Abstract
Background
As osteoarthritis (OA) is a highly heterogeneous disease in terms of progression, establishment of prognostic biomarkers would be highly beneficial for treatment. The present study was performed to identify novel biomarkers capable of predicting the progression of knee OA.
Methods
A total of 69 plasma samples (OA patients undergoing radiographic progression, n = 25; nonprogression, n = 33; healthy donors, n = 11) were analyzed by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF MS), and ion peaks of interest were identified by liquid chromatography and matrix-assisted laser desorption/ionization (MALDI)-TOF MS. The identities of these proteins were further validated by immunoprecipitation combined with SELDI-TOF MS analysis.
Results
SELDI-TOF MS analysis indicated that the intensities of 3 ion peaks differed significantly between progressors and nonprogressors. Subsequent analyses indicated that these peaks corresponded to apolipoprotein C-I, C-III, and an N-terminal truncated form of transthyretin, respectively. The identities of these proteins were confirmed by the loss of ion peaks in SELDI-TOF MS spectra by immunoprecipitation using specific antibodies for the respective proteins.
Conclusions
Three potential biomarkers were identified whose serum levels differed significantly between OA progressors and nonprogressors. These biomarkers are expected to be prognostic biomarkers for knee OA and to facilitate the development of novel disease-modifying treatments for OA.
Keywords: osteoarthritis, biomarker, proteomics, surface-enhanced laser desorption/ionization (SELDI)
Introduction
Osteoarthritis (OA) is the most prevalent form of arthritis and is characterized by a gradual loss of cartilage matrix that often extends over years and decades. Age is the most powerful risk factor for OA. Thus, with increasing longevity, OA has now become a leading cause of disability for older adults in developed countries.
Although OA can affect any synovial joint, OA in knee joints has the highest prevalence rate and greatest association with disability.1,2 Knee OA has now become a large economic and medical burden on society.3,4 Therefore, there is increasing demand to establish effective therapies for this disease. However, no effective treatments have yet been established for OA that can inhibit or even delay disease progression. This may be partly due to the lack of reliable biomarkers to predict disease progression. In knee joints, progression of OA is most often determined by changes in the joint space measured on plain radiographs obtained in weight-bearing positions.5,6 However, as progression of OA occurs gradually over several years, observation over an extended period of time is required to determine whether a given therapy can effectively inhibit progression of the disease. This necessity for a prolonged trial period significantly hinders the development of effective treatments for the disease. Thus, the establishment of reliable prognostic markers would greatly facilitate the process of drug development by reducing the time required for clinical trials. Identification of proteins that are closely associated with disease progression may also be of benefit in research regarding the etiology of OA, which is still unclear.
Proteomic profiling technologies are powerful tools to discover biomarkers of disease.7 The basic strategy to explore biomarkers consists of fractionation of proteins contained in samples and subsequent identification of proteins by mass spectrometry (MS). For the latter step, analysis by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF MS) is now widely used. SELDI-TOF MS is an array-based technology first introduced in 1993.8 In this technology, MS is combined with affinity chromatography to isolate a subset of proteins from complex protein samples, such as blood or urine, taking advantage of the physicochemical properties of the proteins.9 The advantages of SELDI-TOF MS analysis are its high throughput capacity, high degree of accuracy, and good reproducibility. This technology also allows comparison of the entire protein profiles among samples because when using SELDI-TOF MS analysis, various proteins within the samples are detected by their respective ion peaks with intensities correlated with their levels.10 Recent studies have reported significant improvements in the quality and reproducibility of the data acquired by SELDI-TOF MS.11,12 Using this technology, biomarkers for cancers and inflammatory disorders have been successfully identified.13–15
In the present study, we performed a series of analyses to identify biomarkers in plasma that may predict progression of knee OA. SELDI-TOF MS analysis was performed, and the profiles of plasma proteins were compared between knee OA patients who did and did not show disease progression. To increase the sensitivity, 6 high-abundance proteins were depleted from the samples prior to the analysis. The candidate proteins were selected according to the differences in peak intensities between the 2 groups of OA patients. The selected proteins were isolated by multiple liquid chromatography (LC) and finally identified by matrix-assisted laser desorption/ionization (MALDI)-TOF MS analysis. The identities of the proteins were confirmed by immunoprecipitation using specific antibodies for the respective proteins.
Materials and Methods
Subjects and sample collection
This study was approved by the institutional review boards of the participating institutions, and written informed consent was obtained from each subject prior to the study. To obtain plasma samples from OA patients who were undergoing disease progression (progressors) and those in a stable condition (nonprogressors), we performed a 2-year follow-up of 29 patients with knee OA. This study was conducted as part of a 3-year follow-up study of knee OA patients including a larger number of patients.5 In this previous study, knee OA patients were recruited from among those visiting a community medical center seeking medical care for symptomatic knee OA. Inclusion criteria were age 50 years or older, good general health, and primary knee OA with medial involvement in at least 1 knee. The diagnosis of primary knee OA was based on the criteria of the American College of Rheumatology.16 Patients with significant impairment in the spine or lower extremities were excluded from the study, as were those with a history of previous injury or surgery. Patients in whom the joint space of the knee was closed due to the disease were also excluded because progression of the disease could not be evaluated in such cases.
