Abstract
Different kinds of experience during early life can play a significant role in the development of an animal's behavioural phenotype. In natural contexts, this influences behaviours from anti-predator responses to navigation abilities. By contrast, for animals reared in captive environments, the homogeneous nature of their experience tends to reduce behavioural flexibility. Studies with cage-reared rodents indicate that captivity often compromises neural development and neural plasticity. Such neural and behavioural deficits can be problematic if captive-bred animals are being reared with the intention of releasing them as part of a conservation strategy. Over the last decade, there has been growing interest in the use of environmental enrichment to promote behavioural flexibility in animals that are bred for release. Here, we describe the positive effects of environmental enrichment on neural plasticity and cognition in juvenile Atlantic salmon (Salmo salar). Exposing fish to enriched conditions upregulated the forebrain expression of NeuroD1 mRNA and improved learning ability assessed in a spatial task. The addition of enrichment to the captive environment thus promotes neural and behavioural changes that are likely to promote behavioural flexibility and improve post-release survival.
Keywords: enrichment, cognition, neural plasticity, spatial cognitive ability, captive-rearing, Salmo salar
1. Introduction
Animals often express behaviours that appear to be exquisitely adapted to the environment in which they live. In many cases, the development of adaptive behaviour seems to be influenced and refined by early life experiences. For example, male three-spined sticklebacks (Gasterosteus aculeatus) from populations with predators chase their offspring a few days after hatching to help prime appropriate anti-predator responses in the developing juveniles [1]. Similarly, the navigational cues used by different homing pigeons (Columba livia) are influenced by the information most prevalent in the environment as the young birds grow and develop; birds exposed to windy conditions typically learn to navigate using olfactory, air-borne cues [2], whereas pigeons reared in highly visual, but sheltered lofts pay more attention to visual landmarks [3]. In this way, experience during these early life phases plays a significant role in generating behavioural phenotypes that are well suited to different kinds of environment or lifestyle. Changes within the brain, both structural and neurophysiological, are believed to underpin differences in behavioural phenotypes. An indication of this comes from studying the brains and the behavioural repertoires of animals reared in captivity. Captive-bred animals generally have reduced behavioural diversity and less behavioural flexibility, and many regions of the brain are smaller and less active compared with wild counterparts [4–7]. Such reductions appear to be a by-product of the constant, non-demanding rearing environment [8,9].
Several experiments with laboratory rodents have explored the effects of adding environmental enrichment into the cages housing the animals [10–12]. Enrichment, in the form of objects that can be manipulated (such as pieces of rope and toys) or explored and used (such as tubes) provide the animals with more variable experiences. In terms of behaviour, enrichment has been shown to have a positive effect on the ability to learn and remember new tasks [13,14]. Within the brain, enrichment has been shown to affect neurogenesis, synaptic plasticity and long-term potentiation in the hippocampus, a region linked to spatial and other forms of relational memory. The functional relevance of hippocampal neurogenesis has been extensively studied, showing that environmental and physiological conditions such as stress, exercise and learning can modulate hippocampal neurogenesis. The molecular mechanisms of neural plasticity associated with memory have been a major focus for neuroscientists, and this research has now resulted in markers that can indicate up- or downregulation of neural plasticity [15,16]. Recent studies have shown that expression levels of the proneuronal gene neurogenic differentiation 1 (NeuroD1) are a reliable measure of neurogenesis [17,18], and a useful indicator for neurogenic changes associated with learning and memory.
In other fields where animals are bred and reared for release as part of conservation programmes, environmental enrichment has been proposed as a positive addition to the rearing environment [19]. While there has been considerable success in rearing animals for reintroduction programmes, the survival of those animals once released has been generally poor, and in many cases the releases are ineffective at increasing population biomass [20,21]. Enriched rearing environments and training have thus been explored as a means to promote the development of behavioural competence and flexibility, which is presumed to aid post-release survival [22–25].
Over the last decade, studies have explored the effects of different kinds of enrichment in fish reared for release [26–28]. There is now growing (but not always consistent) evidence that the addition of enrichment increases fish behavioural flexibility [28–30]. Other studies have also begun to investigate the kinds of effect that enrichment has on the fish brain. While earlier work described gross morphological changes in the relative size of different brain regions [31], more recent work has begun to examine proliferation activity within the telencephalon or forebrain [32,33]. However, whether changes in neural plasticity are related to differences in cognitive ability has not yet been addressed in fish (nor other animals) reared as part of a re-introduction programme.
To investigate the impact that exposure to enriched early rearing environments has on changes within the brain and cognition, juvenile salmon were reared in contrasting environments (enriched or control) over a period of eight weeks. Differences in forebrain expression of NeuroD1 mRNA and the spatial learning ability of the fish were then compared. As there is now a confirmed link between a fish forebrain region (lateral pallium, lp) and spatial cognitive ability [34,35], we compared the expression of NeuroD1 mRNA in the forebrains of fish. Spatial learning and memory are a complex form of behaviour that requires the animal to integrate multiple pieces of information, and it has been known for rodents with experience of an enriched environment to improve spatial learning [13]. We therefore tested the hypotheses that fish with experience of environmental enrichment would have upregulated expression of genes associated with forebrain neural plasticity and that juvenile fish from this background would have enhanced spatial learning abilities.
