Abstract
The internal temperature of flowers may be higher than air temperature, and warmer nectar could offer energetic advantages for honeybee thermoregulation, as well as being easier to drink owing to its lower viscosity. We investigated the responses of Apis mellifera scutellata (10 colonies) to warmed 10% w/w sucrose solutions, maintained at 20–35°C, independent of low air temperatures, and to 20% w/w sucrose solutions with the viscosity increased by the addition of the inert polysaccharide Tylose (up to the equivalent of 34.5% sucrose). Honeybee crop loads increased with nectar temperature, as did the total consumption of sucrose solutions over 2 h by all bees visiting the feeders. In addition, the preference of marked honeybees shifted towards higher nectar temperatures with successive feeder visits. Crop loads were inversely proportional to the viscosity of the artificial nectar, as was the total consumption of sucrose solutions over 2 h. Marked honeybees avoided higher nectar viscosities with successive feeder visits. Bees thus showed strong preferences for both warmer and less viscous nectar, independent of changes in its sugar concentration. Bees may benefit from foraging on nectars that are warmer than air temperature for two reasons that are not mutually exclusive: reduced thermoregulatory costs and faster ingestion times due to the lower viscosity.
Keywords: nectar temperature, nectar viscosity, crop load, Apis mellifera scutellata, floral microclimate
1. Introduction
Foraging bees require high thoracic temperatures, maintained through metabolic heat production, for flight between flowers and during foraging [1–4]. The energy gain from solar radiation is also important for their thermoregulation during flight [4,5]. In cooler environmental conditions, foraging honeybees have to expend more energy to maintain their body temperatures, and their metabolic rates increase accordingly [2,6]. Thus, there could be energetic advantages to exploiting warm nectar sources.
Although nectar is usually assumed to be at air temperature, the internal temperature of certain flowers may be a few degrees higher than air temperature, depending on the flower size, structure and colour [7–10]. Dyer et al. [11] demonstrated that bumblebees prefer to forage in warmer flowers, which could indicate that food temperature might serve as an additional reward along with the nutrition obtained from nectar. If heat is perceived as a reward, crop loads may increase accordingly; and an increase in crop load of honeybees associated with an increase in air (and presumably nectar) temperature has been demonstrated by Afik & Shafir [12]. Australian stingless bees (Trigona carbonaria) preferred warmer nectars (15% w/v sucrose) at lower air temperatures, but changed their behaviour and selected cooler nectar when the air temperature reached 34°C [13].
The viscosity of sugar solutions increases steeply with concentration but also decreases, less dramatically, with increased temperature [14,15]. The inverse relationship between viscosity and temperature suggests advantages to feeding on warm nectar in addition to energetic benefits [16]. Because higher sugar concentrations increase the viscosity of the nectar, nectar drinkers face a trade-off between energy intake and expenditure [17,18], so that warming of the nectar may alter the optimal nectar concentration for efficient energy intake, depending on the drinking technique that the animal uses. The modelling and empirical data presented by Kim et al. [19] confirm that, for all nectar drinkers, volumetric intake is inversely proportional to nectar viscosity, although the steepness of the relationship depends on drinking technique. Researchers can differentiate between concentration and viscosity effects by adding a cellulose ester polysaccharide (Tylose) to artificial nectar. This increases its viscosity without adding nutritional value, and has enabled study of the effect of viscosity (in isolation from sugar concentration) on trophallaxis in honeybees [20], ingestion rates in the orchid bee Euglossa imperialis [21] and licking rates in sunbirds [22].
In this study, carried out in a natural setting, the effects of warming nectar above air temperature and increasing its viscosity were investigated in separate experiments, carried out on cold winter mornings. For each parameter, we measured the crop loads of individual honeybees and the total volume ingested by colonies visiting the feeders, as well as changes in the preferences of marked individuals with time. We predicted that bees will prefer higher nectar temperatures for the energetic benefits, and food sources of lower viscosity that are easier to ingest.
