Abstract
Nanoporous gold (NPG) was utilized as a support for immobilizing alkaline phosphatase (ALP) conjugated to monoclonal antibodies against either prostate specific antigen (PSA) or carcinoembryonic antigen (CEA). The antibody-ALP conjugates were coupled to self-assembled monolayers of lipoic acid and used in direct kinetic assays. Using the enzyme substrate p-aminophenylphosphate, the product p-aminophenol was detected by its oxidation near 0.1 V (vs. Ag|AgCl) using square wave voltammetry. The difference in peak current arising from oxidation of p-aminophenol before and after incubation with biomarker increased with biomarker concentration. The response to these two biomarkers was linear up to 10 ng mL-1 for CEA and up to 30 ng mL-1 for PSA. The effect of interference on the PSA assay was studied using bovine serum albumin (BSA) as a model albumin protein. The effect of interference from a serum matrix was examined for the PSA assay using newborn calf serum. A competitive version of the immunoassay using antigen immobilized onto the NPG surface was highly sensitive at lower antigen concentration. Estimates of the surface coverage of the antibody-ALP conjugates on the NPG surface are presented.
Introduction
Gold nanostructures are currently actively studied substrates for immunoassay development. Nanoporous gold (NPG) is a nanostructured form of gold consisting of a three dimensional network of interconnected ligaments and pores, of average dimensions typically in the range of 10 – 200 nm depending upon the conditions of preparation. NPG is prepared by selectively leaching less noble metal(s) from an alloy of at least 20% to less than 50% gold most commonly with silver. The resulting three-dimensional network of pores and ligaments creates a vastly increased surface to volume ratio. NPG has been applied for several analytical applications such as for the development of chemical sensors, biosensors, and immunoassays [1,2]. The gold nanostructure can provide a large accessible surface area and can increase antibody loading and sensitivity in immunoassays [3].
Efforts to develop alternatives to traditional ELISA (enzyme-linked immunosorbent assays) have focused on achieving lower limits of detection while maintaining the clinically required linear range of response, elimination of the need for washing or separation steps, more rapid analysis times, use of a single antibody in place of a sandwich complex, and potential application in a lateral flow assay format. A broader range of detection strategies including fluorescence, propagating or localized surface plasmon, mass sensitive, and especially electrochemical methods are under investigation. Electrochemical methods are especially attractive since they are not affected by turbidity or absorbance of the sample. In one of the earlier studies, Meyerhoff and coworkers devised an assay based on microporous nylon membranes onto which gold electrodes were sputtered on one side that served as supports for lipoic acid self-assembled monolayers (SAMs) [4,5]. The pore dimensions of these membranes was near 200 nm, somewhat larger than the typical pore dimensions of the nanoporous gold used in the present study. The assay method involved coupling of the capture antibody to the lipoic acid SAM, and formation of a sandwich complex with the PSA antigen and detection antibody labeled with alkaline phosphatase. The microporous Nylon membrane enabled introduction of the p-aminophenylphosphate enzyme substrate (in pH 10 buffer) from the side of the membrane that had not been sputtered with gold. The assay was conducted in a two chambered cell, and thus the need for a separation step in which unbound conjugate in bulk solution would be removed was eliminated. The detection limit in undiluted serum for the assay was reported as 0.3 ng mL-1. Sample and conjugate were added at the same time and incubated for 27 minutes in this study.
Many examples of immunoassays for PSA or CEA making use of other gold nanostructures have been reported. Garcia et al. reported a dual sensor for the detection of both free and total PSA based on nanogold modified screen printed electrodes and the voltammetric detection of silver deposited onto their surface from Ag+ in solution that was reduced by indigo blue produced from indoxyl generated from indoxyl phosphate by the action of alkaline phosphatase that was a part of the overall antibody-PSA sandwich complex [6]. The sandwich complex in this study was comprised of a capture antibody, PSA, biotinylated detection antibody and streptavidin labeled alkaline phosphatase. A total of three separate one hour incubations times were required, and the detection limit was 1.0 ng mL-1 with a linear range from 1 – 10 ng mL-1. Rusling et al. immobilized anti-PSA antibody on a film of gold nanoparticles and developed an immunoassay based on multiple horseradish peroxidase labeled magnetic beads attached to the detection antibody [7]. The multiple enzyme labeled beads that also were attached to the detection antibody enabled a remarkable detection limit using amperometry (on a rotating disc electrode at 3000 rpm) of 0.5 pg mL-1. The assay required two 75 minute incubations, first with PSA, and then with the detection antibody/HRP labeled beads. Gao et al. reported an amperometric immunoassay for CEA on glassy carbon electrodes modified by the layer by layer assembly of carbon nanotubes onto which gold nanoclusters were electrodeposited, followed by immobilization of anti-CEA antibody, and with detection achieved by measuring the reduction in current due to oxidation of Fe(CN)63- as a result of CEA binding [8]. The assay involved only a capture antibody, an incubation time with CEA sample of 20 minutes, and gave two linear ranges (0.5 – 5.0 ng mL-1, and 5.0 – 160 ng mL-1) with a detection limit of 0.1 ng mL-1. A competitive immunoassay for CEA was reported based on CEA/colloidal gold/chitosan membranes on screen printed electrodes in a flow injection analysis system [9]. The assay provided a linear range from 0.5 – 25.0 ng mL-1 and a detection limit of 0.22 ng mL-1, and could be conducted in 40 minutes.
A range of other methods have been employed for PSA or CEA immunoassays. For example, a method for PSA detection based on antibody immobilization on gold coated cantilevers was reported by Majumdar et al. with a detection limit of 0.2 ng mL-1 and a linear range up to 60 μg mL-1; however, the equilibration time needed was over 3 hours [10]. Choi et al. reported a propagating SPR based assay for PSA based on formation of a sandwich complex between antibody immobilized on the gold surface, PSA, and gold nanoparticles labeled with polyclonal antibodies for PSA with a detection limit of 0.03 ng mL-1 in PBS [11]. An immunoassay for CEA captured in an antibody sandwich on nanoporous gold leaf electrodes was reported in which the secondary antibody was conjugated to CdTe quantum dots [12]. In this assay, a linear range from 0.05 ng mL-1 to 200 ng mL-1 and a detection limit of 0.01 ng mL-1; however, formation of the sandwich assay was reported as requiring a 6 hours followed by 5 more hours for conjugation to the quantum dots that generate the electrochemiluminescent signal. Nanoparticle based methods of detection can enhance sensitivity but introduce the additional tasks of nanoparticle synthesis, functionalization, separation, stabilization prior to immobilization onto the electrode surface.
