Abstract
Aerobic energy production occurs via the oxidative phosphorylation pathway (OXPHOS), which is critically dependent on interactions between the 13 mitochondrial DNA (mtDNA)-encoded and approximately 70 nuclear-encoded protein subunits. Disruptive mutations in any component of OXPHOS can result in impaired ATP production and exacerbated oxidative stress; in mammalian systems, such mutations are associated with ageing as well as numerous diseases. Recent studies have suggested that oxidative stress plays a role in fitness trade-offs in life-history evolution and functional ecology. Here, we show that outcrossing between populations with divergent mtDNA can exacerbate cellular oxidative stress in hybrid offspring. In the copepod Tigriopus californicus, we found that hybrids that showed evidence of fitness breakdown (low fecundity) also exhibited elevated levels of oxidative damage to DNA, whereas those with no clear breakdown did not show significantly elevated damage. The extent of oxidative stress in hybrids appears to be dependent on the degree of genetic divergence between their respective parental populations, but this pattern requires further testing using multiple crosses at different levels of divergence. Given previous evidence in T. californicus that hybridization disrupts nuclear/mitochondrial interactions and reduces hybrid fitness, our results suggest that such negative intergenomic epistasis may also increase the production of damaging cellular oxidants; consequently, mtDNA evolution may play a significant role in generating postzygotic isolating barriers among diverging populations.
Keywords: hybrid breakdown, Tigriopus, speciation, oxidative stress, mitochondrial dysfunction
1. Introduction
Hybrid breakdown is a pattern of postzygotic isolation that occurs during the early stages of allopatric divergence, and it is characterized by markedly reduced fitness in F2 and later generation hybrids [1]. Hybrid breakdown has been observed in a wide array of phenotypes, including fecundity [2], sperm swimming speed [3], offspring viability [4,5], growth rate [6] and stress response [7]. The genes involved in the early stages of reproductive isolation are likely to be found in the cellular and biochemical pathways underlying these phenotypes.
Hybrid breakdown is often explained by the Dobzhansky–Muller (DM) model; evolution results in coadaptation among interacting sets of alleles within diverging isolated populations, but incompatibilities are revealed in recombinant F2 genomes of interpopulation hybrids [8,9]. Although most investigations of DM incompatibilities have focused on interactions among nuclear genes [10], epistasis between nuclear and mitochondrial genomes may be particularly relevant in hybrid breakdown because of the organelle's central role in metabolism [11]; small disruptions in mitochondrial function are likely to be reflected across several phenotypes [2,7,12,13]. Traits such as fertility and longevity, for instance, may be affected by certain combinations of mitochondrial haplotype and nuclear background, even at the intraspecific level [14,15]. Furthermore, systems in which mtDNA evolves rapidly, such as most animals and yeasts, may be predisposed to intergenomic DM incompatibilities [16,17].
Mitochondrial oxidative phosphorylation (OXPHOS) relies on efficient interactions between nuclear- and mtDNA-encoded proteins, making it a candidate pathway for exhibiting mitonuclear incompatibilities. The intertidal copepod Tigriopus californicus has served as an excellent model in which to examine mitonuclear epistasis and hybrid breakdown [2,18–22]. Geographically isolated populations exhibit very high levels of mtDNA divergence (more than 18%) [18], and interpopulation hybrids are viable but frequently exhibit strong fitness breakdown in life-history traits [2,19]. Such low fitness is consistently associated with mitonuclear incompatibilities exposed by hybrid combinations of nuclear and mitochondrial genomes [7,20]. Moreover, recent experiments revealed that T. californicus hybrids often suffer from impaired OXPHOS enzyme activities and lowered ATP production capacity [21,22]. These patterns are concordant with results of mitochondrial dysfunction in other hybrid systems (Nasonia: [23]; Saccharomyces: [24]).
In addition to ATP, the OXPHOS pathway results in the production of reactive oxygen species (ROS). During aerobic respiration, some of the electron-accepting oxygen molecules may remain only partially reduced and form ROS [25]. Controlled ROS leak is an inevitable by-product of OXPHOS activity, but most of the oxidants are quenched by the antioxidant system in healthy individuals [26]. However, a significant imbalance between ROS production and antioxidant capacity may result in cellular oxidative stress [26]. Mutations in proteins of OXPHOS, for instance, have been shown to disturb electron flow and cause oxidative stress [27,28]. Oxidative stress has long been associated with ageing as well as with several mammalian diseases [29], and it has recently received attention from ecologists as a central mechanism driving life-history trade-offs [30–32], providing a link between organismal health and reproductive output [33,34]. Despite its potentially widespread fitness consequences, oxidative stress has been largely ignored as a possible outcome of hybrid incompatibilities.
