Abstract
Rationale: Basic research implicates alveolar endothelial cell apoptosis in the pathogenesis of chronic obstructive pulmonary disease (COPD) and emphysema. However, information on endothelial microparticles (EMPs) in mild COPD and emphysema is lacking.
Objectives: We hypothesized that levels of CD31+ EMPs phenotypic for endothelial cell apoptosis would be elevated in COPD and associated with percent emphysema on computed tomography (CT). Associations with pulmonary microvascular blood flow (PMBF), diffusing capacity, and hyperinflation were also examined.
Methods: The Multi-Ethnic Study of Atherosclerosis COPD Study recruited participants with COPD and control subjects age 50–79 years with greater than or equal to 10 pack-years without clinical cardiovascular disease. CD31+ EMPs were measured using flow cytometry in 180 participants who also underwent CTs and spirometry. CD62E+ EMPs phenotypic for endothelial cell activation were also measured. COPD was defined by standard criteria. Percent emphysema was defined as regions less than −950 Hounsfield units on full-lung scans. PMBF was assessed on gadolinium-enhanced magnetic resonance imaging. Hyperinflation was defined as residual volume/total lung capacity. Linear regression was used to adjust for potential confounding factors.
Measurements and Main Results: CD31+ EMPs were elevated in COPD compared with control subjects (P = 0.03) and were notably increased in mild COPD (P = 0.03). CD31+ EMPs were positively related to percent emphysema (P = 0.045) and were inversely associated with PMBF (P = 0.047) and diffusing capacity (P = 0.01). In contrast, CD62E+ EMPs were elevated in severe COPD (P = 0.003) and hyperinflation (P = 0.001).
Conclusions: CD31+ EMPs, suggestive of endothelial cell apoptosis, were elevated in mild COPD and emphysema. In contrast, CD62E+ EMPs indicative of endothelial activation were elevated in severe COPD and hyperinflation.
Keywords: chronic obstructive pulmonary disease; emphysema; antigens, CD31; endothelium; pulmonary disease
At a Glance Commentary
Scientific Knowledge on the Subject
Prior research using animal models has implicated the primary destruction of the pulmonary capillary bed in the pathogenesis of chronic obstructive pulmonary disease (COPD) and emphysema. The relevance of these findings to clinical disease in humans is incompletely understood. Endothelial microparticles are microscopic vesicles released into the blood in response to endothelial cell perturbation.
What This Study Adds to the Field
This paper demonstrates that endothelial microparticles suggestive of endothelial cell apoptosis are elevated in COPD and, notably, mild COPD and are positively related to percent emphysema on computed tomography. These cellular markers link endothelial cell apoptosis with COPD and emphysema.
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death in the United States (1) and is projected to be the third leading cause of death worldwide by 2020 (2). COPD is defined as airflow obstruction that is not fully reversible (3). Many patients with COPD have emphysema, which is characterized by the destruction of alveolar walls with permanent loss of lung architecture and parenchyma (4).
Cigarette smoking, the primary cause of COPD (3), is known to cause endothelial dysfunction (5). Cigarette smoke is delivered directly to pulmonary endothelial cells and contains multiple factors including acrolein that cause endothelial apoptosis (6). Increased endothelial cell apoptosis has been observed in the lung tissue of patients with emphysema compared with control subjects (7, 8). Additionally, reductions in vascular endothelial growth factor (VEGF) and its receptor have been noted in lung tissue of patients with severe emphysema (8) and COPD (9). In murine models, blockade of VEGF receptor and ceramide up-regulation cause alveolar endothelial apoptosis and emphysema-like changes (10–12); however, the relevance of this work to clinical disease is unclear because the applicability of animal models of COPD to human disease remains controversial (13).
Studies in humans show that endothelial dysfunction, assessed by flow-mediated dilation of the brachial artery, is present in early COPD and is linearly related to decrements in FEV1 and greater percentage of emphysema-like lung (hereafter referred to as percent emphysema) on computed tomography (CT) among smokers with and without COPD (14, 15). Flow-mediated dilation, however, does not provide information at the cellular level.
Endothelial microparticles (EMPs) (0.1 <1.5 μm in diameter) are vesicles shed from endothelial plasma membranes into the circulation in response to endothelial cell perturbation (16). An EMP contains a number of endothelial cell surface proteins, the composition of which is dependent on the stimulus contributing to its release (17). EMPs expressing CD31 (platelet-endothelial cell adhesion marker 1) are phenotypic for endothelial cell apoptosis (16, 17). In contrast, EMPs expressing CD62E (E-selectin) are phenotypic for endothelial activation (16, 17), and EMPs expressing CD51 (vitronectin receptor) are less specific, reflecting chronic injury (18, 19).
Plasma EMP levels are increased in various vascular-related disorders. CD31+ EMPs are elevated in cardiovascular disease (19), end-stage renal disease (20), pulmonary arterial hypertension (21), sleep apnea (22), severe hypertension (23), and type 2 diabetes (24). CD62E+ EMPs are also elevated in cardiovascular disease (25), pulmonary arterial hypertension (21), and sleep apnea (22). CD51+ EMPs are elevated in type 1 diabetes (26) and multiple sclerosis (18). Plasma EMPs are also elevated in symptomatic and asymptomatic smokers compared with nonsmokers, and among nonsmokers exposed to cigarette smoke (27, 28).
CD31+ EMPs were recently associated with an isolated reduction in the diffusing capacity of carbon monoxide (DlCO) (27) and with COPD and its exacerbations (29). The clinical relevance of the former, however, is uncertain and the power of the latter study was not adequate to examine mild COPD or emphysema.
We therefore examined the relationships of circulating levels of EMPs with COPD in a study designed specifically to test the hypothesis that CD31+ EMPs are elevated in mild COPD and emphysema on CT scan. In addition, we examined relationships of EMPs to pulmonary microvascular blood flow (PMBF) assessed on magnetic resonance imaging (MRI), and to DlCO and hyperinflation. Some of the results have previously been reported in abstract form (30, 31).
Methods
Study Sample
The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study enrolled cases of COPD and control subjects from two prospective population-based cohort studies, MESA (32) and the Emphysema and Cancer Action Project (EMCAP) (33), who were 50–79 years old with a 10 or more pack-year smoking history and who did not have clinical cardiovascular disease, stage IIIb-V kidney disease, asthma before age 45 years, other lung disease, prior lung resection, cancer, allergy to gadolinium, claustrophobia, metal in the body, pregnancy, or weight greater than 300 lb. We selected all eligible participants in the MESA Lung Study (34) and oversampled participants with COPD or emphysema from the remainder of MESA and EMCAP, in addition to a small number from neither study. The current report includes participants from the one site (Columbia University) where EMPs were measured.
Protocols were approved by the institutional review boards of the participating institutions and the NHLBI. Written informed consent was obtained from all participants.