During the 2-year study period, plasma samples were obtained from the subjects every 6 months. At the same visits, posteroanterior knee radiographs were obtained in a weight-bearing position, and the progression of knee OA was determined in respective knee joints from the changes in joint space width (JSW) on these radiographs.17,18 In this study, the plasma samples were retrospectively divided into 2 groups, that is, those from the progressors and others from the nonprogressors according to the following strict criteria. Progressors were defined as patients in whom the JSW decreased at a rate of 0.1 mm/year or faster in any 6-month period, or between any 2 consecutive visits, in at least 1 knee. Meanwhile, nonprogressors were patients in whom the JSW did not show any detectable change or reduced at a rate below 0.1 mm/year in the bilateral knees throughout the entire follow-up period (2 years). The progressors in this study often showed JSW reduction only in a specific 6-month period during the 2-year follow-up. Therefore, for progressors, the plasma obtained only at the visit immediately before or after a period in which JSW reduction occurred was chosen for the analysis.
Control plasma samples were obtained from 11 age-matched donors without any significant health problems (Table 1). In these healthy donors, possible involvement of knee OA was excluded by posteroanterior knee radiography in a weight-bearing position.
Table 1.
Demographic characteristics of healthy donors.
| Number of donors | 11 |
| Female | 9 |
| Male | 2 |
| Age* (years) | 61.9 ± 11.7 |
| Height* (cm) | 152.1 ± 6.5 |
| Weight* (kg) | 52.7 ± 7.1 |
| BMI* (kg/m2) | 23.1 ± 3.1 |
Note:
Data are mean ± SD.
All blood samples were obtained from the subjects in the morning under a fasted condition. To obtain plasma samples from the patients and healthy donors, peripheral blood was drawn into tubes containing EDTA, and plasma was separated immediately after acquisition by centrifugation. The plasma samples were aliquoted and stored at −80 °C until use.
SELDI-TOF MS analysis to explore biomarker candidates
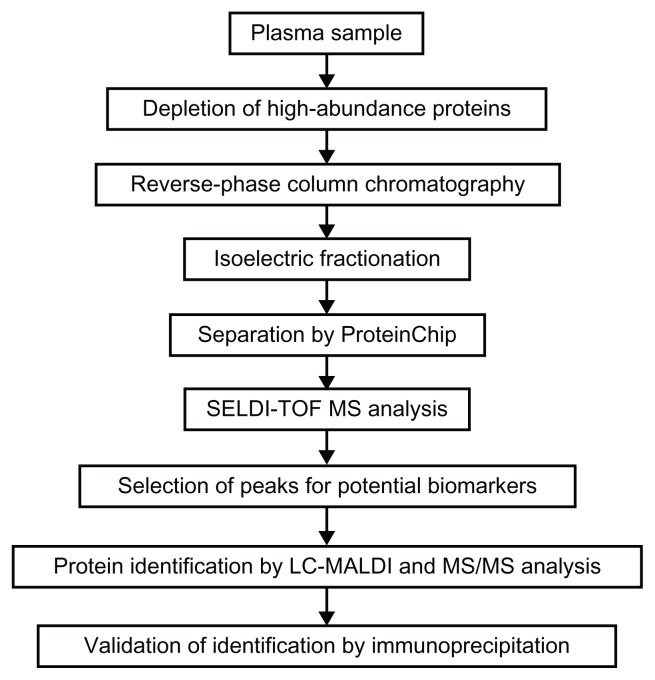
Our procedure for discovering prognostic biomarkers is shown schematically in Figure 1. The plasma samples were first treated with Multiple Affinity Removal System (MARS) Spin Cartridges Human-6 (Agilent Technologies, Santa Clara, CA) to remove 6 high-abundance proteins (albumin, IgG, antitrypsin, IgA, transferrin, and haptoglobin). For this, 30-μL aliquots of plasma samples were passed through the column, and the flow-through fraction was obtained. This fraction was then desalted and purified by SepPak C8 reverse-phase column chromatography (Waters, Milford, MA). Following this step, the samples were dried and then reconstituted with U9 buffer (9.5 M urea, 2% CHAPS, 50 mM Tris-HCl, 1% DTT, pH 9.0), and incubated at 4 °C for 30 minutes to allow denaturation of the proteins. These samples were then separated into 6 fractions by isoelectric point using a ProteinChip Serum Fractionation kit (Ciphergen Biosystems, Freemont, CA) on a Biomek 3000 Laboratory Automation Workstation (Beckman Coulter, Brea, CA). The fraction 1 was obtained at pH 9, and the fraction 2 to 5 were obtained at pH 7, 5, 4, and 3, respectively. The 6th or final fraction (fraction 6) was obtained with buffer containing 33.3% isopropanol, 16.7% acetonitrile, and 0.1% trifluoroacetic acid. Next, with the exception of fraction 1, all the other fractions were allowed to bind to 2 types of ProteinChip, ie, cation-exchange chip (CM10) and reverse-phase chip (H50). Aliquots of 40 μL of the fractionated samples were diluted to 100 μL with the binding buffers for the respective chips and put into each well of the chips. After gentle agitation at room temperature for 1 hour, the chips were washed 3 times with the binding buffers for 5 minutes and rinsed twice with deionized water. Following this step, 1 μL of Energy Absorbing Matrix solution (Ciphergen Biosystems) was applied to each array spot twice and dried at room temperature.
Figure 1.
Schematic diagram of our experimental procedure for identification of prognostic biomarkers for knee OA.