Lateral flow assays designed to be easy to operate and to give results within few minutes have become diagnostic tools for qualitative and semi-quantitative analysis. A lateral flow immunostrip based on change in capacitance was reported for the detection of free and complexed PSA. The electrochemical transducer of the sensor chip consisted of a pH sensitive polymer coated interdigitated carbon electrode and two silver electrodes. The increase in pH in the membrane after reaction between urea and urease in anti-PSA-urease conjugates caused degradation of the polymer membrane and a decrease in capacitance. This immunosensor was reported to be sensitive enough for the detection of PSA in the diagnostic gray zone of 4-10 ng/ml [13]. Another lateral flow assay, with a more limited reproducibility, for the simultaneous detection of free and total PSA was reported with quantitative determination of the PSA based on color development and subsequent densitometric reading. Even though these lateral flow assays are attractive, issues remain with their lack of uniformity in performance, inaccuracy at lower PSA values and difficulty in reading at lower PSA concentration due to faint colors which causes error in end-user read out [14].
Commercial platforms currently being adopted for usage as point of care instruments include the Abbott i-STAT system, which can directly read out results for electrolytes, gases and selected cardiac biomarkers using a drop of blood as a sample (http://www.abbottpointofcare.com/). These devices are designed based on the need of quick result for monitoring components of the blood that can be directly uploaded to a patients chart. The need for a quick result is clearly desirable for the case of cardiac events. Some of the technical details of the microchips, microfluidic system, and detection methods used in earlier versions of the i-STAT have been published [15].
In this paper, we demonstrate application of nanoporous gold as a solid electrode support for an electrochemical immunoassay for CEA or PSA using square wave voltammetry. The assay method makes use of alkaline phosphatase - antibody conjugates covalently coupled to lipoic acid SAMs on NPG. Alkaline phosphatase (ALP) is a widely used enzyme label for immunoassays because of its high activity, relatively low cost, and activity against a range of possible substrates and different methods for detection [14]. A key advantage of square wave voltammetry for electrochemical detection is its high sensitivity due to rejection of background current from double-layer charging [16]. In contrast to traditional sandwich type immunoassays using two or more antibodies, the assay described uses a single antibody present in the conjugate with ALP. The assay described can be implemented in both a direct or competitive format and involves relatively few steps. The direct assay is based on the inhibition due to antigen binding of the rate of conversion of the ALP substrate p-aminophenylphosphate to product p-aminophenol that is then detected by its oxidation in a square wave voltammetric sweep. The high surface are of the NPG electrode makes the demonstrated kinetic assay scheme possible. The competitive assay is based on competition for the antibody-enzyme conjugate in solution between antigen immobilized on NPG and free antigen in solution.
PSA and CEA were chosen as representative protein biomarkers. PSA is a biomarker for prostate cancer, in normal healthy males the PSA level is less than 4 ng mL-1, but depending upon the age, race and family history of prostate cancer this value can vary [17]. There are some controversies regarding the applicability of PSA as a biomarker and the United States Preventive Services Task Force (USPSTF) has recommended against PSA screening for healthy individuals but their recommendation doesn't apply for people already diagnosed with prostate cancer and for the monitoring of PSA after prostatectomy [18]. The other variations of the PSA test such as determination of free vs. bound PSA or the PSA velocity test are also being considered as an alternative to PSA screening based on the absolute scale and cutoff level [19]. In absence of any other reliable biomarker, the PSA test is still recommended by the American Urological Society. A counterargument has been published regarding the flaws on the research of USPSTF [20]. Thus, the PSA test remains routinely applied in many clinics around the world, for informed decision making for prostate cancer diagnosis and further treatment. CEA is a biomarker for colorectal cancer but its closely related proteins has been found in other cancers such as breast and lung cancers [21]. It have been reported that the levels of CEA in healthy individuals without one of these cancers is generally below ∼ 4 ng mL-1, dependent on age and smoking [22]. Estimations of the surface coverage of antibody-enzyme conjugate on the NPG based on solution depletion and protein concentration assays are also presented.
Experimental
Materials and reagents
Gold wire (0.2 mm diameter, 99.99%) was obtained from Electron Microscopy Sciences (Fort Washington, PA, http://www.emsdiasum.com/microscopy/). PSA, CEA, anti-PSA (free PSA) monoclonal antibody, and anti-CEA monoclonal antibody were obtained from Fitzgerald (North Acton, MA, http://www.fitzgerald-fii.com/). Alkaline phosphatase labeling kits were obtained from Dojindo Molecular Technologies Inc. (Rockville, Maryland, http://www.dojindo.com/). P-aminophenyl phosphate (p-APP) was obtained from Gold Biochem (St. Louis, MO, https://www.goldbio.com/). Sodium carbonate (enzyme grade, >99%), N-hydroxysuccinimide (NHS) (≥ 97%), sodium phosphate (certified ACS grade), potassium phosphate (99.6%), sulfuric acid (certified ACS plus), nitric acid (trace metal grade), hydrogen peroxide (50%), and sodium bicarbonate (certified ACS) were all from Fisher Scientific (Pittsburg, PA, http://www.fishersci.com/). Potassium dicyanoargentate (K[Ag(CN)2]) (99.96%) and potassium dicyanoaurate (K[Au(CN)2]) (99.98%), ethanol (HPLC/spectrophotometric grade), acetonitrile (HPLC grade), lipoic acid, N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) (>99%), glycine (99%), sodium bicinchoninate (≥ 98%), sodium tartrate (> 99%), sodium hydroxide (99.99%), cupric sulfate (≥ 99%), zinc chloride (≥ 98%), magnesium chloride (99%), and sodium chloride (99%) were obtained from Sigma-Aldrich (St. Louis, MO, http://www.sigmaaldrich.com/united-states.html). All reagents were used without further purification. Milli-Q water (18.2 MΩ) was used for preparation of all aqueous solutions.
Preparation and characterization of nanoporous gold
Nanoporous gold coated gold wires were prepared by selective removal of silver from an alloy of gold and silver as reported elsewhere, detailed information is also provide in SI [23]. The surface morphology and general structure of NPG was studied by scanning electron microscopy (JEOL JSM-6320F field emission SEM). Electrochemical surface area was determined by the gold oxide stripping method by scanning from 0 V to 1.5 V and back to 0 V (vs. Ag|AgCl) at a scan rate of 100 mV sec-1. The charge under the oxide reduction peak was integrated to estimate the surface area of the NPG covered gold wires used in this study, using the reported conversion factor of 450 μC cm-2 [23].
Preparation of self-assembled monolayers
SAMs of lipoic acid were prepared by immersing 10 NPG coated Au wires at a time into a 10 mM lipoic acid solution, in ethanol, of 10 mL total volume. The wires were left for approximately 17 h and then rinsed with ethanol twice followed by rinsing with acetonitrile. NPG coated wires modified with lipoic acid SAMs were then immersed into EDC (5 mM) and NHS (5 mM) in acetonitrile for 5 hours. After 5 hours, the wires were rinsed with acetonitrile and then twice with 10 mM phosphate buffered saline (PBS) (pH 7.4) and immersed into the antibody-ALP conjugate solution (containing 25 μg of conjugate), in 10 mM PBS (100 μL) in a small glass vial at 4 °C for 24 h followed by rinsing with PBS. For the competitive immunoassay, NPG coated Au wires with NHS activated −COOH groups were prepared as above and then these wires were incubated with 10 μg of antigen in 100 μl of PBS for 2 h at 4 °C in Eppendorf tubes.