Endothelial Microparticles
Preparation of EMP samples and measurement using flow cytometry were performed as previously described (17, 19) and as detailed in the online supplement. To exclude the possibility of the unintended measurement of platelet microparticles, EMPs were defined as microparticles positively labeled by CD31 and negatively labeled by CD42, which is expressed only on platelets (19) (CD31+ EMPs); positively labeled by CD51 and negatively labeled by CD42 (CD51+ EMPs); and positively labeled by CD62E (CD62E+ EMPs).
Spirometry
Spirometry was conducted in accordance with American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines (35) on a dry-rolling-sealed spirometer (Occupational Marketing, Inc., Houston, TX). COPD was defined as a post-bronchodilator ratio of FEV1 to FVC less than 0.70 (2, 3). COPD severity was classified as follows: mild, FEV1 greater than or equal to 80% predicted; moderate, 50–80% predicted; and severe, FEV1 less than 50% predicted (3).
Percent of Emphysema-like Lung
All participants underwent full-lung CTs on General Electric 64-slice helical scanners following the MESA-Lung/SPIROMICS full-inspiration protocol (see online supplement) (36). Image attenuation was assessed using APOLLO software (VIDA Diagnostics, Coralville, IA) at a single reading center by trained readers without knowledge of other participant information. Percent emphysema was defined as the percentage of total voxels within the lung field below −950 Hounsfield units (HU).
Magnetic Resonance Imaging
Images were obtained using a 1.5-T whole-body MR (Signa LX; GE Healthcare, Waukesha, WI) with phased-array coil for signal reception. Participants underwent dynamic first-pass contrast-enhanced MR of the thorax at functional residual capacity using a coronal three-dimensional gradient echo time resolved imaging of contrast kinetics sequence with a temporal resolution of 1.2–1.8 seconds per frame. After a nonenhanced mask scan, a bolus of 0.1 mmol/kg bodyweight gadolinium–diethylenetriamine pentaacetic acid (Magnevist; Berlex, Wayne, NJ) was injected at 5 ml per second, followed by a saline flush of 20 ml at the same injection rate. Regional PMBF was assessed from a γ-variate function fitted to the signal intensity-time curve of the lung parenchyma (37). Slope increase was defined as the maximum signal increase per time interval.
DlCO and Plethysmography
Single-breath DlCO was measured with a Sensormedics Autobox 220 Series instrument (Viasys Healthcare, Yorba Linda, CA) following ATS/ERS guidelines (38). Body plethysmography was performed using a V6200 Series Autobox (Sensormedics, Yorba Linda, CA) following ATS/ERS recommendations (39).
Covariates
Age, sex, race and ethnicity, educational attainment, smoking status, pack-years, and medical history were self-reported. Height, weight, blood pressure, oxygen saturation, high-density lipoprotein, low-density lipoprotein, and fasting plasma glucose were measured using standardized approaches, and smoking status was confirmed by cotinine (see online supplement).
Statistical Analysis
Because EMP counts were skewed in distribution, values were log-transformed to improve normality. Associations between EMPs and COPD severity were initially tested with a linear contrast assuming the ranked categories of COPD severity were equally spaced, in analysis of variance. Linear regression models were then used to adjust for potential confounders, which were selected based on biologic plausibility and examination of correlations with covariates (see Table E1 in the online supplement). The base model was adjusted for age, sex, race and ethnicity, and cohort of selection. We then additionally adjusted for smoking status and pack-years. The full model was additionally adjusted for potential confounders of educational attainment, diabetes, hypertension, oxygen saturation, physician-diagnosed sleep apnea, height, weight, and body mass index in addition to statin use (which may raise EMP levels [40]), high-density lipoprotein (which may affect endothelial health and is related to percent emphysema [41]), and white blood cell count (which, if fragmented, could theoretically be included in CD31+ counts [42]). Models for percent emphysema were additionally adjusted for milliamperes. Models for pulmonary perfusion were additionally adjusted for cardiac output. Additional details on the statistical methods and sensitivity analyses are included in the online supplement.
Results
The study included 180 participants with spirometry, CT, and EMP measures (Figure 1). The mean age of the participants was 68 (SD, 7) years and 58% had COPD (22% mild, 25% moderate, and 11% severe). Thirty-two percent smoked currently and the median pack-years was 38 (interquartile range, 23.3–52.3). The race-ethnic distribution was 57% white, 25% African-American, 16% Hispanic, and 2% Chinese-American. Participants with more severe COPD were more likely to be male, white, and have greater pack-years (Table 1). Of this population, 149 participants completed the gadolinium-enhanced MRI for the perfusion analysis, whereas 118 participants completed DlCO and plethysmography (Figure 1).
Figure 1.
Flowchart of study participants. COPD = chronic obstructive pulmonary disease; CT = computed tomography; EMP = endothelial microparticle; MESA = Multi-Ethnic Study of Atherosclerosis.
TABLE 1.
CLINICAL CHARACTERISTICS OF PARTICIPANTS IN THE MESA COPD STUDY WITH MEASURES OF ENDOTHELIAL MICROPARTICLES STRATIFIED BY COPD SEVERITY
| Control Subjects (n = 76) | COPD |
|||
|---|---|---|---|---|
| Mild (n = 39) | Moderate (n = 46) | Severe/Very Severe (n = 19) | ||
| Age, mean (SD), yr |
68.9 (5.6) |
69.2 (6.7) |
67.3 (8.3) |
66.2 (7.3) |
| Sex, male, No. (%) |
