Abstract
An endocrine-disrupting chemical, diethylstilbestrol (DES), a nonsteroidal estrogen, triggers oocyte maturation in fish. The morphology (the time course of the change in germinal vesicle breakdown) and an intracellular molecular event (the de novo synthesis of cyclin B) induced by DES were indistinguishable from those induced by a natural maturation-inducing hormone, 17α,20β-dihydroxy-4-pregnen-3-one (17,20β-DHP). A synergistic action of DES on 17,20β-DHP-induced oocyte maturation was observed. Both 17,20β-DHP- and DES-induced oocyte maturation was inhibited by an antibody against the maturation-inducing hormone receptor. The structural requirement for the action of DES is discussed based on results obtained with DES analogs.
Keywords: maturation-inducing hormone, endocrine-disrupting chemical, goldfish, zebrafish
Oocyte maturation in lower vertebrates is triggered by maturation-inducing hormone (MIH), which acts on receptors located on the oocyte membrane and induces the activation of maturation-promoting factor in the oocyte cytoplasm (1-4). During the course of maturation, oocytes undergo drastic morphological changes associated with progression of the meiotic cell cycle, in which breakdown of the oocyte nuclear envelope [germinal vesicle breakdown (GVBD)] occurring at the prophase/metaphase transition is usually regarded as a hallmark of the progress of oocyte maturation. Two MIHs, 17α,20β-dihydroxy-4-pregnen-3-one (17,20β-DHP) and 17α,20β,21-trihydroxy-4-pregnen-3-one (20β-S), have been identified in several fish species (5, 6). In goldfish, 17,20β-DHP has been shown to induce oocyte maturation by stimulating the de novo synthesis of cyclin B, a regulatory subunit of maturation-promoting factor (7). Although progestins including 17,20β-DHP and 20β-S are the most potent steroid inducers of oocyte maturation in fish, other hormones such as deoxycorticosterone and testosterone, but not estradiol or its analogs, are also effective (8).
Several endocrine-disrupting chemicals, Kepon and dichlorodiphenyldichloroethane, have been reported to antagonize MIH-induced meiotic maturation of fish oocytes in vitro (9). One of the environmental endocrine-disrupting chemicals (EEDCs), diethylstilbestrol (DES) is a nonsteroidal substance that was prescribed from the late 1940s to the early 1970s to pregnant women to prevent abortion, preeclampsia, and other complications of pregnancy. Male and female offspring exposed in utero to DES may develop multiple and neoplastic lesions of the reproductive tract, along with other changes, during development (10). Here we show that exposing fish oocytes to DES at a dose within a range similar to that used in experimental exposure to 17,20β-DHP induces oocyte maturation. Estradiol-17β has been reported to be ineffective in inducing fish oocyte maturation (11, 12) and even inhibitory in several teleost species (13-15). Thus, the stimulatory effect of DES to induce fish oocyte maturation observed in this study has not been published previously. This report shows that EEDC can potentially induce oocyte maturation like an endogenous MIH, 17,20β-DHP.
Materials and Methods
Materials. Goldfish were purchased from a local supplier and maintained at 15°C until used. Zebrafish were maintained at 28.5°C on a 14-h light/10-h dark cycle (16). 17,20β-DHP, DES, DES dimethyl ether (DM-DES), DES dipropionate (DPDES), and 17β-estradiol were purchased from Sigma. Dimethylstilbestrol (DMS) was a generous gift from J. Katzenellenbogen (University of Illinois, Urbana). 17α-Estradiol, ethynylestradiol, butyl benzyl phthalate, di(2-ethylhexyl)phthalate, and pentachlorophenol were obtained from Wako Pure Chemical (Osaka). Other chemicals were purchased as follows: hexestrol (HEX; ICN); trans,trans-dienestrol(α-dienestrol) (DIES; U.S. Pharmacopeia, Rockville, MD); resveratorol (Calbiochem); DDTs (AccuStandard, New Haven, CT); bisphenol A (Nacalai Tesque, Kyoto); p-nonylphenol (Kanto Chemical, Tokyo); 4-octylphenol (Aldrich).