Electrochemical immunoassays
Square wave voltammetric (SWV) measurements were done using a PARSTAT 2273 (Princeton Applied Research, Oak Ridge, TN, http://www.princetonappliedresearch.com/). The best parameters determined for the square wave voltammetric measurement of the oxidation of p-aminophenol were: pulse height 50 mV, pulse width 0.2 sec, and step height 2 mV. The potential was scanned from -0.1 V to 0.4 V at a rate of 5.0 mV sec-1.
In the direct immunoassay, the peak current for p-aminophenol oxidation was measured before incubating with antigen and again after incubating with antigen. The incubation period with antigen used was 2 h and was carried out in glycine buffer (pH 9, 100 mM). The substrate, p-aminophenyl phosphate (p-APP) was introduced via micropipette into the stirred solution (argon degassed) containing the antibody-ALP conjugate modified NPG covered Au wires and allowed to react for a period of 30 minutes prior to conducting the potential scan. Each measurement on each wire was repeated three times. The difference in peak current (ip (before incubation) − ip (after incubation)) was plotted vs. the antigen concentration to obtain the response plots for PSA and CEA.
For the competitive immunoassay, the NPG covered gold wire electrodes modified with immobilized PSA or CEA antigens were allowed to compete for the conjugate in solution with variable concentrations of free antigens in solution for 24 h in PBS (10 mM, pH 7.4) as significantly smaller signals were obtained after 2 h incubation. The concentration of antibody-ALP conjugate used was 100 ng mL-1. After incubation with conjugate, the NPG coated wires with bound conjugate were incubated in 0.25 mM p-APP in glycine buffer (pH 9.0, 100 mM) for 30 minutes and then a square-wave voltammetry scan was conducted. An antibody-ALP conjugate solution of concentration 100 ng mL-1 was chosen because a much less significant signal was obtained with 50 ng mL-1 and 200 ng mL-1 did not produce a significantly different result from 100 ng mL-1. p-APP concentrations less than 100 μM were not enough to obtain satisfactory currents, and thus 250 μM was chose as a working substrate concentration for the immunoassay. The amount of protein on the NPG surface was determined by a solution depletion method, using the BCA (bicinchoninic acid) assay, detailed procedures for the assay are provided in SI.
Results and discussion
Characterization of the nanoporous gold coated wires
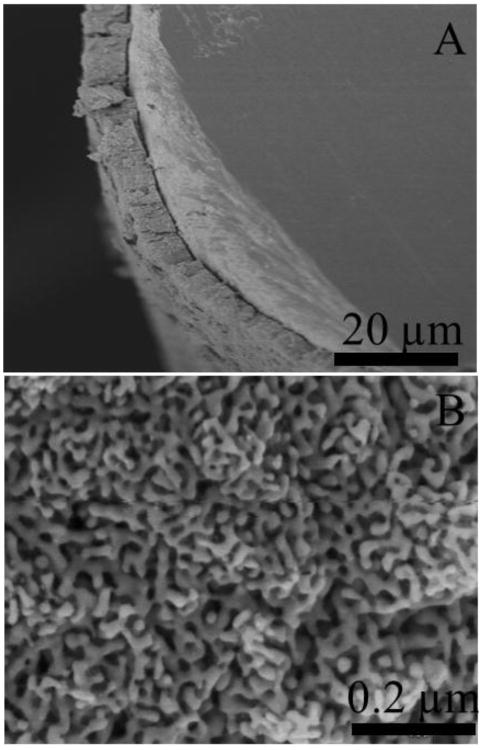
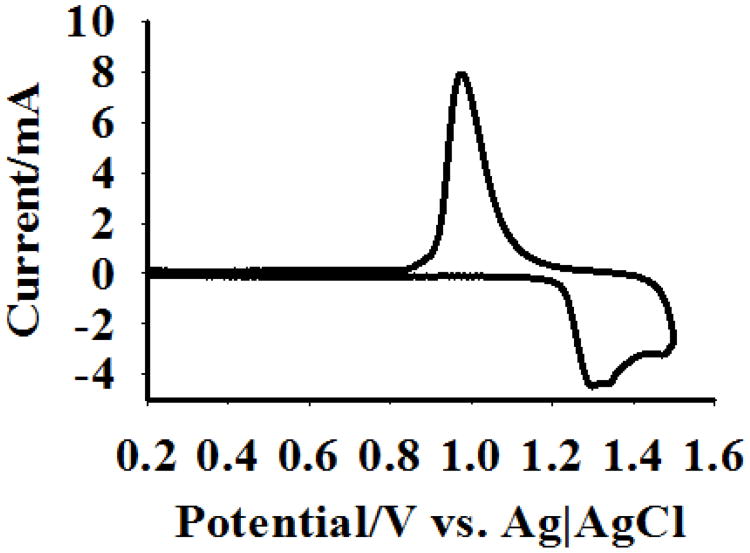
Fig. 1 shows the structure of the NPG coating on the gold wire electrodes used in this study (Fig. 1A), as well as a magnified view of the NPG pore and ligament nanostructure (Fig. 1B). The typical pore size range for the NPG used in this study is 30-50 nm, and is large enough to accommodate diffusion of biomolecules into the NPG interior for both surface modification and recognition interactions. The overall thickness of the NPG coating on the gold wire is approximately 10 microns (Fig. 1A). Surface area determination was conducted using the gold oxide stripping method, and a typical cyclic voltammogram is shown in Fig. 2. The geometric surface area of the gold wire electrode itself is 0.032 cm2 and the average gold surface area determined by oxide stripping for the NPG coated Au electrodes was found to be 12.5 ± 1.3 cm2, n = 3, indicating an increase in surface area by a factor of approximately 300.
Fig. 1.
Scanning electron micrograph of NPG coated gold wire. Figure 1A is the side view of the NPG coated wire. Figure 1B is an SEM micrograph of NPG coating at higher magnification.
Fig. 2.
Cyclic voltammogram of the NPG coated wire in 0.5 M H2SO4. A 5 mm long NPG coated wire was scanned between 0 and 1.5 V at 100 mV sec-1. The charge passed under the oxide reduction peak was used to calculate surface area of NPG.
