L−1 DHT + 100 μmol L−1 ALAPYIP-(arg)8 peptide. Band signals of the DHT-PROTAC-treated group were obviously attenuated compared with the DMSO- or PBS-treated controls in 4 h. Band signals were not attenuated in the DHT or ALAPYIP-(arg)8 single- or combination-treated groups (Figure 1).
Figure 1.
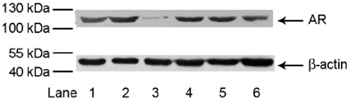
Dihydrotestosterone-based proteolysis-targeting chimeric molecule (DHT-PROTAC)-induced androgen receptor (AR) degradation. LNCaP cells were treated with different agents for 4 h, and cell lysates were prepared for Western blot analysis. Lane 1, PBS as a negative control; lane 2, DMSO control; lane 3, 100 μmol L−1 DHT-PROTAC; lane 4, 100 μmol L−1 DHT; lane 5, 100 μmol L−1 ALAPYIP-(arg)8 peptide (HIF1α); lane 6, 100 μmol L−1 DHT + 100 μmol L−1 HIF1α. As all solutes were dissolved in DMSO, which may be potentially toxic to living cells, a vehicle control was needed in our experiments.
DHT-PROTAC-induced AR degradation is concentration dependent
LNCaP cells were treated with exponentially increasing concentrations of DHT-PROTAC. An equal load of cells was treated with either PBS or DMSO as a negative control. Western blot analysis showed attenuated signals when the concentration of DHT-PROTAC was increased (Figure 2). IHC also depicted that DHT-PROTAC-induced AR degradation was concentration dependent (Figure 3). Nuclear AR expression of the LNCaP cells by IHC was shown by the brown staining (long arrows), whereas the negative staining was shown by blue staining (short arrows). For each concentration, nuclear AR-positive or -negative cells were counted in at least five fields (400 ×). Data were analysed using SAS 6.12 (Cary, NC, USA) statistical software. P < 0.05 was regarded as statistically significant (Table 1).
Figure 2.
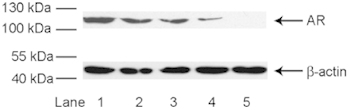
Dihydrotestosterone-based proteolysis-targeting chimeric molecule (DHT-PROTAC)-concentration dependent androgen receptor (AR) degradation detected by Western blot analysis. LNCaP cells treated with exponentially increasing concentrations of DHT-PROTAC for 4 h were examined by western blot analysis. Lane 1, PBS as a negative control; lane 2, DMSO control; lane 3, 10 μmol L−1 DHT-PROTAC; lane 4, 100 μmol L−1 DHT-PROTAC; lane 5, 103 μmol L−1 DHT-PROTAC.
Figure 3.
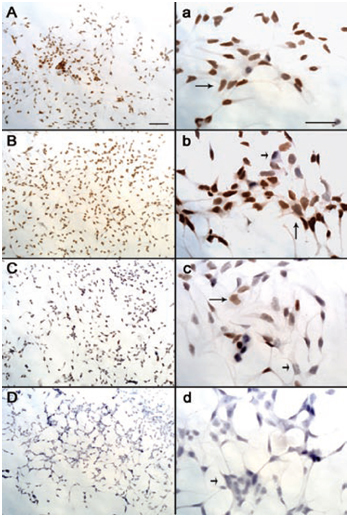
Dihydrotestosterone-based proteolysis-targeting chimeric molecule (DHT-PROTAC) concentration-dependent androgen receptor (AR) degradation detected by immunohistochemistry (IHC). When LNCaP cells were 70% confluent on the coverslips, they were treated with exponentially increasing concentrations of DHT-PROTAC for 4 h and then fixed for IHC. (A/a): PBS as a negative control. (B/b): 10 μmol L−1 DHT-PROTAC. (C/c): 100 μmol L−1 DHT-PROTAC; (D/d): 1 000 μmol L−1 DHT-PROTAC. AR-positive cells were stained brown (long arrows) and AR-negative cells were stained blue (short arrows). Scale bars = 100 μm.
