Abstract
Vitamin C (l-ascorbate) is present in the respiratory lining fluid of human lungs, and local deficits occur during oxidative stress. Here we report a unique function of vitamin C on the cystic fibrosis (CF) transmembrane conductance regulator (CFTR), a cAMP-dependent Cl channel that regulates epithelial surface fluid secretion. Vitamin C (100 μM) induced the openings of CFTR Cl channels by increasing its average open probability from 0 to 0.21 ± 0.08, without a detectable increase in intracellular cAMP levels. Exposure of the apical airway surface to vitamin C stimulated the transepithelial Cl secretion to 68% of forskolin-stimulated currents. The average half-maximal stimulatory constant was 36.5 ± 2.9 μM, which corresponds to physiological concentrations. When vitamin C was instilled into the nasal epithelium of human subjects, it effectively activated Cl transport in vivo. In CF epithelia, previous treatment of the underlying trafficking defect with trimethylamine oxide or expression of WT CFTR restored the activation of Cl transport by vitamin C. Sodium dependency and phloretin sensitivity, as well as the expression of transcripts for sodium-dependent vitamin C transporter (SVCT)-1 and SVCT2, support a model in which an apical vitamin C transporter is central for relaying the effect of vitamin C to CFTR. We conclude that cellular vitamin C is a biological regulator of CFTR-mediated Cl secretion in epithelia. The pool of vitamin C in the respiratory tract represents a potential nutraceutical and pharmaceutical target for the complementary treatment of sticky airway secretions by enhancing epithelial fluid secretion.
Keywords: ascorbate, Cl channel opener, epithelia, ΔF508 CFTR, airway disease
Vitamin C is a required micronutrient for humans because we have lost the ability to synthesize it from d-glucose via the glucuronic pathway (1). Dietary vitamin C crosses the small intestine, enters the plasma, and is transported to its target tissues, where it acts as a cofactor for several intracellular enzymes and scavenges free radicals (2). The lungs and respiratory tract are target tissues for vitamin C (3), where it mainly functions as an electron donor for reactive oxygen species (4). Vitamin C is present in the airway surface liquid (ASL), which is a 10- to 30-μm layer of fluid that forms a critical interface between the respiratory tract epithelial cells and the external environment. The pool of vitamin C in the ASL ranges between 20 and 130 μM in the airways of healthy individuals (5, 6). Epidemiological studies suggested a link between high dietary intake of vitamin C and its protective effects against respiratory symptoms (7); however, an alarming 25% of the U.S. population did not meet the recommended dietary intake levels for vitamin C as published in the Third National Health and Nutrition Examination Survey (8). Insufficient dietary intake of vitamin C (9), environmental pollutants (9, 10), and a number of inflammatory disorders of the airways are known to severely deplete the pools of vitamin C in ASL or plasma. For example, low levels of vitamin C were found in patients with bronchial asthma (6, 11), cystic fibrosis (CF) (12, 13), chronic obstructive pulmonary disease (14), and acute respiratory distress syndrome (15), and in smokers (16) as well as in children exposed to environmental tobacco smoke (17).
In addition to its well known function as an antioxidant, Scott and Cooperstein (18) described a regulatory function of vitamin C on Cl transport across the cornea of amphibians almost 30 years ago. Although these early electrophysiological studies did not propose a cellular target for this effect, they gave precedents for a biological role of vitamin C on salt and water transport. The CF transmembrane conductance regulator (CFTR) Cl channel was cloned in 1989 (19) and has not been considered as a target for vitamin C to date. CFTR, a member of the ATP-binding cassette superfamily, functions as a cAMP-dependent Cl channel that regulates epithelial surface fluid secretion. In the respiratory tract, CFTR mediates the transport of Cl across the apical membrane into the ASL, which is a key process to properly adjust its salt and water composition. The dynamics of the ASL affect its physiological functions, the most important of which are the removal of inhaled particles and the support of mucociliary clearance (20). Mutations in CFTR cause CF, which is characterized by sticky and excessive mucous secretions into the airways and chronic airway infections. The most common mutation of CFTR is a 3-bp deletion that results in the absence of a phenylalanine residue at amino acid position 508 (ΔF508). This mutation accounts for 67% of affected alleles and results in a misfolded protein that is retained in the endoplasmic reticulum, where it is eventually targeted for degradation. However, ΔF508 CFTR can function as a cAMP-dependent Cl channel if it is permitted to reach the apical cell membrane (21-23). Interestingly, CF-like symptoms such as thickened airway secretions are often seen in chronic inflammatory airway diseases without mutations in the CFTR gene, and there is emerging evidence that posttranslational damage to CFTR by reactive oxygen and nitrogen species decreases CFTR function (24).
A report on the total absence of vitamin C in the ASL of asthmatics (6) prompted us to revisit the biological functions of vitamin C as a potential regulator of Cl transport across respiratory epithelia. The present experiments were designed to investigate the role of vitamin C in controlling Cl secretion and to clarify the potential involvement of CFTR as the underlying Cl conductance.
Methods
Human Airway Cell Culture. The human submucosal serous gland-like cell line Calu-3, the CF nasal epithelial cell line homozygous for ΔF508 CFTR (CF15), and normal human tracheal primary cultures (hTE) were cultured as described (25, 26). WT CFTR-corrected CF15 cells were generated through adenovirus-mediated gene transfer. Growth media were nominally free of ascorbic acid (<10 μM; University Pathology, Salt Lake City). For transepithelial measurements, cells were grown on permeable filter inserts and used after 3-10 days. Patch Clamp Analysis. Single-channel patch clamp studies were performed on Calu-3 cells at 37°C. The outside-out recording mode was established through the whole cell mode as described (27). The bath solution contained (in mM) 145 NaCl, 1.7 CaCl2, 1 MgCl2, 10 Hepes, and 10 glucose, pH 7.4. The pipette solution contained (in mM) 15 N-methyl-d-glucamine Cl, 10 EGTA, 1 MgCl2, 10 Hepes, 10 glucose, 120 N-methyl-d-glucamine-gluconate, 5 MgATP, and 0.1 LiGTP, pH 7.4. Open probabilities (Po) for multichannel recordings were calculated for consecutive 20-s records with Po = (I - Ibase)/(N × i), where I is the average current of the respective record, Ibase is the closed-level current, N is the maximal number of channel levels observed in the total recording, and i is the single-channel current.
cAMP Measurements. Confluent Calu-3 cells were exposed to l-ascorbic acid or forskolin for 15 min and lysed with 0.1 M HCl. Cellular cAMP levels were measured in nonacetylated samples by using a competitive immunoassay for cAMP (R & D Systems).