Michaelis-Menten analysis of the immobilized antibody-ALP conjugate
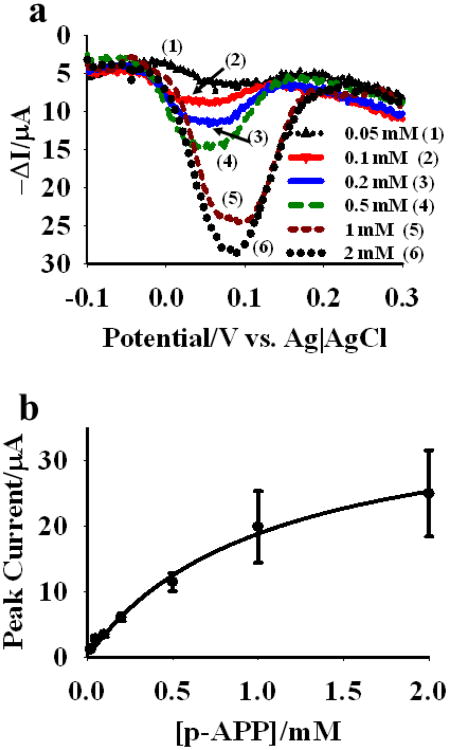
The NPG coated Au wires were modified with lipoic acid SAMs and then the SAMs were activated using the EDS/NHS reaction followed by coupling to the antibody-ALP conjugate, where the antibody is either a monoclonal antibody that binds the free PSA antigen or one that binds the CEA antigen. Square-wave voltammetric sweeps were conducted using these modified NPG coated gold wires as working electrodes. The sweeps were conducted after incubation for 30 minutes with different concentrations of p-APP as the ALP substrate. The incubation period allows time for the ALP enzyme to generate the p-aminophenol product that is then oxidized to p-quinoneimine during the SWV sweep. These SWV sweeps resulted in a prominent peak for the oxidation of p-AP in this concentration range. In contrast, attempts to measure the current peak due to oxidation of p-AP using linear sweep voltammetry were less successful due to the high background currents arising from charging of the electric double layer of NPG. The 30 minute period was found to be the shortest period needed to maximize the current response arising from oxidation of p-AP to p-quinoneimine, which occurs near a potential of 0.1 V (vs. Ag|AgCl). The negative shift in oxidation potential of p-AP in NPG surface is attributed to the better electrocatalytic activity of NPG compared to that of a smooth gold surface [24]. The oxidation potential of p-AP was found to be higher on gold wire surface compared to that of the NPG. p-APP concentrations of 0.05 mM to 2.0 mM were studied, and a set of SWV sweeps are shown in Fig. 3a for an NPG electrode modified by the antibody-ALP conjugate, where the antibodies is the anti-PSA antibodies. A plot of the maximum p-aminophenol oxidation peak current versus initial p-APP concentration is shown in Fig. 3b. Peak current has been reported as proportional to the velocity of the reaction and can be used to create a Michaelis-Menten plot. The rate of a reaction on the electrode surface can be obtained by dividing peak current by the product of nF, where, n is the number of electron involved in the redox processes and F is the Faraday's constant [16]. From this plot, the Km value for the immobilized conjugate was found to be 290 ± 20 μM. A Km value of 56 ± 5 μM was reported for free alkaline phosphatase in solution using p-APP as the substrate [25]. In our prior study using an antibody-ALP conjugate where the antibody binds free PSA, and the substrate used was p-nitrophenyl phosphate with UV-visible detection of the p-nitrophenolate product at 410 nm, an increase in Km from 210 μM to 300 μM was observed upon similar immobilization. The conjugation of the antibodies to the antibody itself in solution was found to increase the measured value of Km from 40 μM to 210 μM [2]. An increase in Km of the enzyme upon conjugation to antibody is expected due to the steric crowding effect of the antibody on the enzymesubstrate interaction.
Fig. 3.
(a) Square wave voltammograms for different concentrations of p-APP incubated for 30 min with the anti-CEA antibody-ALP conjugate immobilized onto NPG. (b) Michaelis-Menten plot for immobilized anti-CEA antibody-ALP conjugate on NPG. Different concentrations of p-APP were incubated with three different wires for 30 min prior to square wave voltammetric measurement. Mean peak currents for three wires were measured and plotted against the p-APP concentrations. SWV measurements were done in 100 mM glycine buffer (pH 9.0).
An increase in Km is anticipated for enzyme immobilization due to restricted conformational freedom of the immobilized enzyme and immobilization in a porous support also introduces tha factor of restricted diffusion of substrate within the pores. Lorenzo et al. reported a Km value of 410 μM for alkaline phosphatase covalently attached to nylon mesh using BSA and glutaraldehyde as cross-linkers [26]. A tenfold increase in Km was reported for alkaline phosphatase immobilized on nylon mesh using p-nitrophenyl phosphate and p-aminophenyl phosphate substrates [26]. A pore size dependent change in enzymatic activity and Km values were reported for ‘gigaporous’ (314 nm), macroporous (104 nm) and mesoporous (14.7 nm) polystyrene microspheres for the lipase from Burkholderia cepacia immobilized by strong hydrophobic interactions [27]. Brinker et al. showed that the change in enzymatic activity and affinity for the corresponding substrate can be enzyme dependent, their study on glucose-6-phosphate dehydrogenase and horseradish peroxidase (HRP) showed that enzymatic activity of immobilized glucose 6-phosphate was 36 % and the enzymatic activity of HRP was 73% of that of the free enzyme in solution. There was a significant increase in Km value for both of these enzymes [28]. Stevenson et al. also reported an increase in Km and decrease in enzymatic activity of HRP immobilized on glass coverslips [16].