2. Material and methods
(a). Experimental fish
Juvenile, pre-smolt River Vosso wild-strain Atlantic salmon (Salmo salar), one of the last wild strains of large salmonids from the North Atlantic, were used. A brood stock is housed in the national genbank in Eidfjord, Norway for conservation purposes. Eggs were hatched at Voss hatchery, and the fish were reared in conventional hatchery tanks where they were provided with river water and a natural photoperiod. The fish were transferred to enriched and control treatments at the age of 10 months and spatial learning experiments were run when the fish were 1 year old. All fish were sexually immature and no sneakers were among the experimental fish.
(b). Treatments
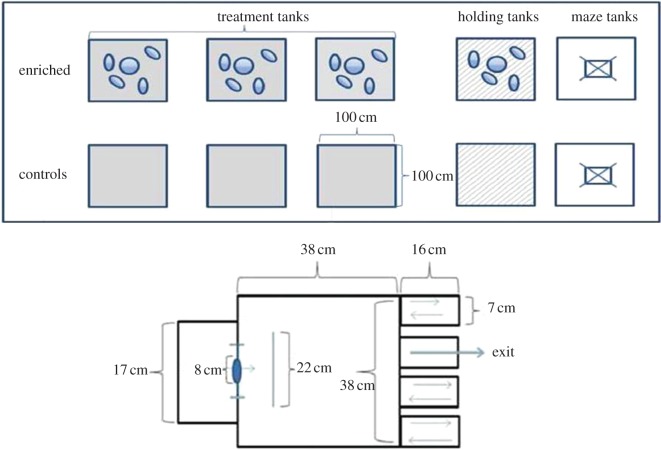
Fish were randomly divided into three control or three enriched tanks (100 × 100 × 60 cm), with 30 fish in each tank, and were held in these conditions for eight weeks (figure 1). Water temperature was maintained at 8 ± 0.5°C and a late autumn daylight schedule was simulated (9 L : 15 D cycle). All fish were tagged using micro PIT-tags (Nonatec Transponder, Lutronic International) under anaesthesia using buffered MS222. Ethical approval for the experiments was given by the Norwegian Veterinary Authorities (site licence no. 18).
Figure 1.
The experimental set-up with treatment tanks, holding tanks, maze tanks and maze design used during the experimental testing of learning ability. All maze arms were open when fish were familiarized with the apparatus for 48 h before the trials began. (Online version in colour.)
Control tanks were standard, plain hatchery tanks, whereas the enriched treatment tanks contained pebbles, cobbles and vertically floating plastic structures [9,28,29]. The pebbles and rocks (diameter 8–12 cm) covered at least 75% of the base of the tank. They were large enough for the fish to move around and hide between. Eight plastic fronds (5 cm wide × 50 cm long) were also added into the enriched tanks. The fronds were weighted at their base using pebbles and the rest of the material then floated vertically in the water column. The fronds provided a place where the fish could hide or seek shelter and were used to mimic a natural habitat for this age of fish where they would normally encounter aquatic plants and woody debris. Fish in the enriched tanks were observed to use all the enrichment items in the tank, and while they sometimes schooled in the water column, at other times individual fish would be seen among the cobbles or swimming between the plastic fronds.
The position of the enrichment items in the tank was randomly changed once a week. To control for disturbance effects, control fish had their water stirred for similar amounts of time. The tanks were situated side-by-side and experienced the same amounts of general disturbance when the tanks were flushed to remove waste every third day or while loading feed onto belt feeders. The fish were not in visual contact between treatment tanks as the tanks were opaque.
(c). Brains samples
A subset of the fish—nine enriched and nine control fish (sampling three fish from each enriched and control tank)—was collected for the NeuroD1 mRNA comparisons. Fish were anaesthetized in buffered MS222 and measured for length (mean ± s.e., enriched: 140.4 ± 3.3 mm; control: 130.8 ± 5.2 mm) and weighed (mean ± s.e., enriched: 28.6 ± 2.4 g; control: 24.0 ± 3.0 g). The brains were rapidly dissected out, immediately placed in RNAlater (Ambion, Austin, TX) and stored at −80°C for subsequent quantification of NeuroD1 mRNA expression.
Frozen whole brains were perfused with RNAlater-Ice at −80°C overnight. The telencephalon was then quickly isolated on ice under a dissecting microscope by cutting away the olfactory bulbs and then cutting vertically between the telencephalon and the rest of the brain. Total RNA was then directly isolated from telencephalon tissue by phenol–chloroform extraction using TRI Reagent (Sigma, St. Louis, MO) as outlined by Chomczynski [36].