Short-Circuit Current Measurement. Filter-grown Calu-3 or CF15 monolayers were mounted in Ussing chambers. Short-circuit current was recorded as described (28). At 20- to 50-s intervals, transepithelial voltage was clamped from 0 to 2 mV, and the transepithelial resistance (Rte) was calculated. A serosa-to-mucosa-directed Cl gradient was applied. The serosal Ussing chamber solution contained (in mM) 120 NaCl, 20 NaHCO3, 5 KHCO3, 1.2 NaH2PO4, 5.6 glucose, 2.5 CaCl2, and 1.2 MgCl2. In mucosal Ussing chamber solutions, all Cl salts were exchanged for gluconate salts.
Nasal Potential Difference (NPD) Measurements. Measurements of NPD were performed in healthy volunteers as described (28). The study protocol was approved by the Internal Review Board at Children's Hospital (Oakland, CA). Solutions and test drugs were perfused into one nostril at ≈5 ml/min at room temperature (23-25°C). The NaCl solution contained (in mM) 145 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, and 10 Hepes, pH 7.4. In Cl-free solutions, all Cl salts were replaced by the respective gluconate salts. All solutions were sterile-filtered before use. NPD was sensed with an Ag/AgCl/agar electrode placed in the perfusing tube with respect to an electrode placed on slightly scratched skin on the forearm (29).
CF Mice and Rectal Potential Difference (RPD) Measurements. Gene-targeted mice homozygous for the ΔF508 mutation (C57BL6/J) received GoLYTELY (Braintree Laboratories), a diarrhetic supplement, in the drinking water to increase their lifespan (30). To correct the maturation defect of ΔF508 CFTR, we used the chemical chaperone trimethylamine oxide (TMAO) (31). CF mice were injected with 4 mg/g TMAO from a 4 M stock solution every 8 h for 24 h, which is a regimen to partially correct the ΔF508 defect in CF mice (22). Controls were water-injected. The RPD assay was performed on mice that were anesthetized with 0.1 mg of ketamine per gram of body weight and 0.01 mg of azepromazine per gram of body weight. RPD was sensed with a 1 M NaCl agar bridge inserted ≈1 cm into the rectum vs. a s.c. needle filled with 1 M NaCl. Measurements were performed in Cl-free solution containing 100 μM amiloride and 5 mM Ba(OH)2 to isolate the Cl-selective RPD.
RT-PCR Analysis and Cloning of Sodium-Dependent Vitamin C Transporter (SVCT)-2. Total RNA was isolated from airway cultures grown on permeable filter inserts by using the RNeasy mini kit (Qiagen, Valencia, CA). All samples were treated with DNase (2 units of DNase, Promega) and RNase inhibitor (100 units of SUPERase-In, Ambion). RT-PCR was performed by using Superscript II RNase reverse transcriptase (Invitrogen) and 2.5 μM random hexamer primers (Applied Biosystems). First-strand cDNA was used as a template in PCRs (REDTaq DNA polymerase, Sigma). The primer sequence for SVCT1 (103 bp) was forward, 5′-TTC TGG TTG TGC TGC TGA CC-3′, and reverse, 5′-TGT ATC AGA CCA CGC TCC TCT-3′. For SVCT2 (97 bp) the sequence was forward, 5′-GCT GTT GCA CAC AGA ACA CA-3′, and reverse, 5′-GAG GAG GCC GAT GAC TAC TTC-3′. Standard PCR was performed by using 30 cycles and annealing at 60°C. The cDNA coding for the ORF of SVCT2 was PCR-amplified from human trachea and was cloned in-frame into the XhoI and EcoRI restriction sites of the enhanced GFP (EGFP) expression vector pEGFP/N1 (Clontech). This construct was transfected into CF15 cells.
Confocal Microscopy. The expression of SVCT2-EGFP fusion proteins was assayed by confocal microscopy. Confluent CF15 monolayers were fixed with 2% paraformaldehyde and immunostained for the tight junction-associated protein ZO-1 as a marker of the apical region by using a monoclonal anti-ZO-1 Ab (BD Biosciences) and Alexa Fluor 546-conjugated secondary Ab (Molecular Probes). Monolayers were embedded in Crystal Mount (Biomedia, Foster City, CA) and observed with a ×63/1.4 numerical aperture oil-immersion objective. A 3D image was produced from a stack of Z sections at 1.1-μm intervals. Image stacks were deconvolved and visualized by using huygens professional software by Scientific Volume Imaging (Hilversum, The Netherlands; www.svi.nl).