39 (51.3) |
27 (69.2) |
26 (56.5) |
14 (73.7) |
| Race–ethnicity |
||||
| White, No. (%) |
40 (52.6) |
25 (64.10) |
25 (55.6) |
13 (68.4) |
| African American, No. (%) |
14 (18.4) |
10 (25.6) |
15 (32.6) |
6 (31.6) |
| Other, No. (%) |
22 (29.0) |
4 (10.3) |
6 (13.0) |
0 (0.0) |
| Educational attainment |
||||
| ≤High school degree, No. (%) |
23 (30.3) |
7 (18.4) |
12 (26.1) |
3 (15.8) |
| Some college/associate degree/vocational school, No. (%) |
22 (29.0) |
8 (21.1) |
11 (23.9) |
8 (42.1) |
| ≥College degree, No. (%) |
31 (40.8) |
24 (61.5) |
23 (50.0) |
8 (42.1) |
| Height, mean (SD), cm |
166.43 (9.76) |
171.15 (8.80) |
169.42 (9.69) |
171.78 (10.61) |
| Weight, mean (SD), kg |
79.60 (18.20) |
79.05 (14.70) |
77.91 (19.89) |
81.26 (20.43) |
| Body mass index, mean (SD), kg/m2 |
28.63 (5.73) |
26.89 (3.89) |
26.90 (5.55) |
27.31 (5.21) |
| Cigarette smoking status |
||||
| Former, No. (%) |
56 (73.7) |
29 (74.4) |
23 (50.0) |
14 (68.42) |
| Current, No. (%) |
20 (26.3) |
10 (25.6) |
23 (50.0) |
5 (26.32) |
| Pack-years of smoking, median (IQR) |
32.0 (20.7–47.5) |
40.4 (25.0–63.0) |
40.0 (36.0–54.7) |
40.0 (20.0, 67.5) |
| Low-density lipoprotein, mean (SD), mg/dl |
110.99 (33.57) |
108.05 (31.03) |
95.54 (29.25) |
102.37 (30.95) |
| High-density lipoprotein, mean (SD), mg/dl |
56.69 (16.69) |
59.54 (18.15) |
59.87 (21.58) |
56.74 (18.64) |
| Triglycerides, mean (SD), mg/dl |
103.92 (42.37) |
99.77 (40.04) |
108.43 (47.53) |
125.11 (70.37) |
| Cholesterol, mean (SD), mg/dl |
188.51 (41.24) |
187.54 (37.54) |
177.09 (32.43) |
184.05 (40.98) |
| Systolic blood pressure, mean (SD), mm Hg |
121.86 (17.28) |
120.80 (14.33) |
125.45 (15.11) |
126.89 (12.19) |
| Diastolic blood pressure, mean (SD), mm Hg |
69.66 (9.93) |
71.59 (9.64) |
72.83 (9.02) |
76.50 (9.72) |
| Hypertension, No. (%) |
25 (32.89) |
14 (35.90) |
19 (41.30) |
8 (42.11) |
| Fasting plasma glucose, median (IQR),mg/dl |
98.5 (92.0–110.0) |
100.0 (89.0–108.0) |
102.0 (97.0–113.0) |
100.0 (87.0–115.0) |
| Diabetes mellitus, No. (%) |
13 (17.1) |
6 (15.4) |
6 (15.4) |
5 (26.3) |
| Medication use |
|
|
|
|
| Statin, No. (%) |
32 (42.1) |
18 (46.2) |
22 (47.8) |
6 (31.6) |
| ACE inhibitors or angiotensin antagonists, No. (%) |
17 (22.4) |
13 (33.3) |
16 (34.8) |
5 (26.3) |
| Calcium channel blockers, No. (%) |
9 (11.8) |
2 (5.1) |
10 (21.7) |
6 (31.6) |
| β-Blockers, No. (%) |
9 (11.8) |
5 (12.8) |
9 (19.6) |
2 (10.5) |
| Omega-3, No. (%) |
12 (15.8) |
3 (7.7) |
6 (13.0) |
0 (0.0) |
| Inhaled or systemic corticosteroids, No. (%) |
3 (4.0) |
3 (7.7) |
6 (13.0) |
17 (89.5) |
| Aspirin, No. (%) |
39 (51.3) |
23 (59.0) |
22 (47.8) |
6 (31.6) |
| Short-acting β agonists, No. (%) |
0 (0.0) |
2 (5.1) |
9 (19.6) |
15 (79.0) |
| Long-acting β agonists, No. (%) |
2 (2.6) |
1 (2.6) |
2 (4.4) |
4 (21.1) |
| Short-acting anticholinergics, No. (%) |
0 (0.0) |
0 (0.0) |
3 (6.5) |
2 (10.5) |
| Long-acting anticholinergics, No. (%) |
1 (1.3) |
2 (5.1) |
6 (13.0) |
15 (79.0) |
| White blood cell count, mean (SD), billion/L |
6.42 (1.67) |
6.31 (1.37) |
7.17 (1.98) |
7.71 (2.43) |
| Neutrophils, mean (SD), % |
58.45 (8.63) |
58.39 (8.65) |
57.09 (11.76) |
62.42 (10.40) |
| Monocytes, mean (SD), % |
7.63 (2.34) |
8.77 (2.55) |
8.14 (2.40) |
8.84 (1.89) |
| Lymphocytes, mean (SD), % |
30.09 (6.87) |
29.51 (7.87) |
31.70 (10.68) |
25.53 (8.35) |
| Hemoglobin, mean (SD), g/L |
13.74 (1.38) |
14.14 (0.89) |
13.80 (1.24) |
14.23 (1.03) |
| Platelet count, mean (SD), billion/L |
223.81 (59.14) |
222.56 (48.45) |
235.87 (57.77) |
231.84 (53.91) |
| FEV1 percent of predicted, mean (SD) |
99.05 (17.77) |
91.42 (10.50) |
68.50 (7.87) |
38.86 (7.16) |
| FVC percent of predicted, mean (SD) |
98.01 (17.19) |
108.68 (13.52) |
91.66 (13.35) |
74.48 (22.17) |
| FEV1/FVC ratio, mean (SD), % |
0.77 (0.04) |
0.63 (0.06) |
0.57 (0.09) |
0.38 (0.07) |
| DlCO % predicted, mean (SD), %, n = 118 |
67.52 (10.98) |
64.31 (11.93) |
56.15 (14.37) |
40.07 (13.91) |
| DlCO VA % predicted, mean (SD), %, n = 118 |
80.21 (13.03) |
70.31 (14.91) |
72.50 (20.94) |
59.02 (19.70) |
| RV % predicted, mean (SD), %, n = 118 |
69.22 (19.41) |
84.15 (19.35) |
96.32 (29.06) |
136.81 (28.57) |
| TLC % predicted, mean (SD), %, n = 118 |
88.72 (12.55) |
100.15 (11.47) |
92.81 (13.33) |
99.49 (12.73) |
| RV/TLC ratio, mean (SD), %, n = 118 |
0.31 (0.08) |
0.31 (0.06) |
0.39 (0.08) |
0.49 (0.08) |
| Percent emphysema−910, median (IQR) |
10.83 (5.02–18.59) |
22.87 (12.78–34.02) |
18.26 (10.31–30.06) |
37.59 (28.07–39.95) |
| Percent emphysema−950, median (IQR) |
0.74 (0.40–1.43) |
2.74 (1.05–5.20) |
2.44 (0.76–6.31) |
14.27 (6.16–26.68) |
| Oxygenation saturation, mean (SD), % |
93.30 (6.78) |
96.70 (2.28) |
94.97 (7.11) |
95.17 (3.01) |
| Home oxygen therapy, No. (%) |
1 (1.32) |
0 (0.00) |
1 (2.17) |
8 (42.11) |
| Sleep apnea, self-reported, No. (%) | 5 (6.58) | 3 (7.69) | 5 (10.87) | 3 (15.79) |
Definition of abbreviations: ACE = angiotensin-converting enzyme; COPD = chronic obstructive pulmonary disease; DlCO = diffusing capacity of the lung for carbon monoxide; IQR = interquartile range; MESA = Multi-Ethnic Study of Atherosclerosis; RV = residual volume; Va = alveolar volume.
EMPs and COPD and Its Severity
CD31+ EMP levels were elevated in COPD compared with control subjects in the fully adjusted model (adjusted mean difference, 0.21 log EMP per microliter; 95% confidence interval [CI], 0.02–0.40; P = 0.03). Levels of CD51+ and CD62E+ EMPs were also higher in COPD compared with control subjects, but these differences did not attain statistical significance in the fully adjusted model (adjusted mean differences of 0.23 log CD51+ EMP per microliter, 95% CI −0.02 to 0.48, P = 0.07; and 0.20 log CD62E+ EMP per microliter, 95% CI −0.03 to 0.42, P = 0.08).
