Abstract
Rationale: Innate immune responses marked by increases in tumor necrosis factor (TNF)-α have been associated with asthma but whether such alterations are evident before symptoms is not yet clear.
Objectives: To determine if prevalence of childhood asthma or asthma-related traits is predicted by perinatal innate immune status and if maternal factors related to pregnancy influence asthma prevalence and innate immune status.
Methods: In the Tucson Infant Immune Study (a nonselected birth cohort), presence of eczema and wheezing in the child's first year and physician-diagnosed asthma through age 9 and asthma in the parents was obtained from parent-completed questionnaires. TNF-α, IL-6, IL-10, and IL-12 were measured in supernatants of LPS-stimulated peripheral blood mononuclear cells at birth and 3 months as was TNF-α in plasma. TNF-α single nucleotide polymorphisms were genotyped by Sequenom. Percent predicted FEV1/FVC was measured at age 9. Maternal weight gain during pregnancy and prepregnancy weight were ascertained from medical records.
Measurements and Main Results: Infants with persistently elevated LPS-induced TNF-α at birth and 3 months were at increased risk for childhood asthma (odds ratio [OR], 4.1; confidence interval [CI], 1.9–8.8; n = 233; P = 0.0003) and had decreased FEV1/FVC ratios at age 9. Children with mothers in the top tertile for pregnancy weight gain had increased risk for asthma (OR, 3.4; CI, 1.7–6.9; n = 225; P = 0.001) and persistently elevated TNF-α in early life (OR, 2.9; CI, 1.4–8.2; n = 195; P = 0.013). These relations were independent of maternal asthma and rhinitis.
Conclusions: Persistently elevated LPS-induced TNF-α production early in life acts as a predictive biomarker for childhood asthma, and excess pregnancy weight gain in the mother seems to contribute to both.
Keywords: biomarker, innate cytokines, asthma etiology
At a Glance Commentary
Scientific Knowledge on the Subject
Although many immune-related alterations have been shown to be associated with childhood asthma once it is established, there are currently no known biomarkers that predict the development of childhood asthma. Maternal asthma is a known risk factor for asthma but there are few if any pregnancy-related risk factors for childhood asthma yet identified.
What This Study Adds to the Field
Evidence is provided that early-life production of LPS-stimulated tumor necrosis factor-α from blood-derived immune cells can serve as a biomarker of risk for childhood asthma and thus very early in life identify infants at risk for asthma. Also, that excess maternal weight gain during pregnancy is associated with increased early-life tumor necrosis factor-α and asthma in the child.
Asthma is the most common chronic disease of childhood and has increased in prevalence over the last several decades in much of the industrialized world (1, 2). Pathways leading to asthma in childhood seem to be initiated early in infancy, possibly even in utero (3, 4), but searches for predictive biomarkers of asthma development have not been successful (5). Although myriad studies support immune-related alterations after asthma is established, whether immune differences precede asthma is much less clear. More than one line of evidence suggests that asthma and allergy may develop by different pathways. Burrows and coworkers (6) found that parental IgE levels (although well established to provide risk for IgE in the child) did not provide risk for asthma in the child, a finding confirmed in a birth cohort, the Children’s Respiratory Study (7). From the Tucson Infant Immune Study (IIS), early-life type-2 cytokine patterns were found to differ in nature and in temporal pattern in relation to childhood asthma and IgE levels (8). Several genome-wide association studies implicate different patterns of genetic variation for asthma and IgE regulation (9) and point toward the possibility that innate immune function may have importance in asthma. In accord with the main goal of the Tucson IIS, we continue to address early-life immune function as related to asthma development. Here we focus on innate immune responses to a common environmentally encountered stimulus, LPS. Given that immune function is undergoing maturation prenatally and postnatally even while responding to myriad environmental exposures, we sought not only to assess variation at individual time points but also to capture response over time from birth to 3 months and determine if such variation provided risk for asthma in the child.
Another objective of the IIS is to determine whether early-life immune function is influenced by in utero influences, and thus mothers enrolled during pregnancy were sampled and queried regarding respiratory health. Maternal asthma is a well-established risk factor for asthma in the child and at least in many studies overshadows risk associated with paternal asthma (10), suggesting the in utero environment associated with maternal asthma may directly influence airway and/or immune development. Nonetheless, although prevalence is lower, most asthma cases develop in children without a parental history. Maternal factors in addition to maternal asthma that might affect asthma risk in the child are maternal weight before pregnancy and the amount of maternal weight gain during pregnancy, which have been increasing, like childhood asthma, in recent decades (11). Variation in prepregnant weight and maternal weight gain during pregnancy clearly influence the fetus (e.g., affecting birth weight [12]), but effects related to immune and subsequent respiratory health status in the child are only beginning to be assessed (13). Recently, several studies have linked such maternal factors to asthma risk (14–18). In addition to seeking evidence for innate immune alterations very early in life that might serve as biomarkers of subsequent asthma diagnoses, this study used the Tucson IIS population to assess whether maternal in utero influences might contribute to early innate immune function and/or asthma.
Some of the results of this study have been previously reported in the form of abstracts (19, 20).
Methods
Subjects
Pregnant women (25–42 wk gestation) were enrolled without selection in IIS between 1996 and 2004 together with their unborn child and the child’s father. Women were eligible if they planned to seek care for their child at 1 of 14 collaborating Tucson pediatricians and if they spoke English, had a telephone, and did not plan to leave Tucson. Su and coworkers (21) provide further details. Maternal and paternal asthma and/or rhinitis at enrollment were defined as physician-diagnosed asthma and/or physician-diagnosed allergic rhinitis with symptoms during the past year. Maternal prepregnant body mass index (BMI) was calculated from medical record information obtained by the physician at the first pregnancy office visit (n = 261). Pregnancy total weight gain was calculated as the (maternal weight at the last office visit before delivery minus prepregnant weight) times the ratio of gestational age at birth/gestational age at the last office visit (n = 258). (The ratio factor was included as an estimate of weight gained from the last office visit to the day of birth but its effect on the weight gain values was small and did not add a significant influence on the relations of weight gain assessed in this study.)
