Abstract
Rationale: High-dose levofloxacin (L) (1,000 mg) was as active as moxifloxacin (M) (400 mg) in an early bactericidal activity trial, suggesting these fluoroquinolones could be used interchangeably. Whether pyrazinamide (Z) contributes sterilizing activity beyond the first 2 months in fluoroquinolone-containing second-line regimens remains unknown.
Objectives: We compared the efficacy of M and high-dose L alone or in combination with ethionamide (Et), amikacin (A), and Z given for 2 or 7 months.
Methods: A pharmacokinetic study was performed to determine the L dose equivalent to 1,000 mg in humans. Treatment started 2 weeks after aerosol infection with Mycobacterium tuberculosis H37Rv. Mice received M or L alone or in combination with 2 months of EtZA followed by 5 months of Et or EtZ.
Measurements and Main Results: After 2 months of treatment, lung colony-forming unit (CFU) counts were similar in mice receiving either fluoroquinolone alone, but, after 4 and 5 months, CFU counts were 2 log10 lower in mice receiving M. Mice receiving 2MEtZA/3MEt and 2LEtZA/3LEt had 1.0 and 2.7 log10 lung CFUs, respectively. When Z was given throughout, both regimens rendered mice culture negative by 5 months, and most mice did not relapse after 7 months of treatment, with fewer relapses observed in the M group after 6 and 7 months of treatment.
Conclusions: In murine tuberculosis, M had superior efficacy compared with L despite lower serum drug exposures and may remain the fluoroquinolone of choice for second-line regimens. Z contributed substantial sterilizing activity beyond 2 months in fluoroquinolone-containing second-line regimens, largely compensating for L’s weaker activity.
Keywords: moxifloxacin, levofloxacin, MDR-TB, pharmacokinetics, mouse model
At a Glance Commentary
Scientific Knowledge on the Subject
Optimizing second-line regimens for treating multidrug-resistant tuberculosis may improve outcomes, enable shortening of the treatment duration, and prevent further resistance. Fluoroquinolones are considered cornerstone agents in second-line regimens. Levofloxacin at 1,000 mg daily is as active as moxifloxacin at 400 mg during the first 7 days of treatment, is less expensive, is more widely available, and may be less likely to prolong the QT interval. However, the two drugs have never been compared head-to-head in combination with other drugs in a clinical trial. Pyrazinamide exerts sterilizing activity only during the first 2 months of treatment with the first-line regimen, but the extent and duration of its contribution to second-line regimens is not well studied.
What This Study Adds to the Field
This study shows that, despite seemingly less favorable pharmacodynamics and comparable activity during the initial 2 months of treatment, moxifloxacin contributes greater bactericidal activity to second-line regimens than levofloxacin during the continuation phase of treatment in a murine tuberculosis model. If these results are reproducible in humans, moxifloxacin may be the fluoroquinolone of choice in the treatment of tuberculosis. The clear benefit of extending pyrazinamide administration during the continuation phase of even the most potent second-line regimens in mice suggests this agent may contribute important sterilizing activity beyond the first 2 months in second-line regimens. These findings warrant further study in clinical trials.
As safe, well-tolerated, orally bioavailable agents with bactericidal activity against Mycobacterium tuberculosis, fluoroquinolones have become cornerstone drugs for treatment of multidrug-resistant tuberculosis (MDR-TB) (1–5). Among currently marketed fluoroquinolones, moxifloxacin (M) is the most potent in vitro (6) and has the best pharmacodynamic profile (7). M also showed the greatest activity against rifampin-tolerant M. tuberculosis in vitro (8), a model of persistent infection. In murine models, M has proven to be the most potent fluoroquinolone (9, 10). Substitution of M at 100 mg/kg for isoniazid (H) in the first-line regimen of isoniazid, rifampin, pyrazinamide (Z), and ethambutol promotes more rapid cure in mice (11, 12). In humans, M showed early bactericidal activity (EBA) similar to that of H or rifampin (13, 14) and promotes similar or more rapid sputum conversion when substituted for H or ethambutol, respectively, although the magnitude of the effect and the endpoint for which the effect was observed differed among trials (15–18).
M has certain limitations that have pushed clinicians to search for alternative fluoroquinolones. M is more expensive, more likely to prolong the QT interval, more susceptible to metabolic induction by rifamycins, and not available in many settings where MDR-TB is prevalent. Levofloxacin (L) is less potent than M in vitro but may be used at higher doses without undue risk of toxicity (19). Indeed, L at 300 mg/kg is more active than L at 200 mg/kg in mice (20). In humans, L at 1,000 mg has EBA over 7 days, which is comparable to that of H or M at 400 mg daily (20). Additionally, L is less expensive, less likely to prolong the QT interval, less susceptible to metabolic induction by rifamycins, and more widely available than M. Veziris and colleagues (21) previously found that M at 100 mg/kg was more active than L at 200 mg/kg when combined with ethionamide (Et), Z, and amikacin (A) in mice, but that study did not include higher doses of L matching exposures obtained with 1,000 mg daily in humans and did not include relapse as the gold standard measure for sterilizing activity. Thus, the first objective of this study was to compare the contribution of high-dose L equivalent to the 1,000 mg human dose to that of the standard M dose in an idealized second-line drug regimen (antituberculosis drug regimens devoid of H and rifampin used for MDR-TB) in a murine model of TB.