Table 1. AR-positive rate after DHT-PROTAC treatment in LNCaP cells.
| DHT-PROTAC concentration (μmol L−1) | Positive | Negative | Total | Positive rate (%) |
|---|---|---|---|---|
| 0 | 165 | 12 | 177 | 93.22 |
| 10 | 191 | 32 | 223 | 85.65 |
| 100 | 179 | 110 | 289 | 61.94 |
| 1 000 | 3 | 172 | 175 | 1.71 |
Abbreviations: AR, androgen receptor; DHT-PROTAC, dihydrotestosterone-based proteolysis-targeting chimeric molecules. LNCaP cells were treated with 0, 10, 100 and 1 000 μmol L−1 DHT-PROTAC for 4 h, respectively, and then fixed for IHC. AR-positive and -negative cells were calculated in at least five random fields (400 ×). Fisher's exact probability test was used to evaluate the positive rate by using SAS 6.12 statistical software (P = 5.27 × 10−99 < 0.05).
Inhibition of DHT-PROTAC-induced AR degradation by competition experiments with DHT or ALAPYIP-(arg)8 peptide
LNCaP cells were treated with DHT-PROTAC and exponentially increasing concentrations of DHT or the ALAPYIP-(arg)8 peptide. After 4 h, cell lysates were prepared for immunoblot analysis. The results showed that AR signals were enhanced with increasing competitor concentrations in both DHT and ALAPYIP-(arg)8 peptide competition experiments (Figure 4).
Figure 4.
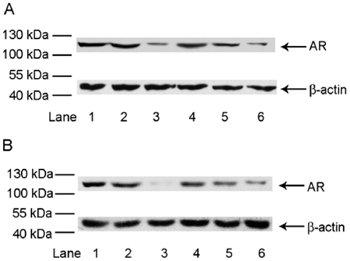
Dihydrotestosterone-based proteolysis-targeting chimeric molecule (DHT-PROTAC) competed with DHT or ALAPYIP-(arg)8 peptide (HIF1α) to facilitate androgen receptor (AR) degradation. LNCaP cells were treated with exponentially increasing concentrations of the DHT-PROTAC competitors, DHT or HIF1α, for 4 h. Cell lysates were prepared for western blot analysis. (A): Lane 1, PBS as a negative control; lane 2, DMSO control; lane 3, 100 μmol L−1 DHT-PROTAC; lane 4, 100 μmol L−1 DHT-PROTAC + 1 000 μmol L−1 DHT; lane 5, 100 μmol L−1 DHT-PROTAC + 100 μmol L−1 DHT; lane 6, 100 μmol L−1 DHT-PROTAC + 10 μmol L−1 DHT. (B): Lane 1, PBS as a negative control; lane 2, DMSO control; lane 3, 100 μmol L−1 DHT-PROTAC; lane 4, 100 μmol L−1 DHT-PROTAC + 1 000 μmol L−1 HIF1α lane 5, 100 μmol L−1 DHT-PROTAC + 100 μmol L−1 HIF1α lane 6, 100 μmol L−1 DHT-PROTAC + 10 μmol L−1 HIF1α.
Inhibition of DHT-PROTAC-induced AR degradation by proteasome inhibitors
MG-132 is a potent membrane-permeable proteasome inhibitor. It can reversibly block proteolytic activity of the 26S proteasome without influencing its ATPase or isopeptidase activities, and inhibit the degradation of lysine-less proteins in living cells 27. Epoxomicin, a natural product isolated from Actinomyces sp., is also a cell-permeable and potent proteasome inhibitor that can covalently modify the N-terminal active site threonine of the β-subunits and exert an irreversible blocking effect 28. E-64 is an effective irreversible inhibitor of cysteine protease that affects lysosomal proteases and certain cytosolic proteases 29. LNCaP cells were pretreated for 0.5 h by these three proteasome or lysosome inhibitors. Subsequently, the cells were treated with DHT-PROTAC for 4 h and analysed by immunoblot analysis. The results showed comparable AR expression signals in the proteasome inhibitor groups (MG-132 and epoxomicin), the PBS control and the vehicle control groups. In contrast, signals in the lysosome inhibitor group and the positive control (DHT-PROTAC) were attenuated (Figure 5).
Figure 5.