(d). Analysis of gene expression levels
Total RNA concentration and purity was determined by the NanoDrop ND-1000 UV-Vis Spectrophotometer (NanoDrop Technologies, Wilmington, DE) and the RNA integrity was evaluated with the Agilent 2100 Bioanalyzer using the RNA 6000 Nano LabChip kit (Agilent Technologies, Palo Alto, CA). Total RNA was treated with RQ1 RNase-free DNase (Promega, Madison, WI) and cDNA reversely transcribed using 500 ng total RNA and random nonamers in conjunction with the Reverse Transcription Core kit (Eurogentec RT-RTCK-05, Liège, Belgium) following the manufacturer's instructions.
We conducted real-time quantitative PCR with gene-specific primers in conjunction SYBR Green Master Mix (ABI; Applied Biosystems, Foster City, CA) using the MJ Research Chromo 4 System Platform (Bio-Rad Laboratories, Hercules, CA). We designed the forward primer for salmon brain NeuroD1 (NCBI account no. GI223647549) as CAATGGACAGCTCCCACATCT and the reverse as CCAGCGCACTTCCGTATGA. For all assays, the thermal cycling protocols contained 5 µl cDNA (125 ng RNA), 200 nM of each primer and 12.5 µl SYBR Green Master Mix in a total volume of 25 µl. The thermal cycling protocol consisted of 10 min at 95°C followed by 45 cycles at 95°C for 15 s and 60°C for 1 min. Melt-curve analysis verified that the primer sets for each Q-PCR assay generated one single product and no primer–dimer artefacts. For each assay, triplicate twofold cDNA dilution series made from different exposure groups were used to determine amplification efficiencies (E) calculated as the slope from the plot of log cDNA concentration versus threshold cycle (Ct) values using the following formula: E = 10(–1/slope). This efficiency was used to correct for differences in amplification efficiency when calculating gene expression according to Pfaffl [37]. Expression was measured in duplicate reactions for each individual and is presented as relative to the endogenous reference gene elongation factor 1 alpha (EF1α, NCBI account no. GI11596419) mRNA expression. EF1α has previously been validated as a reference gene in salmon [38] and was also found not to differ between treatments in this study. The EF1α forward primer was CCCCTCCAGGACGTTTACAAA and the EF1α reverse primer CACACGGCCCACAGGTACA. Data from three of the brains (two control and one enriched fish) were not included in the analysis, because the duplicate differences in the qPCR assay exceeded the accepted threshold. The final sample sizes for the brain screening were thus eight enriched and seven control fish.
(e). Learning assay
Two days before the behaviour trials began, 15 enriched and 15 control fish (five from each replicate enriched and control tank) were sampled randomly and transferred to either a ‘control fish’ or an ‘enriched fish’ holding tank, in which they were maintained during the behaviour trials. These fish were screened to compare how quickly they could locate the correct route out of a maze (figure 1). The fish were initially familiarized with the apparatus by allowing them to swim freely within the maze with all arms open for 48 h before the trials began.
The maze was made of opaque grey plastic; it had an outer start box on one side and four rectangular arms arranged in a row on the opposite wall (figure 1). A barrier was placed between the start box and the arms to prevent fish from having direct visual contact with the exits while in the start box. The maze was placed in the centre of a larger holding tank (100 × 100 × 60 cm). In the outer tank, three stimulus fish (randomly sampled from the main holding tanks) were kept in a cylinder (25 cm diameter) to act as a social stimulus during the trials to motivate the isolated test fish inside the maze to exit. Although salmon of the same age living in natural rivers would tend to be isolated living in independent home ranges (territories), hatchery-reared fish are used to schooling and being in a group. Thus, to motivate the fish to learn the correct route out of the maze, they were trained that on leaving the maze they could interact with the stimulus school. To help ensure similar motivation to leave the maze for both enriched and control fish, we pre-trained the fish before the learning trials began by allowing the fish to explore the experimental set-up over a 48 h period. During this pre-training phase, all the maze arms were open. All 15 enriched fish were pre-trained in one group, and all 15 control fish were in a different group.
There was a flow of water, with fresh water entering both the start box and the container with stimuli fish and leaving via a central drain in the bottom of the holding tank. The water was maintained at a depth at 22 cm in the maze.
At the start of each trial, an individual fish was collected in a hand-held dip-net, their identity was decoded with a hand-held PIT reader, and then they were carefully released into the start box. To keep handling stress to a minimum, we tested the fish in the order in which they were netted. Thus, the order of testing was different for each trial. We noted test fish ID on a label and placed it on the maze so it was visible on the video recording. A Sanyo Xacti VPC-WH1 camera, mounted 1.5 m above the centre of the maze, recorded the trials. Fish could not see the observer, but the observer could view the fish and maze remotely from the display on the camera. After 3 min, the start box door was opened remotely using a pulley. Fish that did not leave the start box within 5 min were encouraged into the maze using opaque plastic paddles. The start box door was closed after fish left the box. The camera was turned off after the fish had left the maze or if maximum trial time (300 s) was reached. Fish that did not find the exit were guided out using the paddles. We tested the fish once per day over seven consecutive days.