Pyrazinamide is the only available drug with clear-cut sterilizing activity that is active against some MDR-TB strains. In combination with rifampin and H in the first-line regimen, its contribution is limited to the first 2 months of treatment because administration beyond that point has been shown to have no added value (22, 23). However, there are limited clinical data regarding the contribution of Z to second-line regimens and no data regarding the duration of therapy necessary for maximal effect. A study in mice indicated that Z continued to contribute sterilizing activity beyond the first 2 months when combined with streptomycin and H (24). The second objective of this study was to determine whether Z contributes sterilizing activity when used in combination with a potent fluoroquinolone and an aminoglycoside and, if so, for what duration of treatment.
To pursue the above-mentioned objectives, we evaluated the contributions of M, high-dose L, and Z to second-line combination regimens in a murine TB model similar to that previously used by Veziris and colleagues (21), except that mice were infected by the aerosol rather than intravenous route and outcomes assessed included relapse after discontinuation of treatment.
Portions of this work were presented as posters and reported in the form of abstracts (25, 26).
Methods
Bacterial Strain
M. tuberculosis H37Rv was passaged in mice and frozen in aliquots at −80°C. After thawing, an aliquot was subcultured in Middlebrook 7H9 broth (Fisher, Pittsburgh, PA) with 10% oleic acid–albumin–dextrose–catalase (Difco, Detroit, MI) and 0.1% Tween 80 (Sigma, St. Louis, MO).
Antimicrobials
M was donated by Bayer (Rolling Meadows, IL). L was donated by Johnson and Johnson (Raritan, NJ). Other drugs were purchased from Sigma. All drugs were prepared in distilled water. Solutions were prepared weekly and stored at 4°C as described previously (27). All drugs were administered by gavage 5 days per week in 0.2 ml by esophageal cannula with the exception of A, which was injected subcutaneously. The dosages of rifampin, H, Z, M, L, Et, and A were 10, 25, 150, 100, 300, 50, and 100 mg/kg of body weight, respectively. Rifampin was given at least 1 hour apart from other drugs to prevent pharmacokinetic antagonism as previously demonstrated (28, 29).
Pharmacokinetics of Levofloxacin
Single-dose pharmacokinetics of L in serum was evaluated in uninfected female BALB/c mice (Charles River, Wilmington, MA) after a 200, 300, or 400 mg/kg oral dose. Groups of three mice were anesthetized with isoflurane and exsanguinated by cardiac puncture at 0.5, 1, 1.5, 2, 4, 6, 8, 10, and 24 hours after dose administration. Serum was harvested and frozen at −80°C before analysis by a validated HPLC method (20). Concentration data were analyzed by standard noncompartmental techniques using WinNonlin (version 5.2.1; Pharsight, Mountain View, CA).
Aerosol Infection
All procedures involving animals were approved by the institutional Animal Care and Use Committee of Johns Hopkins University. Six-week-old female BALB/c mice were used for all experiments. Mice were infected with M. tuberculosis H37Rv as previously described (27) in four aerosol runs of 101 mice. Twelve mice (three mice per run) were killed on the day after infection to determine the number of colony-forming units (CFUs) implanted in the lungs. Another 20 mice (five mice per run) were killed on the day of treatment initiation to determine baseline CFU counts. Ten mice went untreated to confirm the virulence of the infecting strain. The remaining mice were randomly distributed into treatment groups (Table 1).
TABLE 1.
SCHEME OF THE EXPERIEMENT
| Regimens* | Number of mice used to determine lung CFU counts (number of mice held for relapse after treatment completion)† |
||||||
|---|---|---|---|---|---|---|---|
| D−13 | D0 | M2 | M4 | M5 | M6 | M7 | |
| Control groups |
|
|
|
|
|
|
|
| Infected, untreated |
12 |
20 |
+10 monitored for survival |
||||
| 2 mo RHZ + RH |
|
|
5 |
5 |
5 (30) |
5 (30) |
|
| Test groups |
|
|
|
|
|
|
|
| M alone |
|
|
5 |
5 |
5 |
|
|
| L alone |
|
|
5 |
5 |
5 |
|
|
| 2 mo MEtZA + MEt |
|
|
5 |
5 |
5 |
|
|
| 2 mo MEtZA + MEtZ |
|
|
|
5 |
5 (30) |
5 (30) |
5 (30) |
| 2 mo LEtZA + LEt |
|
|
5 |
5 |
5 |
|
|
| 2 mo LEtZA + LEtZ | 5 | 5 (30) | 5 (30) | 5 (30) | |||
Definition of abbreviations: A = amikacin; CFU = colony-forming unit; Et = ethionamide; H = isoniazid; L = levofloxacin; M = moxifloxacin; R = rifampin; Z = pyrazinamide.
Drugs were administered orally, 5 d/wk, at the following doses: R at 10 mg/kg, H at 25 mg/kg, Z at 150 mg/kg, M at 100 mg/kg, L at 300 mg/kg, and Et at 50 mg/kg. A was administered subcutaneously at 100 mg/kg. Mice were killed at the following times: 1 d after infection (D−13); 14 d after infection (D0); and after 2, 4, 5, 6, and 7 mo of treatment (M2, M4, M5, M6, and M7, respectively).
Numbers in parentheses indicate that 30 mice were killed 3 mo after completing the indicated duration of treatment to assess for relapse.
Treatment
The efficacy of M and high-dose L was compared when both drugs were administered alone and in combination with other second-line drugs. Z was administered throughout the treatment or for the first 2 months only to allow us to examine its contribution during the continuation phase. Beginning 13 days after aerosol infection, mice were randomized to receive no treatment (negative controls [group 1]), 2 months of RHZ followed by 4 months of RH (2RHZ/4RH; positive controls [group 2]), M alone (group 3), L alone (group 4), 2MEtZA/3MEt (group 5), 2MEtZA/3MEtZ (group 6), 2LEtZA/5LEt (group 7), or 2LEtZA/5LEtZ (group 8) (Table 1).