Oocyte Preparation and in Vitro Culture. Ovaries of goldfish were isolated from killed females and placed in fresh goldfish Ringer's solution (125 mM NaCl/2.4 mM KCl/0.28 mM MgSO4/0.89 mM MgCl2/2.4 mM CaCl2/2 mM Hepes/5.6 mM glucose/100 units/ml penicillin/0.2 mg/ml streptomycin, pH 7.5) and washed three times with the same solution. Full-grown immature oocytes were exposed in vitro by incubating ovarian fragments (each containing 5-20 oocytes) in 4 ml of goldfish Ringer's solution containing each agent (from a 1,000-fold stock in ethanol) at room temperature with gentle agitation (40 rpm). To assess maturation processes, germinal vesicles in full-grown oocytes were examined under a binocular microscope (SMZ645, Nikon) after placing the oocytes in clearing solution (17). The nuclear state (%) at each time point was determined in 40 oocytes. The morphology of oocytes was photographed with a digital microscope (VH8000, Keyence, Osaka).
Ovaries of zebrafish were isolated from killed females and placed in fresh zebrafish Ringer's solution (116 mM NaCl/2.9 mM KCl/1.8 mM CaCl2/5 mM Hepes, pH 7.2) and washed three times with the same solution. Immature oocytes were exposed in vitro by incubating ovarian fragments (each containing 2-10 oocytes) in 4 ml of zebrafish Ringer's solution containing each agent (from a 1,000-fold stock in ethanol) at room temperature with gentle agitation (40 rpm). To assess maturation processes, germinal vesicles in full-grown oocytes were examined under a binocular microscope (SMZ645, Nikon) after placing the oocytes in clearing solution (17). The nuclear state (%) at each time point was determined in >20 oocytes.
Preparation of Oocyte and Egg Extracts. Groups of 20 oocytes were washed in extraction buffer (0.1 M sodium β-glycerophosphate /15 mM MgCl2/5 mM EGTA/20 mM Hepes/1 mM DTT, pH 7.5) and transferred to a 1.5-ml Eppendorf microcentrifuge tube. After the excess buffer was removed, 200 μl of buffer was added. The samples were crushed with five strokes of a plastic pestle and centrifuged at 13,500 rpm for 10 min at 4°C in a fixed-angle rotor (MX-300 microcentrifuge, Tomy, Tokyo). The clear supernatant (100 μl) was collected for electrophoresis and immunoblotting.
SDS/PAGE and Immunoblotting. Proteins were separated by PAGE under denaturing conditions (SDS/PAGE with 10% gel) by the method of Laemmli (18) and transferred to Immobilon membrane (Millipore). Membranes were blocked in 5% nonfat powdered milk and incubated with primary antibodies for 1 h at room temperature. Immunocomplexes were visualized by using the ECL detection kit (Amersham Biosciences).
cDNA Cloning and Production of Recombinant Proteins. Recently, a strong candidate for an MIH membrane receptor has been identified and characterized in spotted seatrout (19). We cloned cDNA for this membrane progestin receptor α (mPRα) from goldfish. Details of cDNA cloning of a homologue of mPRα will be described elsewhere. GST-tagged recombinant protein of a fragment of 78 N-terminal amino acids of goldfish mPRα was produced. 35S-Labeled mPRα was produced by using a TNT T7-coupled Reticulocyte Lysate System (Promega) according to the manufacturers instructions.
Antibody Production. Full-length goldfish cyclin B was produced in Escherichia coli BL21 (DE3) and 78 N-terminal amino acids of goldfish mPRα was produced in E. coli XL1Blue. These were purified by SDS/PAGE, and subsequently obtained from the gel by electroelution (20). Polyclonal antibodies specific for cyclin B and the receptor protein were raised against purified recombinant proteins according to a procedure described by using guinea pigs (21). Antisera that recognize the 48-kDa band of cyclin B and the 40-kDa band of mPRα were obtained.