The samples bound to the CM10 or H50 chip were analyzed by SELDI-TOF MS (PCS 4000 system; Ciphergen Biosystems) under the following conditions: laser intensity, 170; detector sensitivity, 7; mass deflector, 500 Da; and ion focus mass, 5000 Da. External calibration was performed using All-In-One peptide mass standard (Bio-Rad Laboratories, Hercules, CA). The acquired mass spectra data were analyzed using Ciphergen Express software (Version 3.0.7, Bio-Rad Laboratories). Spectra were normalized by total ion current to an external normalization coefficient of 1.0 within the range of molecular mass-to-charge ratio (m/z) 3000 to 50000. Then, the spectra were manually evaluated and invalid spectra were rejected. Peak intensities were compared between the samples from the progressors and those from the non-progressors by Mann-Whitney U test, and the intensities were considered to be significantly different at P < 0.05.
In our analysis, coefficients of variation (CV) of peak intensities were less than 25% when the peaks were within the above described m/z range. This good reproducibility was achieved by careful optimization of washing conditions and meticulous parameter settings for peak detection.
Identification of candidate proteins by LC-MALDI-TOF MS analysis
Identification of the candidate proteins for biomarkers was carried out using plasma samples obtained from the progressors and nonprogressors. For this, plasma samples were treated with MARS Spin Cartridges and then fractionated with a ProteinChip Serum Fractionation kit, as described in the previous section. The fractions were then subjected to HPLC using an Agilent 1200 HPLC system with a HiQ sil C18 reverse-phase column (0.2 mm i.d. × 100 mm; KYA Technologies, Tokyo, Japan). The mobile phases consisted of 0.1% trifluoroacetic acid (TFA) in 2% acetonitrile (solvent A) and 0.1% TFA in 98% acetonitrile (solvent B). After loading of the sample, the mobile phase was held at 90% solvent A and 10% solvent B for 40 minutes. Linear gradient elution was performed by increasing the mobile phase composition from 10% to 60% solvent B over 55 minutes at a flow rate of 2 μL/minute. The eluted solution was mixed with 0.1% TFA (7 μL/minute) and fractionated on a 384 Prespotted AnchorChip (PAC) MALDI target plate (Bruker Daltonics, Bremen, Germany) at 20-second intervals. These PAC plates were then analyzed by MALDI-TOF MS using an Ultraflex II MALDI-TOF/TOF mass spectrometer (Bruker Daltonics) to find fractions containing the candidate proteins.
Identification of the candidate proteins from the spotted proteins on PAC plates was performed by MS/MS analysis using the Ultraflex II system following proteolytic digestion on the MALDI target plate. That is, 2 μL of 20 ng/μL trypsin solution (sequencing grade; Promega, Madison, WI) containing 100 mM ammonium bicarbonate was added to each well of the PAC plate, which was incubated in a moist chamber at 37 °C for 1 hour. The reaction mixture was then moved onto an AnchorChip MALDI target plate (Bruker Daltonics) and dried under an atmosphere of N2. The sample spot was washed twice with 3 μL of ethanolacetone-0.1% TFA (6:3:1) solution. Finally, the samples were analyzed on an Ultraflex II TOF/TOF mass spectrometer using α-cyano-4-hydroxycinnamic acid (CHCA) according to the manufacturer’s standard procedure for protein identification. The obtained MS/MS spectra data were processed with FlexAnalysis software (Bruker Daltonics) and identified using the Mascot search engine (Matrix Science, Tokyo, Japan) against the UniProt Knowledgebase (UniProtKB/SwissProt; http://www.uniprot.org/) with variable modifications for tryptic peptides.
Confirmation of protein identification by immunoprecipitation
To confirm identification of potential biomarkers, the identified proteins in the samples were immunoprecipitated, and the peaks thought to represent these proteins were analyzed by SELDI-TOF MS. For this experiment, 2 antihuman transthyretin antibodies (C-20, #sc-8104 and N-19, #sc-8105) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and antihuman apolipoprotein C-I (apoC-I) antibody (#31A-R1a) and antihuman apolipoprotein C-III (apoC-III) antibody (#600-101-114) were obtained from Academy Bio-medical Co. (Houston, TX) and Rockland Immunochemicals, Inc. (Gilbertsville, PA), respectively.
For immunoprecipitation, aliquots of 20 μL of plasma samples, which had been treated with a MARS column and ProteinChip Serum Fractionation kit, were diluted with an equal amount of immunoprecipitation buffer (50 mM Tris-HCl, 300 mM NaCl, 0.2% n-octyl glucopyranoside, pH 7.5). A 1.2-μL aliquot of antibody solution (200 μg/mL) was added, and the mixture was agitated at 4 °C for 2 hours. Subsequently, 4.5 μL of Protein A/G PLUS agarose bead suspension (Santa Cruz Biotechnology) was added and the mixture was further agitated at 4 °C for 2 hours. After centrifugation, the residue was washed twice with the immunoprecipitation buffer, and the supernatant was obtained by centrifugation at 6500 × g for 5 minutes at 4 °C. The supernatant was analyzed by SELDI-TOF MS.
Results
Profiling of plasma proteins and exploration of biomarker candidates
In this study, protein profiles were determined by SELDI-TOF MS analysis of 25 plasma samples from 6 progressors, 33 samples from 10 nonprogressors, and 11 samples from healthy donors. The characteristics of OA patients and details of the samples analyzed are shown in Table 2. For each of these samples, approximately 300 ion peaks were identified within the mass range of m/z 3000–50 000. The peak patterns were highly similar among the samples for respective subjects, indicating good reproducibility of the analysis. This might be ascribed in part to the use of an automatic workstation in the analysis.
Table 2A.