Assessment of Treatment Efficacy
The change in lung CFU counts was assessed after 2, 4, and 5 months of treatment by performing quantitative cultures of lung homogenates on oleic acid–albumin–dextrose–catalase enriched 7H11 agar medium (Difco) as previously described (27, 29).
Treatment was continued in three groups for up to 6 months in the case of group 2 2RHZ/4RH or up to 7 months in the case of group 6 2MEtZA/5MEtZ and group 8 2LEtZA/5LEtZ. Cohorts of 30 mice were held without treatment for 3 months after completing 5, 6, or 7 months of treatment before being killed to assess the relapse rate. Relapse was defined by a positive culture upon plating the entire lung homogenate.
Statistical Analysis
CFU counts were log transformed before analysis. Multiple pairwise comparisons of group means at each time point were made by one-way ANOVA with Bonferroni post hoc test. Two-way ANOVA with Bonferroni post hoc test was used to determine whether the effects of M and L treatment were affected by duration of treatment. Group proportions were compared using Fisher’s exact test and adjusting for multiple comparisons using Bonferroni correction. All analyses were performed with Prism v.4.01 (GraphPad, San Diego, CA).
Results
Pharmacokinetics of L
The single-dose serum pharmacokinetics parameters for L are shown in Table 2 alongside prior results for M obtained using similar methods (30). The increases in peak serum concentration (Cmax) and area under the serum concentration–time curve from time zero to infinity (AUC0–∞) were less than proportional with increasing dose. Half-life values after the 300 and 400 mg/kg doses were somewhat longer than those after the 200 mg/kg dose, which may indicate longer absorption or other factors. Because the ratio of AUC to minimum inhibitory concentration (AUC/MIC) is believed to be the pharmacodynamic driver of activity for fluoroquinolones against M. tuberculosis (31) and other pathogens, 300 mg/kg of L in mice was selected to be equivalent to the 1,000 mg dose in humans, which produces a median AUC0–∞ of 137 μg/h/ml (32).
TABLE 2.
PHARMACOKINETIC PARAMETER VALUES FOR LEVOFLOXACIN AND MOXIFLOXACIN IN MICE AFTER SINGLE-DOSE ORAL ADMINISTRATION AND IN PATIENTS WITH TUBERCULOSIS
Treatment Efficacy
All untreated mice became moribund and were killed within 28 days of infection. There were 10 deaths among treated mice, including one mouse each from group 5 2MEtZA/3MEt and group 7 2LEtZA/3LEt and two and six mice each from group 6 2MEtZA/5MEtZ and group 7 2LEtZA/5LEtZ, respectively. All deaths happened within the first week of treatment and appeared to be related to gavage and injection. No death was observed in mice receiving L or M alone or 2RHZ/3RH.
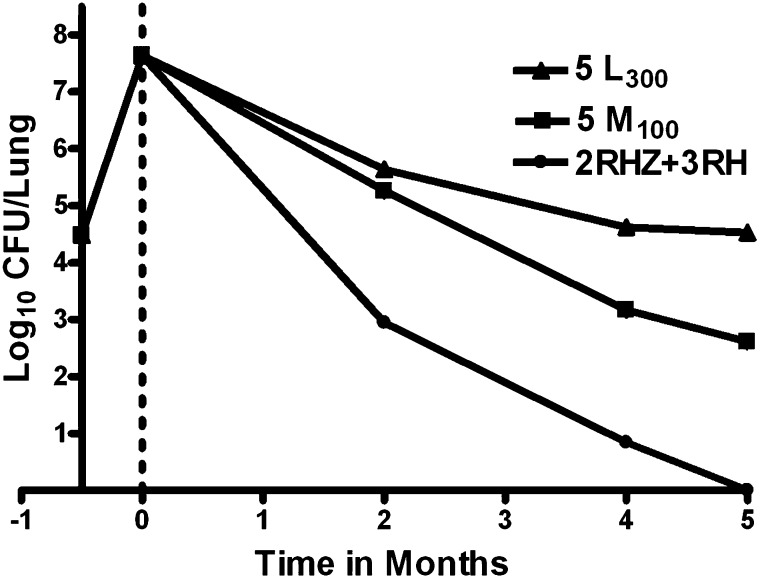
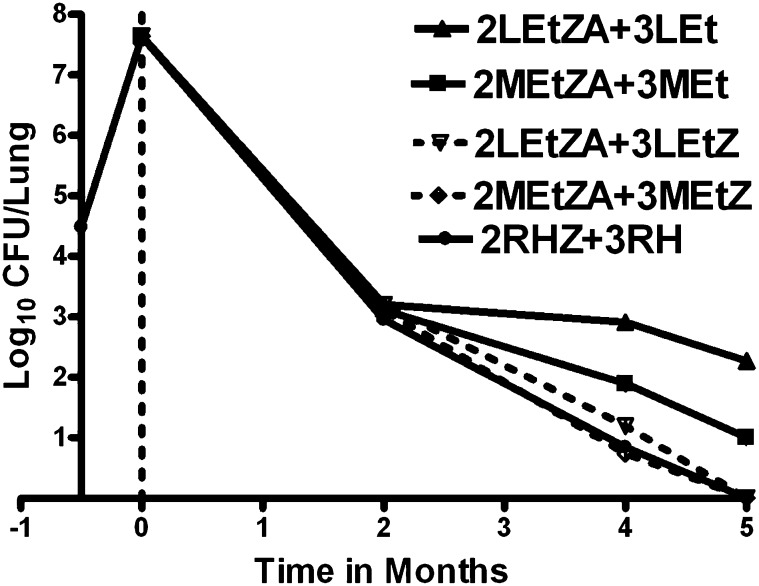
The day after aerosol infection, the mean lung log10 CFU count was 4.48 (SD, 0.11). Two weeks after infection, at initiation of treatment, the mean lung log10 CFU count was 7.64 (SD, 0.33) (Figure 1). After 2 months of treatment, mice receiving L and M alone had 5.64 (SD, 0.20) and 5.26 (SD, 0.08) log10 CFU, respectively, in the lungs. The ≥ 2 log10 reduction in CFU from Day 0 indicates the bactericidal activity of these fluoroquinolones. Mice receiving RHZ had 2.95 (SD, 0.07) log10 CFU in the lungs, compared with 3.20 (SD, 0.07) and 3.11 (SD, 0.18) log10 CFU among mice receiving LEtZA and MEtZA, respectively (Figure 2). Whether the fluoroquinolones were administered alone or in combination with EtZA, there were no significant differences in the activity of regimens containing L or M at 2 months. Furthermore, the activity of the LEtZA and MEtZA combinations was not significantly different from that of RHZ.