Results
Relative Potency of Various Substances in Inducing Fish Oocyte Maturation. The relative effectiveness of MIH and 13 other agents, including EEDCs and several steroid hormones, in inducing GVBD was investigated by using goldfish oocytes (Table 1). Of the 13 agents tested, only DES was effective in inducing GVBD. As in the previous report (8), two other estrogens (β-estradiol and ethynylestradiol) and 17α-estradiol did not induce GVBD at the concentration tested. Eight kinds of EEDCs (DDTs, phthalates, and phenols) did not induce GVBD at the doses tested.
Table 1. In vitro induction of GVBD in goldfish oocytes.
| GVBD, % at concentration of
|
|||
|---|---|---|---|
| Treatment | 0.1 μM | 1 μM | 2 μM |
| EtOH | 0 | ||
| 17,20β-DHP | 83.3 ± 6.3 | 98.3 ± 2.9 | 98.3 ± 2.9 |
| DES | 0 | 18.3 ± 6.3 | 85.0 ± 8.7 |
| β-Estradiol | 0 | 0 | 0 |
| 17α-Estradiol | 0 | 0 | 0 |
| Ethynylestradiol | 0 | 0 | 0 |
| Resveratorol | 0 | 0 | 0.8 ± 1.4 |
| o,p′-DDT | 0 | 0 | 0 |
| p,p′-DDT | 0 | 0 | 0 |
| Butyl benzyl phthalate | 0 | 0 | 0 |
| Di(2-ethylhexyl)phthalate | 0 | 0 | 0 |
| Bisphenol A | 0 | 0 | 0 |
| p-Nonylphenol | 0 | 0 | 0 |
| 4-Octylphenol | 0 | 0 | 0 |
| Pentachlorophenol | 0 | 0 | 0 |
The percentage of GVBD was calculated by determining the percentage of oocytes that had undergone GVBD in a group of 40 oocytes cultured in parallel for 6 h. Each value is the mean (± SD) of three separate experiments with ovaries of three separate females.
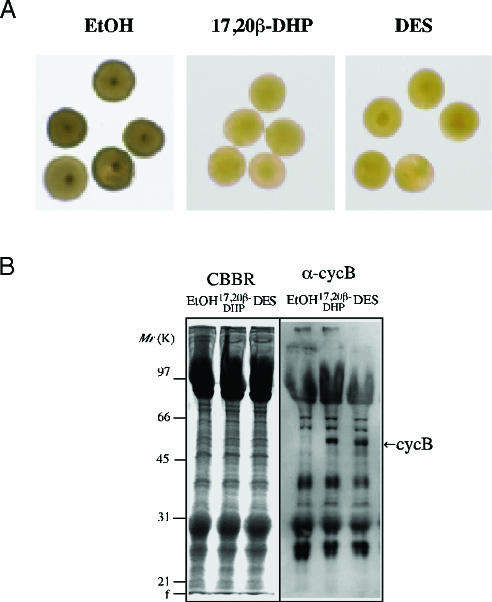
DES Induces Natural Oocyte Maturation. Fig. 1A shows the morphology of oocytes after 6 h of EtOH, 17,20β-DHP, and DES treatment. Germinal vesicles were seen near the center of oocytes after EtOH treatment, whereas they disappeared after the 17,20β-DHP and DES treatments. Also, 17,20β-DHP- and DES-treated oocytes became transparent and changed color to a bright yellow. DES also induced the translation of cyclin B protein (Fig. 1B), a well characterized intracellular molecular event that results in an elevation of the kinase activity of maturation-promoting factor. These results show that the DES-induced maturation was identical with the physiological maturation of oocytes as judged by several criteria.
Fig. 1.