Demographic characteristics of progressors.
| Patients | Number of analyzed samples | Timing of sample acquisition (days after the 1st visit) | Sex (F/M) | Age (years) | Height (cm) | Weight (kg) | BMI (kg/m2) | JSW at 1st visit (right/left; mm) | Narrowing rate of JSW* (mm/year) |
|---|---|---|---|---|---|---|---|---|---|
| #1 | 3 | 0, 184, 392 | F | 66 | 150 | 54.0 | 24.0 | 4.1/3.0 | 1.00, left |
| #2 | 5 | 0, 196, 357, 553, 735 | F | 71 | 150 | 58.0 | 25.8 | 4.1/4.7 | 0.96, left |
| #3 | 5 | 0, 168, 362, 495, 721 | F | 76 | 152 | 65.0 | 28.1 | 4.1/3.4 | 1.59, right |
| #4 | 3 | 0, 189, 357 | F | 77 | 152 | 57.0 | 24.7 | 4.4/1.4 | 1.55, left |
| #5 | 5 | 0, 168, 350, 518, 707 | F | 72 | 153 | 63.0 | 26.9 | 3.9/3.3 | 0.96, right |
| #6 | 4 | 0, 212, 366, 520 | F | 67 | 157 | 56.5 | 22.9 | 4.9/3.4 | 0.97, right |
| Mean ± SD | (n = 25) | – | (6/0) | 71.5 ± 4.5 | 152 ± 2.6 | 58.9 ± 4.2 | 25.4 ± 1.9 | 3.7 ± 0.9 | 1.17 ± 0.31 |
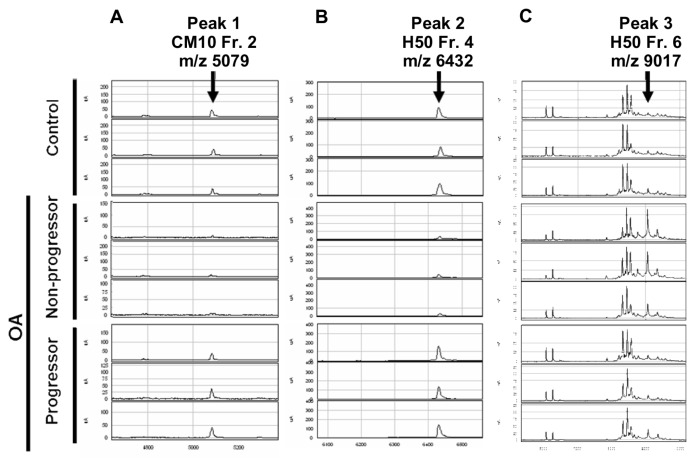
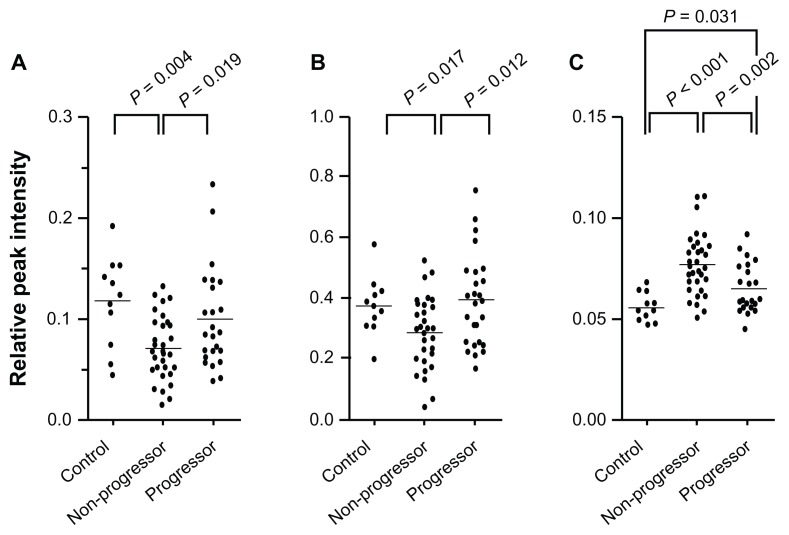
From the results of SELDI-TOF MS analysis, 3 ion peaks were found to exhibit significantly different peak intensities between the samples from the progressors and those from the nonprogressors. These peaks were observed at m/z 5079 of fraction 2 treated with CM10 (peak 1) (Fig. 2A), at m/z 6432 of fraction 4 with H50 (peak 2) (Fig. 2B), and at m/z 9017 of fraction 6 with H50 (peak 3) (Fig. 2C). For peaks 1 and 2, the peak intensities were significantly higher in the progressors compared with the nonprogressors (Fig. 3A and B), while the intensity of peak 3 was significantly lower in the progressors than the non-progressors (Fig. 3C). Among these 3 peaks, peak 3 showed the most obvious difference between the progressors and nonprogressors (P = 0.002, Mann-Whitney U test).
Figure 2.
Results of SELDI-TOF MS analysis. Representative results of plasma samples from healthy donors (Control), nonprogressors, and progressors are shown for each of fraction 2 (Fr. 2) with CM10 (A), fraction 4 (Fr. 4) with H50 (B), and fraction 6 (Fr. 6) with H50 (C). Arrows indicate the peaks with significantly different intensities between progressors and nonprogressors, which were designated as peak 1 (A), 2 (B), and 3 (C), respectively. The m/z values for these peaks are shown.
Figure 3.