Figure 1.
Activity of levofloxacin at 300 mg/kg (5 L300) or moxifloxacin at 100 mg/kg (5 M100), each administered for 5 months, in comparison to a standard multidrug regimen consisting of 2 months of rifampin, isoniazid, and pyrazinamide followed by 3 months of rifampin and isoniazid (2RHZ+3RH).
Figure 2.
Activity of second-line regimens containing high-dose levofloxacin (L) or moxifloxacin (M), with or without pyrazinamide (Z), in combination with ethionamide (Et) and amikacin (A), in comparison to the first-line regimen (2RHZ+3RH).
Mean lung log10 CFU counts among mice receiving L and M alone were 4.62 (SD, 0.12) and 3.17 (SD, 0.33), respectively, after 4 months of treatment (P < 0.001) and 4.52 (SD, 0.13) and 2.61 (SD, 0.45), respectively, after 5 months (P < 0.001) (Figure 1). The difference in lung CFU counts between the two treated groups increased significantly over time (P < 0.001). After 4 and 5 months of treatment, mice receiving 2RHZ/RH were culture negative, with the exception of one mouse with 3 CFUs at 4 months. When Z was omitted from the continuation phase, both fluoroquinolone-containing second-line regimens were inferior to 2RHZ/3RH on the basis of lung CFU counts after 4 and 5 months (P < 0.001) (Figure 2). Among mice receiving 2LEtZA/LEt and 2MEtZA/MEt, mean lung log10 CFU counts were 2.91 (SD, 0.25) and 1.89 (SD, 0.21) after 4 months and 2.27 (SD, 0.23) and 1.00 (SD, 0.15) after 5 months, respectively. These differences were statistically significant at both time points and increased over time (P < 0.001), indicating that, in combination with Et during the continuation phase, M has greater bactericidal activity than L. Taken together with the results with either fluoroquinolone alone, these findings indicate that L at 300 mg/kg and M at 100 mg/kg have similar bactericidal activity over the first 2 months of treatment but that M has significantly greater activity against persisting organisms thereafter. However, even the combination of M and Et was not as active as rifampin and H. Administration of Z in the continuation phase increased activity of both the fluoroquinolone-containing second-line regimens at 4 and 5 months (P < 0.001) and improved the activity of the L-containing regimen such that it was no longer inferior to the M-containing regimen. Both second-line regimens rendered all mice culture negative after 5 months of treatment, an effect indistinguishable from that of 2RHZ/3RH. However, the mean lung CFU count among mice receiving 2LEtZA/2LEtZ was higher than that among mice receiving 2RHZ/2RH (P < 0.001).
Relapse results after treatment for 5, 6, or 7 months are shown in Table 3. Among mice treated with 2RHZ/RH, 23 and 0% relapsed after 5 and 6 months of treatment, respectively. Higher relapse rates were observed in mice treated with 2MEtZA/MEtZ and 2LEtZA/LEtZ after 5 and 6 months (P < 0.0005), although the results after 7 months of treatment with these second-line regimens were comparable to those observed with 5 months of the first-line regimen, further highlighting the sterilizing activity of Z. The M-containing regimen resulted in lower relapse rates than the L-containing regimen after 6 and 7 months of treatment. These differences were not statistically significant when analyzed by time point by Fisher’s exact test (P = 0.16 at 6 and 7 mo). However, when mice treated for 6 or 7 months were combined, the proportion of mice with relapse was significantly lower in the M arm than in the L arm (P = 0.04).
TABLE 3.
RELAPSE* AFTER TREATMENT COMPLETION
| Regimen | % (Proportion) of Mice Relapsing after Treatment for: |
||
|---|---|---|---|
| 5 mo | 6 mo | 7 mo | |
| 2 mo RHZ + RH |
23 (7/30) |
0 (0/30) |
Not done |
| 2 mo MEtZA + MEtZ |
97 (28/29) |
59 (17/29) |
20 (6/30) |
| 2 mo LEtZA + LEtZ | 100 (26/26) | 79 (23/29) | 38 (11/29) |
Definition of abbreviations: A = amikacin; Et = ethionamide; H = isoniazid; L = levofloxacin; M = moxifloxacin; R = rifampin; Z = pyrazinamide.
Relapse was defined by a positive culture upon plating the entire lung homogenate harvested 3 mo after completing the indicated duration of treatment.