DES induces genuine oocyte maturation. (A) The morphology of oocytes after6hof each treatment was photographed. Germinal vesicles were seen near the center of oocytes after EtOH treatment, whereas they disappeared after 17,20β-DHP and DES treatments. (B) Extracts were prepared from 20 oocytes after incubation with EtOH, 17,20β-DHP, or DES. Extracts of each treatment were electrophoresed under denaturing conditions (10.0% gel) and stained with Coomassie brilliant blue (CBBR) or immunostained with anti-goldfish cyclin B polyclonal antibody after electroblotting (α-cycB). The 48-kDa band of cyclin B is indicated by an arrow. Molecular masses of standard proteins are indicated on the left.
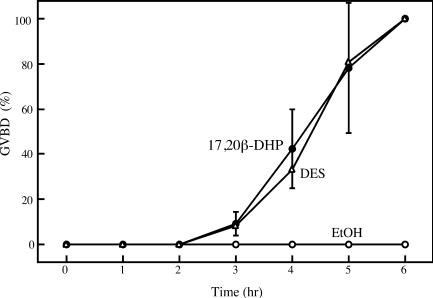
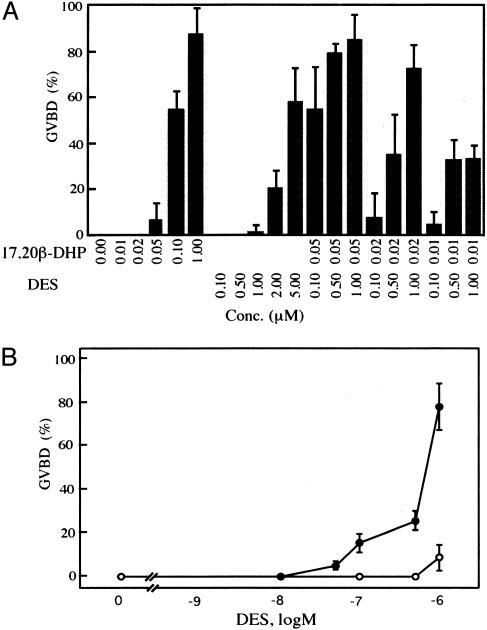
Possible Target of DES in Inducing GVBD. To address the target of DES in the signal transduction pathway to induce GVBD, as a first step, the time-course changes of GVBD induced by DES were compared with those of GVBD induced by 17,20β-DHP. The time course of oocyte maturation induced by DES was the same as that induced by 17,20β-DHP (Fig. 2), suggesting that the most likely candidate for the target of DES is a membrane receptor of 17,20β-DHP. To clarify whether DES induces oocyte maturation through interaction with the MIH receptor, the combined effect of DES and 17,20β-DHP on the induction of GVBD was examined (Fig. 3). When oocytes were treated with a mixture of DES and 17,20β-DHP at concentrations at which each agent could not induce GVBD alone, GVBD was induced: 20 nM 17,20β-DHP and 0.5 μM DES or 10 nM 17,20β-DHP and 0.5 μM DES (Fig. 3A). Also, the magnitude of GVBD was significantly elevated at concentrations at which DES and 17,20β-DHP induced GVBD to only a minor extent (<10%): 50 nM 17,20β-DHP and 1 μM DES or 20 nM 17,20β-DHP and 1 μM DES. These results strongly suggest that DES and 17,20β-DHP are agonists to induce goldfish oocyte maturation.
Fig. 2.
Time-course change of GVBD induced by 17,20β-DHP and DES. Oocyte maturation induced by 0.01% EtOH (○), 1 μM 17,20β-DHP (•), or 2 μM DES (▵). The percentage of GVBD was calculated by determining the percentage of oocytes that had undergone GVBD in a group of 40 oocytes cultured for 6 h. Each value is the mean of three separate experiments with ovaries from three separate females. Vertical lines indicate standard deviation.
Fig. 3.
Synergistic action of DES on 17,20β-DHP-induced oocyte maturation. (A) Oocytes were incubated with various concentrations of 17,20β-DHP and DES. The percentage of GVBD was assessed after 6 h. (B) Oocytes were incubated with (•) or without (○) 20 nM 17,20β-DHP for 6 h in the presence of increasing concentrations of DES. GVBD was 0% at 20 nM 17,20β-DHP alone. Each value is the mean of three separate experiments with ovaries from three separate females. Vertical bars show standard deviation.