Results
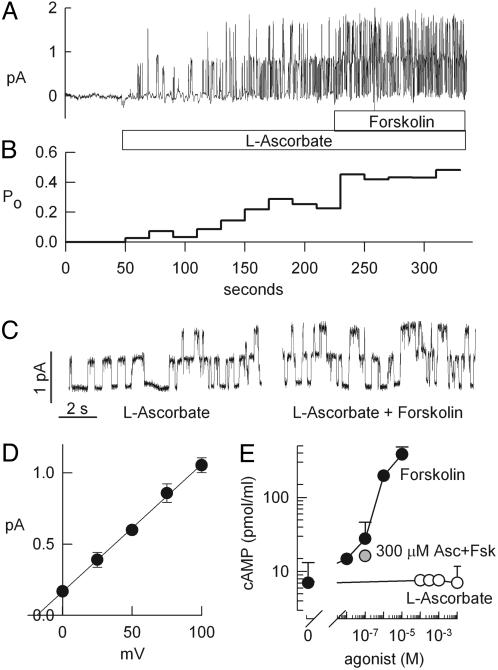
Vitamin C Activates CFTR Cl Channels. The regulatory role of vitamin C on Cl ion channel activity was studied in Calu-3 airway cells, which express large amounts of native CFTR as their major Cl conductance (32). Using the outside-out patch clamp mode, we found that l-ascorbic acid induced openings of CFTR Cl channels when applied to the extracellular surface of the patched membrane. In the recording shown in Fig. 1A, two channels were activated by 100 μM l-ascorbic acid, and the average single-channel open probability (Po) increased from 0 to 0.21 ± 0.08 (n = 4, Fig. 1B). Subsequent addition of 10 μM forskolin (a cAMP-stimulating agonist) in the continued presence of l-ascorbic acid further stimulated Cl channel activity, and average Po increased to 0.54 ± 0.12. Details of the single-channel recording are illustrated in Fig. 1C. The additional cAMP-induced activation of the ascorbate-stimulated Cl channels did not alter its single-channel amplitude or apparent gating kinetics. Fig. 1D shows the current-voltage relationship of the ascorbate-stimulated Cl conductance, and the resulting slope conductance averaged 8.9 ± 0.2 pS (n = 4). At negative potentials no channel openings were resolved, indicating Cl-over-gluconate selectivity. cAMP is the major intracellular messenger for the activation of CFTR. We tested the possibility that l-ascorbic acid increased intracellular cAMP levels [cAMP]i by either turning on cAMP production or preventing cAMP degradation. Fig. 1E compares whole-cell cAMP levels of Calu-3 cells challenged with either increasing concentrations of l-ascorbic acid or the cAMP agonist forskolin. Concentrations of forskolin of 10 nM or greater increased [cAMP]i dose-dependently (Fig. 1E, filled circle). In contrast, concentrations of l-ascorbate from 100 μM to 10 mM did not result in a detectable increase of resting [cAMP]i (Fig. 1E, open circle), and a dose of 300 μM l-ascorbate did not alter the elevated cAMP level that was stimulated by 100 nM forskolin (Fig. 1E, shaded circle). These data suggest that l-ascorbic acid stimulated CFTR activity by a cAMP-independent mechanism.
Fig. 1.
Stimulation of CFTR activity by l-ascorbate. (A) Outside-out patch clamp recording from a Calu-3 airway epithelial cell (150:15 mM Cl gradient from bath to pipette; holding potential = 75 mV). Additive activation of single CFTR Cl channels by l-ascorbate (100 μM, bath) and forskolin (10 μM). (B) Corresponding open probabilities (Po) calculated from 20-s intervals from the recording in A. (C) Details of ascorbate-stimulated CFTR gating from the recording in A. (D) Single-channel current-to-voltage relation. Slope conductance is 8.9 ± 0.2 pS (n = 4). (E) Effects of increasing concentrations of l-ascorbate (open circle), forskolin (filled circle), and 100 nM forskolin in the presence of 300 μM l-ascorbate (300 μM Asc + Fsk, shaded circle) on intracellular cAMP levels.
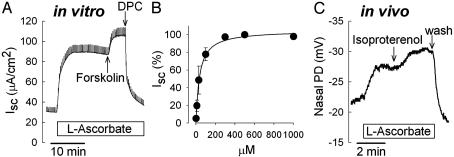
Vitamin C Regulates Cl Transport Across Human Airways in Vitro and in Vivo. Vitamin C activation of CFTR-mediated Cl secretion was studied in Calu-3 cells grown as epithelial monolayers (Fig. 2A). Exposure of the apical membrane to maximal concentrations of l-ascorbic acid stimulated transepithelial Cl currents (Isc) in a sustained fashion to 96 ± 11 μA/cm2 (n = 22). Subsequent addition of forskolin further increased Isc to 141 ± 33 μA/cm2 (n = 9, P = 0.029). On average, Cl secretion was stimulated by ascorbate to 68% of the currents elicited by forskolin. The Cl channel blocker diphenylcarboxylate (4 mM) was added as a measure for the transcellular Cl current, decreasing Isc to 30 ± 4 μA/cm2. When Cl secretion was first stimulated with forskolin (to 106 ± 23 μA/cm2, n = 4), ascorbate had no significant effect on Isc (at 100 μM: ΔICl = -1.5 ± 3.5 μA/cm2, n = 4, not different from 0, one-sample t test). Half-maximal stimulatory constant averaged 36.5 ± 2.9 μM as determined from transepithelial dose-response experiments. Concentrations of >300 μM caused maximal stimulation (Fig. 2B), i.e., concentrations significantly above the physiological plasma concentration [which saturate at ≈90 μM (33)].
Fig. 2.
Stimulation of Cl transport by l-ascorbate. (A) Measurement of transepithelial Cl secretion (Isc) across Calu-3 airway epithelia in vitro. l-ascorbate (100 μM, mucosal) stimulated Isc in a sustained fashion. Ascorbate-stimulated CI secretion is further increased by forskolin (20 μM, serosal) and completely inhibited by the Cl channel blocker DPC (4 mM). (B) Dose-dependency of ascorbate-stimulated Cl currents. Half-maximal stimulatory constant averaged 36.5 ± 2.9 μM (n = 14). (C) Measurement of NPD in a human subject in vivo. Perfusion of the nasal floor with l-ascorbate (300 μM) hyperpolarized NPD. Response to the β-adrenergic agonist isoproterenol (10 μM) is shown for comparison. wash, Washout with saline.