Here, we present experimental evidence that outcrossing between divergent populations may lead to increased oxidative damage, suggesting this cellular process may be relevant in the establishment of postzygotic isolating barriers. After generating recombinant inbred lines among T. californicus populations, we screened lines for evidence of fitness breakdown and quantified oxidative stress associated with each fitness level, also accounting for genetic divergence among parental populations. Considering the negative epistatic interactions in OXPHOS observed in T. californicus [19], we hypothesized that outcrossing between populations with highly divergent mitochondrial genomes may exacerbate ROS leak and oxidative stress in the same way de novo deleterious mutations do. Accordingly, we address two questions: (i) is fitness breakdown across hybrid lines associated with levels of oxidative stress? and (ii) is the level of oxidative stress across hybrid lines related to the degree of divergence between the parental lineages?
2. Material and methods
(a). Generation of experimental lines
In order to generate recombinant genomes with different levels of fitness, we crossed populations exhibiting different levels of divergence. Tigriopus californicus samples were collected from five sites along the California coast: Ocean Beach, San Diego (henceforth SD: 32°45′ N, 117°15′ W), Bird Rock, La Jolla (BR: 32°48′ N, 117°16′ W), Abalone Cove, Los Angeles (AB: 33°44′ N, 118°22′ W), Santa Cruz (SC: 36°57′ N; 122°03′ W) and Bodega Marine Laboratory, Bodega Bay (BB: 38°19′ N, 123°04′ W). Animals were kept in large stock cultures in 400-ml beakers (approx. 500–1000 individuals per beaker) containing 300 ml of filtered seawater. All stock and experimental cultures were maintained in 20°C with a 12 L : 12 D cycle, and were fed ground dried Spirulina wafers.
Hybrids were generated between two genetically similar populations (SD × BR) and multiple genetically divergent populations (SD or BR × AB, SC or BB); divergence estimates were based on sequences of a mitochondrial gene (cytochrome b) and a nuclear-encoded mitochondrial-targeting gene (mitochondrial RNA polymerase; electronic supplementary material, table S1 and table 1). Experimental crosses were performed in Petri dishes and kept in the same conditions as stock cultures. Tigriopus californicus females mate only once. Mature males clasp and guard virgin females until they reach their terminal moult (i.e. when they are reproductively mature), at which time the females are inseminated [21]. Mature males and virgin females from each population were obtained by teasing apart clasped pairs with fine needles. Separated individuals were then used to start intrapopulation parental control crosses (e.g. SD × SD) and interpopulation hybrid crosses (e.g. SD♀ × BR♂ and reciprocal). Crosses were initiated with four replicate dishes, each comprising 20–25 pairs. At each generation from F1 through F3, clasped pairs were separated, and individuals mated with those from replicate dishes at the same stage in order to prevent inbreeding during the first two generations of recombination. As gravid F3 females appeared, they were isolated in individual dishes, and each isofemale line was then propagated through full-sib matings for five generations, with new generations being transferred to fresh culture dishes. A total of 360 hybrid (36 of each cross) and 120 parental (24 of each) isofemale lines were initiated at the F3 generation, and both hybrid and parental sets of crosses were inbred in the same manner. Once each line reached the F9 generation, we made no more efforts to maintain discrete generations, allowing the number of individuals to increase. Density of individuals was maintained similar across lines by transferring highly fecund lines to larger containers (e.g. deeper Petri dishes or beakers). Water quality, salinity and food were monitored and adjusted as required.
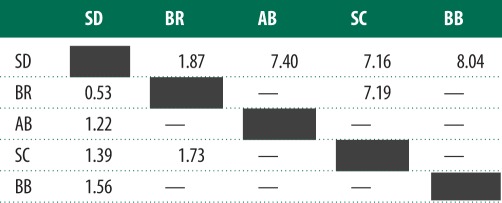
Table 1.
Amino acid sequence divergence (%) among Tigriopus californicus populations. Population abbreviations: SD, San Diego; BR, Bird Rock; AB, Abalone Cove; SC, Santa Cruz; BB, Bodega Bay. Shown are pairwise divergences for cytochrome b (above diagonal) and mitochondrial RNA polymerase (below diagonal). Comparisons marked with ‘—’ were not estimated because hybrids between those populations were not included in this study.
 |
(b). Assessment of fecundity
The number of hatching larvae (nauplii) has been repeatedly shown to suffer breakdown in F2 or later generation hybrids of T. californicus [2,21]. We used this trait as a measure of fitness in each F9 line that had sufficient individuals. Clasped pairs from each F9 inbred line were isolated in fresh culture plates and closely monitored for the hatching of a female's first brood, at which time the number of hatched nauplii was tallied under a dissecting microscope, and the female returned to culture. Fecundity for each inbred line was estimated as the mean number of hatchlings across six to 15 replicate broods. We defined ‘parental-level’ fitness by the lowest mean fecundity across inbred parental lines. Finally, we screened hybrid lines according to their fitness levels, designating lines with fecundity at or higher than the minimum parental-level as ‘high fitness’ and lines below that level as ‘low fitness’ (i.e. exhibiting hybrid breakdown).