Immunoassay using square wave voltammetry and immobilized conjugate
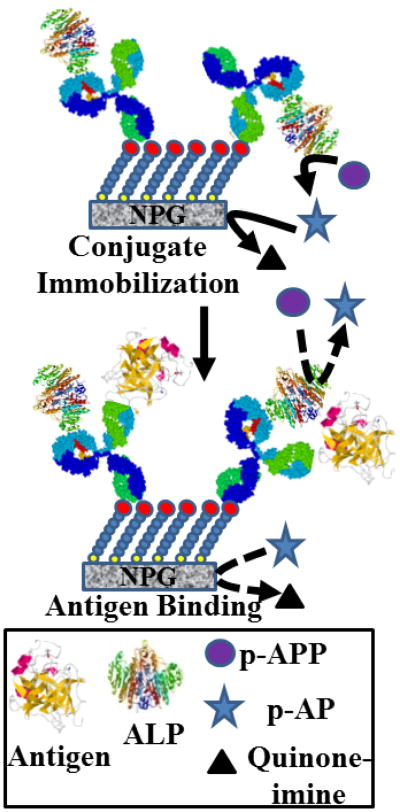
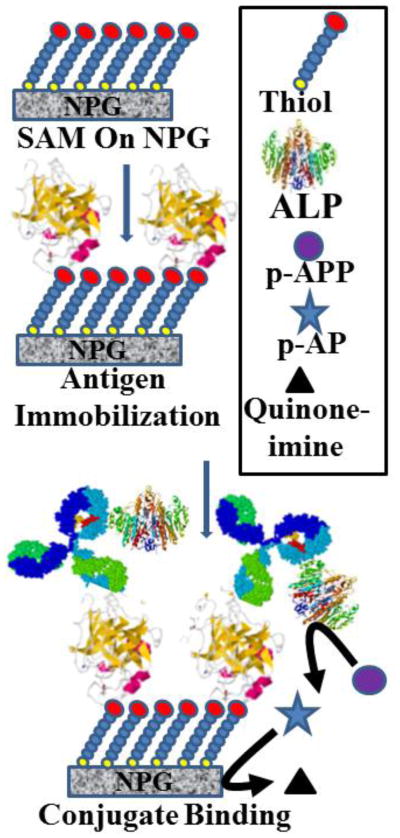
The immunoassay is based on the inhibition of the access of the p-APP substrate to the active site of ALP in the antibody-ALP conjugate upon binding of the antigen, in this case either PSA or CEA. A scheme depicting the working concept for the assay is shown in Fig. 4. The binding of the antigen will reduce the rate at which p-APP substrate is converted to the oxidizable p-AP product. The enzyme reaction rate is assumed to be proportional to the peak current in the square wave voltammogram. The difference in peak current before and after incubation is the response variable for the immunoassay. The concentration of p-APP used is 250 μM, chosen because this is close to the Km value, close to the pseudolinear range of substrate enzyme kinetics, and p-APP concentrations less than 100 μM did not produce sufficient current peaks in the SWV scans. Two hours incubation time was chosen to ensure equilibrium and to achieve steady state conditions but the assay time can be reduced, as almost 80% of the final signal was achieved within 40 min of incubation (Fig. S1). The results obtained for the differences in peak current as a function of antigen concentration are shown in Fig. S2A and S2B for PSA, and in Fig. S2C and S2D for CEA. The response show a trend towards saturation at higher antigen concentration in each case. The linear range for the PSA response plot of difference in peak current (μA) versus antigen concentration (in ng mL-1) extends up to 30 ng mL-1, and linear regression analysis yields y= 0.060 x + 0.58 (R2 = 0.980); for the CEA response plot, the linear range extends up to 10 ng mL-1, and linear regression yields y = 0.27 x + 0.23 (R2 = 0.995). It is interesting to note that the assay is more sensitive to the larger antigen CEA (200 kDa) than to the smaller antigen, free PSA (34 kDa). This is consistent with the proposed mechanism based on steric hindrance of substrate access to the enzyme active site due to antigen binding. The detection limit for the assay was found to be 0.75 ng mL-1 for PSA and 0.015 ng mL-1 for CEA, taking 3 × SD of the lowest limit of the linear range of determination. A small finite intercept is observed in each plot, although it is more evident for the response to PSA than to CEA. We attribute this small finite positive intercept to some small loss of enzyme activity that may be incurred while moving the modified NPG wires back and forth between the glycine and PBS solutions. The linear range and the detection limit of PSA immunoassay extend across and above the so called diagnostic gray zone of 4-10 ng mL-1 [7]. The results are similar to the analytical characteristics mentioned for some commercial ELISA kits such as PSA watch TM, BS-0011 (Mediwatch USA Inc, West Palm Beach, FL, USA, http://psawatch.co.nz/clinicians/) [29] and Human PSA Elisa Kit (cat. No. EL10005) (Abazyme LLC, Needham, MA, USA, http://abazyme.com/ELISA_Kits_Abazyme/)[30] and some of the literature reports on immunoassays for PSA [7,31]. Our immunoassay results for CEA using one antibody is in reasonably good agreement with the other reports in similar studies such as that of Lin et al., who reported an immunoassay for CEA with linear range of 1-25 ng mL-1 and detection limit of 0.5 ng mL-1 [31]. In another study, Tang et al. reported an impedimetric immunoassay for CEA with detection limit of 0.1 ng mL-1 and a linear range of 0.5-20 ng mL-1 [32].
Fig. 4.
Schematic depiction of the direct immunoassay. The conjugate of alkaline phosphatase and a monoclonal antibody was prepared and coupled to the activated SAM on the NPG surface. These modified NPG coated electrodes were incubated with varying concentrations of antigen and then the enzymatic activity of alkaline phosphatase with and without antigen were compared. The presence of antigen on the antibody creates a steric hindrance to the substrate access to the active site of the enzyme, and hence the enzymatic activity is reduced with an increase in the concentration of the antigen bound to antibody on the surface.
The effect on the PSA assay of the introduction of 5.0 mg mL-1 of bovine serum albumin into the PBS containing the antigen and into the glycine buffer in which the measurements take place was examined. Fig. S3a shows the effect of the presence of BSA in the immunoassay. The presence of this amount of BSA had little effect on the slope of the response plot and the performance of the assay for PSA. The linear regression fit to the data yields y = 0.064 x + 1.28, (R2 = 0.964). This increase in intercept in the presence of BSA is due to the nonspecific adsorption of BSA onto the NPG surface which decreases the current response by blocking some of the exposed Au area or sites conducive for electron transfer.
Newborn calf serum was also used for the study of effect of a serum matrix on the immunoassay (Fig. S3b). For the range of the PSA concentrations for which linear response was observed in PBS, a linear response was also observed in the serum matrix. The linear regression fit to these data yield y = 0.14 x + 5.12, (R2 = 0.960). The higher intercept can be attributed to the presence of some additional components in the serum matrix that adsorb onto the surface and contribute to a reduction in current independent of the addition of PSA. The more interesting observation is that in the serum matrix, the slope and response are higher than in buffer alone or in buffer with added BSA. This could suggest that some protein component in the serum is binding to the PSA protein and increasing the effective mass bound during the experiment since binding of a higher mass to the antibody would result in greater steric hindrance and an increased response in the direct kinetic assay.
Competitive immunoassay using square wave voltammetry and immobilized antigen
An electrochemical immunoassay using square wave voltammetry was also implemented in a competitive format. The NPG was modified by a SAM of lipoic acid and then activated with EDC/NHS, as noted. The activated lipoic acid esters were then coupled to either the CEA or PSA antigen. A scheme depicting the working concept for the competitive assay is shown in Fig. 5. The results for the competitive assay for PSA and CEA using a conjugate concentration of 100 ng mL-1 are shown in Fig. 6. The addition of BSA at 5.0 mg mL-1 to the PBS or glycine buffer used was also found to not affect the response for the CEA assay (Fig. S4). The competitive assay is sensitive at lower concentrations of antigen, below 4 ng mL-1 and is somewhat complimentary in response to the direct assay presented above. The amount of conjugate bound to antigen on the NPG surface, as measured from the activity of ALP, is greater at low antigen concentration and then decreases rapidly with antigen concentration. A residual and nearly constant ALP activity is observed at higher antigen concentrations which we attribute to non-specific adsorption of the antibody-ALP conjugate.
Fig. 5.