Discussion
The two main findings of this study are that high-dose L was less efficacious than M and that Z contributed sterilizing activity well beyond the first 2 months of treatment in the idealized second-line regimen tested in this murine model of TB. A recent EBA study showed that L at 1,000 mg daily has an EBA similar to a 400 mg daily dose of M or gatifloxacin (20), raising hopes that this less expensive and more widely available fluoroquinolone that is less likely to prolong the QT interval and less vulnerable to metabolic induction by rifamycins may substitute for M, the more potent fluoroquinolone in vitro. This expectation is in line with the comparable AUC/MIC and Cmax/MIC values achieved with high-dose L and M (20). However, the present study suggests that matching the two fluoroquinolones on these parameters does not assure similar sterilizing activity. Despite producing AUC/MIC and Cmax/MIC values greater than those observed with M at 100 mg/kg/d (30, 31), L at 300 mg/kg/d was less efficacious than M when used alone or in combination with second-line drugs, especially after the first 2 months of treatment.
The basis for the superior overall efficacy of M over L after comparable initial bactericidal activity in this murine model is not clear. Two in vitro studies have suggested that M may be more active than L against persistent M. tuberculosis. Hu and colleagues found M to be more potent than L than predicted by MIC alone in two in vitro models of bacterial persistence (8). Furthermore, Malik and colleagues found that M may be unique among marketed fluoroquinolones in its ability to kill M. tuberculosis in the presence of the protein synthesis inhibitor chloramphenicol (33, 34). Improved drug delivery to the site of infection may be an additional reason for the greater potency of M. M concentrates inside macrophages and neutrophils to a greater extent than L (35, 36). This may lead to higher exposures for M relative to L in our murine model in which M. tuberculosis resides almost entirely intracellularly. M has been shown to accumulate preferentially in the cellular cuff of rabbit lung granulomas (37). However, the implications of such preferential intracellular penetration for the comparative sterilizing activity in human TB lesions are unclear because the location of persistent M. tuberculosis remains to be determined. Whether our results are relevant to treatment of pre-XDR and XDR-TB with fluoroquinolone resistance is uncertain. Several clinical studies have suggested that M still lends activity when resistance to ofloxacin has been demonstrated (38, 39), likely because achievable M concentrations at the site of infection still exceed the MIC. However, immunomodulatory effects of fluoroquinolones may also play a role (40, 41).
The second main finding that Z contributed sterilizing activity throughout the length of treatment with the second-line regimens is evident when comparing the efficacy of 2MEtZA/3MEt and 2LEtZA/3LEt with that of 2MEtZA/3MEtZ and 2LEtZA/3LEtZ, respectively. In each case, the continuation of Z was the key determinant of lung culture conversion within 5 months. Indeed, the use of Z throughout largely abrogated the difference observed between MEt and LEt continuation phase regimens. The sterilizing activity of Z under acidic conditions in vitro, in mice, and in humans is well documented (22, 42). Indeed, the addition of Z to regimens containing rifampin and H permits shortening the duration of TB treatment from 9 to 6 months and constitutes one basis of modern short-course TB therapy (22, 23). However, continuation of Z beyond the first 2 months of treatment with combinations containing rifampin and H does not further shorten the duration of treatment in mice or in humans, whereas continuation of Z beyond the first 2 months of the second-line regimen studied here shortened the duration of treatment necessary to render lung cultures negative. Whether longer courses of Z in this or other second-line regimens in mice will reduce the treatment duration required to prevent relapse requires further experimentation. The reason(s) Z does not contribute sterilizing activity to the first-line regimen when administered beyond the first 2 months is unclear but may be related to overlapping sterilizing effects of Z and rifamycins against some persistent bacilli and/or possibly antagonistic effects of H (12, 24, 29, 43). Alongside recent clinical observations, our results support current WHO recommendations to include Z alongside later-generation fluoroquinolones throughout the first 8 months of treatment with second-line regimens and highlight the important role that reliable Z susceptibility testing could play in determining the optimum duration of treatment (44, 45). If the clinical situation mirrors our results in mice, susceptibility of the infecting isolate to Z may indicate the potential for short-course therapy of 9 months or less if Z is continued. On the other hand, continuing Z in the face of resistance may not provide any treatment-shortening benefit while increasing the risk of intolerance and/or toxicity, as observed with fluoroquinolone-Z combinations used to treat latent TB infection among MDR-TB contacts (46–49). Given the favorable interactions between Z and new TB drugs in development and rising concerns over Z resistance among MDR-TB isolates, improved susceptibility testing methods for Z, including genotypic resistance testing, will continue to require attention in the future (50–53).
Our study has several limitations. It is based on an experimental murine model that does not recapitulate all aspects of human TB. The contribution of Z and the relative potency of M and L may be inordinately influenced by the predominantly intracellular nature of infection in mice. To date, however, murine models have provided an accurate representation of the contribution of Z to existing TB regimens (24) and the comparative EBA of H, M, and L (3, 9, 20). However, results in this model have not always been confirmed in clinical trials using sputum culture conversion as a surrogate endpoint (17, 54) for cure. Second, it may be argued that the more rapid clearance of the fluoroquinolones in mice than in humans results in different serum pharmacokinetic profiles that make extrapolation of the results to humans difficult. However, we evaluated drug doses that matched the median AUC/MIC for L and biased the achieved AUC/MIC and Cmax/MIC values (which correlate best with fluoroquinolone activity in experimental models) in favor of L in this comparative study (31).