CD31+ EMPs differed by COPD severity (Table 2; see Figure E1) and were significantly elevated not only in severe COPD but also in mild COPD compared with control subjects in adjusted analyses. The magnitude of the association of CD31+ EMPs with mild COPD increased with adjustment particularly for age and race-ethnicity, differences that had attenuated the association in the unadjusted analysis. In contrast, CD51+ EMPs were not significantly elevated and CD62E+ EMPs were only elevated in severe COPD compared with control subjects.
TABLE 2.
ENDOTHELIAL MICROPARTICLE COUNTS BY COPD SEVERITY
| Control Subjects (n = 76) | Mild (n = 39) | Moderate (n = 45) | Severe (n = 19) | P Value for Linear Trend Across COPD Severity | |
|---|---|---|---|---|---|
| CD31+ endothelial microparticles per microliter, log-transformed | |||||
| Mean (SD) |
7.11 (0.58) |
7.12 (0.49) |
7.26 (0.71) |
7.52 (0.51) |
0.01 |
| Predicted mean, model 1* |
7.10 |
7.30 |
7.27 |
7.49† |
0.02 |
| Predicted mean, model 2‡ |
7.10 |
7.32† |
7.30 |
7.51† |
0.01 |
| Predicted mean, model 3§ |
7.07 |
7.31† |
7.27 |
7.40† |
0.03 |
| CD51+ endothelial microparticles per microliter, log-transformed | |||||
| Mean (SD) |
6.80 (0.79) |
6.91 (0.68) |
6.94 (0.78) |
7.17 (0.83) |
0.07 |
| Predicted mean, model 1 |
6.80 |
7.06 |
6.89 |
7.00 |
0.43 |
| Predicted mean, model 2 |
6.80 |
7.08 |
6.94 |
7.02 |
0.80 |
| Predicted mean, model 3 |
6.71 |
7.12† |
6.85 |
6.83 |
0.47 |
| CD62E+ endothelial microparticles per microliter, log-transformed | |||||
| Mean (SD) |
5.98 (0.62) |
6.09 (0.68) |
6.02 (0.71) |
6.35 (0.69†) |
0.047 |
| Predicted mean, model 1 |
5.98 |
6.15 |
6.11 |
6.53† |
0.05 |
| Predicted mean, model 2 |
5.98 |
6.16 |
6.12 |
6.54† |
0.05 |
| Predicted mean, model 3 | 5.98 | 6.22 | 6.11 | 6.51† | 0.03 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease.
Model 1 adjusted for age, sex, race and ethnicity, and cohort.
P value less than 0.05, compared with control subjects.
Model 2 adjusted for variables in model 1 in addition to smoking status, and pack-years.
Model 3 adjusted for variables in model 2 in addition to educational attainment, body mass index, height, weight, diabetes mellitus, hypertension, oxygen saturation, white blood cell count, sleep apnea, high-density lipoprotein, and statin use.
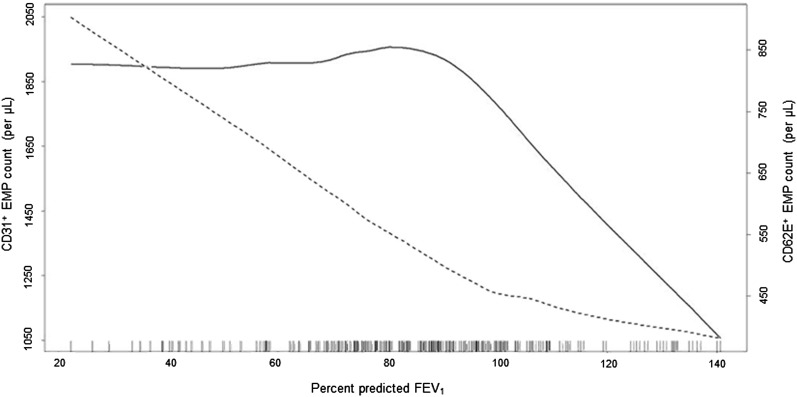
CD31+ EMPs were inversely related to the percent predicted FEV1 (P = 0.04), as were CD62E+ EMPs (P = 0.02). However, depiction of these relationships using generalized additive models, which do not force the multivariate relationship to be linear, showed different relationships of EMPs to the percent predicted FEV1 with an early increase in CD31+ EMPs and a late increase for CD62E+ EMPs (Figure 2).
Figure 2.
Endothelial microparticles and severity of chronic obstructive pulmonary disease. Smoothed regression plots of the relationship of counts of CD31+ (dark line) and CD62E+ (dashed line) endothelial microparticles to the percent predicted FEV1. The plots were obtained from regression models adjusted for age, sex, race and ethnicity, cohort, smoking status, pack-years, educational attainment, body mass index, height, weight, diabetes mellitus, hypertension, oxygen saturation, white blood cell count, sleep apnea, high-density lipoprotein, and statin use. The hash marks denote data points. EMP = endothelial microparticle.
EMPs and Percent Emphysema
CD31+ EMPs were significantly associated with percent emphysema in adjusted models (Table 3). CD31+ EMPs were increased monotonically across categories of percent emphysema and the relationship of the continuous measures was linear without evidence of a threshold effect (Figure 3). In contrast, there was no significant increase in CD51+ EMPs or CD62E+ EMPs with percent emphysema.
TABLE 3.