Inhibition of dihydrotestosterone-based proteolysis-targeting chimeric molecule (DHT-PROTAC)-induced androgen receptor (AR) degradation by proteasome inhibitors. LNCaP cells were pretreated with different proteasome or lysosome inhibitors for 0.5 h, followed by treatment with 100 μmol L−1 DHT-PROTAC for 4 h. AR expression was detected by western blot analysis. Lane 1, PBS as a negative control, without DHT-PROTAC; lane 2, DMSO control without DHT-PROTAC; lane 3, proteasome inhibitor MG-132 (50 μmol L−1) pretreatment; lane 4, lysosome inhibitor E-64 (50 μmol L−1) pretreatment; lane 5, proteasome inhibitor epoxomicin (10 μmol L−1) pretreatment; lane 6, no inhibitor pretreatment.
Inhibition of LNCaP cell growth after DHT-PROTAC-induced AR degradation
The growth curve of LNCaP cells was nearly parallel to the time axis after periodic treatment with DHT-PROTAC, whereas the PBS or vehicle control showed an ascending trend (Figure 6A). For PC-3 cells that are AR-negative, live cell counts of the three groups at 24-h intervals steadily increased (Figure 6B). Three cell lines, LNCaP, PC-3 and 786-O, were involved in the MTT cell viability assay. The 786-O cell line was derived from renal carcinoma and western blot analysis showed that this cell line was AR-negative in our earlier study. It served as a non-prostate cancer cell control. Only the LNCaP cells treated with DHT-PROTAC showed decreasing values of optical density after a 24-h incubation (Figure 7).
Figure 6.
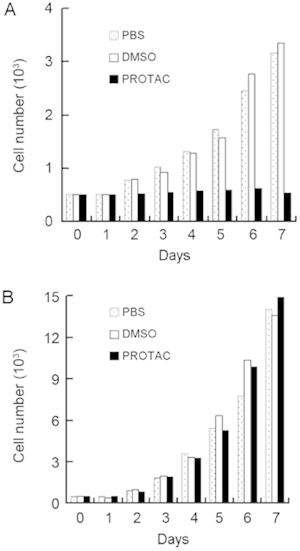
Repression of cell proliferation after dihydrotestosterone-based proteolysis-targeting chimeric molecule (DHT-PROTAC)-induced androgen receptor (AR) degradation. A total of 5 000 LNCaP or PC-3 cells were seeded into each well of a 24-well plate. PBS, DMSO or 100 μmol L−1 DHT-PROTAC was added to each well every 4 h, and living cells negatively stained by trypan blue were counted at 24-h intervals. Growth curves of LNCaP (A) and PC-3 cells (B) after 7 days are shown.
Figure 7.
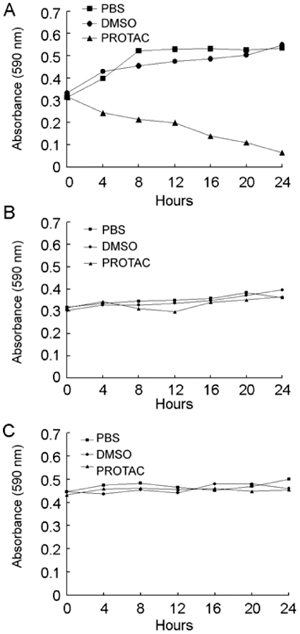
Inhibition of cell viability after dihydrotestosterone-based proteolysis-targeting chimeric molecule (DHT-PROTAC)-induced androgen recetor (AR) degradation. In all, 5 000 LNCaP (A), PC-3 (B) or 786-O (C) cells were seeded into each well of a 96-well plate. After 12 h, cells were treated with PBS, DMSO or 100 μmol L−1 DHT-PROTAC at intervals of 2 h for 24 h and then incubated with 5 mg mL−1 MTT for 4 h. The optical density at 590 nm relative to the blank well control was measured for each sample using a microplate spectrophotometer.