Occurrences of Year 1 wheezing and/or physician-diagnosed eczema in the child were obtained from parental responses on questionnaires. Childhood asthma was defined as physician-diagnosed asthma with active symptoms or asthma medications prescribed in the past year reported at least once on questionnaires at ages 2, 3, 5, or 9. Diagnosing physicians did not have knowledge of study data. Spirometry was measured as described in the online supplement. Allergen skin test reactivity was assessed as any positive response to 17 local aeroallergen skin tests applied at age 5 as previously described (22). The University of Arizona Institutional Review Board approved the study and informed consent was obtained for all participants.
Sample Processing, Stimulation, Assessment of Cytokine Production, and Genotyping
Heparinized blood samples were collected at birth; 3 months; and 1, 2, 3, and 5 years of age. Cord and peripheral blood mononuclear cells (CBMCs and PBMCs) were isolated as previously described (23). See the online supplement for methods for culture, LPS stimulation, assays of cytokines and leptin, and tumor necrosis factor (TNF)-α single nucleotide polymorphism (SNP) genotyping.
Data Management and Statistical Analysis
Data availability and integrity are maintained in a data warehouse created by Epi-Logs, a system developed at the Arizona Respiratory Center. Statistical analyses were performed by SPSS version 19.0 (IBM Corp, Amonk, NY). LPS induced detectable levels of TNF-α, IL-6, IL-10, and IL-12 in 99%, 100%, 99%, and 29% of CBMC supernatants and 99%, 100%, 97%, and 72% of 3-month PBMC supernatants, respectively. To capture persistence of cytokine production in early life, variables for TNF-α, IL-6, and IL-10 production were created by dichotomizing birth and 3-month values at the median and pairing to yield four groups of temporally matched (cord/3 mo) values: low/low, low/high, high/low, and high/high. Preliminary analysis demonstrated the first three groups shared a low risk for asthma (Table 1) and these groups were combined for comparison with the high/high group. Another variable of larger size was created by including subjects with one low and one missing data point in the “at least one low” group (n = 205). Children with one high and one missing value formed a separate “high/missing” group (n = 88). The third group, high/high (n = 38) was the same as above. LPS-induced IL-12 values from CBMCs (with 72% of values undetectable) were divided as low and high based on detectability and 3-month values were divided at the median. For comparisons of cytokine relations with childhood asthma, a Bonferroni correction for multiple comparisons was used: 0.05/4 = 0.012.
TABLE 1.
RELATION OF CYTOKINE PRODUCTION IN EARLY LIFE TO CHILDHOOD ASTHMA IN TUCSON INFANT IMMUNE STUDY SUBJECTS
Path analysis was conducted in Stata version 10.1 (StataCorp LP, College Station, TX) using the pathreg command (see path analysis [24]). For this analysis, TNF-α (high/high vs. other) was modeled as a function of maternal weight gain (upper tertile vs. two lower tertiles), and childhood asthma was modeled as a function of both maternal weight gain and TNF-α. Resulting regression coefficients (βs) are reported and the contribution of the indirect pathway (maternal weight gain→TNF-α→asthma) calculated as the product of βs along that pathway.
Results
Early-life temporal patterns of LPS-stimulated production differed among innate cytokines. From birth to 3 months of age, log mean TNF-α production did not change, whereas production of IL-6 and IL-10 both decreased and the proportion of detectable values of IL-12 increased (see Table E1 in the online supplement). Demographic characteristics of the subjects who did not have cytokine data from the cord or 3-month LPS-stimulated samples were compared with those with data (see Table E2) and found not to differ.
Prevalence of physician-diagnosed childhood asthma in our nonselected IIS birth cohort followed through age 9 was 17.9% (77 of 430). Table E3 provides a comparison of features in IIS children with and without asthma. A positive skin test at age 5 to any allergen occurred in 44.0% (144 of 327).
Relation of Early-Life Cytokine Production to Childhood Asthma and to Lung Function
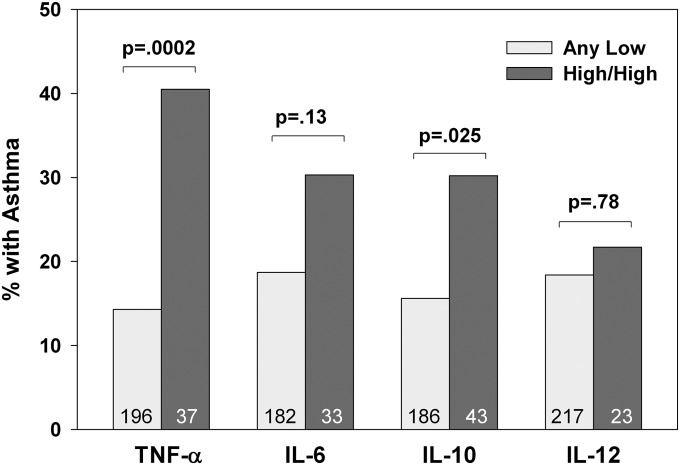
LPS-stimulated TNF-α production in early life, assessed by combining the two time points of birth and 3 months (see Methods), provided a discriminating signal for childhood asthma with subjects having consistently high values at greatest risk (Table 1). Post hoc analysis of this relation on combining the three groups with any low value and comparing with the high/high group yielded an odds ratio (OR) of 3.5 (confidence interval [CI], 1.5–8.3; n = 129; P = 0.004). Including subjects with one low and one missing data point in the “at least one low” group, the OR was 4.1 (CI, 1.9–8.8; n = 233; P = 0.0003). This relation is portrayed in Figure 1 together with the other LPS-stimulated cytokines similarly assessed for early-life persistent production. Although production of the other cytokines correlated significantly with TNF-α (see Table E4), none of the other cytokines related to childhood asthma (Table 1, subjects limited to no missing data and Figure 1, at least one low and high/high). Placing the four cytokines in a logistic regression model yielded an OR for the association of asthma with persistently high TNF-α of 4.6 (CI, 1.7–12.0; n = 163; P = 0.002) and none of the other cytokines contributed significantly.
Figure 1.