Effect of DES Analogs on Inducing Oocyte Maturation. In further analysis, we checked the potency of DES in inducing oocyte maturation in another species of fish, the zebrafish. Although the goldfish is suitable for biochemical analyses, its spawning season is limited to the spring. In zebrafish, full-grown oocytes that can be induced to undergo maturation by MIH are collectable in all seasons.
Zebrafish oocytes were found to be more sensitive to 17,20β-DHP and DES than goldfish oocytes (Table 2). Zebrafish oocytes can be induced to undergo maturation at concentrations approximately one order of magnitude lower than those needed for goldfish oocytes. We therefore decided to use zebrafish in further analyses.
Table 2. In vitro induction of GVBD in zebrafish oocytes.
| GVBD, % at concentration, μM of
|
||||||
|---|---|---|---|---|---|---|
| Treatment | 0.001 | 0.01 | 0.1 | 1 | 2 | |
| EtOH | 0 | |||||
| 17,20β-DHP | 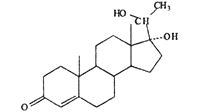 |
28.1 ± 17.4 | 86.5 ± 11.9 | 92.6 ± 4.1 | 92.1 ± 6.9 | − |
| DES | 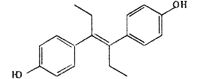 |
− | 2.9 ± 2.6 | 72.5 ± 10.3 | 84.9 ± 9.3 | 91.2 ± 6.4 |
| DMS | 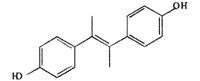 |
− | 0 ± 0.0 | 1.8 ± 3.1** | 7.9 ± 6.3** | 11.5 ± 8.1** |
| HEX | 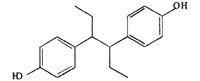 |
− | 0 ± 0.0 | 38.3 ± 21.2** | 82.4 ± 5.8 | 86.3 ± 8.2 |
| DIES | 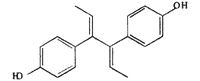 |
− | 0 ± 0.0 | 4.5 ± 3.8** | 49.2 ± 3.4** | 73.2 ± 2.2** |
| DM-DES | 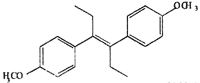 |
− | 0 ± 0.0 | 0 ± 0.0** | 0 ± 0.0** | − |
| DP-DES | 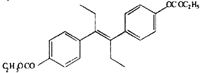 |
− | 5.0 ± 0.5 | 16.7 ± 7.1** | 63.5 ± 4.5** | 74.4 ± 2.8* |
The percentage of GVBD was calculated by determining the percentage of oocytes that had undergone GVBD in a group composed of >20 oocytes cultured in parallel for 3 h. Each value in the mean (± SD) of three separate experiments with ovaries of three separate females. ** and * indicate statistically significant differences between the percentage of GVBD induced by the same concentration of DES and DES analogues at the P < 0.01 and P < 0.05 levels, respectively.
The relative effectiveness of DES analogs in inducing GVBD was determined (Table 2). We examined two groups of DES derivatives. The compounds in the first group had a change in the center part of DES (olefinic double bond and ethyl groups), whereas those in the second group had a hydroxyl changed. Among the first group, HEX induced GVBD almost the same as DES. DIES induced GVBD at a concentration one order of magnitude higher than HEX or DES. These compounds are altered at the olefinic double bond compared with DES. Another compound in the first group, DMS, induced GVBD only at 10% even at concentrations 100 times greater than DES. The ethyl groups in DES are changed to methyl groups in DMS. The results suggest that ethyl groups are important for interaction between DES and the MIH receptor and the length of the ethyl group is necessary. They also suggest that the flexibility of side chains is important for appropriate interaction. In the second group, although DP-DES induced GVBD at 50%, DM-DES did not have any effect. These results suggest that hydroxides also contribute to the interaction of DES with the MIH receptor.