Discussion
The UPP is one of two major systems for protein degradation in eukaryotic cells. The bulk of proteins in mammalian cells, including abnormal proteins, short- or long-lived proteins, ER-associated proteins and even AR proteins, are hydrolysed via the UPP. In this pathway, most substrates are first marked for degradation by their covalent linkage to multiple molecules of ubiquitin, followed by recognition by the 26S proteasome, which leads to rapid and effective protein cleavage. The E3 ubiquitin ligation step often determines the specificity of protein ubiquitination and serves as a rate-limiting step. Each E3 ligase or recognition subunit of a multiprotein E3 ligase complex binds specifically to a limited number of protein targets that share a particular destruction sequence 30. Constitutive recognition, post-translational modification and ancillary protein mediation seem to be the signals for the onset of ubiquitination of the substrates 31. The PROTAC molecule makes E3 approach the substrate, resulting in conjugation of ubiquitin with the exposed lysine in the substrate, and can be considered as another mechanism for the onset of ubiquitination. Different PROTACs can be designed to target various kinds of proteins to explore their functions. Ligands for the target proteins can be natural molecules or synthesized substances.
Our experiments demonstrated that AR was tagged to be eliminated via the UPP by DHT-PROTAC and highlighted the applicability of a novel strategy to target and degrade AR in prostate cancer cells. Although DHT-PROTAC has earlier been shown to be effective in AR degradation 12, this is the first example of synthesized molecules that are capable of inducing AR degradation in prostate cancer cells upon their addition to cells. We observed that only when the two functional ligands were conjugated together can DHT-PROTAC induce AR degradation in LNCaP cells (Figure 1, lane 3). We also observed an increasing effect in AR degradation with exponentially increasing DHT-PROTAC concentrations (Figure 2, lanes 3–5; Figure 3). A competitive effect of either ligand presented with DHT-PROTAC was observed (Figure 4A, lanes 4–6; Figure 4B, lanes 4–6). Blockage of the UPP by specific proteasome inhibitors protected the AR from degradation by the proteasome and confirmed that DHT-PROTAC induces AR degradation via the UPP (Figure 5, lanes 3–6). Interestingly, E-64, a lysosomal protease inhibitor, can weakly block AR degradation by the UPP (Figure 5, lane 4), but the underlying mechanism needs to be explored further. Under normoxic conditions, the central proline in the ALAPYIP sequence of HIF1α is catalysed by a proline hydroxylase 32, 33, 34, and HIF1α is subsequently degraded in a VHL- and ubiquitin-dependent manner 35, 36. It is assumed that the ALAPYIP sequence in the DHT-PROTAC molecule is ubiquitinated and degraded by the proteasome; this could explain the need to administer DHT-PROTAC every 4 h because considerable amounts of DHT-PROTAC molecules permeating the membrane are degraded and out-of-run before their anticipated function.
Proliferation and viability of LNCaP cells were inhibited after AR elimination by DHT-PROTAC, suggesting that AR degradation represses the growth of LNCaP cells. As the effect of DHT-PROTAC on PC-3 and 786-O cells was negligible, we concluded that DHT-PROTAC affected only AR-positive, not AR-negative, cells. Suppression of AR expression and inactivation of AR function in prostate cancer cells have been achieved using an AR antisense oligonucleotide 37, a hammerhead robozyme 38, synthesized double-stranded small interfering RNA (siRNA) duplex 39 or a vector-based siRNA 40 targeting AR messenger RNA. Microinjections of an AR-neutralizing antibody 41 and corepressor 42 have also been reported to block AR signalling in LNCaP cells. All of the published results are consistent with our observation that the disruption of AR signalling adversely affected androgen-sensitive LNCaP cell proliferation. DHT-PROTAC is the only post-translational strategy that blocks AR signalling in LNCaP cells.
For disease intervention, proteins involved in the pathogenesis, regression or progression of the disease are targets in drug design. For example, both gene knockout and RNA interference can prevent the synthesis of AR. Although these strategies are useful for exploring AR function, they are impractical in conventional treatment. Clinically, patients can be treated with drugs only when they have been diagnosed with prostate cancer. Our strategy of using PROTAC via the UPP for targeted AR degradation is different from that of disrupting AR expression at the DNA or RNA level. Without genetically modifying the host cell, AR was eliminated after its natural expression in prostate cancer cells, and this is similar to the clinical management for prostate cancer. In the future, DHT-PROTAC may be used as a drug to remove AR and may play an important role in the treatment of prostate cancer.