Schematic depiction of the competitive immunoassay. Antigen was immobilized on the activated SAM on NPG and then incubated with a mixture of antibody-antigen conjugate and different concentrations of antigen in solution. Electrodes were removed from the solution and enzymatic activity was measured for the relative amount of conjugate bound to the surface as a result of competition between free and bound antigens.
Fig. 6.
Competitive immunoassay for (a) PSA and (b) CEA were carried out using square wave voltammetry (parameters as above). NPG coated gold wire electrodes with PSA or CEA antigen immobilized on the surface were incubated in 100 ng mL-1 anti-PSA antibody/anti-CEA antibody-ALP conjugate in pH 7.4 PBS buffer and variable amounts of PSA or CEA for 24 h. Electrodes were then removed from the incubating solution and rinsed with an excess of pH 9.0 glycine buffer and then incubated with 250 μM p-APP for 30 min before taking SWV measurements.
Estimation of surface coverage of antibody-ALP conjugate on nanoporous gold
The surface coverage of lipoic acid on the NPG electrodes was estimated by reductive desorption of the SAMs in 0.5 M NaOH solution. The average (n =5) surface coverage for lipoic acid SAMs was found to be 2.42 × 10-10 mol cm-2. The number of lipoic acid molecules was estimated from the charge passed under the reductive desorption peak and the gold surface area was taken as the 12.5 cm2 average determined from the oxide stripping experiments. The thiol atom surface coverage for alkanethiol SAMs on flat gold surfaces has been reported to be 7.6 × 10-10 mol cm-2 and for lipoic acid it is 7.1 × 10-10 mol (of thiols) cm-2, which is equivalent to 3.5 × 10-10 mol cm-2 of lipoic acid molecules cm-2, assuming two thiol groups per lipoic acid molecule [33]. This indicated that the surface coverage of lipoic acid on NPG surface is approximately 70% and is thus less than a monolayer and is likely disordered. Estimation of the surface coverage of the conjugate on the lipoic acid modified SAMs was sought by solution depletion measurements. The amount of either antibody-ALP conjugate or of antigen PSA or CEA immobilized onto the surface was estimated by using a solution depletion measurement of protein concentration and the BCA protein concentration assay. 10 NPG wires modified with lipoic acid SAMs, and with the terminal −COOH groups activated by EDC/NHS chemistry, were added to the 25 μg of antibody-ALP conjugate or to 10 μg of the antigen in 100 μL PBS. Wires were incubated for 24 h at 4 °C. Wires were then removed from the incubation solution and rinsed with 100 μL of PBS three times. The PBS used for rinsing was collected and the amount of protein in it was determined by the BCA assay. The amount of protein remaining in the incubation solution was determined independently and added to the amount of protein leached during washing. The difference between the amount of protein initially present in solution and the final amount gave the estimated amount of protein attached to the NPG wires. Table I presents the amounts of these biomolecules found on average immobilized on an NPG wire as determined from the BCA assay. Given the surface coverage of lipoic acid, equivalent to 2.42 × 10-10 mol cm-2 and the surface coverage in mol cm-2 of either the antibody-ALP conjugate (1.9 × 10-13 mol cm-2) or of the PSA (9.7 × 10-13 mol cm-2) or CEA (3 × 10-13 mol cm-2) antigens, it is evident that a small fraction of the lipoic acid molecules are conjugated to protein in either case.
Table 1.
Summary of surface coverage for different proteins on NPG, calculated by solution depletion studies (BCA assay).
| Protein | Mol wt. kDa | Surface Coverage | ||
|---|---|---|---|---|
|
| ||||
| Mass μg cm-2 | mol cm-2 | Molecules cm-2 (mol cm-2 × Navo) | ||
|
|
||||
| PSA Antigen | 34 | 0.033 | 9.7 × 10-13 | 5.8 × 1011 |
| Conjugate | 146+69 | 0.041 | 1.9 × 10-13 | 1.1 × 1011 |
| (IgG+ALP) | ||||
| CEA Antigen | 180 | 0.054 | 3 × 10-13 | 1.0 × 1011 |
The area occupied by these antigens or by the conjugate is subject to variability considering that they can occupy a range of orientations. Hence, any estimate of surface coverage is approximate in the absence of orientational information. However, using the dimensions of these proteins, estimations can be made. Using the structures found in the Protein Data Bank (2PSA), the dimensions of the PSA antigen are 4.1 nm × 4.5 nm × 5.1 nm. The footprint on the surface for the PSA antigen is thus estimated as ranging from 18.5 (4.1 × 4.5) nm2 − 23.0 (5.1 × 4.5) nm2. The corresponding dimensions for the CEA antigen (1E07) are 28 nm × 3.4 nm × 3.7 nm, those for ALP (1B8J) are 7.8 nm × 8.6 nm × 10 nm, and those for a typical IgG (1HZH) are 11.4 nm × 10.5 nm × 16 nm. The information on the dimensions of these proteins is summarized in table I, along with the ranges of their possible surface footprint areas.
Approximate dimensions of the antibody-ALP conjugate can be estimated assuming that there is one enzyme attached to per antibody. The labeling process is expected to add 1-2 ALP enzymes per IgG [34]. The estimated footprint for the antibody-ALP conjugate thus ranges from 86 nm2 (10 nm × 8.6 nm) for the possible situation of the ALP enzyme attached at the bottom of the Fc region of the IgG to a maximum for the ALP attached to the side of the Fv regions of 282 nm2 ((15.9 nm + 10 nm) × 10.5 nm). It is also likely that there is some range of attachment points to the surface, resulting in a range of footprints on the surface for the antibody-ALP conjugate. Using these dimensions, the maximum theoretical surface coverage would range from (0.355 - 1.16) × 1012 molecules cm-2. The surface coverage that resulted from the protein concentration assay solution depletion measurements for the antibody-ALP conjugate is 0.115 × 1012 molecules cm-2, suggesting a fractional surface coverage in the range of 10 – 32%. For the PSA antigen, the range of estimated maximum theoretical surface coverage is (4.35 – 5.41) × 1012 molecules cm-2 compared to the coverage estimated from solution depletion measurements of 0.588 × 1012 molecules cm-2 suggesting a fractional coverage of 11-42%. For the CEA antigen, the range of estimated maximum theoretical surface coverage is (1.03 – 0.97) × 1012 molecules cm-2 compared to the coverage estimated from solution depletion measurements of 0.1 × 1012 molecules cm-2, suggesting an approximate fractional coverage of 10 % or less. The estimated ranges for surface coverages are summarized in Table II.
Table 2.