Interrelations of LPS-induced cytokine production in early-life and childhood asthma. See Methods for definitions of grouped variables of cytokines from LPS-stimulated blood mononuclear cells showing persistence of production from birth to 3 months. The “any low” group (subjects with low/low, low/missing, and missing/low values) is compared with the “high/high” group (subjects with high values at both cord and 3 mo). The “high/missing” group is excluded from this analysis. Group sizes are given in the bars. Childhood asthma is defined as physician-diagnosed asthma between ages 2 and 9. The P values are based on chi-square. TNF = tumor necrosis factor.
We examined the relation of TNF-α in the context of other known early-life risk factors for asthma. As anticipated, eczema and wheeze in the first year of life and a history of maternal asthma and rhinitis were found by marginal analysis to show significant relations to childhood asthma (Table 2). Assessing the cord and 3-month TNF-α production together with these variables revealed that persistently high TNF-α was a significant independent risk factor for asthma. The analysis in Table 2 also demonstrates that the TNF-α “high/missing” group does not differ from the “at least one low” (reference) group and these two groups are combined for comparison with the “high/high” group in analyses of Tables 3–5. The same relations are revealed if active asthma at age 5 and/or 9 is the outcome rather than our more age-inclusive “childhood asthma” variable (see Table E5). Neither gestational age at birth nor paternal asthma and rhinitis was related to early-life TNF-α production or childhood asthma (data not shown).
TABLE 2.
ODDS OF CHILDHOOD ASTHMA BY EARLY-LIFE TNF-α PRODUCTION BEFORE AND AFTER ADJUSTMENT FOR FACTORS IN EARLY LIFE KNOWN TO RELATE TO ASTHMA
| Unadjusted OR (95% CI)* | P Value | Adjusted OR (95% CI)† | P Value | |
|---|---|---|---|---|
| Cord/3-mo TNF-α | ||||
| Any low |
Reference |
|
Reference |
|
| High/msg |
1.1 (0.52–2.3) |
0.82 |
1.0 (0.45–2.4) |
0.95 |
| High/high |
4.1 (1.9–8.8) |
0.0003 |
3.4 (1.4–8.1) |
0.006 |
| Eczema, Year 1 | ||||
| No |
Reference |
|
Reference |
|
| Yes |
3.2 (1.7–6.3) |
0.0005 |
2.9 (1.4–5.8) |
0.003 |
| Wheeze, Year 1 | ||||
| No |
Reference |
|
Reference |
|
| Yes |
2.9 (1.5–5.4) |
0.001 |
2.6 (1.3–5.1) |
0.005 |
| Maternal asthma and/or rhinitis | ||||
| No |
Reference |
|
Reference |
|
| Yes | 2.5 (1.4–4.8) | 0.003 | 2.4 (1.2–4.6) | 0.011 |
Definition of abbreviations: any low = group with at least one low value for persistent TNF-α in cord and 3-month LPS-stimulated blood mononuclear cell supernatants; CI = confidence interval; high/high = group with high values for persistent TNF-α in both cord and 3-month LPS-stimulated blood mononuclear cell supernatants; high/msg = group with one high TNF-α value at cord or 3 months and the other missing; OR = odds ratio; TNF = tumor necrosis factor.
Population for marginal analysis (n = 283) limited to those with data for persistent TNF-α and parent-reported first-year physician-diagnosed eczema, first-year wheeze, and maternal asthma and rhinitis.
Multivariable analysis model.
TABLE 3.
ODDS OF HIGH PERSISTENT TUMOR NECROSIS FACTOR-α* IN EARLY LIFE BEFORE AND AFTER ADJUSTMENT FOR CANDIDATE MATERNAL INFLUENCES
| Maternal Influences | Unadjusted OR (95% CI)† | P Value | Adjusted OR (95% CI)‡ | P Value |
|---|---|---|---|---|
| Maternal pregnancy weight gain |
||||
| Low/mid tertiles |
Reference |
|
Reference |
|
| High tertile |
3.4 (1.4–8.2) |
0.008 |
3.2 (1.3–7.9) |
0.012 |
| Maternal prepregnant body mass index |
|
|
|
|
| Low tertile |
Reference |
|
Reference |
|
| Mid tertile |
1.5 (0.5–4.5) |
0.47 |
1.4 (0.4–4.3) |
0.57 |
| High tertile |
1.3 (0.4–4.0) |
0.64 |
1.4 (0.5–4.5) |
0.54 |
| Maternal asthma and/or rhinitis |
|
|
|
|
| No |
Reference |
|
Reference |
|
| Yes | 1.8 (0.8–4.4) | 0.17 | 1.7 (0.7–4.3) | 0.23 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Persistent tumor necrosis factor-α as outcome is in the model as the high/high (cord/3 mo) group versus all other.
Population for marginal (univariate) analysis (n = 195) is limited to those with data for maternal pregnancy weight gain, prepregnant body mass index, and maternal asthma and/or rhinitis.
Multivariable analysis model applied after determining other maternal factors (age, ethnicity, previous births) and parental smoking and child sex were without independent effects.
TABLE 5.
ODDS FOR CHILDHOOD ASTHMA WITH MATERNAL PREGNANCY WEIGHT GAIN AND EARLY-LIFE TNF-α PRODUCTION IN THE MODEL
| Unadjusted OR* (95% CI) | P | Adjusted OR† (95% CI) | P | |
|---|---|---|---|---|
| Cord/3 mo TNF-α |
|
|
|
|
| All other |
Reference |
|
Reference |
|
| High/high |
3.8 (1.5–9.8) |
0.005 |
3.1 (1.2–8.2) |
0.020 |
| Maternal pregnancy weight gain |
|
|
|
|
| Low 2 tertiles |
Reference |
|
Reference |
|
| High tertile | 3.1 (1.4–7.0) | 0.006 | 2.7 (1.2–6.1) | 0.021 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio; TNF = tumor necrosis factor.
Population for marginal analysis (n = 191) is limited to those with data for early-life TNF-α values and maternal weight gain.
Multivariable analysis model applied after determining other maternal factors (age, ethnicity, previous births), parental smoking, and child sex were without independent effects.