In vivo effects of ascorbic acid on Cl transport were evaluated by using the NPD assay for Cl channel function. The Cl-selective NPD was measured in amiloride-containing (100 μM) and Cl-free solutions. These experiments served as an important control to verify the significance of the transepithelial results obtained with ascorbic acid in Calu-3 cells. In close agreement with the transepithelial measurements, NPD was progressively hyperpolarized during the exposure of the nasal mucosa to l-ascorbic acid (by -6.7 ± 0.4 mV, n = 3) and the cAMP-stimulating agonist isoproterenol (by -5.5 ± 0.5 mV, n = 4) (Fig. 2C). A dose of 300 μM l-ascorbic acid hyperpolarized NPD to 72% of the NPD in the presence of the cAMP agonist isoproterenol (10 μM). This in vivo assay showed that vitamin C activated Cl transport across the nasal mucosa in human subjects, similar to its in vitro effects on transepithelial Cl currents.
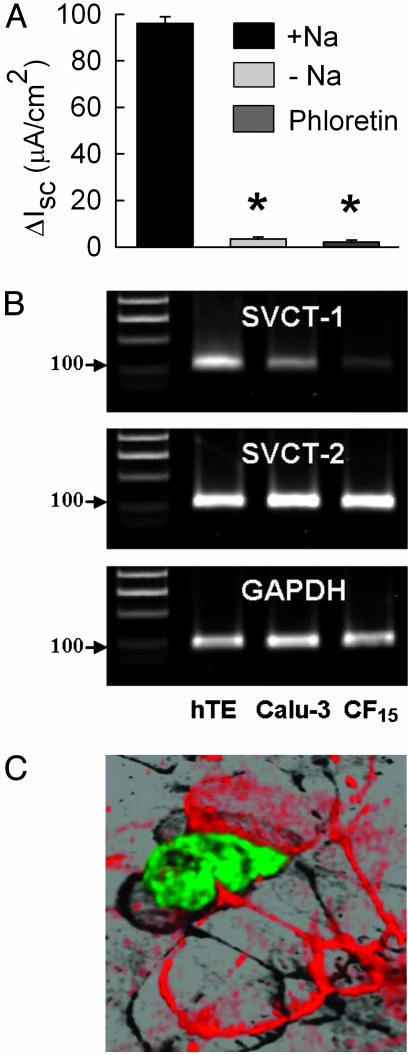
Function and Expression of SVCTs in Human Airway Epithelia. Ascorbate-stimulated Cl secretion was significantly reduced in the absence of extracellular sodium on the mucosal side (Fig. 3A). Furthermore, the response to ascorbate in sodium-containing medium was blunted in the presence of phloretin, a known inhibitor of SVCT1 and SVCT2 (34). These data suggested the involvement of SVCT1 and/or SVCT2 during the activation of ascorbate-stimulated Cl secretion. We verified the molecular expression of SVCT1 and SVCT2 transcripts in Calu-3 cells by performing RT-PCR and DNA sequencing analysis. For comparison, we included hTE and a nasal CF airway cell line (CF15). Comparison of the intensities of the specific PCR products suggested that SVCT2 was equally expressed among all tested cell types (Fig. 3B Middle), whereas SVCT1 was less abundant in Calu-3 and CF15 compared with hTE (Fig. 3B Top). DNA sequencing of the ORF of SVCT2 from hTE revealed that SVCT2 from trachea (GenBank accession no. AY380556) was identical to the published sequence from kidney (GenBank accession no. AJ269478) with the exception of 1 nt exchanged at position 1,807, T→C. The recombinantly expressed SVCT2-EGFP fusion protein was targeted exclusively to the apical membrane pole of CF15 epithelia (Fig. 3C, shown in green). The tight-junction protein of the zonula occludens-1 (ZO-1) is shown as a marker of the apical region (Fig. 3C, shown in red).
Fig. 3.
SVCT in human airway epithelia. (A) Summary of ascorbate-stimulated Cl currents (ΔIsc) across Calu-3 monolayers in the presence (+Na) or absence (-Na) of apical sodium, or after pretreatment with 200 μM phloretin. *, Significant difference from +Na, P ≤ 0.05; n = 6-14 experiments. (B) RT-PCR analysis of SVCT1 and SVCT2 mRNA expression in hTE, Calu-3, and CF15. GAPDH is shown as control. The 100-bp size markers are indicated. (C) Localization of recombinantly expressed SVCT2-EGFP fusion protein (in green) in polarized CF15 cells. The 3D reconstruction from a confocal Z series displays dominant expression of SVCT2 in the apical membrane pole. Zonula occludens protein-1 (ZO-1) marks the apical membrane region and is visualized in red by immunostaining with a ZO-1/Texas red conjugate.
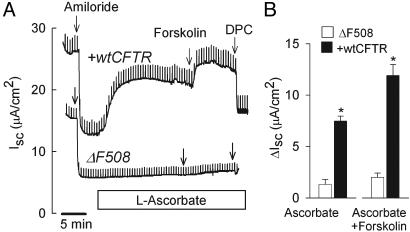
Vitamin C Is Specific for CFTR. We investigated whether ascorbate-stimulated Cl currents were solely mediated by CFTR Cl conductance. This was done by using the CF15 cell line, which is characterized by the absence of functional CFTR in the apical plasma membrane and the presence of other non-CFTR Cl conductances (26). We applied a concentration of l-ascorbic acid that lay within the upper plateau of the dose-response curve (500 μM) and compared ascorbate-stimulated Cl currents in CF15 vs. WT CFTR-corrected CF15 monolayers (Fig. 4A). The l-ascorbic acid did not significantly increase Isc (ΔIsc = 1.3 ± 0.5 μA/cm2, n = 5), whereas calcium-elevating agonists effectively stimulated the calcium-activated Cl conductance in these cells (data not shown). The defective response to l-ascorbic acid was reversed in WT CFTR-corrected CF15 epithelia such that Isc responded promptly to l-ascorbic acid (ΔIsc = 7.5 ± 0.5 μA/cm2, n = 6). The ascorbate-stimulated current was further activated by the cAMP agonist forskolin, and total stimulated Isc averaged 12.1 ± 1.1 μA/cm2 (Fig. 4B). The ascorbate-stimulated Cl current reached 60 ± 15% of the forskolin-stimulated current in CFTR-corrected CF15 monolayers, which was close to the corresponding findings in Calu-3 monolayers (68%; see Fig. 2A). The absence of ascorbate-stimulated Cl currents in CF15 monolayers supports the notion that non-CFTR Cl conductances were not activated by l-ascorbic acid. These experiments demonstrated a causal relationship between CFTR expression and ascorbate stimulation in epithelia.