(c). Oxidative damage assay
Oxidative stress is the outcome of an imbalance between ROS production and total antioxidant defence capacity, with a net increase in unquenched ROS resulting in damage to proteins, lipids and DNA [25,26]. The observed level of damage to macromolecules hence provides better quantification of oxidative stress than commonly used assays of antioxidant enzyme activities or gene expression [35]. For each inbred line, we quantified the concentration of 8-hydroxy-2′-deoxyguanosine (8-OH-dG), which is the oxidized derivative of guanine and a highly specific biomarker of ROS-mediated damage to DNA [36]. Tissue samples were obtained by collecting the broods initiated for Assessment of fecundity. After fecundity was measured, broods were allowed to grow to age 21–24 days, when they were transferred to a 1.5-ml centrifuge tube and rinsed twice with fresh filtered seawater. The water was then removed by pipetting, and the copepods were flash-frozen by immersing the tube in a slurry dry-ice : ethanol mixture. Tissues were kept at −80°C until all replicates were obtained for the assay. By maintaining similar culture conditions and by collecting copepods at the same age, we attempted to control for environmental and age effects among lines.
We determined through preliminary trials that a minimum of 15 individuals is required in a sample in order to obtain sufficient genomic DNA (gDNA) for the 8-OH-dG assay. When collecting copepods for tissue, as described above, we aimed to have 15–25 individuals per sample. Because some hybrid lines had consistently low fecundity, we pooled different replicate broods as necessary to obtain the minimum number. gDNA was extracted from frozen tissues using the Qiagen DNeasy kit (cat. 69506), extract purity assessed by measuring absorbance ratios A260/A280 and A260/A230, and gDNA concentration determined with a sensitive fluorescence method (PicoGreen assay kit, Invitrogen cat. P11496). For each line, two to three high-quality gDNA samples were used in an enzyme-linked immunosorbent assay (ELISA, Cayman Chemical cat. 589320) to quantify 8-OH-dG, and resulting values were normalized to respective DNA concentrations.
(d). Statistical analyses
All statistical analyses were performed in R v. 2.6.2 (R Development Core Team). An initial comparison of overall difference in 8-OH-dG levels between parental and hybrid lines was performed with linear mixed-effects models, with cross type (parental/hybrid) included as fixed effect and replicate as a random effect. A model with equal variances not assumed between cross types performed better than a model with equal variances (log-likelihood ratio = 45.2, p < 0.001); hence, we report results from the former model. Subsequent comparisons between groups (parental versus hybrids, with hybrid lines categorized by fitness level and degree of interpopulation genetic divergence) for differences in 8-OH-dG and fecundity were performed with t-tests, using mean values for each inbred line as individual data points. Finally, Pearson's correlation was used to examine the association between oxidative damage and fecundity.
3. Results
A total of 12 parental and 28 hybrid lines were still viable at the F9 generation, after five generations of inbreeding. Hybrid lines exhibited significantly lower fecundity than parental lines (t19.90 = 3.39, p < 0.01; mean ± s.e.m. number of first-brood larvae, parental: 21.3 ± 2.3, hybrid: 13.9 ± 0.8). Variance in fecundity across hybrid lines was high, with 16 lines showing breakdown (i.e. below-parental fecundity) and 12 lines well within the range of parental fitness (figure 1). As expected [2], crosses between genetically close populations yielded proportionately fewer low-fitness lines than those between divergent populations (Fisher's exact test, p = 0.015), but this result is tempered by the fact that only a single pair of genetically close populations was used in the study.
Figure 1.
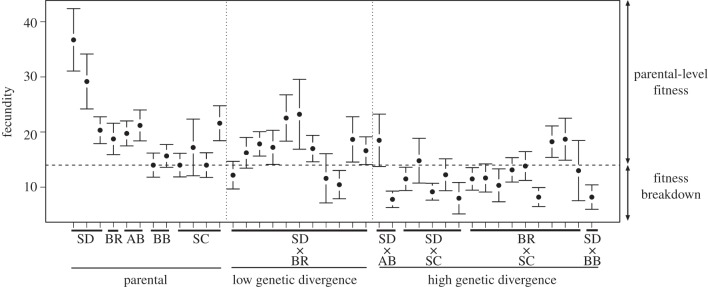
Distribution of fecundity across inbred lines of Tigriopus californicus. Fecundity was measured as the number of larvae hatching from a female's first brood. Data points are means ± s.e.m. for each experimental line. Population abbreviations are as listed in table 1. The horizontal dashed line separates hybrid samples according to their fitness level, using the lowest parental mean (n = 14 hatchlings) as threshold. Reciprocal crosses (e.g. SD♀ × SC♂ and SC♀ × SD♂) are pooled under the same designation (SD × SC).