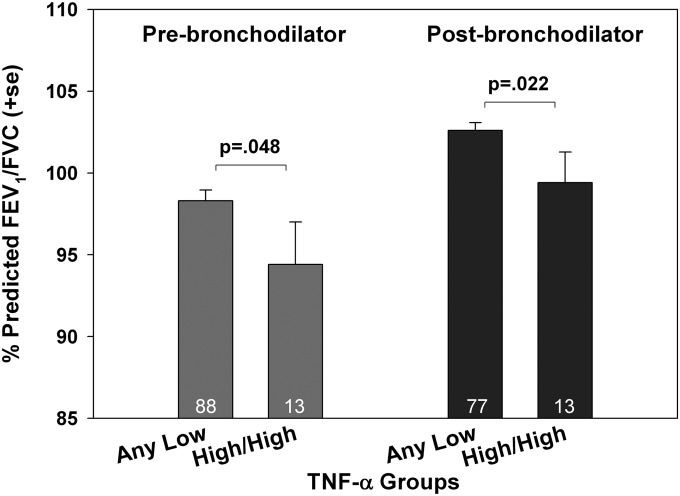
Early-life TNF-α production related inversely to FEV1/FVC ratio assessed at age 9 before and after administering a bronchodilator (Figure 2). This relation was restricted to the FEV1/FVC ratio per se, because neither the mean log values of FEV1 nor FVC were significantly associated with early-life TNF-α production. None of the other cytokines showed a relation to lung function. In contrast to the capacity to predict asthma and decreased lung function, early-life TNF-α production was not significantly related to allergen skin test reactivity tested at age 5 (data not shown).
Figure 2.
Relation of early-life tumor necrosis factor (TNF)-α groups to lung function assessed at age 9. Mean and SEM FEV1/FVC ratio for the children in the TNF-α high/high group (subjects with high values at both cord and 3 mo) compared with all others (with at least one value at cord and 3 mo) are shown for lung function assessments performed before and after bronchodilator (two puffs albuterol). The P values are based on Student unpaired t test.
The capacity of TNF-α production to predict childhood asthma assessed by the dichotomized cord and 3-month combined variable provided a signal stronger than values at either time point alone (see Table E6). Interestingly, this relation to asthma was restricted to TNF-α production in this early-life period: no relation of 12-, 24-, 36-, or 60-month LPS-induced TNF-α production to asthma was evident (see Table E6). Median plasma concentrations of TNF-α at birth and 3 months were approximately three orders of magnitude lower than the LPS-stimulated supernatant concentrations (see Table E7). Plasma levels of TNF-α (assessed as a combined cord/3-mo variable) did not correlate with LPS-induced TNF-α production in PBMCs and were unrelated to childhood asthma (data not shown).
Capacity of Maternal Factors to Predict Early-Life LPS-induced TNF-α in the Child
To assess whether genetic variation might regulate LPS-induced TNF-α, five bin-tagging SNPs were genotyped. None of the SNPs showed a relation to either TNF-α production or to asthma.
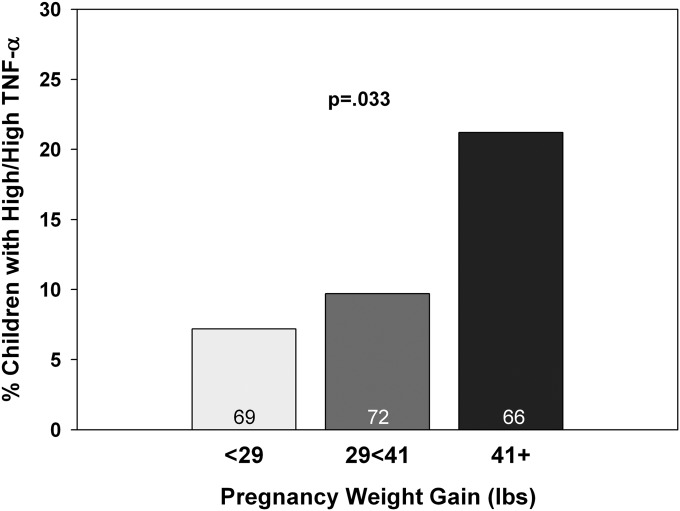
To address possible influences of the maternal milieu, we assessed maternal age, ethnicity, previous births, weight gain during pregnancy, prepregnancy BMI, and parental smoking for relation to early-life TNF-α production in the child. Only one relation was identified: tertiles of pregnancy weight gain revealed a direct relation to TNF-α (Figure 3). Marginal analysis of percent with high/high TNF-α yielded an OR of 3.4 (1.2–10.2; n = 207; P = 0.025) for the top tertile. This relation remained when adjusted for the factors mentioned previously and for maternal asthma and/or rhinitis (Table 3). Early-life TNF-α production was unrelated to LPS-induced TNF-α production in PBMCs of the mother (data not shown).
Figure 3.
The relation of maternal weight gain to early-life LPS-induced tumor necrosis factor (TNF)-α production in the child. The outcome variable is the percent of subjects in the TNF-α high/high group for maternal weight gain during pregnancy (in tertiles). The P values are based on chi-square and Fisher exact test.
The maternal pregnancy weight gain values were distributed normally and ranged from −11 to 78+ lb. Comparison with pregnancy weight gain recommendations of the Institute of Medicine (25) revealed that 58% of IIS mothers gained excess weight during pregnancy (see Table E8).