Distribution of ion peak intensities. Intensities of peak 1 (A), 2 (B), and 3 (C) of the samples obtained from healthy donors (control, n = 11), nonprogressors (n = 33), and progressors (n = 25) are shown. Bars indicate means. P values were determined by Mann-Whitney U test.
Protein identification of biomarker candidates
To identify the proteins corresponding to the 3 peaks described above, 6 high-abundance proteins (albumin, IgG, antitrypsin, IgA, transferrin, and haptoglobin) were removed from the samples, followed by fractionation according to isoelectric point prior to LCMALDI-TOF MS analysis. By these treatments, the ion peaks for the 3 potential biomarkers could be identified as isolated peaks on MS spectra. The proteins represented by these peaks were then identified by MS/MS analysis. Identification was first conducted using plasma samples that showed the highest peak intensities and then confirmed by analysis of 2 other samples. The protein represented by peak 1 was identified as transthyretin, and those for peaks 2 and 3 were identified as apoC-I and apoC-III, respectively (Table 2). For peaks 2 and 3, the molecular weights of apoC-I (6631 Da) and apoC-III (8765 Da without glycosylation) calculated from amino acid sequences were very close to the observed m/z values for the respective peaks. However, for peak 1, the m/z value (m/z 5079) differed substantially from the calculated molecular weight of transthyretin (13762 Da). Thus, peak 1 was considered not to represent the entire transthyretin molecule, but a truncated form of the protein.
Confirmation of protein identification by immunoprecipitation
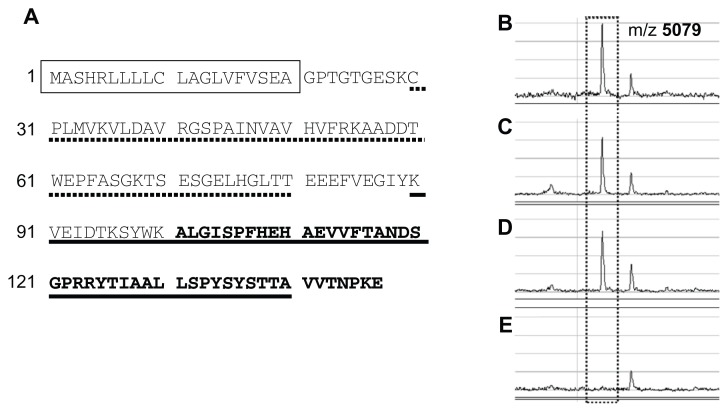
Next, identification of the proteins was validated by immunoprecipitation. In this experiment, a protein was considered to be correctly identified if the intensity of the peak was significantly reduced by depletion of that protein within the sample by immunoprecipitation. For peak 1, immunoprecipitation was performed using 2 types of antitransthyretin antibody specific for N- and C-terminal regions of the protein, respectively (Fig. 4A). Peak 1 was almost completely abolished by treatment of the sample with the antibody for the C-terminal region of transthyretin, while the intensity changed little following treatment with control IgG or the other antibody specific for the N-terminal region (Fig. 4B–E). From this result, peak 1 was considered to represent a C-terminal fragment(s) of transthyretin in plasma samples.
Figure 4.
(A) Amino acid sequence of human transthyretin (P02766, UniProtKB/SwissProt). Amino acids contained in the fragment detected by SELDI-TOF MS as peak 1 are shown in bold (101A-147E), and sequences of the peptides used to raise antibodies against the N-terminal and C-terminal regions of transthyretin are shown by broken (29C-80T) and solid lines (90K-140A), respectively. The predicted signal peptide is shown in a box. (B–E) SELDI-TOF MS spectra containing peak 1 (at m/z 5079) of untreated sample (B) or samples immunoprecipitated with control IgG (C), an antibody against the N-terminal region of transthyretin (D), or another antibody against the C-terminal region of the protein (E).
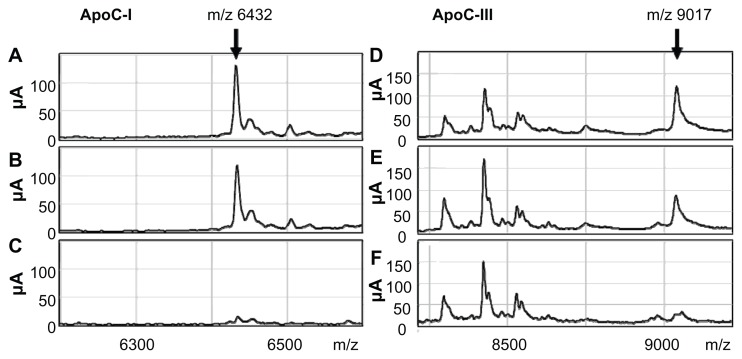
This experiment was repeated for peaks 2 and 3 using antibodies for apoC-I and apoC-III, respectively. As anticipated, these peaks were almost completely abolished by depletion of the respective proteins by immunoprecipitation, which confirmed the correct identification of these proteins (Fig. 5).
Figure 5.
Changes in peak 2 (A–C) and peak 3 (D and E) by depletion of the identified proteins by immunoprecipitation. SELDI-TOF MS spectra of untreated samples (A and D) and samples immunoprecipitated with control IgG (B and E) are shown together with those of the samples immunoprecipitated with anti-apoC-I (C) or anti-apoC-III antibody (F).