As fish left the maze, they swam into the larger body of water in the holding tank. The opaque walls prevented test fish within the maze from being in visual contact with fish on the outside. The maze was brightly lit, but there were shaded areas available underneath it for use as shelter. No fish ever re-entered the maze. After the trial on the last day, the fish were anaesthetized using buffered MS222, and their length (mean ± s.e., enriched: 12.79 ± 0.17 cm; control: 12.88 ± 0.18 mm) and weight (mean ± s.e., enriched: 21.97 ± 0.88 g; control: 21.97 ± 1.02 g) were measured. From playback of videos, we noted the time the test fish took to leave the start box, the time to the first error (which was defined as trying to exit through a dead-end), the number of errors made and the time to exit the maze.
(f). Data analysis
All statistics were performed using R v. 2.15.1 (R Development Core Team, http://www.r-project.org). We tested for differences in length and weight using a linear mixed-effects model [39]. We specified ‘tank’ as a random effect factor to account for tank effects and used ‘treatment’ (enriched and control) as a fixed effect factor.
(g). Gene expression data
We investigated differences between enriched and control fish in telencephalic NeuroD1 mRNA expression relative to the endogenous reference gene expression (EF1α) using linear mixed-effects modelling [39] with ‘treatment’ (enriched or control) as fixed factor. We included ‘tank’ as a random effect factor to account for the dependency of observations within tanks.
(h). Learning in the maze trials
(i). Errors and time to exit
For individual fish, we first calculated the cumulative number of errors (‘cumul.errors’) and cumulative time (‘cumul.time’) to leave the maze for successive experimental days. We then fitted linear mixed-effects models assuming first-order autocorrelation [39] and specified ‘fish’ (fish ID) nested under ‘tank’ as random effect factors to account for tank effect and repeated observations of individual fish. ‘Treatment’ (enriched or control) and ‘day’ (experimental day) were specified as fixed effects. The following equation in R was used:
To test for differences in time spent before leaving the maze, we used a polynomial model to allow for a curved relationship between cumulative time before leaving the maze and days. The R equation was [39]
3. Results
The fish reared in the tanks with enrichment had higher levels of NeuroD1 mRNA expression relative to EF1α expression in the telencephalon compared with the control fish reared in standard hatchery conditions (lme, F1,4 = 10.18, p = 0.03; figure 2). These enriched and control fish did not differ in their weight (lme, F1,4 = 0.92, p = 0.39) or length (lme, F1,4 = 1.29, p = 0.32).
Figure 2.
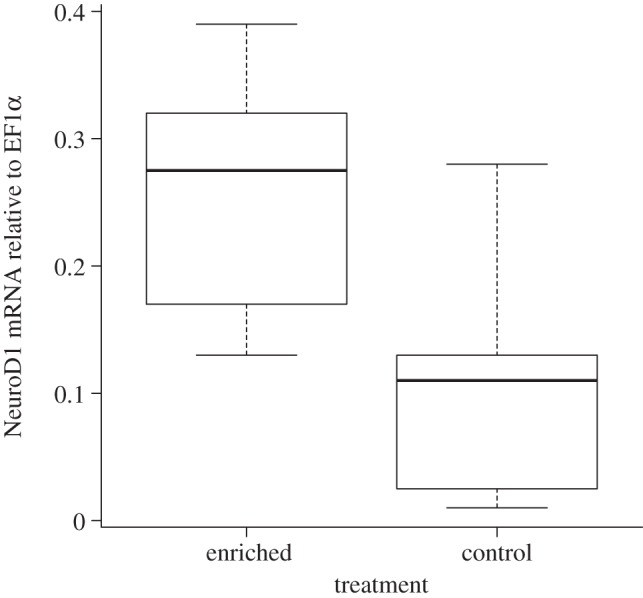
Juvenile Atlantic salmon reared in tanks with spatial structures (enriched) had higher levels of NeuroD1 mRNA expression relative to EF1α (p = 0.03). The values portrayed are the standard box-and-whisker plot in R (i.e. the box shows median, and first and third quartiles, and the whiskers represent min and max values). There were no outliers in the data.
Over the 7 day testing period, enriched fish made fewer mistakes as they searched for the correct exit compared with the control fish (lme, comparisons of slopes, F1,153 = 11.15, p < 0.01; figure 3). Although the performance of the fish was not different on the first test day (F1,21 = 0.85, p = 0.37), the enriched fish became increasingly more accurate as the trials progressed. In terms of time taken to leave the maze, again the enriched fish exited the maze faster than control fish as experimental days progressed (lme, comparisons of slopes, F2,159 = 8.24, p ≪ 0.01; figure 4). Hence, enriched fish outperformed control fish as experimental days went by. There were no differences in escape performance at the beginning of the trials (lme, comparisons of intercepts, F1,4 = 2.68, p = 0.12; figure 4), but the second-order polynomials of the models were significantly negative (p = 0.03; figure 4), indicating that the fish became faster at leaving the maze over experimental days.
Figure 3.