Capacity of Maternal-related Factors to Predict Asthma in the Child
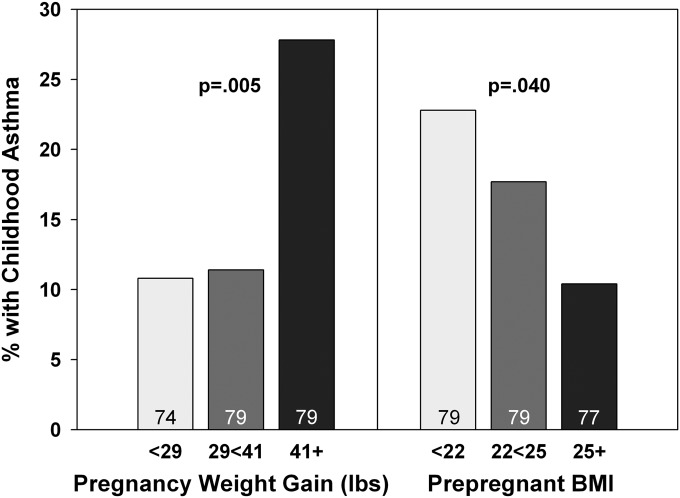
We examined the maternal-related factors described previously for relation to childhood asthma. In addition to the known relation with maternal asthma and/or rhinitis, only maternal weight gain during pregnancy was found to have a direct relation to asthma prevalence in the child (Figure 4). Marginal analysis yielded an OR of 3.2 (CI, 1.3–7.7; n = 232; P = 0.01) for the top tertile. Only maternal prepregnant BMI revealed an inverse relation to asthma (Figure 4). Modeled together, pregnancy weight gain remained a strong predictor of childhood asthma, independent of maternal asthma and/or rhinitis, whereas the inverse relation to prepregnant BMI was no longer statistically significant (Table 4). These relations were the same if outcome was limited to active asthma at ages 5 and/or 9 (see Table E9).
Figure 4.
Maternal weight gain during pregnancy (in tertiles) relates directly and maternal prepregnant body mass index (BMI; in tertiles) relates inversely to the prevalence of childhood asthma. The P values are based on chi-square and Fisher exact test.
TABLE 4.
ODDS OF CHILDHOOD ASTHMA BY CANDIDATE MATERNAL RISK FACTORS
| Maternal Factors | Unadjusted OR (95% CI)* | P Value | Adjusted OR (95% CI)† | P Value |
|---|---|---|---|---|
| Maternal pregnancy weight gain |
|
|
|
|
| Low 2 tertiles |
Reference |
|
Reference |
|
| High tertile |
3.4 (1.7–6.9) |
0.001 |
3.4 (1.6–7.2) |
0.002 |
| Maternal prepregnant body mass index |
|
|
|
|
| Low tertile |
Reference |
|
Reference |
|
| Mid tertile |
0.6 (0.3–1.4) |
0.32 |
0.5 (0.2–1.3) |
0.16 |
| High tertile |
0.4 (0.2–1.0) |
0.057 |
0.4 (0.2–1.1) |
0.072 |
| Maternal asthma and/or rhinitis |
|
|
|
|
| No |
Reference |
|
Reference |
|
| Yes | 3.3 (1.6–6.8) | 0.001 | 2.9 (1.4–6.2) | 0.004 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Population for marginal analysis (n = 225) is limited to those with data for pregnancy weight gain, prepregnancy body mass index, and maternal asthma and/or rhinitis.
Multivariable analysis model applied after determining other maternal factors (age, ethnicity, previous births), parental smoking, and child sex were without independent effects.
Leptin levels assessed in maternal prenatal plasma correlated strongly with pregnancy weight gain and prepregnant BMI. However, leptin levels did not show a relation to childhood asthma or to the child’s TNF-α production (data not shown).
Models of Childhood Asthma Risk Including Early Persistent TNF-α and Maternal Weight Gain
When maternal weight gain during pregnancy and the child’s early-life LPS-induced production of TNF-α were examined together for their relation to childhood asthma (Table 5), the OR for excess pregnancy weight gain and persistent early-life TNF-α were somewhat reduced, but both remained as independent predictors of childhood asthma.
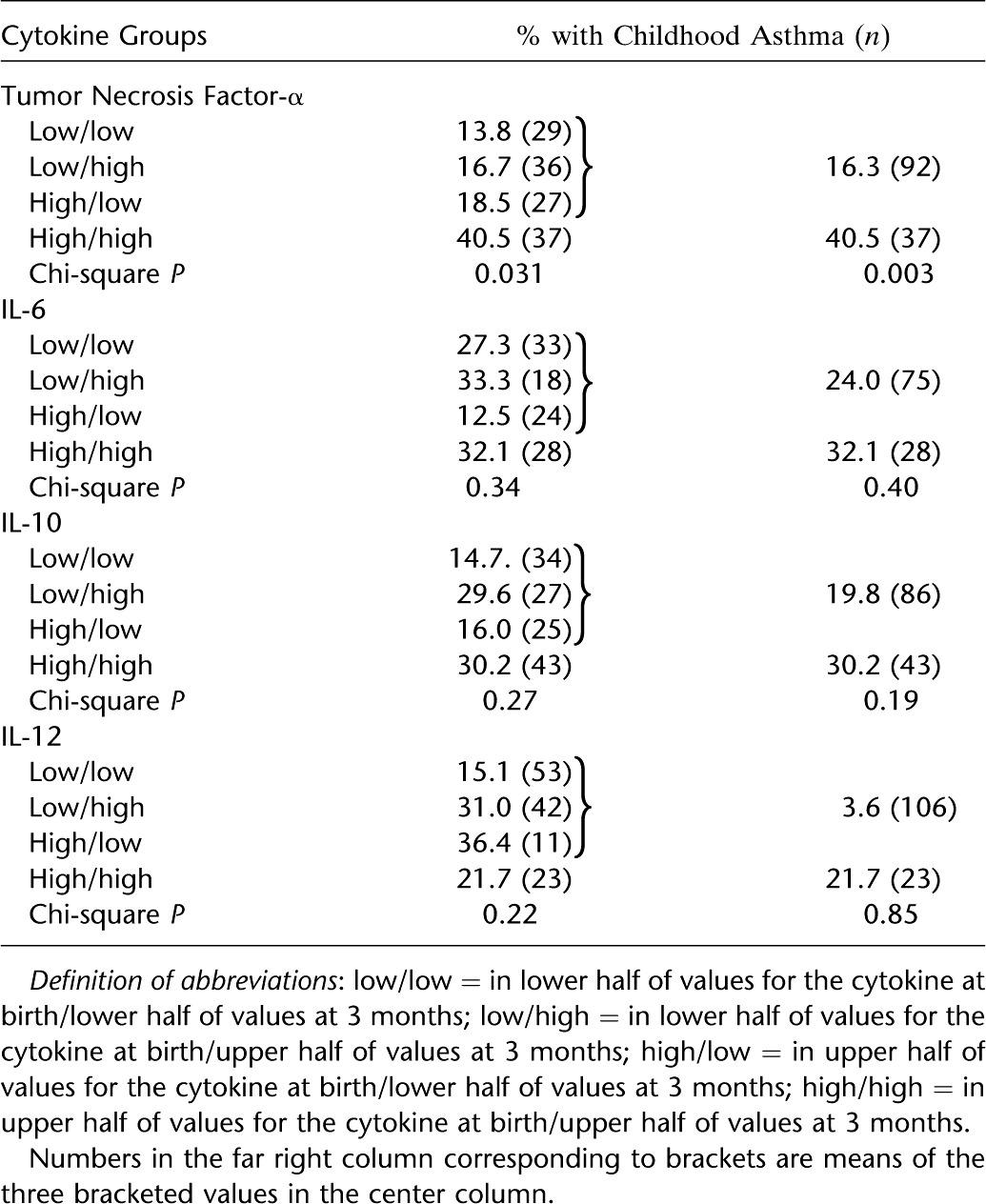
A path analysis was performed to assess further the possibility that excess maternal weight gain is associated with increased risk for asthma by influencing the early-life TNF-α production of the child (Figure 5). The direct path (maternal weight gain to childhood asthma) has a regression coefficient of 0.17. The two standardized regression coefficients for the indirect path (maternal weight gain to TNF-α relation, and the TNF-α to asthma relation) when multiplied equal 0.03. Thus, the indirect path represents 15% of the total (direct + indirect or 0.17 + 0.03) path. Replacing the outcome variable with asthma at age 5 and/or 9 increases the indirect path to 26% (see Figure E1).