Discussion
Despite many previous studies, biomarkers that can reliably predict progression of OA have not yet been discovered. This is partly because it is not easy to obtain blood or urine samples from OA patients who are definitely undergoing disease progression. In previous studies, progression of the disease was most often determined by the changes in radiographs between the beginning and the end of the study period.19–25 Blood or urine samples were obtained only once at the beginning of most of those studies. In such studies, it could not be guaranteed that the progressors were indeed undergoing disease progression at the time of sample acquisition—in such subjects, progression may have occurred after the samples had been obtained. To overcome this problem, we followed knee OA patients for 2 years, and obtained radiographs and plasma samples repeatedly during this period. This strategy allowed us to recognize samples that had been obtained during disease progression. This solid method of sample collection was one of the advantages of the present study.
Another advantage of this study was the use of state-of-art technologies for proteomic analysis. To find prognostic markers in plasma, we performed SELDI-TOF MS analysis. Compared with other proteomic procedures, this method has several unique advantages, such as ease of determination of protein profiles and high-throughput capacity.26 On the other hand, SELDI-TOF MS analysis has certain limitations in reproducibility and quantitativeness of the data. To minimize these problems, we first meticulously set the parameters and optimized the experimental conditions very carefully in all steps of the analysis.
Difficulty in protein identification is another limitation with this technology. In the present study, we took several measures to overcome this problem. Plasma samples were treated by MARS columns to remove more than 90% of 6 high-abundance proteins (albumin, IgG, antitrypsin, IgA, transferrin, and haptoglobin) from plasma samples. This treatment is known to be particularly effective in the identification of small amounts of proteins within plasma samples.27 Following column treatment, samples were fractionated according to their isoelectric points before identification. Finally, MS/MS analysis was performed in combination with LC-MALDI-TOF MS. These strategies allowed successful identification of proteins in this study.
Taking advantage of the high-throughput capacity of SELDI-TOF MS analysis, we analyzed as many as 69 plasma samples, in each of which we identified about 300 ion peaks. Among them, 3 ion peaks were found to have significantly different intensities between the samples from the progressors and those from the nonprogressors. In subsequent analyses, these peaks were shown to correspond to 2 proteins and 1 peptide. Among them, apoC-I was found to have higher peak intensity in samples from the progressors compared with the nonprogressors, while apoC-III showed higher peak intensities in the samples from the nonprogressors compared with the progressors. To our knowledge, there have been no previous studies suggesting the involvement of these apolipoproteins in the pathology of OA. ApoC-I and C-III are proteins related to lipid metabolism. A recent study suggested that altered lipid metabolism may be involved in the development of knee OA.28 Disturbance of lipid metabolism has been observed in osteoarthritic chondrocytes.29 Therefore, it is possible that the changes in apolipoprotein concentrations in plasma are associated with the progression of knee OA. Further studies are required to clarify how these proteins are related to the loss of cartilage matrix within joints affected by OA.
In the present study, we also found that transthyretin was significantly associated with the progression of OA. Transthyretin is present in plasma as a tetramer of identical subunits and acts as a carrier protein for retinol and thyroid hormones. Previously, several investigators reported that transthyretin may be a possible biomarker for rheumatoid arthritis (RA).30,31 In these studies, the plasma level of transthyretin was reduced by treatment with anti-TNF-α antibody, suggesting that the level of transthyretin in plasma may be correlated with the severity of inflammation in RA patients. In contrast, in the present study, the level of transthyretin was reduced in OA patients while the disease was in an active phase. This may reflect the differences in pathology between RA and OA. However, caution should be exercised in interpreting these results, because the change in concentration was observed with the entire transthyretin for RA patients, while for OA patients, the change was detected with an N-terminal truncated form of the protein.
Interestingly, truncated forms of transthyretin have been found to be associated with several other pathologies, such as senile systemic amyloidosis and ovarian cancer.32,33 The truncation site identified in the present study differed from those reported previously and may be unique to OA. Although speculative, the transthyretin fragment identified here may be generated by an endogenous protease(s) within OA joints. The activity of such enzyme(s) may be elevated in OA knees during disease progression, which could also promote the cleavage of transthyretin at the identified site. Again, it should be noted that it is SELDI-TOF MS that makes it possible to discover such fragments as possible biomarkers because such truncated peptides are unlikely to be detected by conventional methods of analysis, such as enzyme-linked immunosorbent assay.
For these proteins, comparison with the control plasma samples revealed an interesting trend: for all 3 identified factors, peak intensities of the control plasma samples were closer to those of the progressors than the nonprogressors. The mechanisms for these seemingly paradoxical changes are currently unknown. We assume that such changes might reflect the shift in the balance between expression levels and degradation rates of the candidate proteins because both of these can change with the activity of the disease. Hopefully, subsequent studies will clarify the mechanisms underlying the change of the identified proteins.
In the present study, we performed a series of proteomic analyses and identified 3 potential biomarkers whose serum levels differed significantly between progressors and nonprogressors. None of them have been reported previously, and these represent novel potential prognostic markers for knee OA. Although the prognostic values of these factors should be validated in future studies, our strategy to identify progressors among knee OA patients and the methods used to identify biomarker proteins are worth considering in future studies to explore biomarkers for OA. We hope that our methods and results will be of help in future attempts to establish reliable prognostic markers for knee OA.
Table 2B.