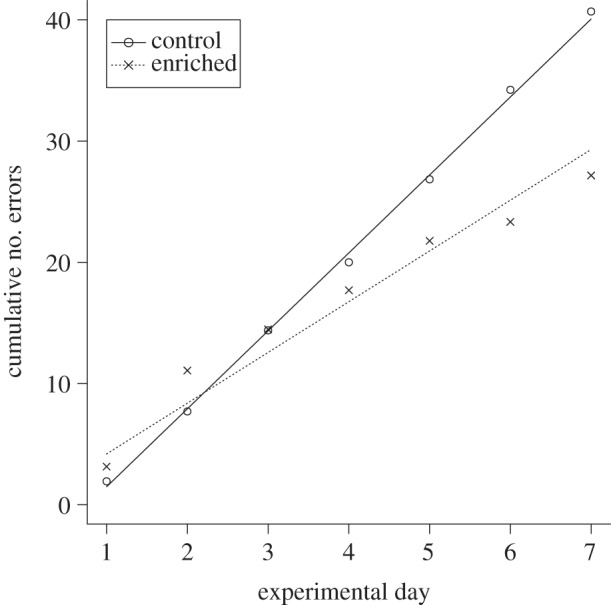
Cumulative number of errors of enriched and control fish during trials in the maze. Lines represent model predictions based on individual data and points represent mean values.
Figure 4.
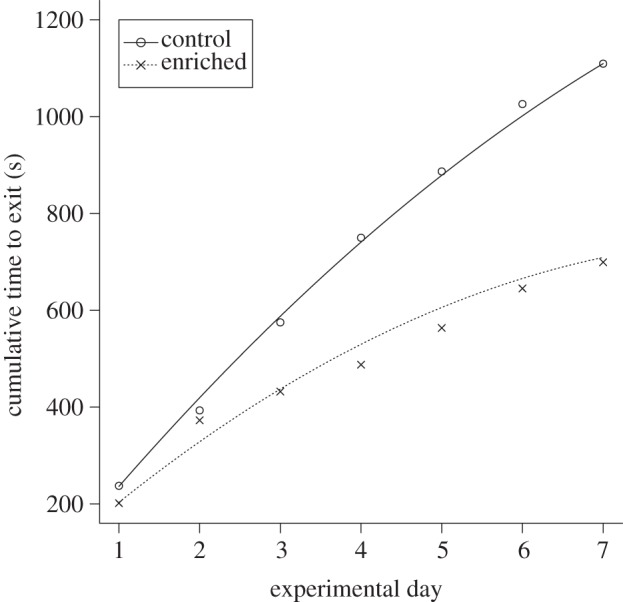
Change in cumulative time taken by enriched and control fish to leave the maze. Lines represent model predictions based on individual data and points represent mean values.
Enriched and control fish used did not differ in their weight (F1,4 = 0.03, p = 0.88) or length (F1,4 = 0.001, p = 0.97).
4. Discussion
In comparison with fish reared in impoverished tanks, juvenile salmon that had eight weeks in an enriched environment, from 10 to 12 months after hatching, had increased neural plasticity in the telencephalon, with upregulated NeuroD1 mRNA expression, and exposure to tank enrichment also produced fish that had a superior spatial learning ability, allowing the fish to correctly locate the exit of the maze more efficiently. That enriched fish made fewer mistakes suggests that they were better at learning and then improving their performance through a trial-and-error process during the 7 days of testing. Experience with enrichment thus produced juvenile fish that quickly found their way out of the maze. By contrast, the fish reared in the plain impoverished control environments were slower to exit the maze. Together, our results indicate that exposure to enrichment during the rearing period has a positive effect on fish performance in a maze task. These results are similar to those previously reported for rodents, where exposure to cage enrichment was found to increase spatial learning ability and to increase levels of neurotrophic factors within the brain, particularly within the hippocampus, a region associated with spatial and other forms of relational memory functions [13,14,40]. However, this is the first time an effect of enrichment has been found to positively facilitate both neural plasticity and spatial learning in fish.
In mammals, neural plasticity is upregulated within the hippocampus as a result of exposure to environmental enrichment [16,41,42]. Environmental enrichment increases neurogenesis in the hippocampus, and is correlated with improved learning and memory tasks [41,42]. NeuroD1 is a member of the basic helix–loop–helix transcription factor family involved in the development of the central nervous system [43], hippocampal neurogenesis [44] and dendritic spine stability [45]. Thus, the increased NeuroD1 mRNA expression in the telencephalon of fish that experienced an enriched environment, and its correlation with enhanced spatial learning in the maze task in the present study, suggest a conserved mechanism across vertebrate taxa.
Our understanding of the fish brain is expanding rapidly at this time [34,45,46]. Studies from domesticated species such as the goldfish (Carassius auratus) are allowing us to refine our understanding of the function of different brain regions [34,35]. Research from species reared in aquaculture is further providing insight into the way domestication affects the fish brain [32,47]. Comparative studies of closely related species now living in contrasting natural habitats (e.g. cichlids in the African Rift Valley lakes) are revealing how different environments rapidly change the fish brain in terms of sensory processing structures (e.g. olfactory lobes), or the relative size of areas such as the telencephalon, cerebellum and hypothalamus [48]. Also, recent studies with the zebrafish (Danio rerio) are beginning to explore proliferation and neural mechanisms within this model species [33,46]. The results we report here highlight that, as in mammals, there is a link between neural plasticity gene expression markers and spatial behaviour, and we suggest that this further validates the use of fish as model organisms for studies of vertebrate brain and behaviour.