Theoretical and experimental surface coverage of proteins on NPG.
| Proteins | Maxa | Minb | Surface Coverage (calculated) (molec cm-2) | ||
|---|---|---|---|---|---|
|
| |||||
| Surface area (cm2) | Surface coverage (molec cm-2) | Surface area (cm2) | Surface coverage (molec cm-2) | ||
| PSA antigen | 57.42 × 10-14 | 1.44 × 1012 | 18.50 × 10-14 | 5.40 × 1012 | 5.80 × 1011 |
| IgG-ALP conjugate | 282.00 × 10-14 | 3.55 × 1011 | 86.0 × 10-14 | 1.16 × 1012 | 1.10 × 1011 |
| CEA antigen | 103.60 × 10-14 | 9.65 × 1011 | 12.60 10-14 | 7.94 × 1012 | 1.0 × 1011 |
Theoretically possible maximum surface areas were calculated by confining molecules inside a bound box and maximum combination of dimensions were takes to obtain maximum surface areas.
Minimum surface area was calculated from minimum values of dimensions.
Conclusions
The presented assay strategy demonstrates use of the high surface area of NPG and of its ability to be used as an electrode for SWV detection of an oxidizable enzyme product, in this case p-aminophenol. The high surface area allows for implementation of a kinetics based assay approach that would not be feasible on a flat electrode. The use of SWV is required to overcome the large double-layer charging effect in the NPG. The assay approach can be applied to a wide range of protein antigens, and should respond better to those of larger molar mass since these introduce greater steric hindrance upon binding. In the present study, PSA and CEA were used as readily available biomarkers for testing the strategy of using SWV and a NPG electrode. The use of other enzymes in the conjugate, such as horseradish peroxidase, could also be considered. The assay makes use of one antibody, which while it may reduce specificity relative to that achieved with a two antibody sandwich strategy, reduces the cost of required antibodies. In the case of PSA, appropriate choice of antibody can allow response to either the free PSA protein by targeting an epitope that is masked when the PSA is bound to anti-chymotyrpsin, or to total PSA, by targeting an epitope exposed in either form [35]. The present studies were carried out using free PSA. In real samples, the dominant form will be the PSA-ACT complex, even more so in samples for which cancer is likely and where the fraction of free PSA is reduced. The assay is likely to be more sensitive to the larger antigen, the PSA-ACT complex in which the PSA protein (34 kDa) is bound to anti-chymotrypsin (68 kDa), just as it was found here to be more sensitive to CEA than to free PSA. The time for this assay can be approximately 90 minutes, and possibly reduced further if stirring of the solution or a flow-through or infusion arrangement was introduced during incubation so that binding and substrate access would no longer be solely diffusion limited. Further improvement could involve running two electrode reactions in parallel, where one has been exposed to the antigen containing sample and one has not.
A feature of this assay strategy that should be noted is that access of the p-AP product to the gold surface where it is oxidized must be possible, and as such perfect, well-packed monolayers would not be conducive to the presented assay since they would block the oxidation of p-AP. As such, disordered monolayers presenting sites at which pAP can be oxidized are required. The p-AP produced by the enzyme action during prior to the SWV sweep is likely building up inside the NPG pores and beginning to diffuse out into the bulk solution. Additional substrate will be diffusing into the NPG pores as p-APP is depleted within NPG by the action of the ALP enzyme. The oxidation current observed should be arising from oxidation of p-AP within the NPG interior and also from PAP located around the NPG electrode some distance into the bulk solution.
NPG electrodes can be prepared in a range of sizes and formats, with a range of pore sizes, and are suitable for miniaturization or use in flow-through electrochemical devices. The properties of NPG electrodes present opportunities for the development of new electrochemical assay formats. The greatly enhanced surface to volume ratio of NPG facilitates strategies such as the one described here which relies upon the enhancement in peak current in a square wave voltammetry sweep following production formation within a nanoporous electrode. The high surface area of NPG allows new possibilities for assay development based on enzyme activity differences in the presence or absence of bound antigens.
Supplementary Material
Acknowledgments
The authors thank Professor Fraundorf, Jay K. Bhattarai, Dr. David Osborn and Dr. Dan Zhou of the UM-St. Louis Center for Nanoscience for usage and discussion of SEM. This work was supported by UM-St. Louis and by the NIGMS award R01-GM090254.
References
- 1.Seker E, Reed M, Begley M. Nanoporous Gold: Fabrication, Characterization, and Applications. Materials. 2009;2(4):2188–2215. [Google Scholar]
- 2.Shulga OV, Zhou D, Demchenko AV, Stine KJ. Detection of free prostate specific antigen (fPSA) on a nanoporous gold platform. Analyst. 2008;133(3):319–322. doi: 10.1039/b712760j. [DOI] [PubMed] [Google Scholar]
- 3.Tan Yih Horng, P B, Abeera Sharma, Jay Bhattarai, Stine Keith J. Bioconjugation reactions for covalent coupling of proteins to gold surfaces. Global journal of Biochemistry 2010 [Google Scholar]
- 4.Duan C, Meyerhoff ME. Separation-Free Sandwich Enzyme Immunoassays Using Microporous Gold Electrodes and Self-Assembled Monolayer/Immobilized Capture Antibodies. Anal Chem. 1994;66(9):1369–1377. doi: 10.1021/ac00081a003. [DOI] [PubMed] [Google Scholar]
- 5.Meyerhoff ME, Duan C, Meusel M. Novel nonseparation sandwich-type electrochemical enzyme immunoassay system for detecting marker proteins in undiluted blood. Clin Chem. 1995;41(9):1378–1384. [PubMed] [Google Scholar]