ELISAs revealed that T. californicus hybrid lines exhibited, on average, elevated levels of oxidative damage to DNA when compared with parental lines (F1,75 = 7.19, p = 0.009, figure 2 and electronic supplementary material, figure S1). Average levels of 8-OH-dG between high and low divergence crosses were very similar, but the variance across low divergence lines was greater than across high divergence lines (figure 2). High-divergence hybrid lines exhibited significantly higher levels of 8-OH-dG than parental lines (t24.6 = 2.30, p = 0.015), whereas lines in the single low-divergence cross showed no significant difference from parentals (t10.7 = 1.19, p = 0.13). Furthermore, the highest levels of DNA damage were consistently found in lines suffering hybrid breakdown (t-test versus parental lines: t21.7 = 2.54, p = 0.009), whereas hybrid lines with parental-level fecundity showed no significant difference in damage when compared with parentals (t14.7 = 0.57, p = 0.29, figure 2). Finally, we detected a significant negative relationship between fecundity and 8-OH-dG levels across all lines (r = −0.37, p = 0.018, figure 3).
Figure 2.
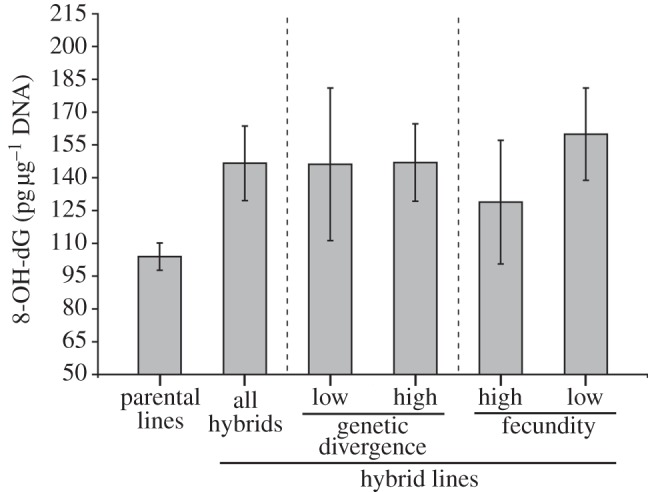
Levels of oxidative damage to DNA in Tigriopus californicus inbred lines. Shown are means ± s.e.m. picograms of 8-OH-dG, normalized to DNA concentrations, for each category of inbred line. For genetic divergence, reciprocal crosses between SD × BR are considered ‘low’, whereas crosses between (SD or BR) × (AB, SC or BB) are considered ‘high’. Fecundity levels were assigned based on the mean fecundity of each hybrid line compared with the parental-level threshold, as described in figure 1. In other words, hybrid lines showing breakdown were pooled as ‘low’, and those with parental-level fecundity were pooled as ‘high’.
Figure 3.
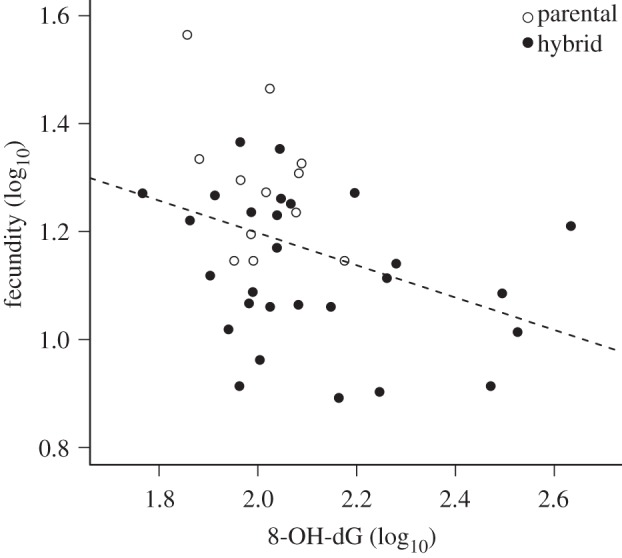
Correlation between fecundity and oxidative damage to DNA across inbred lines of Tigriopus californicus. Fecundity was measured in females, whereas oxidative damage was quantified in their offspring.