Acknowledgments
We are grateful to the National Natural Science Foundation of China (Grant No. 30600618) and the Science and Technology Commission of Shanghai Municipality (Grant No. 07QA14037) for their financial support. We thank Professor Qi-Xiang Guo and Liang Ding for providing the DHT-PROTAC.
References
- Andriole G, Bruchovsky N, Chung LW, Matsumoto AM, Rittmaster R, et al. Dihydrotestosterone and the prostate: the scientific rationale for 5α-reductase inhibitors in the treatment of benign prostatic hyperplasia. J Urol. 2004;172:1399–403. doi: 10.1097/01.ju.0000139539.94828.29. [DOI] [PubMed] [Google Scholar]
- Ntais C, Polycarpou A, Tsatsoulis A. Molecular epidemiology of prostate cancer: androgens and polymorphisms in androgen-related genes. Eur J Endocrinol. 2003;149:469–77. doi: 10.1530/eje.0.1490469. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Ueda T, Ichikawa T, Ito H. Androgen receptor involvement in the progression of prostate cancer. Endocr Relat Cancer. 2003;10:209–16. doi: 10.1677/erc.0.0100209. [DOI] [PubMed] [Google Scholar]
- Edwards J, Bartlett JM. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 1: Modifications to the androgen receptor. BJU Int. 2005;95:1320–6. doi: 10.1111/j.1464-410X.2005.05526.x. [DOI] [PubMed] [Google Scholar]
- Tyagi RK, Lavrovsky Y, Ahn SC, Song CS, Chatterjee B, et al. Dynamics of intracellular movement and nucleocytoplasmic recycling of the ligand-activated androgen receptor in living cells. Mol Endocrinol. 2000;14:1162–74. doi: 10.1210/mend.14.8.0497. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin–proteasome pathway: on protein death and cell life. EMBO J. 1998;17:7151–60. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar S, Bugg CE, Wilkinson KD, Vierstra RD, Hatfield PM, et al. Comparison of the three-dimensional structures of human, yeast, and oat ubiquitin. J Biol Chem. 1987;262:6396–9. [PubMed] [Google Scholar]
- Breitschopf K, Bengal E, Ziv T, Admon A, Ciechanover A. A novel site for ubiquitination: the N-terminal residue, and not internal lysines of MyoD, is essential for conjugation and degradation of the protein. EMBO J. 1998;17:5964–73. doi: 10.1093/emboj/17.20.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;3:503–33. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- Cogoni C, Macino G. Post-transcriptional gene silencing across kingdoms. Curr Opin Genet Dev. 2000;10:638–43. doi: 10.1016/s0959-437x(00)00134-9. [DOI] [PubMed] [Google Scholar]
- Schneekloth JS, Jr, Fonseca FN, Koldobskiy M, Mandal A, Deshaies R, et al. Chemical genetic control of protein levels: selective in vivo targeted degradation. J Am Chem Soc. 2004;126:3748–54. doi: 10.1021/ja039025z. [DOI] [PubMed] [Google Scholar]
- Schneekloth JS, Jr, Crews CM. Chemical approaches to controlling intracellular protein degradation. Chembiochem. 2005;6:40–6. doi: 10.1002/cbic.200400274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Aihara K, Sato T, Akaike M, Yoshizumi M, et al. Androgen receptor gene knockout male mice exhibit impaired cardiac growth and exacerbation of angiotensin II-induced cardiac fibrosis. J Biol Chem. 2005;280:29661–6. doi: 10.1074/jbc.M411694200. [DOI] [PubMed] [Google Scholar]
- Bao BY, Hu YC, Ting HJ, Lee YF. Androgen signaling is required for the vitamin D-mediated growth inhibition in human prostate cancer cells. Oncogene. 2004;23:3350–60. doi: 10.1038/sj.onc.1207461. [DOI] [PubMed] [Google Scholar]
- Wright ME, Tsai MJ, Aebersold R. Androgen receptor represses the neuroendocrine transdifferentiation process in prostate cancer cells. Mol Endocrinol. 2003;17:1726–37. doi: 10.1210/me.2003-0031. [DOI] [PubMed] [Google Scholar]
- Zhou P, Bogacki R, McReynolds L, Howley PM. Harnessing the ubiquitination machinery to target the degradation of specific cellular proteins. Mol Cell. 2000;6:751–6. doi: 10.1016/s1097-2765(00)00074-5. [DOI] [PubMed] [Google Scholar]