- 6.Escamilla-Gómez V, Hernández-Santos D, González-García MB, Pingarrón-Carrazón JM, Costa-García A. Simultaneous detection of free and total prostate specific antigen on a screen-printed electrochemical dual sensor. Biosensors Bioelectron. 2009;24(8):2678–2683. doi: 10.1016/j.bios.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 7.Mani V, Chikkaveeraiah BV, Patel V, Gutkind JS, Rusling JF. Ultrasensitive Immunosensor for Cancer Biomarker Proteins Using Gold Nanoparticle Film Electrodes and Multienzyme-Particle Amplification. ACS Nano. 2009;3(3):585–594. doi: 10.1021/nn800863w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao X, Zhang Y, Chen H, Chen Z, Lin X. Amperometric immunosensor for carcinoembryonic antigen detection with carbon nanotube-based film decorated with gold nanoclusters. Anal Biochem. 2011;414(1):70–76. doi: 10.1016/j.ab.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Tang J, Dai Z, Yan F, Ju H, Murr NE. A disposable electrochemical immunosensor for flow injection immunoassay of carcinoembryonic antigen. Biosensors Bioelectron. 2006;22(1):102–108. doi: 10.1016/j.bios.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Wu G, Datar RH, Hansen KM, Thundat T, Cote RJ, Majumdar A. Bioassay of prostate-specific antigen (PSA) using microcantilevers. Nat Biotech. 2001;19(9):856–860. doi: 10.1038/nbt0901-856. [DOI] [PubMed] [Google Scholar]
- 11.Choi JW, Kang DY, Jang YH, Kim HH, Min J, Oh BK. Ultra-sensitive surface plasmon resonance based immunosensor for prostate-specific antigen using gold nanoparticle–antibody complex. Colloids Surf Physicochem Eng Aspects. 2008;313-314(0):655–659. doi: 10.1016/j.colsurfa.2007.05.057. [DOI] [Google Scholar]
- 12.Li X, Wang R, Zhang X. Electrochemiluminescence immunoassay at a nanoporous gold leaf electrode and using CdTe quantun dots as labels. Microchimica Acta. 2011;172(3):285–290. doi: 10.1007/s00604-010-0487-x. [DOI] [Google Scholar]
- 13.Fernández-Sánchez C, McNeil CJ, Rawson K, Nilsson O. Disposable Noncompetitive Immunosensor for Free and Total Prostate-Specific Antigen Based on Capacitance Measurement. Anal Chem. 2004;76(19):5649–5656. doi: 10.1021/ac0494937. [DOI] [PubMed] [Google Scholar]
- 14.Gosling JP. A decade of development in immunoassay methodology. Clin Chem. 1990;36(8):1408–1427. [PubMed] [Google Scholar]
- 15.Lauks IR. Microfabricated Biosensors and Microanalytical Systems for Blood Analysis. Acc Chem Res. 1998;31(5):317–324. doi: 10.1021/ar9700670. [DOI] [Google Scholar]
- 16.Lyon JL, Stevenson KJ. Picomolar Peroxide Detection Using a Chemically Activated Redox Mediator and Square Wave Voltammetry. Anal Chem. 2006;78(24):8518–8525. doi: 10.1021/ac061483d. [DOI] [PubMed] [Google Scholar]
- 17.Stenman UH, Leinonen J, Zhang WM, Finne P. Prostate-specific antigen. Semin Cancer Biol. 1999;9(2):83–93. doi: 10.1006/scbi.1998.0086. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Preventive Services Task Force. [Accessed 04/16/2012];Screening for Prostate Cancer: Draft Recommendation Statement. http://www.uspreventiveservicestaskforce.org/uspstf12/prostate/draftrec3.htm.
- 19.Djavan B, Zlotta AR, Byttebier G, Shariat S, Omar M, Schulman CC, Marberger M. Prostate Specific Antigen Density of the Transition Zone for Early Detection of Prostate Cancer. The Journal of Urology. 1998;160(2):411–418. doi: 10.1016/s0022-5347(01)62911-2. [DOI] [PubMed] [Google Scholar]
- 20.Catalona WJ. The United States Preventive Services Task Force Recommendation against Prostate-Specific Antigen Screening–Counterpoint. Cancer Epidemiology Biomarkers & Prevention. 2012 doi: 10.1158/1055-9965.epi-12-0059. [DOI] [PubMed] [Google Scholar]
- 21.Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989;57(2):327–334. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- 22.Duffy MJ. Carcinoembryonic Antigen as a Marker for Colorectal Cancer: Is It Clinically Useful? Clin Chem. 2001;47(4):624–630. [PubMed] [Google Scholar]
- 23.Pandey B, Tan YH, Fujikawa K, Demchenko AV, Stine KJ. Comparative Study of the Binding of Concanavalin A to Self-Assembled Monolayers Containing a Thiolated α-Mannoside on Flat Gold and on Nanoporous Gold. J Carbohydr Chem. 2012;31(4-6):466–503. doi: 10.1080/07328303.2012.683909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan X, Meng F, Cui S, Liu J, Gu J, Zou Z. Effective and rapid electrochemical detection of hydrazine by nanoporous gold. J Electroanal Chem. 2011;661(1):44–48. doi: 10.1016/j.jelechem.2011.07.011. [DOI] [Google Scholar]
- 25.Thompson RQ, Barone GC, Iii, Halsall HB, Heineman WR. Comparison of methods for following alkaline phosphatase catalysis: Spectrophotometric versus amperometric detection. Anal Biochem. 1991;192(1):90–95. doi: 10.1016/0003-2697(91)90190-5. [DOI] [PubMed] [Google Scholar]
- 26.Pariente F, Hernández L, Lorenzo E. Amperometric sensor based on the alkaline phosphatase activity. Bioelectrochem Bioenerget. 1992;27(1):73–87. doi: 10.1016/0302-4598(92)85014-7. [DOI] [Google Scholar]
- 27.Li Y, Gao F, Wei W, Qu JB, Ma GH, Zhou WQ. Pore size of macroporous polystyrene microspheres affects lipase immobilization. J Mol Catal B: Enzym. 2010;66(1-2):182–189. doi: 10.1016/j.molcatb.2010.05.007. [DOI] [Google Scholar]
- 28.Bhatia RB, Brinker CJ, Gupta AK, Singh AK. Aqueous Sol–Gel Process for Protein Encapsulation. Chem Mater. 2000;12(8):2434–2441. doi: 10.1021/cm000260f. [DOI] [Google Scholar]
- 29.Device Technologies New Zealand Limited, PSAwatch™. Device Technologies New Zealand Limited; [Accessed 4/16/2012]. http://psawatch.co.nz/clinicians/ [Google Scholar]
- 30.For the Quantitative Determination of Human Prostate-Specific Antigen (PSA) Concentrations in Serum. Abazyme, LLC; [Accessed 4/16/2012]. Human PSA ELISA Kit. http://abazyme.com/ELISA_Kits_Abazyme//Human%20PSA%20ELISA%20Abazyme.pdf. [Google Scholar]
- 31.Niwa O, Xu Y, Halsall HB, Heineman WR. Small-volume voltammetric detection of 4-aminophenol with interdigitated array electrodes and its application to electrochemical enzyme immunoassay. Anal Chem. 1993;65(11):1559–1563. doi: 10.1021/ac00059a013. [DOI] [PubMed] [Google Scholar]
- 32.Tang H, Chen J, Nie L, Kuang Y, Yao S. A label-free electrochemical immunoassay for carcinoembryonic antigen (CEA) based on gold nanoparticles (AuNPs) and nonconductive polymer film. Biosensors Bioelectron. 2007;22(6):1061–1067. doi: 10.1016/j.bios.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 33.Dong Y, Abaci S, Shannon C, Bozack MJ. Self-Assembly and Electrochemical Desorption of Thioctic Acid Monolayers on. Gold Surfaces Langmuir. 2003;19(21):8922–8926. doi: 10.1021/la0261141. [DOI] [Google Scholar]
- 34.Alkaline Phosphatase Labeling Kit-NH2. Dojindo; [Accessed 04/16/2012]. http://www.dojindo.com/store/p/47-Alkaline-Phosphatase-Labeling-Kit-NH2.aspx. [Google Scholar]
- 35.Allard WJ, Zhou Z, Yeung KK. Novel immunoassay for the measurement of complexed prostate-specific antigen in serum. Clin Chem. 1998;44(6):1216–1223. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.