4. Discussion
Experiments with genetic model systems have shown that defects in any of the OXPHOS enzyme complexes exacerbate ROS production, presumably by stalling electron flow and accumulating superoxide anions (O2−), the precursor of most ROS [37–39]. In many of these systems, the mitochondrial dysfunctions are associated with human diseases and cancers, and have been traced to point mutations in OXPHOS genes [28,40,41]. In T. californicus, populations are highly divergent across mtDNA genes [18] and interpopulation hybrids suffer from impaired OXPHOS functions [21,22]. Hence, we hypothesized that mitochondrial dysfunction in T. californicus exacerbate levels of cellular oxidative stress and promote increased damage to macromolecules.
(a). Oxidative damage in Tigriopus californicus
Overall, our results show that oxidative damage in hybrids was, on average, more than 30% higher than the background levels in parental populations. Support for our hypothesis, however, does not require this overall difference, because intergenic incompatibilities are expected to occur in only a fraction of recombinant genomes. Indeed, variance in oxidative damage across hybrid lines was large. Although previous studies strongly suggest that hybrid breakdown in T. californicus is due predominantly to mitonuclear incompatibilities [20–22], the influence of negative epistasis in nuclear–nuclear interactions cannot be ruled out [42]. Nevertheless, our main goal was to test whether recombinant genomes exhibiting these incompatibilities also suffer from elevated oxidative stress. Recombinant lines showing breakdown in fecundity showed the highest levels of DNA damage, even in the cross involving less-divergent populations. With a single cross type within the ‘low-divergence’ category, our study has limited power to address the influence of divergence. The presence of high oxidative damage in some low-divergence hybrid lines, however, at least suggests that ROS-inducing negative epistasis may appear early in allopatric divergence.
We argue that the observed values of damage in hybrids may be an underestimate. Because of limitations in assay sensitivity, we could not quantify damage in individual copepods, and pooling F2 hybrids would conceal associations between phenotypes and different recombinant genomes. Our approach was to allow inbreeding of isofemale lines for five generations in order to increase genomic homogeneity within lines, permitting pooling of individuals for quantification of damage as well as fecundity. One consequence of waiting for multiple generations before assaying damage is that recombinant lines with the strongest degree of breakdown were likely lost due to intrinsic selection before reaching the F9 stage. Therefore, levels of oxidative damage in hybrids are potentially even higher soon after the first generation of recombination.
(b). Oxidative stress and reproduction
Recent ecological studies have suggested that oxidative stress may be a key link in trade-offs between reproduction and other life-history traits, but this relationship is still poorly understood [26,30,31]. For instance, experimental manipulations of reproductive investment in zebra finches (Taeniopygia guttata) revealed a decrease in antioxidant capacity with increasing clutch sizes [33], whereas the reverse was observed in field colonies of a swift species (Alpus melba) [43], and no correlations were found between measures of damage and litter size in Mus musculus [44]. These studies aimed to test the general prediction that elevated oxidative stress is a costly consequence of reproductive efforts [30,31]. Our experiment was not designed to examine increases in oxidative stress owing to reproduction, which would require quantifying pre- and post-reproduction oxidative status. Nevertheless, we found a significant negative relationship between fecundity of females and 8-OH-dG (i.e. oxidative damage) levels of their offspring across all lines. While the proximate cause of this correlation cannot be inferred from our data, it is consistent with findings in mice and humans that accumulation of oxidative damage may directly impair reproduction by injuring oocytes, reducing fertilization success, or affecting early embryonic development [45,46].
(c). Possible sources and consequences of oxidative stress in Tigriopus californicus
The mitochondrial OXPHOS pathway is the main source of ROS [25,26]. Given previous experimental evidence of mitochondrial dysfunction in T. californicus hybrids, at least some genetic incompatibilities responsible for increased oxidative damage in these hybrids are likely components of OXPHOS complexes [20,22]. OXPHOS dysfunction may in turn also be due to disruption of other intergenomic pathways, especially of mtDNA transcription [7] or translation [47], which are required for producing OXPHOS proteins. Elevated oxidative damage in hybrids with mitonuclear mismatches could result from either a direct increase in basal ROS leakage from a dysfunctional OXPHOS system or from reduced antioxidant capacity. It seems likely that both mechanisms can contribute to reductions in hybrid fitness. Damage, owing to increased ROS, in turn, requires the repair or replacement of macromolecules resulting in higher maintenance costs and consequently reduced hybrid fertility and viability. Because previous work has shown that hybrid mitochondria in T. californicus have reduced ATP synthetic capacity [21], energy for preventive antioxidant activity and energy to repair damage is possibly reduced in hybrids.
In addition to causing damage to cellular components, increased ROS levels activate numerous retrograde signalling pathways from mitochondria to the nucleus, which alter expression of many genes in both genomes and can affect important metabolic processes [48,49]. Moreover, dysfunctional ROS metabolism may undergo a positive feedback loop: increased oxidative damage can increase mitochondrial dysfunction, promoting further ROS accumulation [25,29].