In conclusion, our results indicate that M has greater bactericidal activity and may contribute greater sterilizing activity than high-dose L during the continuation phase of treatment in this murine model of TB. If this finding is confirmed in longer relapse-based mouse experiments evaluating fluoroquinolone-containing second-line regimens that lack other sterilizing agents such as Z or in clinical trials, M may be the preferred fluoroquinolone for second-line TB treatment. A trial comparing the efficacy of these two potent fluoroquinolones in the context of MDR-TB treatment is warranted. Inclusion of Z, when it is active against the infecting isolate, is expected to add important sterilizing activity and may compensate for differences in the fluoroquinolone component.
Footnotes
This work was supported by National Institutes of Health grants N01-AI40007 and K08-AI58993; by a Ramalingaswami fellowship grant from the Department of Biotechnology, Government of India; and by MLP grants GAP-1160 and MLP-6010 of CSIR, Government of India, through the Indian Institute of Integrative Medicine (Z.A.).
Author Contributions: Conception and design of the study: Z.A., J.H.G., and E.L.N. Acquisition, analysis, and interpretation of the data: Z.A., S.T., A.M., C.A.P., J.H.G., and E.L.N. Drafting of the manuscript: Z.A., J.H.G., and E.L.N.
Originally Published in Press as DOI: 10.1164/rccm.201212-2328OC on April 17, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Yew WW, Chan CK, Chau CH, Tam CM, Leung CC, Wong PC, Lee J. Outcomes of patients with multidrug-resistant pulmonary tuberculosis treated with ofloxacin/levofloxacin-containing regimens. Chest. 2000;117:744–751. doi: 10.1378/chest.117.3.744. [DOI] [PubMed] [Google Scholar]
- 2.Chan ED, Laurel V, Strand MJ, Chan JF, Huynh ML, Goble M, Iseman MD. Treatment and outcome analysis of 205 patients with multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2004;169:1103–1109. doi: 10.1164/rccm.200308-1159OC. [DOI] [PubMed] [Google Scholar]
- 3.Tahaoglu K, Torun T, Sevim T, Atac G, Kir A, Karasulu L, Ozmen I, Kapakli N. The treatment of multidrug-resistant tuberculosis in Turkey. N Engl J Med. 2001;345:170–174. doi: 10.1056/NEJM200107193450303. [DOI] [PubMed] [Google Scholar]
- 4.Yew WW, Chan CK, Leung CC, Chau CH, Tam CM, Wong PC, Lee J. Comparative roles of levofloxacin and ofloxacin in the treatment of multidrug-resistant tuberculosis: preliminary results of a retrospective study from Hong Kong. Chest. 2003;124:1476–1481. doi: 10.1378/chest.124.4.1476. [DOI] [PubMed] [Google Scholar]
- 5.Rieder HL. Fourth-generation fluoroquinolones in tuberculosis. Lancet. 2009;373:1148–1149. doi: 10.1016/S0140-6736(09)60559-6. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez JC, Ruiz M, Lopez M, Royo G. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis. Int J Antimicrob Agents. 2002;20:464–467. doi: 10.1016/s0924-8579(02)00239-x. [DOI] [PubMed] [Google Scholar]
- 7.Lubasch A, Keller I, Borner K, Koeppe P, Lode H. Comparative pharmacokinetics of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, trovafloxacin, and moxifloxacin after single oral administration in healthy volunteers. Antimicrob Agents Chemother. 2000;44:2600–2603. doi: 10.1128/aac.44.10.2600-2603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Y, Coates AR, Mitchison DA. Sterilizing activities of fluoroquinolones against rifampin-tolerant populations of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2003;47:653–657. doi: 10.1128/AAC.47.2.653-657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji B, Lounis N, Maslo C, Truffot-Pernot C, Bonnafous P, Grosset J. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:2066–2069. doi: 10.1128/aac.42.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nuermberger E, Grosset J. Pharmacokinetic and pharmacodynamic issues in the treatment of mycobacterial infections. Eur J Clin Microbiol Infect Dis. 2004;23:243–255. doi: 10.1007/s10096-004-1109-5. [DOI] [PubMed] [Google Scholar]
- 11.Nuermberger EL, Yoshimatsu T, Tyagi S, O'Brien RJ, Vernon AN, Chaisson RE, Bishai WR, Grosset JH. Moxifloxacin containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am J Respir Crit Care Med. 2004;169:421–426. doi: 10.1164/rccm.200310-1380OC. [DOI] [PubMed] [Google Scholar]
- 12.Nuermberger EL, Yoshimatsu T, Tyagi S, Williams K, Rosenthal I, O'Brien RJ, Vernon AA, Chaisson RE, Bishai WR, Grosset JH. Moxifloxacin containing regimens of reduced duration produce a stable cure in murine tuberculosis. Am J Respir Crit Care Med. 2004;170:1131–1134. doi: 10.1164/rccm.200407-885OC. [DOI] [PubMed] [Google Scholar]
- 13.Pletz MW, De Roux A, Roth A, Neumann KH, Mauch H, Lode H. Early bactericidal activity of moxifloxacin in treatment of pulmonary tuberculosis: a prospective, randomized study. Antimicrob Agents Chemother. 2004;48:780–782. doi: 10.1128/AAC.48.3.780-782.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gosling RD, Uiso LO, Sam NE, Bongard E, Kanduma EG, Nyindo M, Morris RW, Gillespie SH. The bactericidal activity of moxifloxacin in patients with pulmonary tuberculosis. Am J Respir Crit Care Med. 2003;168:1342–1345. doi: 10.1164/rccm.200305-682OC. [DOI] [PubMed] [Google Scholar]