Figure 5.
Path analysis of maternal weight gain during pregnancy estimating direct and indirect (through early-life tumor necrosis factor [TNF]-α) paths to childhood asthma. Multiplying the standardized regression coefficient for the relation of maternal weight gain to early-life TNF-α (0.18) by the coefficient for early-life TNF-α to asthma (0.18) equals 0.03, and thus the indirect path represents 15% (0.03/[0.17 + 0.03]) of the total (direct plus indirect) path. Variables are entered dichotomously (1/0): maternal weight gain as upper tertile compared with two lower tertiles; early-life TNF-α as high/high versus other; childhood asthma as physician-diagnosed active asthma (ages 2–9) or not.
Discussion
Our study demonstrates that persistently increased production of TNF-α in response to LPS stimulation of blood mononuclear cells at birth and at 3 months is predictive of the development of childhood asthma in our birth cohort of unselected children. The strength of signal was greater than that for, and independent of, maternal asthma and/or rhinitis. Increased TNF-α production was also associated with a decreased age 9 FEV1/FVC ratio but was unrelated to aeroallergen skin test reactivity. A strong in utero predictor of the child’s production of TNF-α and of childhood asthma was excess maternal weight gain during pregnancy.
Ours is not the first study to suggest that TNF-α is related to asthma, but to our knowledge it is the first to show prospectively a relation to asthma of early-life TNF-α. Others have shown this cytokine to be in higher concentrations in the sputum (26) and in more cells in bronchoalveolar lavage (27) and bronchial biopsies (28) of subjects with versus without asthma. Direct administration of TNF-α to the airways of normal volunteers induced an increase in bronchial responsiveness (29). Clinical trials with anti–TNF-α or TNF receptor analogs, however, have yielded conflicting results in asthma (20, 30) and shown risk of serious side effects (31). Interestingly, although we find that TNF-α production very early in life is predictive of childhood asthma, TNF-α production at ages later than 3 months (between ages 1 and 5 yr) showed no relation to asthma. These data suggest that a role for TNF-α in asthma development may involve events initiated very early in life and thus suggest a possible rationale as to why therapeutic approaches that reduce TNF-α after the disease is manifest may not be effective. There is also a report of substantially different effects of acute versus chronic exposure to TNF-α, with only the latter being critical to invoking chronic inflammatory states (32). To our knowledge, there are no reports that focus directly on early-life TNF-α production as it relates to asthma per se. Wood and coworkers (33) reported that LPS-induced TNF-α from cord blood was unrelated to frequent wheeze in the first year of life. Lappalainen and coworkers (34) found that dog ownership (studied as a potential immune modifier) was associated with decreased production of TNF-α production at birth.
Specificity of the asthma predictive capacity of TNF-α was explored by similar assessment of persistence for three other innate immune cytokines. Only TNF-α (and not IL-6, IL-10, or IL-12) showed a significant relation to asthma by logistic regression. This specificity raises the possibility that TNF-α may have a preferential or even unique biologic role in asthma development. Although asthma is most often not diagnosed until age 3 or later, this does not mean that the process is not initiated much earlier in life.
It is also possible that the relation of TNF-α to asthma does not have a direct biologic basis and simply serves as a biomarker. However, even if it should be found to be uninvolved mechanistically, the identification of the high persistent production of this cytokine as a biomarker could provide a major advance in therapeutic design of prevention strategies, because currently there are no known perinatal biomarkers capable of predicting asthma.
Another risk factor for childhood asthma identified in our study is that of excess maternal weight gain during pregnancy, and this was independent of maternal asthma and rhinitis. The weight gained by most of the mothers of our study was above the range recommended by the Institute of Medicine (25). Increased weight gain in pregnancy also related directly to increased capacity of infant blood cells to produce TNF-α. This relation is intriguing and suggests that the maternal milieu may vary with weight gain and may in turn influence the capacity of the infant to produce TNF-α to stimuli in the perinatal period. In addition, the models in Table 5 and Figure 5 suggest that pregnancy weight gain may be associated with risk for asthma by mechanisms only partially involving TNF-α production and thus emphasize the need for further mechanistic studies in this area.
Both prevalence of asthma and excessive maternal weight gain during pregnancy have been increasing over the last few decades (12, 35). Harpsoe and coworkers (16), in a recent study from Denmark, examined maternal weight gain in relation to childhood asthma and similarly identified risk. Although Rusconi and coworkers (36) did not find an association of persistent wheezing in offspring with maternal pregnancy weight gain, the Italian women in that study gained much less weight than the mothers in our population.