Fig. 4.
l-ascorbate is specific for CFTR-mediated Cl secretion. (A) Transepithelial Cl currents across nasal CF15 epithelia (ΔF508) and CFTR-corrected CF15 epithelia (+wtCFTR) were measured in the presence of the sodium channel blocker amiloride (20 μM). Gene transfer of WT CFTR recovered the defective Cl secretory response to l-ascorbate (500 μM, mucosal). Ascorbate-stimulated CFTR Cl currents were further activated by forskolin (20 μM) and blocked by DPC (4 mM) in +wtCFTR but no ΔF508. (B) Summary of ascorbate-stimulated Cl currents before (Ascorbate) and after (Ascorbate+Forskolin) addition of forskolin in +wtCFTR vs. ΔF508 epithelia. *, Significantly different from ΔF508, P ≤ 0.05; n = 5-7 experiments.
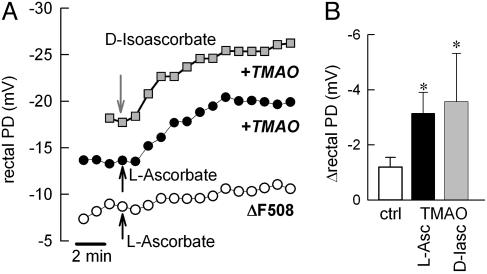
Stimulation of RPD in Rescue Compound-Treated CF Mice in Vivo. Next we determined whether l-ascorbic acid affected the functional activation of ΔF508 CFTR in the context of a living organism. Using gene-targeted mice homozygous for the ΔF508 mutation, we have previously shown that the osmolyte TMAO effectively supported ΔF508 CFTR trafficking in vivo (22). Accordingly, the effect of l-ascorbic acid was tested in homozygous ΔF508 CF mice treated with TMAO by using the RPD assay as a functional end point measure for mutant ΔF508 CFTR function. Fig. 5A illustrates that TMAO-treated CF mice manifested a detectable response to a maximal dose of l-ascorbic acid (1 mM) or its epimer d-isoascorbic acid (300 μM), and RPD hyperpolarized on average by -3.2 ± 0.8 mV (n = 4) and -3.6 ± 1.8 mV (n = 3), respectively (Fig. 5B). In contrast, perfusion with l-ascorbic acid or d-isoascorbic acid did not substantially alter RPD in water-injected control CF mice (ΔRPD = -1.1 ± 0.4 mV, n = 9). TMAO treatment increased the ascorbate- and isoascorbate-stimulated RPD ≈3-fold when compared with untreated CF mice (Fig. 5B), indicating the activation of outward Cl ion movement through functionally restored ΔF508 CFTR channels in the rectal mucosa of CF mice. We conclude that both l-ascorbate and d-isoascorbate are pharmacological tools for the activation of ΔF508-mutated CFTR after its trafficking defect has been corrected.
Fig. 5.
Activation of ΔF508 CFTR-mediated Cl transport by l-ascorbate and d-isoascorbate after correction of the trafficking defect of ΔF508 CFTR with TMAO in a CF mouse model. (A) Measurements of RPD in CF mice homozygous for ΔF508 CFTR. Arrows indicate onset of perfusion with l-ascorbate (1 mM) or d-isoascorbate (300 μM). RPD was hyperpolarized by both ascorbates in CF mice treated with 4 mg/g TMAO for 24 hours (filled circle and shaded square), but not in water-injected mice (open circle). (B) Summary of ascorbate-induced changes in RPD in TMAO-treated and control CF mice. ctrl, Control; L-Asc, 1 mM l-ascorbate; D-Iasc, 300 μM d-isoascorbate. *, Significantly different from control CF mice, P ≤ 0.05; n = 3-9 experiments.
Discussion
This study discussed a unique biological function of vitamin C as an activator of the epithelial CFTR Cl channel in the human respiratory tract. We used (i) single-channel patch clamp recordings, (ii) transepithelial short-circuit current measurements, and (iii) NPD and RPD assays to investigate the effect of vitamin C on single CFTR Cl channels, transepithelial Cl secretion, and in vivo CFTR function. We demonstrate that 100 μM l-ascorbic acid stimulated CFTR Cl channel activity by increasing its Po to ≈40% of the cAMP-stimulated CFTR. Application of ascorbic acid to the epithelial surface stimulated transepithelial Cl secretion in a sustained fashion to 68% (in Calu-3 monolayers) and 60% (in CFTR-corrected CF15 monolayers) of the currents elicited by maximally stimulated cAMP levels. Local exposure of the nasal mucosa to vitamin C activated the Cl-selective potential in healthy human subjects, and the magnitude of the ascorbate-stimulated NPD reached ≈72% of the cAMP-stimulated response. The half-maximal effective concentration for the stimulation of CFTR (36.5 μM) was within the range of normal plasma vitamin C levels (23-90 μM) (8, 33) and estimated concentrations of vitamin C in the ASL (20-130 μM) (5, 6).
Over the years there has been a surge of interest in discovering activators of Cl secretion primarily as drug candidates for CF therapy, but also as a means of increasing the hydration of excessive mucous secretions in asthma, chronic bronchitis, and chronic obstructive pulmonary disease (COPD). Several candidate molecules have been shown to be effective CFTR activators in vitro or in vivo (35); however, the majority of compounds that activate CFTR have additional pharmacological effects, whereas vitamin C is on the FDA's list of substances generally recognized as safe, and mega-doses of vitamin C (up to 2.5 g/day) are well tolerated (33). Our study highlights the potential therapeutic use of vitamin C as a safe, nontoxic, and efficacious small molecule for the activation of CFTR-mediated Cl transport in epithelia.