Evolution within populations promotes coadaptation between the nuclear and mitochondrial genomes. We propose that hybridization breaks up this coadaptation, specifically in the OXPHOS pathway, where nuclear- and mtDNA-encoded proteins interact most closely [14,17,50]. Disruption of OXPHOS function results in elevated basal levels of ROS and potentially reduced antioxidant defence. Besides causing damage to cellular components and increasing maintenance and repair costs, accumulation of ROS has been shown to promote mtDNA rearrangements [38] and to reduce protein translation fidelity [51]. The net impact will be reduced hybrid fecundity, viability and growth rate. Consequently, our data suggest that elevated oxidative stress resulting from hybridization between genetically differentiated natural populations may be a significant molecular mechanism underlying the establishment of postzygotic isolating barriers.
Acknowledgements
We thank R. Pereira and G. Moy for helpful discussions of our results and technical advice. We are also grateful to Associate Editor Damian Dowling and two anonymous referees for their thoughtful suggestions that greatly improved the paper.
Funding statement
This study was supported by grants from the National Science Foundation to R.S.B. (grants nos. DEB-1051057 and IOS-1155030).
References
- 1.Harrison RG. 1990. Hybrid zones: windows on evolutionary process. In Oxford surveys in evolutionary biology (eds Futuyma DJ, Antonovics DJJ.), pp. 69–128 New York, NY: Oxford University Press [Google Scholar]
- 2.Edmands S. 1999. Heterosis and outbreeding depression in interpopulation crosses spanning a wide range of divergence. Evolution 53, 1757–1768 (doi:10.2307/2640438) [DOI] [PubMed] [Google Scholar]
- 3.Whiteley AR, Persaud KN, Derome N, Montgomerie R, Bernatchez L. 2009. Reduced sperm performance in backcross hybrids whitefish species-pairs (Coregonus sp.). Can. J. Zool. 87, 566–572 (doi:10.1139/Z09-042) [Google Scholar]
- 4.Breeuwer AJ, Werren JH. 1995. Hybrid breakdown between two haplodiploid species: the role of nuclear and cytoplasmic genes. Evolution 49, 705–717 (doi:10.2307/2410324) [DOI] [PubMed] [Google Scholar]
- 5.Rogers SM, Bernatchez L. 2006. The genetic basis of intrinsic and extrinsic post-zygotic reproductive isolation jointly promoting speciation in the lake whitefish species complex (Coregonus clupeaformis). J. Evol. Biol. 19, 1979–1994 (doi:10.1111/j.1420-9101.2006.01150.x) [DOI] [PubMed] [Google Scholar]
- 6.Burton RS. 1990. Hybrid breakdown in developmental time in the copepod Tigriopus californicus. Evolution 44, 1814–1822 (doi:10.2307/2409510) [DOI] [PubMed] [Google Scholar]
- 7.Ellison CK, Burton RS. 2008. Genotype-dependent variation of mitochondrial transcriptional profiles in interpopulation hybrids. Proc. Natl Acad. Sci. USA 105, 15 831–15 836 (doi:10.1073/pnas.0804253105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobzhanksy T. 1936. Studies on hybrid sterility. II. Localization of sterility factors in Drosophila pseudoobscura hybrids. Genetics 21, 113–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller HJ. 1942. Isolating mechanisms, evolution, and temperature. Biol. Symp. 6, 71–125 [Google Scholar]
- 10.Orr HA. 2005. The genetic basis of reproductive isolation: insights from Drosophila. Proc. Natl Acad. Sci. USA 102, 6522–6526 (doi:10.1073/pnas.0501893102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gershoni M, Templeton AR, Mishmar D. 2009. Mitochondrial bioenergetics as a major motive force of speciation. Bioessays 31, 642–650 (doi:10.1002/bies.200800139) [DOI] [PubMed] [Google Scholar]
- 12.Fishman L, Willis JH. 2006. A cytonuclear incompatibility causes anther sterility in Mimulus hybrids. Evolution 60, 1372–1381 (doi:10.1554/05-708.1) [DOI] [PubMed] [Google Scholar]
- 13.Gibson JD, Niehuis O, Peirson BRE, Cash EI, Gadau J. 2013. Genetic and developmental basis of F2 hybrid breakdown in Nasonia parasitoid wasps. Evolution 67, 2124–2132 (doi:10.1111/evo.12080) [DOI] [PubMed] [Google Scholar]