Demographic characteristics of the non-progressors whose samples were used for the analysis.
| Patients | Number of analyzed samples | Timing of sample acquisition (days after the 1st visit) | Sex (F/M) | Age (years) | Height (cm) | Weight (kg) | BMI (kg/m2) | JSW at 1st visit (right/left; mm) | Narrowing rate of JSW* (mm/year) |
|---|---|---|---|---|---|---|---|---|---|
| #1 | 3 | 0, 163, 387 | F | 62 | 160 | 68.0 | 26.6 | 3.9/4.3 | 0.04, left |
| #2 | 5 | 0, 175, 343, 565, 721 | F | 60 | NA | NA | NA | 2.6/3.9 | 0.06, left |
| #3 | 3 | 0, 182, 343 | F | 56 | 153 | 50.0 | 21.4 | 3.1/4.0 | 0.06, right |
| #4 | 2 | 0, 168 | F | 67 | 155 | 64.0 | 26.6 | 4.0/6.7 | 0.00, right and left |
| #5 | 2 | 0, 168 | F | 71 | 148 | 50.0 | 22.8 | 4.4/5.7 | 0.07, left |
| #6 | 3 | 0, 210, 385 | F | 72 | 158 | 54.0 | 21.6 | 5.1/4.4 | 0.05, left |
| #7 | 4 | 0, 219, 366, 541 | F | 74 | 150 | 46.0 | 20.4 | 4.9/4.7 | 0.09, left |
| #8 | 4 | 0, 149, 415, 583 | M | 75 | 165 | 70.0 | 25.7 | 6.6/6.3 | 0.09, right |
| #9 | 4 | 0, 163, 317, 506 | F | 66 | 151 | 60.0 | 26.3 | 4.0/3.7 | 0.05, right |
| #10 | 3 | 0, 164, 359 | F | 72 | 145 | 57.0 | 27.1 | 3.6/3.3 | 0.10, left |
| Mean ± SD | (n = 33) | – | (9/1) | 67.5 ± 6.4 | 154 ± 6.3 | 57.7 ± 8.5 | 24.3 ± 2.7 | 4.5 ± 1.1 | 0.06 ± 0.03 |
Notes:
The greater of the rates for right and left knees is shown with laterality.
Abbreviations: JSW, joint space width; NA, not available.
Table 3.
Results of protein identification.
| m/z | Protein name | Accession no.* | Mascot score | Identified peptide | |
|---|---|---|---|---|---|
| Peak 1 | 5079 | Transthyretin | P02766 | 118 | K.ALGISPFHEHAEVVFTANDSGPR |
| 62 | R.RYTIAALLSPYSYSTTAVVTNPKE | ||||
| Peak 2 | 6432 | Apolipoprotein C-I | P02654 | 42 | K.MREWFSETFQK |
| Peak 3 | 9017 | Apolipoprotein C-III | P02656 | 48 | K.DALSSVQESQVAQQAR |
Note:
UniProt Knowledgebase (UniProtKB/SwissProt; http://www.uniprot.org/uniprot/).
Footnotes
Author Contributions
Conceived and designed the experiments: HO, NF. Analysed the data: YT, SYo, TU. Wrote the first draft of the manuscript: YT, NF. Contributed to the writing of the manuscript: TTM, AM, SYa. Agreed on the results and conclusions of the manuscript: TI, IF, HO, YN. Jointly developed the structure and arguments for the paper: TTM, SYo, HO, YN. Made critical revisions and approved final version: AM, NF. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
Author(s) disclose no funding sources.
References
- 1.Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–13. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jinks C, Jordan K, Croft P. Osteoarthritis as a public health problem: the impact of developing knee pain on physical function in adults living in the community: (KNEST 3) Rheumatology (Oxford) 2007;46:877–81. doi: 10.1093/rheumatology/kem013. [DOI] [PubMed] [Google Scholar]
- 3.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: Evidence from national survey data. Arthritis Rheum. 2009;60:3546–53. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 4.Kim S. Changes in surgical loads and economic burden of hip and knee replacements in the US: 1997–2004. Arthritis Rheum. 2008;59:481–8. doi: 10.1002/art.23525. [DOI] [PubMed] [Google Scholar]
- 5.Fukui N, Yamane S, Ishida S, et al. Relationship between radiographic changes and the symptoms or physical examination findings in the subjects with symptomatic medial knee osteoarthritis: a three-year prospective study. BMC Musculoskelet Disord. 2010;11:269–78. doi: 10.1186/1471-2474-11-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emrani PS, Katz JN, Kessler CL, et al. Joint space narrowing and Kellgren-Lawrence progression in knee osteoarthritis: an analytic literature synthesis. Osteoarthritis Cartilage. 2008;16:873–82. doi: 10.1016/j.joca.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruiz-Romero C, Blanco FJ. Proteomics role in the search for improved diagnosis, prognosis and treatment of osteoarthritis. Osteoarthritis and Cartilage. 2010;18:500–9. doi: 10.1016/j.joca.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Hutchens T, Yip T. New desorption strategies for the mass spectrometric analysis of macromolecules. Rapid Commun Mass Spectrom. 1993;7:576–80. [Google Scholar]
- 9.Reddy G, Dalmasso EA. SELDI ProteinsChip®Array Technology: protein-based predictive medicine and drug discovery applications. J Biomed Biotechnol. 2003;4:237–41. doi: 10.1155/S1110724303210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petricon EF, Liotta LA. SELDI-TOF-based serum proteomic pattern diagnostics for early detection of cancer. Curr Opin Biotechnol. 2004;15:24–30. doi: 10.1016/j.copbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Harezlak J, Wang M, Christiani D, Lin X. Quantitative quality-assessment techniques to compare fractionation and depletion methods in SELDI-TOF mass spectrometry experiments. Bioinformatics. 2007;23:2441–8. doi: 10.1093/bioinformatics/btm346. [DOI] [PubMed] [Google Scholar]