PREDICTED MEAN COUNTS OF ENDOTHELIAL MICROPARTICLES BY QUINTILE OF PERCENTAGE OF EMPHYSEMATOUS LUNG ON COMPUTED TOMOGRAPHY
| N = 180 | Computed Tomography Percent Emphysema |
Difference Per Log Increase in Percent Emphysema (95% CI) | P Value | ||||
|---|---|---|---|---|---|---|---|
| Quintile 1 (n = 36) | Quintile 2 (n = 36) | Quintile 3 (n = 36) | Quintile 4 (n = 36) | Quintile 5 (n = 36) | |||
| CD31+ endothelial microparticles per microliter, log-transformed | |||||||
| Mean (SD) |
7.09 (0.50) |
7.27 (0.49) |
7.07 (0.59) |
7.13 (0.55) |
7.41 (0.79) |
|
0.13 |
| Predicted mean, model 1* |
7.09 |
7.14 |
7.22 |
7.31 |
7.57 |
0.082 (0.019 to 0.145) |
0.01 |
| Predicted mean, model 2† |
7.07 |
7.13 |
7.21 |
7.32 |
7.60 |
0.089 (0.026 to 0.152) |
0.006 |
| Predicted mean, model 3‡ |
7.01 |
7.06 |
7.11 |
7.18 |
7.27 |
0.075 (0.002 to 0.149) |
0.045 |
| CD51+ endothelial microparticles per microliter, log-transformed | |||||||
| Mean (SD) |
6.86 (0.69) |
7.13 (0.68) |
6.75 (0.77) |
6.74 (0.67) |
7.02 (0.98) |
|
0.85 |
| Predicted mean, model 1 |
6.81 |
6.85 |
6.90 |
6.98 |
7.17 |
0.059 (−0.032 to 0.150) |
0.20 |
| Predicted mean, model 2 |
6.80 |
6.84 |
6.91 |
6.99 |
7.20 |
0.068 (−0.023 to 0.159) |
0.14 |
| Predicted mean, model 3 |
6.88 |
6.91 |
6.93 |
6.96 |
7.00 |
0.032 (−0.088 to 0.152) |
0.60 |
| CD62E+ endothelial microparticles per microliter, log-transformed | |||||||
| Mean (SD) |
5.94 (0.60) |
5.92 (0.68) |
6.05 (0.73) |
6.11 (0.56) |
6.22 (0.71) |
|
0.03 |
| Predicted mean, model 1 |
5.91 |
6.00 |
6.05 |
6.12 |
6.24 |
0.078 (−0.001 to 0.156) |
0.05 |
| Predicted mean, model 2 |
5.94 |
6.03 |
6.08 |
6.15 |
6.25 |
0.079 (0.00 to 0.158) |
0.05 |
| Predicted mean, model 3 | 5.98 | 6.03 | 6.07 | 6.12 | 6.20 | 0.058 (−0.039 to 0.155) | 0.24 |
Definition of abbreviation: CI = confidence interval.
Model 1 adjusted for age, sex, race and ethnicity, and cohort.
Model 2 adjusted for variables in model 1 in addition to smoking status, and pack-years.
Model 3 adjusted for variables in model 2 in addition to educational attainment, body mass index, height, weight, diabetes mellitus, hypertension, oxygen saturation, white blood cell count, sleep apnea, high-density lipoprotein, statin use, and high milliamperes.
Figure 3.
CD31+ endothelial microparticle count and percent emphysema on computed tomography. Smoothed regression plot of the relationship of counts of CD31+ endothelial microparticles to the percentage of emphysema-like lung on computed tomography (dark line). The lighter lines are 95% confidence intervals. The plot was obtained from a regression model adjusted for age, sex, race and ethnicity, cohort, smoking status, pack-years, educational attainment, body mass index, height, weight, diabetes mellitus, hypertension, oxygen saturation, white blood cell count, sleep apnea, high-density lipoprotein, statin use, and high milliamperes. The hash marks denote data points. EMP = endothelial microparticle; HU = Hounsfield unit.
EMPs, PMBF, and Diffusing Capacity
CD31+ EMPs were inversely related to pulmonary microvascular perfusion as assessed by slope increase on contrast-enhanced MR among the 149 participants who completed (Table 4). No significant associations were found between changes in slope increase and the mean number of CD51+ or CD62E+ EMPs.
TABLE 4.
THE ASSOCIATION OF ENDOTHELIAL MICROPARTICLES WITH PULMONARY MICROVASCULAR BLOOD FLOW, DIFFUSING CAPACITY, AND HYPERINFLATION
| Slope Increase* (n = 149) (per AU/s increase) | P Value | DlCO (n = 118) (per ml CO/min/mm Hg increase) | P Value | RV (n = 118) (per milliliter increase) | P Value | RV/TLC ratio (n = 118) (per unit increase) | P Value | |
|---|---|---|---|---|---|---|---|---|
| CD31+ endothelial microparticles per microliter, log-transformed | ||||||||
| Mean difference, model 1† |
−0.014 (−0.027 to −0.001) |
0.04 |
−0.034 (−0.056 to −0.012) |
0.003 |
0.065 (−0.097 to 0.228) |
0.43 |
0.653 (−0.495 to 1.802) |
0.26 |
| Mean difference, model 2‡ |
−0.015 (−0.028 to −0.002) |
0.02 |
−0.038 (−0.061 to −0.015) |
0.001 |
0.106 (−0.057 to 0.270) |
0.23 |
0.910 (−0.237 to 2.057) |
0.12 |
| Mean difference, model 3§ |
−0.015 (−0.029 to −0.001) |
0.047 |
−0.030 (−0.053 to −0.007) |
0.01 |
0.141 (−0.052 to 0.333) |
0.15 |
1.089 (−0.212 to 2.390) |
0.10 |
| CD51+ endothelial microparticles per microliter, log-transformed | ||||||||
| Mean difference, model 1 |
−0.007 (−0.025 to 0.010) |
0.42 |
−0.030 (−0.063 to −0.002) |
0.07 |
0.113 (−0.121 to 0.347) |
0.34 |
0.519 (−1.143 to 2.182) |
0.54 |
| Mean difference, model 2 |
−0.009 (−0.027 to 0.009) |
0.33 |
−0.033 (−0.066 to −0.001) |
0.046 |
0.161 (−.076 to 0.397) |
0.35 |
0.808 (−0.864 to 2.481) |
0.34 |
| Mean difference, model 3 |
−0.011 (−0.031 to 0.001) |
0.26 |
−0.029 (−0.062 to 0.004) |
0.08 |
0.074 (−0.020 to 0.356) |
0.58 |
0.738 (−1.267 to 2.742) |
0.47 |
| CD62E+ endothelial microparticles per microliter, log-transformed | ||||||||
| Mean difference, model 1 |
0.011 (−0.004 to 0.027) |
0.15 |
−0.025 (−0.055 to 0.006) |
0.10 |
0.202 (−0.009 to 0.414) |
0.06 |
1.821 (0.335 to 3.306) |
0.016 |
| Mean difference, model 2 |
0.011 (−0.005 to 0.027) |
0.17 |
−0.029 (−0.059 to 0.001) |
0.06 |
0.240 (0.026 to 0.455) |
0.03 |
2.043 (0.545 to 3.540) |
0.008 |
| Mean difference, model 3 | 0.012 (−0.005 to 0.028) | 0.17 | −0.022 (−0.053 to 0.009) | 0.17 | 0.390 (0.180 to 0.601) | <0.001 | 2.853 (1.147 to 4.558) | 0.001 |
Definition of abbreviations: AU = arbitrary units; DlCO = diffusing capacity of carbon monoxide; RV = residual volume.
Adjusted for variables in model 3 in addition to cardiac output.
Model 1 adjusted for age, sex, race and ethnicity, and cohort.
Model 2 adjusted for variables in model 1 in addition to smoking status, and pack-years.
Model 3 adjusted for variables in model 2 in addition to educational attainment, body mass index, height, weight, diabetes mellitus, hypertension, oxygen saturation, white blood cell count, sleep apnea, high-density lipoprotein, and statin use.
CD31+ EMPs were inversely associated with DlCO and DlCO/Va, whereas there was no association of CD51+ or CD62E+ EMPs with diffusing capacity in the fully adjusted model (Table 4, Figure 4a).