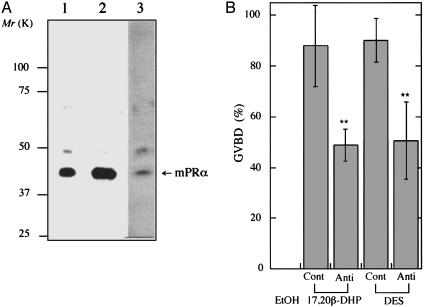
Inhibition of DES-Induced Oocyte Maturation by Anti-mPR Antibody. We cloned a cDNA for mPRα from goldfish showing overall 80% homology with spotted sea trout. A polyclonal antibody against its N-terminal fragment (79 amino acids), which is predicted as an external cellular region, was prepared. The antibody cross-reacted with the 40-kDa band of goldfish and zebrafish extracts from immature oocytes (Fig. 4A). Then, the effects of the antibody on the 17,20β-DHP- and DES-induced oocyte maturation were examined. Immature oocytes were treated with the antibody before addition of 17,20β-DHP and DES. The percentage of GVBD was lowered to ≈50% in both 17,20β-DHP and DES groups, compared with that of the control IgG group (Fig. 4B). These results strongly suggested that DES-induced oocyte maturation is by way of the MIH-mediated pathway.
Fig. 4.
Inhibition of 17,20β-DHP- and DES-induced oocyte maturation by anti-mPRα antibody. (A) Extracts were prepared from 20 oocytes of goldfish (lane 1) and zebrafish (lane 3). Extracts were electrophoresed under denaturing conditions (10.0% gel) and immunostained with anti-goldfish mPRα polyclonal antibody after electroblotting. The 35S-labeled goldfish mPRα was produced in vitro in rabbit reticulocyte lysate (lane 2). After the translation, 35S-labeled proteins were resolved by SDS/PAGE followed by autoradiography on imaging plates (Fuji). The 40-kDa band of mPRα is indicated by an arrow. Molecular masses of standard proteins are indicated on the left. (B) Oocytes were incubated with 100 μg/ml anti-goldfish mPRα polyclonal antibody (Anti) or control IgG (Cont) for1hat25°C, then oocytes were treated with 0.1 μM 17,20β-DHP or 1 μM DES for 3 h. The percentage of GVBD was calculated by determining the percentage of oocytes that had undergone GVBD in a group of >20 oocytes. Each value is the mean of three separate experiments with ovaries from three separate females. Vertical bars show standard deviation. **, statistically significant differences between the percentage of GVBD induced in control and anti-mPRα antibody-treated oocytes at the P < 0.01 level.
Discussion
In this study, the effects of endocrine-disrupting chemicals on the maturation of fish oocytes were examined. We found that treatment of oocytes with a nonsteroidal substance, DES, alone induces maturation. The morphology and an intracellular molecular event induced by DES and 17,20β-DHP were indistinguishable and suggest that, at least qualitatively, DES and 17,20β-DHP induced the same type of maturation. However, a difference occurred between the relative potency of DES to induce oocyte maturation and that of 17,20β-DHP. Other EEDCs did not induce GVBD at the doses examined in this study, although we cannot rule out the possibility that these agents might have effects at higher doses.
In general, the potency and biological properties of a compound can be predicted from its structure. It is possible, therefore, to dissect the contribution of a particular structural modification of a compound that binds to the 17,20β-DHP receptor to the biological response of a target. To study the structural requirement for the MIH action of DES, the relative potency of DES analogs to induce GVBD was compared. All of the compounds except DM-DES induced a dose-related stimulation of oocyte maturation similar to that induced by 17,20β-DHP, although potencies were significantly different (17,20β-DHP > DES, HEX > DIES, and DP-DES > DMS). This analytical approach to the structure-activity relationships of compounds revealed that ethyl groups and hydroxyls of DES contribute to the interaction between DES and the MIH receptor. Other compounds, such as 4-hydroxytamoxifen and bisphenol, have structural features similar to DES necessary for their potential interaction with the MIH receptor. These compounds might also have the ability to induce oocyte maturation in fish.