- 14.Camus MF, Clancy DJ, Dowling DK. 2012. Mitochondria, maternal inheritance, and male aging. Curr. Biol. 22, 1717–1721 (doi:10.1016/j.cub.2012.07.018) [DOI] [PubMed] [Google Scholar]
- 15.Yee WKW, Sutton KL, Dowling DK. 2013. In vivo male fertility is affected by naturally occurring mitochondrial haplotypes. Curr. Biol. 23, R55–R56 (doi:10.1016/j.cub.2012.12.002) [DOI] [PubMed] [Google Scholar]
- 16.Rand DM, Haney RA, Fry AJ. 2004. Cytonuclear coevolution: the genomics of cooperation. Trends Ecol. Evol. 19, 645–652 (doi:10.1016/j.tree.2004.10.003) [DOI] [PubMed] [Google Scholar]
- 17.Burton RS, Barreto FS. 2012. A disproportionate role for mtDNA in Dobzhansky–Muller incompatibilities? Mol. Ecol. 21, 4942–4957 (doi:10.1111/mec.12006) [DOI] [PubMed] [Google Scholar]
- 18.Burton RS, Byrne RJ, Rawson PD. 2007. Three divergent mitochondrial genomes from California populations of the copepod Tigriopus californicus. Gene 403, 53–59 (doi:10.1016/j.gene.2007.07.026) [DOI] [PubMed] [Google Scholar]
- 19.Burton RS, Ellison CK, Harrison JS. 2006. The sorry state of F2 hybrids: consequences of rapid mitochondrial DNA evolution in allopatric populations. Am. Nat. 168, S14–S24 (doi:10.1086/509046) [DOI] [PubMed] [Google Scholar]
- 20.Harrison JS, Burton RS. 2006. Tracing hybrid incompatibilities to single amino acid substitutions. Mol. Biol. Evol. 23, 559–564 (doi:10.1093/molbev/msj058) [DOI] [PubMed] [Google Scholar]
- 21.Ellison CK, Burton RS. 2006. Disruption of mitochondrial function in interpopulation hybrids of Tigriopus californicus. Evolution 60, 1382–1391 [PubMed] [Google Scholar]
- 22.Rawson PD, Burton RS. 2002. Functional coadaptation between cytochrome c and cytochrome c oxidase within allopatric populations of a marine copepod. Proc. Natl Acad. Sci. USA 99, 12 955–12 958 (doi:10.1073/pnas.202335899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellison CK, Niehuis O, Gadau J. 2008. Hybrid breakdown and mitochondrial dysfunction in hybrids of Nasonia parasitoid wasps. J. Evol. Biol. 21, 1844–1851 (doi:10.1111/j.1420-9101.2008.01608.x) [DOI] [PubMed] [Google Scholar]
- 24.Lee H-Y, Chou J-Y, Cheong L, Chang N-H, Yangm S-Y, Leu J-Y. 2008. Incompatibility of nuclear and mitochondrial genomes causes hybrid sterility between two yeast species. Cell 135, 1065–1073 (doi:10.1016/j.cell.2008.10.047) [DOI] [PubMed] [Google Scholar]
- 25.Andreyev AY, Kushnareva YE, Starkov AA. 2005. Mitochondrial metabolism of reactive oxygen species. Biochemistry 70, 200–214 (doi:10.1007/s10541-005-0102-7) [DOI] [PubMed] [Google Scholar]
- 26.Balaban RS, Nemoto S, Finkel T. 2005. Mitochondria, oxidants, and aging. Cell 120, 483–495 (doi:10.1016/j.cell.2005.02.001) [DOI] [PubMed] [Google Scholar]
- 27.Gusdon AM, Votyakova TV, Reynolds IJ, Mathews CE. 2007. Nuclear and mitochondrial interaction involving mt-Nd2 leads to increased mitochondrial reactive oxygen species production. J. Biol. Chem. 282, 5171–5179 (doi:10.1074/jbc.M609367200) [DOI] [PubMed] [Google Scholar]
- 28.Petros JA, et al. 2005. mtDNA mutations increase tumorigenicity in prostate cancer. Proc. Natl Acad. Sci. USA 102, 719–724 (doi:10.1073/pnas.0408894102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin MT, Beal MF. 2006. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 (doi:10.1038/nature05292) [DOI] [PubMed] [Google Scholar]
- 30.Dowling DK, Simmons LW. 2009. Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. B 276, 1737–1745 (doi:10.1098/rspb.2008.1791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monaghan P, Metcalfe NB, Torres R. 2009. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 12, 75–92 (doi:10.1111/j.1461-0248.2008.01258.x) [DOI] [PubMed] [Google Scholar]
- 32.Metcalfe NB, Alonso-Álvarez C. 2010. Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct. Ecol. 24, 984–996 (doi:10.1111/j.1365-2435.2010.01750.x) [Google Scholar]
- 33.Bertrand S, Alonso-Álvarez C, Devevey G, Faivre B, Prost J, Sorci G. 2006. Carotenoids modulate the trade-off between egg production and resistance to oxidative stress in zebra finches. Oecologia 147, 576–584 (doi:10.1111/j.1365-2435.2006.01191.x) [DOI] [PubMed] [Google Scholar]