Figure 4.
CD31+ and CD62+ endothelial microparticles and chronic obstructive pulmonary disease (COPD) subphenotypes of pulmonary diffusing capacity and hyperinflation. (a) Smoothed regression plot of the relationship of counts of CD31+ endothelial microparticles to diffusing capacity. (b) Smoothed regression plot of the relationship of counts of CD62E+ endothelial microparticles to the ratio of residual volume to total lung capacity. The plots were obtained from regression models adjusted for age, sex, race and ethnicity, cohort, smoking status, pack-years, educational attainment, body mass index, height, weight, diabetes mellitus, hypertension, oxygen saturation, white blood cell count, sleep apnea, high-density lipoprotein, and statin use. The dark lines are the regression lines; the lighter lines are 95% confidence intervals. The hash marks denote data points. DlCO = diffusing capacity of the lung for carbon monoxide; EMP = endothelial microparticle; RV/TLC = residual volume/total lung capacity.
EMPs and Hyperinflation
As Table 4 shows, in contrast to findings for percent emphysema and pulmonary perfusion, CD62E+ EMPs were highly significantly related to hyperinflation characterized by both higher RV and RV/TLC ratio (Figure 4b), whereas CD31+ EMPs displayed no association with RV or RV/TLC ratio (Table 4).
Sensitivity Analyses
Sensitivity analyses demonstrated similar associations for CD31+ EMPs and COPD with additional adjustment for use of long-acting β agonists, inhaled corticosteroids, long-acting anticholinergics, and omega-3 polyunsaturated fatty acids, and an interaction term between cohort and case status. The results also did not change after restriction to MESA and EMCAP cohorts; former smokers; white participants; and those without hypertension, diabetes, asthma after the age of 45, or sleep apnea (see Figure E2). The relationship of CD31+ EMPs to mild COPD and percent emphysema were also consistent across these sensitivity analyses (see Figures E3 and E4, respectively), as was the relationship of CD62E+ EMPs to RV/TLC ratio (see Figure E5). Restriction to participants without subclinical cardiovascular disease strengthened the association of CD31+ EMPs and percent emphysema (see Figure E4) and slightly attenuated the association of CD31+ EMPs with COPD status and attenuated the association with mild COPD (see Figures E2 and E3, respectively). Such restriction yielded similar associations between CD62E+ EMPs and the RV/TLC ratio (see Figure E5).
To better define the apoptotic phenotypes, we additionally adjusted analyses of CD31+ EMPs with COPD and percent emphysema for CD62E+ EMPs. Such further adjustment yielded similar although slightly attenuated associations for COPD status (P = 0.11) and percent emphysema (P = 0.06).
Discussion
CD31+ EMPs, which are suggestive of endothelial cell apoptosis, were elevated in COPD compared with control subjects and this elevation was observed not only in severe COPD but also in mild COPD. Higher levels of CD31+ EMPs were also associated with the percent emphysema on CT scan, reduced PMBF, and lower DlCO. In contrast, elevations in CD62E+ EMPs were observed only in severe COPD and with hyperinflation. These findings suggest endothelial cell apoptosis early in the pathogenesis of COPD and emphysema, and endothelial activation in severe, hyperinflated COPD.
This is the first study of which we are aware to demonstrate that EMPs are increased in mild COPD and are related to a measure of emphysema. The findings, obtained using precise cellular measures linked to state-of-the-art structural and functional imaging in a general-population sample, are consistent with prior work in murine models that suggests a mechanistic role of VEGFR blockade and ceramide up-regulation as a cause of alveolar endothelial apoptosis to epithelial apoptosis and emphysema-like changes (10, 12). Together, these findings suggest a role of endothelial damage and potentially apoptosis in the pathogenesis of emphysema-predominant COPD.
Most prior work on endothelial cells in COPD has been limited to small studies using specimens collected at autopsy or surgery. Reductions in the level of VEGF, a key cytokine involved in endothelial cell survival, reductions in VEGFR, and increased endothelial apoptosis have been observed in the lung tissue of patients with emphysema or COPD compared with those without (8, 43). Peinado and coworkers (44) demonstrated increased endothelial progenitor cells (EPCs) in the pulmonary arteries of patients with COPD, suggesting endothelial injury and repair in early COPD. Reductions in circulating EPCs (45), however, may reflect reduced reparative capacity caused by smoking-related suppression of EPC generation in the bone marrow or increased margination of EPCs with increased repair.
EMPs, by contrast, directly reflect endothelial perturbation unrelated to the bone marrow. Consistent with our findings for COPD, Takahashi and coworkers (29) recently showed that CD31+ EMPs were elevated in COPD compared with control subjects and during COPD exacerbations. The current study expands on their findings and demonstrates both that CD31+ EMPs are elevated in mild COPD and that there is a strong, graded, and specific relationship of CD31+ EMPs to percent emphysema, findings that are consistent with animal models and that suggest that EMPs are not merely a biomarker in COPD but that endothelial apoptosis may be involved in the pathogenesis of emphysema and COPD.
Unlike CD31+ EMPs, CD62E+ EMPs in the current study were elevated predominantly in severe COPD and related to functional measures of pulmonary hyperinflation rather than structural measures of pulmonary emphysema. Elevations in CD62E+ EMPs are suggestive of endothelial activation (17), particularly in response to inflammatory cytokines and specifically in response to tumor necrosis factor-α (17). Elevated tumor necrosis factor-α is well-described in severe COPD (46) and we speculate that in contrast to CD31+ EMPs, CD62E+ EMPs were elevated as a secondary, late response caused by inflammation in severe COPD.
The present study has several strengths including precisely measured EMPs by flow cytometry; relatively large, population-based sample size; and state-of-the-art assessment of the major phenotypes by spirometry, CT scan, gadolinium-enhanced MRI, diffusing capacity, and plethysmography. Still, there are several reasons why the present results may not support the translation of experimental murine findings on endothelial apoptosis to the human diseases of COPD and emphysema.
First, it is not certain that pulmonary circulation was the origin of the EMP elevation as we sampled EMPs in the peripheral venous circulation. Cell-surface or other markers that definitively label EMPs as pulmonary or systemic are, unfortunately, lacking. Recently, the absence of von Willebrand factor was proposed as a marker for alveolar capillary endothelial cells (29), as has the presence of angiotensin-converting enzyme (CD143) (27). Although we did not use these markers, three lines of reasoning suggest that the origin of the excess EMPs is pulmonary. First, CD31+ EMPs were specifically associated with novel measures of PMBF on contrast-enhanced MRI in addition to DlCO, the latter association being previously observed in smokers without COPD (27). Second, patients in this study were specifically selected for COPD and we excluded patients with diseases likely to increase EMPs of systemic origin, such as clinical cardiovascular disease and significant renal disease. Third, the findings were similar in secondary analyses restricted to patients free of hypertension, diabetes, and sleep apnea, which may increase EMPs of systemic origin. Furthermore, restriction to patients free of subclinical cardiovascular disease slightly attenuated the association with COPD status, attenuated that of mild COPD, and strengthened the relationship with percent emphysema.