To understand whether DES acts by means of the MIH receptor specific for 17,20β-DHP, we used indirect strategy by raising an antibody against goldfish mPRα. Both 17,20β-DHP- and DES-induced oocyte maturation was inhibited by this antibody. These findings, together with data from time-course studies and synergistic effects, suggest that DES may interact with the MIH receptor. Further evidence to support these mechanisms of DES action is required.
It is well known that DES is a ligand for estrogen receptors (22, 23). However, interaction between DES and other physiological receptors has been reported recently (24-26). Orphan nuclear receptors are members of the nuclear receptor family that lack identified ligands (27). Recent studies have identified several natural and synthetic ligands for orphan nuclear receptors, which led to new hormone-response systems implicated in the control of various aspects of development and adult physiology (28, 29). Among orphan nuclear receptors, the estrogen-related receptors have been reported to be inhibited in their binding with coactivator protein by DES. In trophoblast stem cells, DES promotes coactivator release from estrogen-related receptor β and inhibits its transcriptional activity (25). By using fluorescence resonance energy transfer assay, it has also been shown that DES binds to estrogen-related receptor γ (26). These findings suggest a pathway for DES action as a ligand for receptors besides estrogen receptors. Likewise, this study shows that DES may interact with the MIH receptor.
Many EEDCs, including DES, may mimic the action of the sex hormone estradiol and are therefore referred to as xenoestrogens. We show here the possibility that they mimic other steroids. Our findings emphasize the need for studies to examine more widely the various nonestrogenic effects of endocrine disrupters.
Acknowledgments
We thank Dr. J. A. Katzenellenbogen for the gift of DMS. This work was supported by the CREST Research Project of Japan Science and Technology Corporation (to Y.N.) and by grants-in-aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Part of this study was performed as the National Institute for Basic Biology Cooperative Research Program (00-121 and 01-112 to T.T). M.T. is a Research Fellow of the Japan Science and Technology Corporation.
Abbreviations: 17,20β-DHP, 17α, 20β-dihydroxy-4-pregnen-3-one; MIH, maturation-inducing hormone; EEDC, environmental endocrine-disrupting chemical; DES, diethylstilbestrol; HEX, hexestrol; DIES, trans,trans-dienestrol(α-dienestrol); DMS, dimethylstilbestrol;, DM-DES, DES dimethyl ether; DP-DES, DES dipropionate; GVBD, germinal vesicle breakdown; DDT, dichlorodiphenyltrichloroethane; mPRα, membrane progestin receptor α.
References
- 1.Ishikawa, K., Hanaoka, Y., Kondo, Y. & Imai, K. (1977) Mol. Cell. Endocrinol. 9, 91-100. [DOI] [PubMed] [Google Scholar]
- 2.Godeau, J. F., Schorderet-Slatkine, S., Hubert, P. & Baulieu, E. E. (1978) Proc. Natl. Acad. Sci. USA 75, 2353-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshikuni, M., Shibata, N. & Nagahama, Y. (1993) Fish Physiol. Biochem. 11, 15-24. [DOI] [PubMed] [Google Scholar]
- 4.Masui, Y. & Clarke, H. J. (1979) Int. Rev. Cytol. 57, 185-282. [DOI] [PubMed] [Google Scholar]
- 5.Nagahama, Y. & Adachi, S. (1985) Dev. Biol. 109, 428-435. [DOI] [PubMed] [Google Scholar]
- 6.Patino, R. & Thomas, P. (1990) Gen. Comp. Endocrinol. 78, 474-478. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita, M., Kajiura, H., Tanaka, T., Onoe, S. & Nagahama, Y. (1995) Dev. Biol. 168, 62-75. [DOI] [PubMed] [Google Scholar]
- 8.Nagahama, Y., Hirose, K., Young, G., Adachi, S., Suzuki, K. & Tamaoki, B. (1983) Gen. Comp. Endocrinol. 51, 15-23. [DOI] [PubMed] [Google Scholar]
- 9.Thomas, P. (1999) in Endcrine Disruptors: Effects on Male and Female Reproductive Systems, ed. Naz. R. K. (CRC, Boca Raton, FL), pp. 3-38.