Marginal analysis revealed an inverse relation of prepregnant maternal BMI to asthma in the child. However, this relation lost significance when adjusted for pregnancy weight gain and maternal asthma and rhinitis and others have not reported a relation of this type. Indeed, several studies reported a direct relation (14–18). The relations to asthma, however, in most of these studies are limited to asthma subgroups that vary in phenotype by study. Perhaps the nature of maternal nutrition may contribute to variability.
Our study is limited in terms of population size and reliance on a single geographic location and thus its general applicability awaits additional corroborative studies. For the analyses performed using the post hoc grouped variables, our conclusions are considered exploratory. Although our results suggest that early-life TNF-α may relate to asthma by altering the FEV1/FVC ratio, our findings are epidemiologic in nature and do not address directly a biologic role for the early-life LPS-induced TNF-α. Longitudinal population studies, perhaps especially those in birth cohorts, have logistical difficulties in obtaining samples at all time points from every subject, further emphasizing the importance for these findings to be assessed for generalizability through additional studies. Nonetheless, we suggest that the LPS-induced TNF-α in the first 3 months of life serves as a remarkably strong predictive biomarker for childhood asthma in our population in a relation that is independent of maternal asthma and/or rhinitis, maternal production of TNF-α, polymorphisms in the TNF-α gene, and the production of some other innate immune cytokines. A factor influencing the capacity to produce TNF-α in the child is the amount of weight gained by the mother during pregnancy. Childhood asthma is also related to maternal pregnancy weight gain, a finding that confirms another recent report (16). Thus, these data open a new area of study exploring a possible asthma prevention strategy through interventions designed to regulate optimal maternal weight gain during pregnancy.
Acknowledgments
Acknowledgment
The authors thank Dr. Penelope Graves for overseeing the genotyping analysis; Heidi Erickson, R.N., for subject enrollment and follow-up; Susan Solomon, David Spies, and Bruce Saul for information technology support; and Amber Spangenberg, Dayna Anderson, and Sara Mobley for blood cell preparations, stimulations, and preservations. They are grateful for the assistance of Dr. Dean Billheimer and Isaac Jenkins with whom they consulted regarding the path analysis interpretation.
Footnotes
Supported by Arizona Disease Control Research Commission, Science Foundation of Arizona, and the National Institutes of Health (AI 42268).
Author Contributions: M.H. and A.L.W. designed the study. I.C.L. and W.L.E. performed laboratory cell cultures and assays with supervision by M.H. A.L.W. supervised epidemiologic data acquisition. M.H., D.A.S., and J.R. performed data analysis. M.H. drafted and I.C.L., W.L.E., D.A.S., J.R., and A.L.W. edited the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201207-1265OC on April 12, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wang LY, Zhong Y, Wheeler L. Direct and indirect costs of asthma in school-age children. Prev Chronic Dis. 2005;2:A11. [PMC free article] [PubMed] [Google Scholar]
- 2.Wong GW, Chow CM. Childhood asthma epidemiology: insights from comparative studies of rural and urban populations. Pediatr Pulmonol. 2008;43:107–116. doi: 10.1002/ppul.20755. [DOI] [PubMed] [Google Scholar]
- 3.Kumar R. Prenatal factors and the development of asthma. Curr Opin Pediatr. 2008;20:682–687. doi: 10.1097/MOP.0b013e3283154f26. [DOI] [PubMed] [Google Scholar]
- 4.Martinez FD. The origins of asthma and chronic obstructive pulmonary disease in early life. Proc Am Thorac Soc. 2009;6:272–277. doi: 10.1513/pats.200808-092RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savenije OE, Kerkhof M, Koppelman GH, Postma DS. Predicting who will have asthma at school age among preschool children. J Allergy Clin Immunol. 2012;130:325–331. doi: 10.1016/j.jaci.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Burrows B, Martinez FD, Cline MG, Lebowitz MD. The relationship between parental and children’s serum IgE and asthma. Am J Respir Crit Care Med. 1995;152:1497–1500. doi: 10.1164/ajrccm.152.5.7582283. [DOI] [PubMed] [Google Scholar]
- 7.Halonen M, Stern DA, Lohman C, Wright AL, Brown MA, Martinez FD. Two subphenotypes of childhood asthma that differ in maternal and paternal influences on asthma risk. Am J Respir Crit Care Med. 1999;160:564–570. doi: 10.1164/ajrccm.160.2.9809038. [DOI] [PubMed] [Google Scholar]
- 8.Rothers J, Halonen M, Stern DA, et al. Adaptive cytokine production in early life differentially predicts total IgE levels and asthma through age 5 years J Allergy Clin Immunol 2011128397–402.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008;8:169–182. doi: 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- 10.Lim RH, Kobzik L, Dahl M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS ONE. 2010;5:e10134. doi: 10.1371/journal.pone.0010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schieve LA, Cogswell ME, Scanlon KS. Trends in pregnancy weight gain within and outside ranges recommended by the Institute of Medicine in a WIC population. Matern Child Health J. 1998;2:111–116. doi: 10.1023/a:1022992823185. [DOI] [PubMed] [Google Scholar]
- 12.Rode L, Hegaard HK, Kjaergaard H, Møller LF, Tabor A, Ottesen B. Association between maternal weight gain and birth weight. Obstet Gynecol. 2007;109:1309–1315. doi: 10.1097/01.AOG.0000266556.69952.de. [DOI] [PubMed] [Google Scholar]
- 13.O'Reilly JR, Reynolds RM. The risk of maternal obesity to the long term health of the offspring. Clin Endocrinol. 2013;78:9–16. doi: 10.1111/cen.12055. [DOI] [PubMed] [Google Scholar]