For obvious reasons, unlike in animal studies, human studies of COPD pathology are limited to observation and cannot include experimentation (i.e., inducing COPD). Therefore, the results may be potentially biased by unmeasured explanatory factors that elevate EMPs and also cause COPD. We adjusted, however, for precise measures of multiple potential confounders and, if anything, the results of the fully adjusted models were of greater significance than the unadjusted results. Furthermore, this potential limitation is offset by the fact that the results apply directly to patients with clinical disease from the general population.
Elevated CD31+ EMP levels in mild COPD is not necessarily synonymous with CD31+ EMP elevations in early COPD, because not all patients with mild COPD progress to severe COPD (47). However, low lung function is the major determinant of accelerated decline in lung function characteristic of COPD (48, 49), and percent emphysema predicts decline in lung function (48). Longitudinal studies are needed to definitively confirm or refute whether elevated CD31+ EMPs contributes to lung function decline and progression of emphysema.
Annexin V on CD31+ EMPs has been used to confirm the apoptotic nature of endothelial cells of origin (50). We did not measure CD31+/annexin V+ EMPs, which limits a definitive statement on the apoptotic nature of the CD31+ EMPs. However, Jimenez and coworkers (17) showed a distinct elevation of CD31+ EMPs in response to the presence of apoptotic agents which, along with our observed findings for CD31+ EMPs that were relatively independent of CD62E+ EMPs, implies that the elevations of CD31+ EMPs discussed herein are suggestive of apoptosis.
Finally, case-control studies can be subject to selection bias; however, the nested design of the current study, in which the sampling probabilities within MESA and EMCAP were known, minimized the possibility of this bias. A small number of participants were recruited from outside the two cohorts and exclusion of these participants yielded consistent results.
In conclusion, CD31+ EMPs were elevated in COPD in a pattern consistent with endothelial apoptosis in mild COPD. CD31+ EMPs were also positively related to percent emphysema and correlated with reductions in pulmonary microvascular perfusion assessed by MRI and diffusing capacity. In contrast, CD62E+ EMPs suggestive of endothelial activation were elevated in severe COPD and with hyperinflation. These cellular markers may implicate endothelial apoptosis in the pathogenesis of COPD and emphysema.
Acknowledgments
Acknowledgment
The authors thank Shuqing Zhao, the other investigators, staff, and participants of the MESA chronic obstructive pulmonary disease study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This manuscript has been reviewed by the MESA investigators for scientific content and consistency of data interpretation with previous MESA publications and significant comments have been incorporated prior to submission for publication.
Footnotes
Supported by National Institutes of Health R01-HL093081, R01-HL077612, R01-HL075476, and N01-HC95159-HC95169, UL1 RR024156.
Author Contributions: M.A.T. and D.S. contributed equally to the writing of the manuscript. D.S. and R.G.B. designed the research protocol, obtained funding, and supervised the study. M.A.P. performed the statistical analysis. D.S. and E.A.H. conducted the quantitative assessment of percent emphysema. J.V.-C. and K.H. performed pulmonary perfusion measures. J.F. performed the endothelial microparticle flow cytometry. C.-Y.L. and D.A.B. designed the magnetic resonance imaging protocol. C.E.V. and M.F.D. assisted in study implementation. All authors provided input into the final manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201209-1697OC on April 19, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hoyert D, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2012;61:1–52. [PubMed] [Google Scholar]
- 2.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease, GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 3.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 4.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JA, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 6.Misonou Y, Asahi M, Yokoe S, Miyoshi E, Taniguchi N. Acrolein produces nitric oxide through the elevation of intracellular calcium levels to induce apoptosis in human umbilical vein endothelial cells: implications for smoke angiopathy. Nitric Oxide. 2006;14:180–187. doi: 10.1016/j.niox.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M. Upregulation of gelatinases A and b, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest. 2000;117:684–694. doi: 10.1378/chest.117.3.684. [DOI] [PubMed] [Google Scholar]
- 8.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med. 2001;163:737–744. doi: 10.1164/ajrccm.163.3.2002117. [DOI] [PubMed] [Google Scholar]
- 9.Santos S, Peinado VI, Ramirez J, Morales-Blanhir J, Bastos R, Roca J, Rodriguez-Roisin R, Barbera JA. Enhanced expression of vascular endothelial growth factor in pulmonary arteries of smokers and patients with moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1250–1256. doi: 10.1164/rccm.200210-1233OC. [DOI] [PubMed] [Google Scholar]
- 10.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, Salvemini D, Voelkel NF, Flores SC. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol. 2003;29:88–97. doi: 10.1165/rcmb.2002-0228OC. [DOI] [PubMed] [Google Scholar]
- 12.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11:491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright JL, Cosio M, Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2008;295:L1–L15. doi: 10.1152/ajplung.90200.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr RG, Mesia-Vela S, Austin JH, Basner RC, Keller BM, Reeves AP, Shimbo D, Stevenson L. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the Emphysema and Cancer Action Project (EMCAP) Study. Am J Respir Crit Care Med. 2007;176:1200–1207. doi: 10.1164/rccm.200707-980OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eickhoff P, Valipour A, Kiss D, Schreder M, Cekici L, Geyer K, Kohansal R, Burghuber OC. Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:1211–1218. doi: 10.1164/rccm.200709-1412OC. [DOI] [PubMed] [Google Scholar]
- 16.Horstman LL, Jy W, Jimenez JJ, Ahn YS. Endothelial microparticles as markers of endothelial dysfunction. Front Biosci. 2004;9:1118–1135. doi: 10.2741/1270. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez JJ, Jy W, Mauro LM, Soderland C, Horstman LL, Ahn YS. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res. 2003;109:175–180. doi: 10.1016/s0049-3848(03)00064-1. [DOI] [PubMed] [Google Scholar]
- 18.Minagar A, Jy W, Jimenez JJ, Sheremata WA, Mauro LM, Mao WW, Horstman LL, Ahn YS. Elevated plasma endothelial microparticles in multiple sclerosis. Neurology. 2001;56:1319–1324. doi: 10.1212/wnl.56.10.1319. [DOI] [PubMed] [Google Scholar]