Previous experiments that have specifically addressed the effect of the early rearing environment in fish have reported a range of behavioural benefits that exposure to enrichment confers. For example, enrichment promotes a number of different flexible behaviours in terms of more adaptive foraging abilities, decreased levels of aggression, more flexible shoaling responses, a faster ability to recover from stressful experiences and improved social learning skills [9,26,28–30,49]. All of these changes in behaviour are likely to be associated with increased survival of individuals released into a natural environment. Our current observations extend these earlier results to spatial learning. Being able to find a way around an environment, to avoid areas that associated with danger, to rapidly locate shelter when threatened or to be able to return to a profitable feeding location are the kinds of challenge that juvenile salmon will face on a daily basis. Having sufficiently well-developed spatial skills that allow fish to solve these kinds of problem will be an important factor contributing to post-release survival. As we used a small group of fish as a social stimulus to motivate the test fish into escaping from the maze, it is possible that the effect we found could be a combination of both spatial learning and social motivation. If this is the case, then a possible difference in social motivation between enriched and control fish may underpin the maze performance differences. We believe that this explanation is unlikely, as both enriched and control test fish were observed to move towards and interact with the stimuli fish on leaving the maze.
Animals that grow and develop in a natural, non-captive environment have the advantage that direct experience helps to refine and adapt behaviour so that it fits the demands of local environments. In this way, an animal learns the adaptive value of being wary of predators, or it learns how to most efficiently move between different resources. By contrast, animals that are reared in captivity and subsequently released are at a considerable disadvantage because they are behaviourally ill-equipped to deal with the novel environment. We suggest that use of environmental enrichment in the captive environment helps to prime fish in terms of their underlying neural mechanisms and their behavioural plasticity, and that together the benefits of these kinds of priming process help to confer a greater chance of post-release survival.
Acknowledgements
We thank the Voss hatchery and Eidfjord Genbank for the fish, and Frank Midtøy, Diep Mach Ellertsen and Valentina Tronci for technical assistance. We appreciate the constructive comments from Nadia Aubin-Horth on an earlier version of the paper.
Data accessibility
All data used in this paper are provided in the electronic supplementary material.
Funding statement
We are grateful to the University of Bergen (www.uib.no) and the Research Council of Norway (Independent Basic Research Grant Nos. 177888 and 190469) for funding.
References
- 1.Huntingford FA, Wright PJ, Tierney JF. 1994. Adaptive variation in antipredator behaviour in threespine stickleback. In The evolutionary biology of the threespine stickleback (eds Bell MA, Foster SA.), pp. 345–380 Oxford, UK: Oxford University Press [Google Scholar]
- 2.Wiltschko R, Schöps M, Kowalski U. 1989. Pigeon homing: wind exposition determines the importance of olfactory input. Naturwissenschaften 76, 229–231 (doi:10.1007/BF00627698) [DOI] [PubMed] [Google Scholar]
- 3.Braithwaite VA, Guilford T. 1995. A loft with a view: does exposure to natural landmarks during development encourage adult pigeons to use visual landmarks during homing? Anim. Behav. 49, 252–254 (doi:10.1016/0003-3472(95)80176-6) [Google Scholar]
- 4.Clayton NS, Krebs JR. 1994. Hippocampal growth and attrition in birds affected by experience. Proc. Natl Acad. Sci. USA 91, 7410–7414 (doi:10.1073/pnas.91.16.7410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Healy SD, Gwinner E, Krebs JR. 1996. Hippocampal volume in migratory and non-migratory warblers: effects of age and experience. Behav. Brain Res. 81, 61–68 (doi:10.1016/S0166-4328(96)00044-7) [DOI] [PubMed] [Google Scholar]
- 6.Mathews F, Orros M, McLaren G, Gelling M, Foster R. 2005. Keeping fit on the ark: assessing the suitability of captive-bred animals for release. Biol. Conserv. 121, 569–577 (doi:10.1016/j.biocon.2004.06.007) [Google Scholar]