- 12.Keshishian H, Addona T, Burgess M, Kuhn E, Carr SA. Quantitative, multiplexed assays for low abundance proteins in plasma by targeted mass spectrometry and stable isotope dilution. Mol Cell Proteomics. 2007;6:2212–29. doi: 10.1074/mcp.M700354-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee IN, Chen CH, Sheu JC, et al. Identification of complement C3a as a candidate biomarker in human chronic hepatitis C and HCV-related hepatocellular carcinoma using a proteomics approach. Proteomics. 2006;6:2865–73. doi: 10.1002/pmic.200500488. [DOI] [PubMed] [Google Scholar]
- 14.Melle C, Ernst G, Scheibner O, et al. Identification of specific protein markers in microdissected hepatocellular carcinoma. J Proteome Res. 2007;6:306–15. doi: 10.1021/pr060439b. [DOI] [PubMed] [Google Scholar]
- 15.Gray RD, MacGregor G, Noble D, et al. Sputum proteomics in inflammatory and suppurative respiratory diseases. Am J Respir Crit Care Med. 2008;178:444–52. doi: 10.1164/rccm.200703-409OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 17.Peterfy C, Li J, Zaim S, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32:128–32. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 18.Kothari M, Guermazi A, von Ingersleben G, et al. Fixed-flexion radiography of the knee provides reproducible joint space width measurements in osteoarthritis. Eur Radiol. 2004;14:1568–73. doi: 10.1007/s00330-004-2312-6. [DOI] [PubMed] [Google Scholar]
- 19.Vilím V, Olejárová M, Machácek S, et al. Serum levels of cartilage oligomeric matrix protein (COMP) correlate with radiographic progression of knee osteoarthritis. Osteoarthritis Cartilage. 2002;10:707–13. doi: 10.1053/joca.2002.0819. [DOI] [PubMed] [Google Scholar]
- 20.Deberg MA, Labasse AH, Collette J, et al. One-year increase of Coll 2-1, a new marker of type II collagen degradation, in urine is highly predictive of radiological OA progression. Osteoarthritis Cartilage. 2005;13:1059–65. doi: 10.1016/j.joca.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Sharif M, Shepstone L, Elson CJ, Dieppe PA, Kirwan JR. Increased serum C reactive protein may reflect events that precede radiographic progression in osteoarthritis of the knee. Ann Rheum Dis. 2000;59:71–4. doi: 10.1136/ard.59.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharif M, George E, Shepstone L, et al. Serum hyaluronic acid level as a predictor of disease progression in osteoarthritis of the knee. Arthritis Rheum. 1995;38:760–7. doi: 10.1002/art.1780380608. [DOI] [PubMed] [Google Scholar]
- 23.Pavelka K, Forejtová S, Olejárová M, et al. Hyaluronic acid levels may have predictive value for the progression of knee osteoarthritis. Osteoarthritis Cartilage. 2004;12:277–83. doi: 10.1016/j.joca.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Nelson AE, Golightly YM, Kraus VB, et al. Serum transforming growth factor-beta 1 is not a robust biomarker of incident and progressive radiographic osteoarthritis at the hip and knee: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage. 2010;18:825–9. doi: 10.1016/j.joca.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botha-Scheepers S, Watt I, Slagboom E, et al. Innate production of tumour necrosis factor alpha and interleukin 10 is associated with radiological progression of knee osteoarthritis. Ann Rheum Dis. 2008;67:1165–9. doi: 10.1136/ard.2007.084657. [DOI] [PubMed] [Google Scholar]
- 26.De Bock M, de Seny D, Meuwis MA, et al. Challenges for biomarker discovery in body fluids using SELDI-TOF-MS. J Biomed Biotechnol. 2010;2010:906082. doi: 10.1155/2010/906082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–67. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 28.Sowers M, Karvonen-Gutierrez CA, Palmieri-Smith R, et al. Knee osteoarthritis in obese women with cardiometabolic clustering. Arthritis Rheum. 2009;61:1328–36. doi: 10.1002/art.24739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsezou A, Iliopoulos D, Malizos KN, Simopoulou T. Impaired expression of genes reguilating cholesterol effulux in human osteoarthritic chondrocytes. J Orthopaedic Res. 2010;28:1033–9. doi: 10.1002/jor.21084. [DOI] [PubMed] [Google Scholar]
- 30.Naishiro Y, Suzuki C, Kimura M, et al. Plasma analysis of rheumatoid arthritis by SELDI. Jpn J Clin Immunol. 2007;30:145–50. doi: 10.2177/jsci.30.145. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi T, Nakanishi T, Tabushi Y, et al. Serum protein profile of rheumatoid arthritis treated with anti-TNF therapy (infliximab) J Chromatography B Analyt Technol Biomed Life Sci. 2007;855:66–70. doi: 10.1016/j.jchromb.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 32.Mizuguchi M, Hayashi A, Takeuchi M, et al. Unfolding and aggregation of transthyretin by the truncation of 50 N-terminal amino acids. Proteins. 2008;72:261–9. doi: 10.1002/prot.21919. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Bast RC, Jr, Yu Y, et al. Three biomarkers identified from serum proteomic analysis for the detection of early stage ovarian cancer. Cancer Res. 2004;64:5882–90. doi: 10.1158/0008-5472.CAN-04-0746. [DOI] [PubMed] [Google Scholar]