A major function of the respiratory tract is to filter the inhaled air and to keep the distal lung clean and sterile. The ciliated cells of the airway epithelium and the ASL are the basic components that support mucociliary clearance of inhaled particles. A great deal of work has identified the height, viscosity, and composition of the ASL as critical parameters for normal airway function (33-35). The ionic composition of the ASL is determined by the ion transport mechanisms of the airway epithelium. In CF, CFTR-mediated Cl secretion is not functional, owing to mutations in the CFTR gene. This results in abnormal ASL height, viscosity, and composition, as well as decreased mucociliary clearance causing massive airway infections (36-39). In other pulmonary diseases, such as COPD and asthma, there is emerging evidence that posttranslational damage to normal CFTR by reactive oxygen and nitrogen species may contribute to the development of thickened airway secretions (24). Thus, functional CFTR-mediated Cl transport is a key mechanism for maintaining normal airway function and healthy lungs.
Based on the fact that the ASL is deficient in vitamin C during chronic inflammatory disorders of the airways, it appears prudent to supplement the ASL with vitamin C. Unfortunately plasma vitamin C concentrations do not exceed 90 μM even at high oral doses of >1 g (33). Therefore, local delivery of vitamin C to the airway lumen via inhalation may be more advantageous, because this route will yield considerably higher concentrations at the target tissue. Based on our study, we predict that supplementation of the airway surface with pharmacological doses of vitamin C will activate the secretion of Cl, which is followed by fluid movement into the ASL. This transport process may loosen sticky mucous secretions and increase mucociliary clearance of obstructed airways. This concept is already supported by the fact that high doses of vitamin C cause secretory diarrhea as a side effect (40), which is readily explained by its stimulatory effect on CFTR-mediated Cl secretion in intestinal epithelia. Our findings provide a platform for future studies that will evaluate the pharmacological recovery of CFTR function by vitamin C for a variety of clinical conditions such as chronic airway obstructions, intestinal constipation, or dry eye syndrome.
The most common CF-causing mutation of CFTR, ΔF508, results in a misfolded protein that gets intracellularly recognized and degraded. Thus, little or no ΔF508 CFTR is functional in the apical plasma membrane of epithelial cells. We found that vitamin C had no significant effect on Cl transport in CF epithelia homozygous for ΔF508 CFTR, whereas WT CFTR-corrected CF epithelia were effectively stimulated by vitamin C (Fig. 4). These data indicated that surface expression of CFTR must occur for its effective activation by vitamin C. In the current study, CF mice were similarly treated with the chemical chaperone TMAO (22, 41) to support ΔF508 CFTR trafficking to the plasma membrane, and we applied RPD measurements as a reporting assay. We found that both l-ascorbate and its epimer d-isoascorbate significantly stimulated the Cl-selective potential in TMAO-treated mice, indicating that “membrane-rescued” ΔF508 CFTR was successfully activated by vitamin C. Thus, primary strategies that are aimed at correcting the Cl channel defect in CF (such as compounds that rescue CFTR to the plasma membrane or gene therapy) are likely to be supported by complementary treatment with vitamin C.
The stimulatory effect of vitamin C on CFTR-mediated Cl secretion across Calu-3 monolayers was dependent on extracellular sodium and blunted in the presence of phloretin (Fig. 3A), indicating that a specific cellular uptake mechanism for vitamin C was involved. SVCT1 and SVCT2 have been recently cloned. It was pointed out that the expression of SVCT1 was largely confined to epithelia (e.g., kidney and small intestine), whereas SVCT2 served a host of metabolically active cells and specialized tissues in the brain, eye, and other organs (34). The expression of vitamin C transporters has not been reported in the human respiratory tract. Using RT-PCR analysis, we did not detect an isoform-specific expression pattern in three different types of human airway epithelia. Our findings are in line with a study that reported the expression for both SVCT1 and SVCT2 in bronchiolar epithelium of rats (34) and with the discovery that SVCT2 has a vital function in the lung of mice where it appears to be critical for the initial expansion of the lungs at birth (42). Whether knockout of SVCT2 results in defective activation of CFTR by vitamin C has not been considered and will need further investigation. Selective sorting to the apical membrane compartment has been recently demonstrated for SVCT1 in Caco-2 intestinal cells (43). Here we show the localization of recombinant SVCT2-EGFP fusion protein to the apical membrane pole of nasal epithelial cells (Fig. 3C), suggesting that SVCT2 is a prime candidate for mediating the uptake of vitamin C from the airway lumen. This finding highlights the feasibility for the pharmacological delivery of vitamin C via inhalation.
Our data support a model in which an apical vitamin C transporter is central for relaying the effect of ascorbic acid to CFTR. We suggest that an increase in the intracellular ascorbate concentration stimulated the activity of CFTR, and we consider two possible mechanisms for this activation. First, we propose that vitamin C activated CFTR by a cAMP-independent mechanism, because vitamin C did not result in an elevation of cellular cAMP levels. However, a signaling cascade that leads to the activation of an apical adenylate cyclase in close proximity to the CFTR channel has been proposed for the CFTR activator adenosine in Calu-3 cells (44). Compartmentalized changes in cAMP levels are not detected by conventional cellular cAMP measurements; therefore we cannot exclude the possibility that ascorbate modulated cAMP levels in the apical microdomain in our study. We also reported that ascorbate failed to stimulate Cl currents at high intracellular cAMP levels, suggesting that ascorbate and forskolin share elements of a common signaling pathway for the activation of CFTR. Second, vitamin C is well known for its properties as an antioxidant, and we considered the notion that ascorbate activated CFTR directly by modifying its redox state. Previous work has shown that redox reagents alter the kinetics of CFTR gating such that reducing conditions (e.g., 2-mercaptoethanol and dithiotreitol) speed up gating and increase the open probability of CFTR (45), whereas oxidizing conditions slow gating of CFTR, presumably through cysteine residues present in the nucleotide-binding domains of CFTR (46). A variety of reducing and sulfhydryl-modifying agents activate CFTR in a similar manner. For example, N-ethylmaleimide or N-acetyl-l-cysteine increased CFTR-mediated currents (47, 48) involving a critical cysteine residue in the regulatory domain of CFTR (47). Taken together, the cellular pool of vitamin C may serve as a key biological factor for redox-dependent regulation of CFTR activity.