- 7.Kihslinger RL, Lema SC, Nevitt GA. 2006. Environmental rearing conditions produce forebrain differences in wild Chinook salmon Oncorhynchus tshawytscha. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 145, 145–151 (doi:10.1016/j.cbpa.2006.06.041) [DOI] [PubMed] [Google Scholar]
- 8.Price EO. 1999. Behavioral development in animals undergoing domestication. Appl. Anim. Behav. Sci. 65, 245–271 (doi:10.1016/S0168-1591(99)00087-8) [Google Scholar]
- 9.Salvanes AGV, Braithwaite VA. 2005. Exposure to variable spatial information in the early rearing environment generates asymmetries in social interactions in cod (Gadus morhua). Behav. Ecol. Sociobiol. 59, 250–257 (doi:10.1007/s00265-005-0031-x) [Google Scholar]
- 10.Mohammed AK, Winblad B, Ebendal B, Lärkfors L. 1990. Environmental influence on behaviour and nerve growth factor in the brain. Brain Res. 528, 62–72 (doi:10.1016/0006-8993(90)90195-H) [DOI] [PubMed] [Google Scholar]
- 11.Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, Petrosini L. 2005. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav. Brain Res. 163, 78–90 (doi:10.1016/j.bbr.2005.04.009) [DOI] [PubMed] [Google Scholar]
- 12.Harburger LL, Nzerem CK, Frick KM. 2007. Single enrichment variables differentially reduce age-related memory decline in female mice. Behav. Neurosci. 121, 679–688 (doi:10.1037/0735-7044.121.4.679) [DOI] [PubMed] [Google Scholar]
- 13.Falkenberg T, Mohammed AK, Henriksson B, Persson H, Winblad B, Lindfors N. 1992. Increased expression of brain-derived neurotrophic factor mRNA in rat hippocampus is associated with improved spatial memory and enriched environment. Neurosci. Lett. 138, 153–1156 (doi:10.1016/0304-3940(92)90494-R) [DOI] [PubMed] [Google Scholar]
- 14.Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. 2000. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp. Neurol. 164, 45–52 (doi:10.1006/exnr.2000.7415) [DOI] [PubMed] [Google Scholar]
- 15.Shaw C, McEachern J. (eds) 2001. Toward a theory of neuroplasticity. London, UK: Psychology Press [Google Scholar]
- 16.Rossi C, et al. 2006. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur. J. Neurosci. 24, 1850–1856 (doi:10.1111/j.1460-9568.2006.05059.x) [DOI] [PubMed] [Google Scholar]
- 17.Guillemot F. 2007. Spatial and temporal specification of neural fates by transcription factor codes. Development 134, 3771–3780 (doi:10.1242/dev.006379) [DOI] [PubMed] [Google Scholar]
- 18.Okamoto M, Inoue K, Terashima K, Soya H, Asashima M, Kuwabara T. 2011. Reduction in paracrine Wnt3 factors during aging causes impaired neurogenesis. FASEB J. 25, 3570–3582 (doi:10.1096/fj.11-184697) [DOI] [PubMed] [Google Scholar]
- 19.Rabin LA. 2003. Maintaining behavioural diversity in captivity for conservation: natural behaviour management. Anim. Welfare 12, 85–94 [Google Scholar]
- 20.Seddon PJ. 1999. Persistence without intervention: assessing success in wildlife introductions. Trends Ecol. Evol. 14, 503 (doi:10.1016/S0169-5347(99)01720-6) [DOI] [PubMed] [Google Scholar]
- 21.Fischer J, Lindenmayer DB. 2000. An assessment of the published results of animal relocations. Biol. Conserv. 96, 1–11 (doi:10.1016/S0006-3207(00)00048-3) [Google Scholar]
- 22.Maloney RF, McLean IG. 1995. Historical and experimental learned predator recognition in free-living New Zealand robins. Anim. Behav. 50, 1193–1201 (doi:10.1016/0003-3472(95)80036-0) [Google Scholar]
- 23.Dobson A, Lyles A. 2000. Black-footed ferret recovery. Science 288, 985–988 (doi:10.1126/science.288.5468.985) [DOI] [PubMed] [Google Scholar]
- 24.Shier DM, Owings DH. 2006. Effects of predator training on behaviour and post-release survival of captive prairie dogs (Cynomys ludovicanus). Biol. Conserv. 132, 126–135 (doi:10.1016/j.biocon.2006.03.020) [Google Scholar]
- 25.Seddon PJ, Armstrong DP, Maloney RF. 2007. Developing the science of reintroduction biology. Conserv. Biol. 21, 303–312 (doi:10.1111/j.1523-1739.2006.00627.x) [DOI] [PubMed] [Google Scholar]
- 26.Berejikian BA, Tezak EP, Riley SC, LaRae AL. 2001. Competitive ability and social behaviour of juvenile steelhead reared in enriched and conventional hatchery tanks and a stream environment. J. Fish Biol. 59, 1600–1613 (doi:10.1111/j.1095-8649.2001.tb00224.x) [Google Scholar]
- 27.Brown C, Davidson T, Laland K. 2003. Environmental enrichment and prior experience improve foraging behaviour in hatchery-reared Atlantic salmon. J. Fish Biol. 63, 187–196 (doi:10.1111/j.1095-8649.2003.00208.x) [Google Scholar]
- 28.Salvanes AG, Moberg VO, Braithwaite VA. 2007. Effects of early experience on group behaviour in fish. Anim. Behav. 74, 805–811 (doi:10.1016/j.anbehav.2007.02.007) [Google Scholar]