- 15.Burman WJ, Goldberg S, Johnson JL, Muzanye G, Engle M, Mosher AW, Choudhri S, Daley CL, Munsiff SS, Zhao Z, et al. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am J Respir Crit Care Med. 2006;174:331–338. doi: 10.1164/rccm.200603-360OC. [DOI] [PubMed] [Google Scholar]
- 16.Conde MB, Efron A, Loredo C, De Souza GR, Graça NP, Cezar MC, Ram M, Chaudhary MA, Bishai WR, Kritski AL, et al. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet. 2009;373:1183–1189. doi: 10.1016/S0140-6736(09)60333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorman SE, Johnson JL, Goldberg S, Muzanye G, Padayatchi N, Bozeman L, Heilig CM, Bernardo J, Choudhri S, Grosset JH, et al. Tuberculosis Trials Consortium. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am J Respir Crit Care Med. 2009;180:273–280. doi: 10.1164/rccm.200901-0078OC. [DOI] [PubMed] [Google Scholar]
- 18.Rustomjee R, Lienhardt C, Kanyok T, Davies GR, Levin J, Mthiyane T, Reddy C, Sturm AW, Sirgel FA, Allen J, et al. Gatifloxacin for TB (OFLOTUB) study team. A phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2008;12:128–138. [PubMed] [Google Scholar]
- 19.Johnson JL, Hadad DJ, Boom WH, Daley CL, Peloquin CA, Eisenach KD, Jankus DD, Debanne SM, Charlebois ED, Maciel E, et al. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2006;10:605–612. [PubMed] [Google Scholar]
- 20.Klemens SP, Sharpe CA, Rogge MC, Cynamon MH. Activity of levofloxacin in a murine model of tuberculosis. Antimicrob Agents Chemother. 1994;38:1476–1479. doi: 10.1128/aac.38.7.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veziris N, Truffot-Pernot C, Aubry A, Jarlier V, Lounis N. Fluoroquinolone-containing third-line regimen against Mycobacterium tuberculosis in vivo. Antimicrob Agents Chemother. 2003;47:3117–3122. doi: 10.1128/AAC.47.10.3117-3122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Mitchison D. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis. 2003;7:6–21. [PubMed] [Google Scholar]
- 23.East African/British Medical Research Councils. Controlled clinical trial of four short-course (6-month) regimens of chemotherapy for treatment of pulmonary tuberculosis: second report. Lancet. 1973;1:1331–1339. [PubMed] [Google Scholar]
- 24.Grosset J. The sterilizing value of rifampicin and pyrazinamide in experimental short-course chemotherapy. Bull Int Union Tuberc. 1978;53:5–12. [PubMed] [Google Scholar]
- 25.Nuermberger EL, Ahmad Z, Tyagi S, Minkowski A, Grosset J. Contribution of potent fluoroquinolones and pyrazinamide to second-line regimens in murine TB. Int J Tuberc Lung Dis. 2010;14:S319. [Google Scholar]
- 26.Ahmad Z, Tyagi S, Nuermberger EL, Grosset JH. Continuation of pyrazinamide beyond the first 2 months improves the efficacy of second-line regimens in the mouse model of tuberculosis. Am J Respir Crit Care Med. 2010;181:A5451. [Google Scholar]
- 27.Ahmad Z, Nuermberger EL, Tasneen R, Pinn ML, Williams KN, Peloquin CA, Grosset JH, Karakousis PC. Comparison of the ‘Denver regimen’ against acute tuberculosis in the mouse and guinea pig. J Antimicrob Chemother. 2010;65:729–734. doi: 10.1093/jac/dkq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grosset J, Truffot-Pernot C, Lacroix C, Ji B. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob Agents Chemother. 1992;36:548–551. doi: 10.1128/aac.36.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almeida D, Nuermberger E, Tasneen R, Rosenthal I, Tyagi S, Williams K, Peloquin C, Grosset J. Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis. Antimicrob Agents Chemother. 2009;53:4178–4184. doi: 10.1128/AAC.00830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenthal IM, Williams K, Tyagi S, Vernon AA, Peloquin CA, Bishai WR, Grosset JH, Nuermberger EL. Weekly moxifloxacin and rifapentine is more active than the denver regimen in murine tuberculosis. Am J Respir Crit Care Med. 2005;172:1457–1462. doi: 10.1164/rccm.200507-1072OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shandil RK, Jayaram R, Kaur P, Gaonkar S, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharath S, Balasubramanian V. Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob Agents Chemother. 2007;51:576–582. doi: 10.1128/AAC.00414-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peloquin CA, Hadad DJ, Molino LP, Palaci M, Boom WH, Dietze R, Johnson JL. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob Agents Chemother. 2008;52:852–857. doi: 10.1128/AAC.01036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malik M, Drlica K. Moxifloxacin lethality against Mycobacterium tuberculosis in the presence and absence of chloramphenicol. Antimicrob Agents Chemother. 2006;50:2842–2844. doi: 10.1128/AAC.00250-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drlica K, Malik M, Kerns RJ, Zhao X. Quinolone-mediated bacterial death. Antimicrob Agents Chemother. 2008;52:385–392. doi: 10.1128/AAC.01617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garraffo R, Lavrut T, Durant J, Héripret L, Sérini MA, Dunais B, Dellamonica P. In vivo comparative pharmacokinetics and pharmacodynamics of moxifloxacin and levofloxacin in human neutrophils. Clin Drug Investig. 2005;25:643–650. doi: 10.2165/00044011-200525100-00003. [DOI] [PubMed] [Google Scholar]