- Belshaw PJ, Ho SN, Crabtree GR, Schreiber SL. Controlling protein association and subcellular localization with a synthetic ligand that induces heterodimerization of proteins. Proc Natl Acad Sci USA. 1996;93:4604–7. doi: 10.1073/pnas.93.10.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto KM, Kim KB, Verma R, Ransick A, Stein B, et al. Development of Protacs to target cancer-promoting proteins for ubiquitination and degradation. Mol Cell Proteom. 2003;2:1350–8. doi: 10.1074/mcp.T300009-MCP200. [DOI] [PubMed] [Google Scholar]
- Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, et al. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci USA. 2001;98:8554–9. doi: 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobaugh ME, Blickenstaff RT. Synthesis and androgen receptor binding of dihydrotestosterone hemisuccinate homologs. Steroids. 1990;55:259–62. doi: 10.1016/0039-128x(90)90041-9. [DOI] [PubMed] [Google Scholar]
- Hon WC, Wilson MI, Harlos K, Claridge TD, Schofield CJ, et al. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature. 2002;417:975–8. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2002;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Derossi D, Joliot AH, Chassaing G, Prochiants A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269:10444–50. [PubMed] [Google Scholar]
- Fawell S, Seery J, Daikh Y, Moore C, Chen LL, et al. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA. 1994;91:664–8. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai Y, Irie K, Ohigashi, Wender PA. Synthesis and characterization of the first cysteine-rich domain of novel protein kinase C. Bioorg Med Chem. 1997;5:117–22. doi: 10.1016/s0968-0896(97)00116-8. [DOI] [PubMed] [Google Scholar]
- Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologies. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- Meng L, Mohan R, Kwok BH, Elofsson M, Sin N, et al. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci USA. 1999;96:10403–8. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AJ, Kembhavi AA, Brown MA, Kirschke H, Knight CG, et al. ℓ-trans-epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982;201:189–98. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 2002;21:4037–48. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp DM, Schaefer LK, Ye X, Keyomarsi K, Chu C, et al. Phosphorylation-dependent ubiquitination of cyclin E by SCFFbw7 ubiquitin ligase. Science. 2001;294:173–7. doi: 10.1126/science.1065203. [DOI] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Heritson KS, O'Rourke J, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–40. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Yu F, White SB, Zha Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci USA. 2001;98:9630–5. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohh M, Park CW, Ivan M, Hoffmann MA, Kim TY, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–7. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder IE, Culig Z, Ramoner R, Thurnher M, Putz T, et al. Inhibition of LncaP prostate cancer cells by means of androgen receptor antisense oligonucleotides. Cancer Gene Ther. 2000;7:997–1007. doi: 10.1038/sj.cgt.7700202. [DOI] [PubMed] [Google Scholar]
- Chen S, Song CS, Lavrovsky Y, Bi B, Vellanoweth R, et al. Catalytic cleavage of the androgen receptor messenger RNA and functional inhibition of androgen receptor activity by a hammerhead ribozyme. Mol Endocrinol. 1998;12:1558–66. doi: 10.1210/mend.12.10.0186. [DOI] [PubMed] [Google Scholar]
- Furutani T, Takeyama K, Koutoku H, Ito S, Taniguchi N, et al. A role of androgen receptor protein in cell growth of an androgen-independent prostate cancer cell line. Biosci Biotechnol Biochem. 2005;69:2236–9. doi: 10.1271/bbb.69.2236. [DOI] [PubMed] [Google Scholar]
- Yang Q, Fung KM, Day WV, Kropp BP, Lin HK. Androgen receptor signaling is required for androgen-sensitive human prostate cancer cell proliferation and survival. Cancer Cell Int. 2005;5:8. doi: 10.1186/1475-2867-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62:1008–13. [PubMed] [Google Scholar]
- Moehren U, Papaioannou M, Reeb CA, Hong W, Baniahmad A. Alien interacts with the human androgen receptor and inhibits prostate cancer cell growth. Mol Endocrinol. 2007;21:1039–48. doi: 10.1210/me.2006-0468. [DOI] [PubMed] [Google Scholar]