Acknowledgments
We thank Dr. J. H. Widdicombe (University of California, Davis) for providing human tracheobronchial cultures and for support with confocal microscopy, Dr. T. E. Machen (University of California, Berkeley) for CFTR-corrected CF cells, Dr. M. Yaffee for deconvolution and visualization of confocal images, and G. Borcanski and L. Sachs for technical assistance. This work was supported by National Institutes of Health Grant HL-071829, Cystic Fibrosis Foundation Grant ILLEK02G0, and Cystic Fibrosis Research, Inc.
Abbreviations: ASL, airway surface liquid; CF, cystic fibrosis; CFTR, CF transmembrane conductance regulator; Isc, short-circuit current; NPD, nasal potential difference; RPD, rectal potential difference, SVCT, sodium-dependent vitamin C transporter; TMAO, trimethylamine oxide; hTE, normal human tracheal primary cultures; EGFP, enhanced GFP.
Data deposition: SVCT2 was cloned from human tracheal epithelial cultures, and the nucleotide sequence reported in this article has been deposited in the GenBank database (accession no. AY380556).
References
- 1.Nishikimi, M., Fukuyama, R., Minoshima, S., Shimizu, N. & Yagi, K. (1994) J. Biol. Chem. 269, 13685-13688. [PubMed] [Google Scholar]
- 2.Rumsey, S. C. & Levine, M. (1998) J. Nutr. Biochem. 9, 116-130. [Google Scholar]
- 3.Willis, R. J. & Kratzing, C. C. (1976) Biochim. Biophys. Acta 444, 108-111. [DOI] [PubMed] [Google Scholar]
- 4.Slade, R., Crissman, K., Norwood, J. & Hatch, G. (1993) Exp. Lung Res. 19, 469-484. [DOI] [PubMed] [Google Scholar]
- 5.van der Vliet, A., O'Neill, C. A., Cross, C. E., Koostra, J. M., Volz, W. G., Halliwell, B. & Louie, S. (1999) Am. J. Physiol. 276, L289-L296. [DOI] [PubMed] [Google Scholar]
- 6.Kelly, F. J., Mudway, I., Blomberg, A., Frew, A. & Sanstorm, T. (1999) Lancet 354, 482-483. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz, J. & Weiss, S. T. (1994) Am. J. Clin. Nutr. 59, 110-114. [DOI] [PubMed] [Google Scholar]
- 8.Food and Nutrition Board Institute of Medicine (2002) in Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids (Natl. Acad. Press, Washington, DC), pp. 95-185.
- 9.Kodavanti, U. P., Costa, D. L., Richards, J., Crissman, K. M., Slade, R. & Hatch, G. E. (1996) Exp. Lung Res. 22, 435-448. [DOI] [PubMed] [Google Scholar]
- 10.Cross, C. E., Motchnik, P. A., Bruener, B. A., Jones, D. A., Kaur, H., Ames, B. N. & Halliwell, B. (1992) FEBS Lett. 298, 269-272. [DOI] [PubMed] [Google Scholar]
- 11.Menzel, D. B. (1992) Ann. N.Y. Acad. Sci. 669, 141-155. [DOI] [PubMed] [Google Scholar]
- 12.Winklhofer-Roob, B. M., Ellemunter, H., Fruhwirth, M., Schlegel-Haueter, S. E., Khoschsorur, G., van't Hof, M. A. & Shmerling, D. H. (1997) Am. J. Clin. Nutr. 65, 1858-1866. [DOI] [PubMed] [Google Scholar]
- 13.Brown, L. A. S., Harris, F. L. & Jones, D. P. (1997) Am. J. Physiol. 273, L782-L788. [DOI] [PubMed] [Google Scholar]
- 14.Calikoglu, M., Unlu, A., Tamer, L., Ercan, B., Bugdayci, R. & Atik, U. (2002) Clin. Chem. Lab. Med. 40, 1028-1031. [DOI] [PubMed] [Google Scholar]
- 15.Cross, C. E., Forte, T., Stocker, R., Louie, S., Yamamoto, Y., Ames, B. N. & Frei, B. (1990) J. Lab. Clin. Med. 115, 396-404. [PubMed] [Google Scholar]
- 16.Lykkesfeldt, J., Christen, S., Wallock, L. M., Chang, H. H., Jacob, R. A. & Ames, B. N. (2000) Am. J. Clin. Nutr. 71, 530-536. [DOI] [PubMed] [Google Scholar]
- 17.Preston, A. M., Rodriguez, C., Rivera, C. E. & Sahai, H. (2003) Am. J. Clin. Nutr. 77, 167-172. [DOI] [PubMed] [Google Scholar]
- 18.Scott, W. N. & Cooperstein, D. F. (1975) Invest. Ophthalmol. 14, 763-766. [PubMed] [Google Scholar]
- 19.Riordan, J. R., Rommens, J. M., Kerem, B., Alon, N., Rozmahel, R., Grzelczak, Z., Zielenski, J., Lok, S., Plavsic, N., Chou, J. L., et al. (1989) Science 245, 1066-1073. [DOI] [PubMed] [Google Scholar]
- 20.Widdicombe, J. G. (1995) Am. J. Respir. Crit. Care Med. 151, 2088-2092. [DOI] [PubMed] [Google Scholar]
- 21.Dalemans, W., Barbry, P., Champigny, G., Jallat, S., Dott, K., Dreyer, D., Crystal, R. G., Pavirani, A., Lecocq, J. P. & Lazdunski, M. (1991) Nature 354, 526-528. [DOI] [PubMed] [Google Scholar]
- 22.Fischer, H., Barbry, P., Illek, B., Sartori, C., Fukuda, N. & Matthay, M. A. (2001) Am. J. Physiol. 281, L52-L57. [DOI] [PubMed] [Google Scholar]
- 23.Egan, M. E., Glockner-Pagel, J., Ambrose, C. A., Cahill, P. A., Pappoe, L., Balamuth, N., Cho, E., Canny, S., Wagner, C. A., Geibel, J., et al. (2002) Nat. Med. 8, 485-492. [DOI] [PubMed] [Google Scholar]
- 24.Bebok, Z., Varga, K., Hicks, J. K., Venglarik, C. J., Kovacs, T., Chen, L., Hardiman, K. M., Collawn, J. F., Sorscher, E. J. & Matalon, S. (2002) J. Biol. Chem. 277, 43041-43049. [DOI] [PubMed] [Google Scholar]
- 25.Sachs, L. A., Finkbeiner, W. E. & Widdicombe, J. H. (2003) In Vitro Cell Dev. Biol. Anim. 39, 56-62. [DOI] [PubMed] [Google Scholar]
- 26.Jefferson, D. M., Valentich, J. D., Marini, F. C., Grubman, S. A., Iannuzzi, M. C., Dorkin, H. L., Li, M., Klinger, K. W. & Welsh, M. J. (1990) Am. J. Physiol. 259, L496-L505. [DOI] [PubMed] [Google Scholar]
- 27.Fischer, H. (2001) in Methods in Molecular Medicine, eds. Skatch, W. R. & Walker, J. M. (Humana, Totowa, NJ), pp. 49-66.