- 36.Michot JM, Seral C, Van Bambeke F, Mingeot-Leclercq MP, Tulkens PM. Influence of efflux transporters on the accumulation and efflux of four quinolones (ciprofloxacin, levofloxacin, garenoxacin, and moxifloxacin) in J774 macrophages. Antimicrob Agents Chemother. 2005;49:2429–2437. doi: 10.1128/AAC.49.6.2429-2437.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prideaux B, Dartois V, Staab D, Weiner DM, Goh A, Via LE, Barry CE, III, Stoeckli M. High-sensitivity MALDI-MRM-MS imaging of moxifloxacin distribution in tuberculosis-infected rabbit lungs and granulomatous lesions. Anal Chem. 2011;83:2112–2118. doi: 10.1021/ac1029049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobson KR, Tierney DB, Jeon CY, Mitnick CD, Murray MB. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis. 2010;51:6–14. doi: 10.1086/653115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dheda K, Shean K, Zumla A, Badri M, Streicher EM, Page-Shipp L, Willcox P, John MA, Reubenson G, Govindasamy D, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet. 2010;375:1798–1807. doi: 10.1016/S0140-6736(10)60492-8. [DOI] [PubMed] [Google Scholar]
- 40.Dalhoff A. Immunomodulatory activities of fluoroquinolones. Infection. 2005;33:55–70. doi: 10.1007/s15010-005-8209-8. [DOI] [PubMed] [Google Scholar]
- 41.Dalhoff A, Shalit I.Immunomodulatory effects of quinolones Lancet Infect Dis 20033359–371.(Review) [DOI] [PubMed] [Google Scholar]
- 42.Chang KC, Leung CC, Yew WW, Leung EC, Leung WM, Tam CM, Zhang Y. Pyrazinamide may improve fluoroquinolone-based treatment of multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2012;56:5465–5475. doi: 10.1128/AAC.01300-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grosset J, Truffot C, Fermanian J, Lecoeur H. [Sterilizing activity of the main drugs on the mouse experimental tuberculosis. Pathol Biol (Paris) 1982;30:444–448. [PubMed] [Google Scholar]
- 44.Chang KC, Yew WW, Zhang Y. Pyrazinamide susceptibility testing in Mycobacterium tuberculosis: a systematic review with meta-analyses. Antimicrob Agents Chemother. 2011;55:4499–4505. doi: 10.1128/AAC.00630-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health OrganizationGuidelines for the programmatic management of drug resistant tuberculosis, 2011 update [accessed 2012 Dec 28]. Available from: http://whqlibdoc.who.int/publications/2011/9789241501583_eng.pdf [PubMed]
- 46.Horn DL, Hewlett D, Jr, Alfalla C, Peterson S, Opal SM. Limited tolerance of ofloxacin and pyrazinamide prophylaxis against tuberculosis. N Engl J Med. 1994;330:1241. doi: 10.1056/nejm199404283301718. [DOI] [PubMed] [Google Scholar]
- 47.Lou HX, Shullo MA, McKaveney TP. Limited tolerability of levofloxacin and pyrazinamide for multidrug-resistant tuberculosis prophylaxis in a solid organ transplant population. Pharmacotherapy. 2002;22:701–704. doi: 10.1592/phco.22.9.701.34065. [DOI] [PubMed] [Google Scholar]
- 48.Papastavros T, Dolovich LR, Holbrook A, Whitehead L, Loeb M. Adverse events associated with pyrazinamide and levofloxacin in the treatment of latent multidrug-resistant tuberculosis. CMAJ. 2002;167:131–136. [PMC free article] [PubMed] [Google Scholar]
- 49.Ridzon R, Meador J, Maxwell R, Higgins K, Weismuller P, Onorato IM. Asymptomatic hepatitis in persons who received alternative preventive therapy with pyrazinamide and ofloxacin. Clin Infect Dis. 1997;24:1264–1265. doi: 10.1093/clinids/24.6.1264. [DOI] [PubMed] [Google Scholar]
- 50.Tasneen R, Li SY, Peloquin CA, Taylor D, Williams KN, Andries K, Mdluli KE, Nuermberger EL. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother. 2011;55:5485–5492. doi: 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nuermberger E. Using animal models to develop new treatments for tuberculosis. Semin Respir Crit Care Med. 2008;29:542–551. doi: 10.1055/s-0028-1085705. [DOI] [PubMed] [Google Scholar]
- 52.Wallis RS, Jakubiec W, Kumar V, Bedarida G, Silvia A, Paige D, Zhu T, Mitton-Fry M, Ladutko L, Campbell S, et al. Biomarker-assisted dose selection for safety and efficacy in early development of PNU-100480 for tuberculosis. Antimicrob Agents Chemother. 2011;55:567–574. doi: 10.1128/AAC.01179-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stoffels K, Mathys V, Fauville-Dufaux M, Wintjens R, Bifani P. Systematic analysis of pyrazinamide-resistant spontaneous mutants and clinical isolates of mycobacterium tuberculosis. Antimicrob Agents Chemother. 2012;56:5186–5193. doi: 10.1128/AAC.05385-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dorman SE, Goldberg S, Stout JE, Muzanyi G, Johnson JL, Weiner M, Bozeman L, Heilig CM, Feng PJ, Moro R, et al. Tuberculosis Trials Consortium. Substitution of rifapentine for rifampin during intensive phase treatment of pulmonary tuberculosis: study 29 of the tuberculosis trials consortium. J Infect Dis. 2012;206:1030–1040. doi: 10.1093/infdis/jis461. [DOI] [PubMed] [Google Scholar]