- 10.Bern, H. A. (1992) in Chemically Induced Alterations in Sexual and Functional Development: The Wildlife/Human Connection, eds. Collborn. T. & Clement. C. (Princeton Scientific Publishing, Princeton, NJ.), pp. 9-15.
- 11.Young, G., Kagawa, H. & Nagahama, Y. (1982) J. Exp. Zool. 224, 265-275. [DOI] [PubMed] [Google Scholar]
- 12.Trant, J. M. & Thomas, P. (1988) Gen. Comp. Endocrinol. 71, 307-317. [DOI] [PubMed] [Google Scholar]
- 13.Pandey, S. & Hoar, W. S. (1972) Can. J. Zool. 50, 1679-1680. [DOI] [PubMed] [Google Scholar]
- 14.Jalabert, B. (1975) C. R. Hebd. Seances Acad. Sci. D 281, 811-814. [PubMed] [Google Scholar]
- 15.Sundararaj, B. I., Goswami, S. V. & Lamba, V. (1979) J. Steroid Biochem. 11, 701-707. [DOI] [PubMed] [Google Scholar]
- 16.Westerfield, M. (1995) in The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) (Univ. of Oregon Press, Eugene).
- 17.Lessman, C. A. & Kavumpurath, S. (1984) Gamete Res. 10, 21-29. [Google Scholar]
- 18.Laemmli, U. K. (1970) Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- 19.Zhu, Y., Rice, C. D., Pang, Y., Pace, M. & Thomas, P. (2003) Proc. Natl. Acad. Sci. USA 100, 2231-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirai, T., Yamashita, M., Yoshikuni, M., Lou, Y. H. & Nagahama, Y. (1992) Mol. Reprod. Dev. 33, 131-140. [DOI] [PubMed] [Google Scholar]
- 21.Tokumoto, T., Tokumoto, M., Seto, K., Horiguchi, R., Nagahama, Y., Yamada, S., Ishikawa, K. & Lohka, M. J. (1999) Exp. Cell Res. 247, 313-319. [DOI] [PubMed] [Google Scholar]
- 22.Katzenellenbogen, B. S., Iwamoto, H. S., Heiman, D. F., Lan, N. C. & Katzenellenbogen, J. A. (1978) Mol. Cell. Endocrinol. 10, 103-113. [DOI] [PubMed] [Google Scholar]
- 23.Prins, G. S., Birch, L., Couse, J. F., Choi, I., Katzenellenbogen, B. & Korach, K. S. (2001) Cancer Res. 61, 6089-6097. [PubMed] [Google Scholar]
- 24.Lu, D., Kiriyama, Y., Lee, K. Y. & Giguere, V. (2001) Cancer Res. 61, 6755-6761. [PubMed] [Google Scholar]
- 25.Tremblay, G. B., Kunath, T., Bergeron, D., Lapointe, L., Champigny, C., Bader, J. A., Rossant, J. & Giguere, V. (2001) Genes Dev. 15, 833-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coward, P., Lee, D., Hull, M. V. & Lehmann, J. M. (2001) Proc. Natl. Acad. Sci. USA 98, 8880-8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giguere, V. (1999) Endocr. Rev. 20, 689-725. [DOI] [PubMed] [Google Scholar]
- 28.Blumberg, B. & Evans, R. M. (1998) Genes Dev. 12, 3149-3155. [DOI] [PubMed] [Google Scholar]
- 29.Kliewer, S. A., Lehmann, J. M. & Willson, T. M. (1999) Science 284, 757-760. [DOI] [PubMed] [Google Scholar]