- 28.Illek, B. & Fischer, H. (1998) Am. J. Physiol. 275, L902-L910. [DOI] [PubMed] [Google Scholar]
- 29.Alton, E. W. F. W., Currie, A. D., Logan-Sinclair, R., Warner, J. O., Hodson, M. E. & Geddes, D. M. (1990) Eur. Resp. J. 3, 922-926. [PubMed] [Google Scholar]
- 30.Clarke, L. L., Gawenis, L. R., Franklin, C. L. & Harline, M. C. (1996) Lab. Anim. Sci. 46, 612-618. [PubMed] [Google Scholar]
- 31.Brown, C. R., Hong-Brown, L. Q., Biwersi, J., Verkman, A. S. & Welch, W. J. (1996) Cell Stress Chaperones 1, 117-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haws, C., Finkbeiner, W. E., Widdicombe, J. H. & Wine, J. J. (1994) Am. J. Physiol. 266, L502-L512. [DOI] [PubMed] [Google Scholar]
- 33.Levine, M., Conry-Cantilena, C., Wang, Y., Welch, R. W., Washko, P. W., Dhariwal, K. R., Park, J. B., Lazarev, A., Graumlich, J. F., King, J., et al. (1996) Proc. Natl. Acad. Sci. USA 93, 3704-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsukaguchi, H., Tokui, T., Mackenzie, B., Berger, U. V., Chen, X. Z., Wang, Y., Brubaker, R. F. & Hediger, M. A. (1999) Nature 399, 70-75. [DOI] [PubMed] [Google Scholar]
- 35.Illek, B., Fischer, H. & Machen, T. E. (1998) Am. J. Physiol. 275, G1221-G1226. [DOI] [PubMed] [Google Scholar]
- 36.Widdicombe, J. H. (1999) News Physiol. Sci. 14, 126-127. [DOI] [PubMed] [Google Scholar]
- 37.Boucher, R. C. (2003) Pflügers Arch. 445, 495-498. [DOI] [PubMed] [Google Scholar]
- 38.Jayaraman, S., Joo, N. S., Reitz, B., Wine, J. J. & Verkman, A. S. (2001) Proc. Natl. Acad. Sci. USA 98, 8119-8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCray, P. B., Jr., Zabner, J., Jia, H. P., Welsh, M. J. & Thorne, P. S. (1999) Am. J. Physiol. 277, L183-L190. [DOI] [PubMed] [Google Scholar]
- 40.Diplock, A. T. (1995) Am. J. Nutr. 62, 1510S-1516S. [DOI] [PubMed] [Google Scholar]
- 41.Brown, C. R., Hong-Brown, L. Q. & Welch, W. J. (1997) J. Bioenerg. Biomembr. 29, 491-502. [DOI] [PubMed] [Google Scholar]
- 42.Sotiriou, S., Gispert, S., Cheng, J., Wang, Y., Chen, A., Hoogstraten-Miller, S., Miller, G. F., Kwon, O., Levine, M., Guttentag, S. H., et al. (2002) Nat. Med. 8, 514-517. [DOI] [PubMed] [Google Scholar]
- 43.Maulen, N. P., Henriquez, E. A., Kempe, S., Carcamo, J. G., Schmid-Kotsas, A., Bachem, M., Grunert, A., Bustamante, M. E., Nualart, F. & Vera, J. C. (2003) J. Biol. Chem. 278, 9035-9041. [DOI] [PubMed] [Google Scholar]
- 44.Huang, P., Lazarowski, E. R., Tarran, R., Milgram, S. L., Boucher, R. C. & Stutts, M. J. (2001) Proc. Natl. Acad. Sci. USA 98, 14120-14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrington, M. A., Gunderson, K. L. & Kopito, R. R. (1999) J. Biol. Chem. 274, 27536-27544. [DOI] [PubMed] [Google Scholar]
- 46.Harrington, M. A. & Kopito, R. R. (2002) Biophys. J. 82, 1278-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cotten, J. F. & Welsh, M. J. (1997) J. Biol. Chem. 272, 25617-25622. [DOI] [PubMed] [Google Scholar]
- 48.Kottgen, M., Busch, A. E., Hug, M. J., Greger, R. & Kunzelmann, K. (1996) Pflügers Arch. 431, 549-555. [DOI] [PubMed] [Google Scholar]