- 34.Lane N. 2011. Mitonuclear match: optimizing fitness and fertility over generations drives ageing within generations. Bioessays 33, 860–869 (doi:10.1002/bies.201100051) [DOI] [PubMed] [Google Scholar]
- 35.Constantini D, Verhulst S. 2009. Does high antioxidant capacity indicate low oxidative stress? Funct. Ecol. 23, 506–509 (doi:10.1111/j.1365-2435.2009.01546.x) [Google Scholar]
- 36.Guetens G, De Boeck G, Highley M, van Oosterom AT, de Bruijn EA. 2002. Oxidative DNA damage: biological significance and methods of analysis. Crit. Rev. Clin. Lab. Sci. 39, 331–457 (doi:10.1080/10408360290795547) [DOI] [PubMed] [Google Scholar]
- 37.Pitkänen S, Robinson BH. 1996. Mitochondrial complex I deficiency leads to increased production of superoxide radicals and induction of superoxide dismutase. J. Clin. Invest. 98, 345–351 (doi:10.1172/JCI118798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esposito LA, Melov S, Panov A, Cottrell BA, Wallace DC. 1999. Mitochondrial disease in mouse results in increased oxidative stress. Proc. Natl Acad. Sci. USA 96, 4820–4825 (doi:10.1073/pnas.96.9.4820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwong LK, Sohal RS. 1998. Substrate and site specificity of hydrogen peroxide generation in mouse mitochondria. Arch. Biochem. Biophys. 350, 118–126 (doi:10.1006/abbi.1997.0489) [DOI] [PubMed] [Google Scholar]
- 40.Mattiazzi M, Vijayvergiya C, Gajewski CD, DeVivo DC, Lenaz G, Wiedmann M, Manfredi G. 2004. The mtDNA T8993G (NARP) mutation results in an impairment of oxidative phosphorylation that can be improved by antioxidants. Hum. Mol. Genet. 13, 869–879 (doi:10.1093/hmg/ddh103) [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J-I. 2008. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320, 661–664 (doi:10.1126/science.1156906) [DOI] [PubMed] [Google Scholar]
- 42.Edmands S, Burton RS. 1999. Cytochrome c oxidase activity in interpopulation hybrids of a marine copepod: a test for nuclear–nuclear or nuclear–cytoplasmic coadaptation. Evolution 53, 1972–1978 (doi:10.2307/2640456) [DOI] [PubMed] [Google Scholar]
- 43.Bize P, Devevey G, Monaghan P, Doligez B, Christe P. 2008. Fecundity and survival in relation to resistance to oxidative stress in a free-living bird. Ecology 89, 2584–2593 (doi:10.1890/07-1135.1) [DOI] [PubMed] [Google Scholar]
- 44.Garratt M, Vasilaki A, Stockely P, McArdle F, Jackson M, Hurst JL. 2011. Is oxidative stress a physiological cost of reproduction? An experimental test in house mice. Proc. R. Soc. B 278, 1098–1106 (doi:10.1098/rspb.2010.1818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarín JJ. 1996. Potential effects of age-associated oxidative stress on mammalian oocytes/embryos. Mol. Hum. Reprod. 2, 717–724 (doi:10.1093/molehr/2.10.717) [DOI] [PubMed] [Google Scholar]
- 46.Agarwal A, Gupta S, Sharma RK. 2005. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 3, 28 (doi:10.1186/1477-7827-3-28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barreto FS, Burton RS. 2013. Evidence for compensatory evolution of ribosomal proteins in response to rapid divergence of mitochondrial rRNA. Mol. Biol. Evol. 30, 310–314 (doi:10.1093/molbev/mss228) [DOI] [PubMed] [Google Scholar]
- 48.Chae S, et al. 2013. A systems approach for decoding mitochondrial retrograde signaling pathways. Sci. Signal. 6, RS4 (doi:10.1126/scisignal.2003266) [DOI] [PubMed] [Google Scholar]
- 49.Woodson JD, Chory J. 2008. Coordination of gene expression between organellar and nuclear genomes. Nat. Rev. Genet. 9, 383–395 (doi:10.1038/nrg2348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dowling DK, Friberg U, Lindell J. 2008. Evolutionary implications of non-neutral mitochondrial genetics variation. Trends Ecol. Evol. 23, 546–554 (doi:10.1016/j.tree.2008.05.011) [DOI] [PubMed] [Google Scholar]
- 51.Ling J, Söll D. 2010. Severe oxidative stress induces protein mistranslation through impairment of an aminoacyl-tRNA synthetase editing site. Proc. Natl Acad. Sci. USA 107, 4028–4033 (doi:10.1073/pnas.1000315107) [DOI] [PMC free article] [PubMed] [Google Scholar]