- 29.Braithwaite VA, Salvanes AGV. 2005. Environmental variability in the early rearing environment generates behaviourally flexible cod: implications for rehabilitating wild populations. Proc. R. Soc. B 272, 1107–1113 (doi:10.1098/rspb.2005.3062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JSF, Berejikian BA. 2008. Effects of the rearing environment on average behaviour and behavioural variation in steelhead. J. Fish Biol. 72, 1736–1749 (doi:10.1111/j.1095-8649.2008.01848.x) [Google Scholar]
- 31.Marchetti MP, Nevitt GA. 2003. Effects of hatchery rearing on brain structures of rainbow trout, Oncorhynchus mykiss. Environ. Biol. Fish. 66, 9–14 (doi:10.1023/A:1023269221678) [Google Scholar]
- 32.Lema SC, Hodges MJ, Marchetti MP, Nevitt GA. 2005. Proliferation zones in the salmon telencephalon and evidence for environmental influence of proliferation rate. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 141, 327–335 (doi:10.1016/j.cbpb.2005.06.003) [DOI] [PubMed] [Google Scholar]
- 33.von Krogh K, Sørensen C, Nilsson GE, Ø Øverli. 2010. Forebrain cell proliferation, behavior, and physiology of zebrafish, Danio rerio, kept in enriched or barren environments. Physiol. Behav. 101, 32–39 (doi:10.1016/j.physbeh.2010.04.003) [DOI] [PubMed] [Google Scholar]
- 34.Broglio C, Rodríguez F, Gómez A, Arias JL, Salas C. 2010. Selective involvement of the goldfish lateral pallium in spatial memory. Behav. Brain Res. 210, 191–201 (doi:10.1016/j.bbr.2010.02.031) [DOI] [PubMed] [Google Scholar]
- 35.Durán E, Ocaña FM, Broglio C, Rodríguez F, Salas C. 2010. Lateral but not medial telencephalic pallium ablation impairs the use of goldfish spatial allocentric strategies in a ‘hole-board’ task. Behav. Brain Res. 214, 480–487 (doi:10.1016/j.bbr.2010.06.010) [DOI] [PubMed] [Google Scholar]
- 36.Chomczynski P. 1993. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 15, 532–537 [PubMed] [Google Scholar]
- 37.Pfaffl MW. 2004. Quantification strategies in real-time PCR. In A–Z of quantitative PCR (ed. Bustin SA.), ch. 3, pp. 1–23 San Diego, CA: International University Line [Google Scholar]
- 38.Olsvik PA, Lie KK, Jordal AEO, Nilsen TO, Hordvik I. 2005. Evaluation of potential reference genes in real-time RT-PCR studies of Atlantic salmon. BMC Mol. Biol. 6, 21 (doi:10.1186/1471-2199-6-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer [Google Scholar]
- 40.Pinaud R, Tremere LA, Penner MR, Hess FF, Barnes S, Robertson HA, Currie RW. 2002. Plasticity-driven gene expression in the rat retina. Mol. Brain Res. 98, 93–101 (doi:10.1016/S0169-328X(01)00328-X) [DOI] [PubMed] [Google Scholar]
- 41.Van Praag H, Kempermann G, Gage FH. 2000. Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 1, 191–198 (doi:10.1038/35044558) [DOI] [PubMed] [Google Scholar]
- 42.Veena JBN, Srikumar K, Mahati V, Bhagya TR, Raju BS, Shankaranarayana R. 2009. Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J. Neurosci. Res. 87, 831–843 (doi:10.1002/jnr.21907) [DOI] [PubMed] [Google Scholar]
- 43.Cho JH, Tsai MJ. 2004. The role of BETA2/NeuroD1 in the development of the nervous system. Mol. Neurobiol. 30, 35–47 (doi:10.1385/MN:30:1:035) [DOI] [PubMed] [Google Scholar]
- 44.von Bohlen Und Halbach O. 2007. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 329, 409–420 (doi:10.1007/s00441-007-0432-4) [DOI] [PubMed] [Google Scholar]
- 45.Gonda A, Herczeg G, Merilä J. 2009. Habitat-dependent and -independent plastic responses to social environment in the nine-spined stickleback (Pungitius pungitius) brain. Proc. R. Soc. B 276, 2085–2092 (doi:10.1098/rspb.2009.0026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panula P, Chen Y-C, Priyadarshini M, Kudo H, Semenova S, Sundvik M, Sallinen V. 2010. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol. Dis. 40, 46–57 (doi:10.1016/j.nbd.2010.05.010) [DOI] [PubMed] [Google Scholar]
- 47.Mayer I, Meager J, Skjæraasen JE, Rodewald P, Sverdrup G, Fernö A. 2011. Domestication causes rapid changes in heart and brain morphology in Atlantic cod (Gadus morhua). Environ. Biol. Fish. 92, 181–186 (doi:10.1007/s10641-011-9831-1) [Google Scholar]
- 48.Shumway CA. 2010. The evolution of complex brains and behaviours in African chiclid fishes. Curr. Zool. 56, 144–156 [Google Scholar]
- 49.Strand DA, Utne-Palm AC, Jakobsen P, Braithwaite VA, Jensen KH, Salvanes AGV. 2010. Enrichment promotes learning in fish. Mar. Ecol. Prog. Ser. 412, 273–282 (doi:10.3354/meps08682) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this paper are provided in the electronic supplementary material.