- 19.Bernal-Mizrachi L, Jy W, Jimenez JJ, Pastor J, Mauro LM, Horstman LL, de Marchena E, Ahn YS. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J. 2003;145:962–970. doi: 10.1016/S0002-8703(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 20.Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, Boulanger CM. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–3388. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 21.Amabile N, Heiss C, Real WM, Minasi P, McGlothlin D, Rame EJ, Grossman W, De Marco T, Yeghiazarians Y. Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:1268–1275. doi: 10.1164/rccm.200710-1458OC. [DOI] [PubMed] [Google Scholar]
- 22.Yun CH, Jung KH, Chu K, Kim SH, Ji KH, Park HK, Kim HC, Lee ST, Lee SK, Roh JK. Increased circulating endothelial microparticles and carotid atherosclerosis in obstructive sleep apnea. J Clin Neurol. 2010;6:89–98. doi: 10.3988/jcn.2010.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, Aime G, Ahn YS. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension. 2003;41:211–217. doi: 10.1161/01.hyp.0000049760.15764.2d. [DOI] [PubMed] [Google Scholar]
- 24.Esposito K, Ciotola M, Giugliano F, Schisano B, Improta L, Improta MR, Beneduce F, Rispoli M, De Sio M, Giugliano D. Endothelial microparticles correlate with erectile dysfunction in diabetic men. Int J Impot Res. 2007;19:161–166. doi: 10.1038/sj.ijir.3901500. [DOI] [PubMed] [Google Scholar]
- 25.Garcia S, Chirinos J, Jimenez J, Del Carpio Munoz F, Canoniero M, Jy W, Horstman L, Ahn Y. Phenotypic assessment of endothelial microparticles in patients with heart failure and after heart transplantation: switch from cell activation to apoptosis. J Heart Lung Transplant. 2005;24:2184–2189. doi: 10.1016/j.healun.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Sabatier F, Darmon P, Hugel B, Combes V, Sanmarco M, Velut JG, Arnoux D, Charpiot P, Freyssinet JM, Oliver C, et al. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes. 2002;51:2840–2845. doi: 10.2337/diabetes.51.9.2840. [DOI] [PubMed] [Google Scholar]
- 27.Gordon C, Gudi K, Krause A, Sackrowitz R, Harvey BG, Strulovici-Barel Y, Mezey JG, Crystal RG. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am J Respir Crit Care Med. 2011;184:224–232. doi: 10.1164/rccm.201012-2061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heiss C, Amabile N, Lee AC, Real WM, Schick SF, Lao D, Wong ML, Jahn S, Angeli FS, Minasi P, et al. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J Am Coll Cardiol. 2008;51:1760–1771. doi: 10.1016/j.jacc.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi T, Kobayashi S, Fujino N, Suzuki T, Ota C, He M, Yamada M, Suzuki S, Yanai M, Kurosawa S, et al. Increased circulating endothelial microparticles in COPD patients: a potential biomarker for COPD exacerbation susceptibility. Thorax. 2012;67:1067–1074. doi: 10.1136/thoraxjnl-2011-201395. [DOI] [PubMed] [Google Scholar]
- 30.Barr RG, Shimbo D, Liu CY, Bluemke DA, Ventetuolo CE, Doyle M. Endothelial microparticiples in early chronic obstructive pulmonary disease (COPD): the MESA COPD Study [abstract] Am J Respir Crit Care Med. 2011;183:A2288. [Google Scholar]
- 31.Thomashow MA, Shimbo D, Parikh M, Hoffman EA, Liu CY, Bluemke DA, Ventetuolo CE, Doyle M, Barr RG. Endothelial microparticles and emphysema on CT scan: the MESA COPD Study [abstract] Am J Respir Crit Care Med. 2012;161:A466. [Google Scholar]
- 32.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 33.Mesia-Vela S, Yeh CC, Austin JH, Dounel M, Powell CA, Reeves A, Santella RM, Stevenson L, Yankelevitz D, Barr RG. Plasma carbonyls do not correlate with lung function or computed tomography measures of lung density in older smokers. Biomarkers. 2008;13:422–434. doi: 10.1080/13547500802002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez J, Jiang R, Johnson WC, MacKenzie BA, Smith LJ, Barr RG. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med. 2010;152:201–210. doi: 10.1059/0003-4819-152-4-201002160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 36.Sieren JP, Hoffman EA, Baumhauer H, Barr RG, Goldin JG, Rennard S. Chicago: Radiological Society of North America; 2011. CT imaging protocol standardization for use in a multicenter study: spiromics. [Google Scholar]
- 37.Hueper KPM, Prince MR, Schoenfeld C, Liu CY, Bluemke DA, Dashnaw SM, Goldstein TA, Hoffman EA, Lima JA, Skrok J, et al. Quantitative and semi-quantitative measures of regional pulmonary parenchymal perfusion by magnetic resonance imaging and their relationships to global lung perfusion and lung diffusing capacity: the MESA COPD Study. Invest Radiol. 2013;48:140–144. doi: 10.1097/RLI.0b013e318281057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 39.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 40.Diamant M, Tushuizen ME, Abid-Hussein MN, Hau CM, Boing AN, Sturk A, Nieuwland R. Simvastatin-induced endothelial cell detachment and microparticle release are prenylation dependent. Thromb Haemost. 2008;100:489–497. [PubMed] [Google Scholar]
- 41.Burkart KM, Ahmed FS, Watson K, Hoffman EA, Burke GL, Barr RG. Association between high density lipoproteins (HDL) cholesterol and CT percent emphysema. The MESA Lung Study. Am J Respir Crit Care Med. 2010;181:A2878. [Google Scholar]
- 42.Simmons DL, Walker C, Power C, Pigott R. Molecular cloning of CD31, a putative intercellular adhesion molecule closely related to carcinoembryonic antigen. J Exp Med. 1990;171:2147–2152. doi: 10.1084/jem.171.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marwick JA, Stevenson CS, Giddings J, MacNee W, Butler K, Rahman I, Kirkham PA. Cigarette smoke disrupts VEGF165-VEGFR-2 receptor signaling complex in rat lungs and patients with COPD: morphological impact of VEGFR-2 inhibition. Am J Physiol Lung Cell Mol Physiol. 2006;290:L897–L908. doi: 10.1152/ajplung.00116.2005. [DOI] [PubMed] [Google Scholar]
- 44.Peinado VI, Ramirez J, Roca J, Rodriguez-Roisin R, Barbera JA. Identification of vascular progenitor cells in pulmonary arteries of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2006;34:257–263. doi: 10.1165/rcmb.2005-0255OC. [DOI] [PubMed] [Google Scholar]
- 45.Palange P, Testa U, Huertas A, Calabro L, Antonucci R, Petrucci E, Pelosi E, Pasquini L, Satta A, Morici G, et al. Circulating haemopoietic and endothelial progenitor cells are decreased in COPD. Eur Respir J. 2006;27:529–541. doi: 10.1183/09031936.06.00120604. [DOI] [PubMed] [Google Scholar]
- 46.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 47.Anthonisen NR. Lessons from the Lung Health Study. Proc Am Thorac Soc. 2004;1:143–145. doi: 10.1513/pats.2306033. [DOI] [PubMed] [Google Scholar]
- 48.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 49.Drummond MB, Hansel NN, Connett JE, Scanlon PD, Tashkin DP, Wise RA. Spirometric predictors of lung function decline and mortality in early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:1301–1306. doi: 10.1164/rccm.201202-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Werner N, Wassmann S, Ahlers P, Kosiol S, Nickenig G. Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:112–116. doi: 10.1161/01.ATV.0000191634.13057.15. [DOI] [PubMed] [Google Scholar]