- 14.Lowe A, Braback L, Ekeus C, Hjern A, Forsberg B.Maternal obesity during pregnancy as a risk for early-life asthma J Allergy Clin Immunol 20111281107–1109.e1–e2 [DOI] [PubMed] [Google Scholar]
- 15.Kumar R, Story RE, Pongracic JA, Hong X, Arguelles L, Wang G, Kuptsova-Clarkson N, Pearson C, Ortiz K, Bonzagni A, et al. Maternal pre-pregnancy obesity and recurrent wheezing in early childhood. Pediatr Allergy Immunol Pulmonol. 2010;23:183–190. doi: 10.1089/ped.2010.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harpsøe MC, Basit S, Bager P, Wohlfahrt J, Benn CS, Nøhr EA, Linneberg A, Jess T. Maternal obesity, gestational weight gain, and risk of asthma and atopic disease in offspring: a study within the Danish National Birth Cohort. J Allergy Clin Immunol. 2013;131:1033–1040. doi: 10.1016/j.jaci.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Scholtens S, Wijga AH, Brunekreef B, Kerkhof M, Postma DS, Oldenwening M, de Jongste JC, Smit HA. Maternal overweight before pregnancy and asthma in offspring followed for 8 years. Int J Obes (Lond) 2010;34:606–613. doi: 10.1038/ijo.2009.194. [DOI] [PubMed] [Google Scholar]
- 18.Patel SP, Rodriguez A, Little MP, Elliott P, Pekkanen J, Hartikainen AL, Pouta A, Laitinen J, Harju T, Canoy D, et al. Associations between pre-pregnancy obesity and asthma symptoms in adolescents. J Epidemiol Community Health. 2012;66:809–814. doi: 10.1136/jech.2011.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohman ICSD, Wright AL, Liu A, Martinez FD, Halonen M. The relation of LPS-induced TNF-alpha production to asthma and to household dust LPS exposure [abstract] Am J Respir Crit Care Med. 2009;179:A4847. [Google Scholar]
- 20.Wright ALLI, Stern DA, Graves P, Halonen M. Excess weight gain in pregnancy is associated with LPS-stimulated TNF-alpha from neonatal blood cells and with asthma in the child at age 5 [abstract] Am J Respir Crit Care Med. 2011;183:A1409. [Google Scholar]
- 21.Su Y, Rothers J, Stern DA, Halonen M, Wright AL. Relation of early antibiotic use to childhood asthma: confounding by indication? Clin Exp Allergy. 2010;40:1222–1229. doi: 10.1111/j.1365-2222.2010.03539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothers J, Wright AL, Stern DA, Halonen M, Camargo CA., JrCord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona J Allergy Clin Immunol 20111281093–1099.e1–e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halonen M, Lohman IC, Stern DA, Spangenberg A, Anderson D, Mobley S, Ciano K, Peck M, Wright AL. Th1/Th2 patterns and balance in cytokine production in the parents and infants of a large birth cohort. J Immunol. 2009;182:3285–3293. doi: 10.4049/jimmunol.0711996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pathreg: command that allows the user to specify all of the equations in a path analysis mode. 2009. Available from: http://www.ats.ucla.edu/stat/stata/faq/pathreg.htm
- 25.Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: National Academies Press; 2009 [PubMed]
- 26.Obase Y, Shimoda T, Mitsuta K, Matsuo N, Matsuse H, Kohno S. Correlation between airway hyperresponsiveness and airway inflammation in a young adult population: eosinophil, ECP, and cytokine levels in induced sputum. Ann Allergy Asthma Immunol. 2001;86:304–310. doi: 10.1016/S1081-1206(10)63303-0. [DOI] [PubMed] [Google Scholar]
- 27.Ying S, Robinson DS, Varney V, Meng Q, Tsicopoulos A, Moqbel R, Durham SR, Kay AB, Hamid Q. TNF alpha mRNA expression in allergic inflammation. Clin Exp Allergy. 1991;21:745–750. doi: 10.1111/j.1365-2222.1991.tb03205.x. [DOI] [PubMed] [Google Scholar]
- 28.Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH, Holgate ST. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994;10:471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- 29.Thomas PS. Tumour necrosis factor-alpha: the role of this multifunctional cytokine in asthma. Immunol Cell Biol. 2001;79:132–140. doi: 10.1046/j.1440-1711.2001.00980.x. [DOI] [PubMed] [Google Scholar]
- 30.Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, Bradding P, Brightling CE, Wardlaw AJ, Pavord ID. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006;354:697–708. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]
- 31.Wenzel SE, Barnes PJ, Bleecker ER, Bousquet J, Busse W, Dahlén SE, Holgate ST, Meyers DA, Rabe KF, Antczak A, et al. T03 Asthma Investigators. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-alpha blockade in severe persistent asthma. Am J Respir Crit Care Med. 2009;179:549–558. doi: 10.1164/rccm.200809-1512OC. [DOI] [PubMed] [Google Scholar]
- 32.Clark J, Vagenas P, Panesar M, Cope AP. What does tumour necrosis factor excess do to the immune system long term? Ann Rheum Dis. 2005;64:iv70–iv76. doi: 10.1136/ard.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood RA, Bloomberg GR, Kattan M, et al. Relationships among environmental exposures, cord blood cytokine responses, allergy, and wheeze at 1 year of age in an inner-city birth cohort (Urban Environment and Childhood Asthma study) J Allergy Clin Immunol 2011127913–919.e1–e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lappalainen MH, Huttunen K, Roponen M, Remes S, Hirvonen MR, Pekkanen J. Exposure to dogs is associated with a decreased tumour necrosis factor-α-producing capacity in early life. Clin Exp Allergy. 2010;40:1498–1506. doi: 10.1111/j.1365-2222.2010.03566.x. [DOI] [PubMed] [Google Scholar]
- 35.Helms E, Coulson CC, Galvin SL. Trends in weight gain during pregnancy: a population study across 16 years in North Carolina. Am J Obstet Gynecol. 2006;194:e32–e34. doi: 10.1016/j.ajog.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 36.Rusconi F, Galassi C, Forastiere F, Bellasio M, De Sario M, Ciccone G, Brunetti L, Chellini E, Corbo G, La Grutta S, et al. Maternal complications and procedures in pregnancy and at birth and wheezing phenotypes in children. Am J Respir Crit Care Med. 2007;175:16–21. doi: 10.1164/rccm.200512-1978OC. [DOI] [PubMed] [Google Scholar]