2. Material and methods
(a). General methods
This study was conducted on 10 colonies of Apis mellifera scutellata at the experimental farm of the University of Pretoria. The honeybees were trained before the experiment to collect food from feeders containing 50% w/w sucrose solution. Experiments were conducted during the winter of 2010 (temperature experiments in July and viscosity experiments in August) because we needed cool air temperatures and little competing forage. Pretoria winters are dry and sunny and all tests were conducted with the colonies in the sun. Feeding stations were placed approximately 30 cm from each tested colony in order to avoid robbing from neighbouring colonies. Experiments were replicated five times, at 4-day intervals, for each colony. In all replicates the order of the feeders remained the same, i.e. lowest to highest temperature or lowest to highest viscosity.
(b). Testing temperature effects
A feeding station was constructed with four feeders (20 ml Petri dishes). To test for temperature effects, the feeders were warmed to 20°C, 25°C, 30°C and 35°C by enclosing them in heating baths. Temperatures were maintained using aquarium heaters, with submerged aquarium pumps to circulate warm water to the feeders. Gravity feeders consisted of 500 ml honey jars, containing 250 ml of a 10% w/w sucrose solution, inverted on the Petri dishes. Owing to the scarcity of food, we used 10% w/w sucrose solutions to prevent overcrowding at the feeders. Solutions were warmed to different temperatures 30 min before the start of the experiment. To correct for evaporation, the set of temperature-controlled feeders was placed in similar environmental conditions, with honeybees and other insects excluded by means of wire mesh. The rates of evaporation during the 2 h trials were low, ranging from 7.6 ± 0.9 ml (mean ± s.d.) at a feeder temperature of 20°C to 12.8 ± 1.8 ml at a feeder temperature of 35°C. All consumption values were corrected for evaporation. Temperatures of the sucrose solutions and adjacent air temperatures were monitored to the nearest 0.1°C using a type T thermocouple (IT-18, Physitemp Instruments, Clifton, NJ, USA) and a digital thermometer (APPA51, APPA Technology Corp., Taiwan).
(c). Testing viscosity effects
To determine the effect of viscosity on crop load, the same feeder setup was used except that none of the solutions was heated. The viscosity of a 20% w/w sucrose solution was increased by the addition of different quantities of Tylose H 10000 P2 (SE Tylose GmbH & Co. KG, Wiesbaden, Germany). The amounts of Tylose added were calculated from a regression equation based on data in Josens & Farina [23], up to the viscosity equivalent of a 34.5% w/w sucrose solution at 20°C (table 1). The sucrose solutions were changed from 10% to 20% for this set of tests because in preliminary runs the honeybees demonstrated a lack of willingness to feed at the lower concentration, probably due to an increase in natural food resources. Temperatures of these solutions were assumed to follow adjacent air temperatures, which were monitored during the experimental period (see electronic supplementary material, figure S1). Evaporation controls were included as for the temperature trials and consumption values were corrected for evaporation.
Table 1.
Quantities of Tylose added to increase viscosity of artificial nectar. Tylose was added to 20% w/w sucrose solutions to give viscosities equivalent to those of 27.5%, 31% and 34.5% w/w sucrose solutions. The amounts of Tylose were calculated from a regression equation based on data in Josens & Farina [23].
| Tylose added (% w/w) | viscosity equivalent as w/w sucrose (%) |
|---|---|
| 0 | 20 |
| 0.046 | 27.5 |
| 0.07 | 31 |
| 0.093 | 34.5 |
(d). Experimental protocol
Each replicate was conducted over a 2 h period. In the first hour, foragers were marked according to the feeder they visited. Each individual was marked a maximum of two times, allowing for three preferences to be obtained over the 2 h period. Markings were made with water-based acrylic paints (Genuine Heritage Craft Products, Johannesburg, South Africa), with different colours representing the four feeders. During the second hour, individual workers on all feeders were captured when they were about to return to the hive, and gently squeezed to obtain their crop loads by regurgitation. Similar numbers of bees were squeezed from all four feeders, with their preferences and crop loads being recorded. Crop loads were measured by collecting the regurgitated liquid in capillary tubes and measuring the length of the column (80 µl: 75 mm, O.D.: 1.4–1.6 mm, Lasec; Cape Town, South Africa). At the end of each trial, the amount of solution remaining in each feeder was measured, using syringes, to determine the total food intake, and subsequently corrected for evaporation. Three colonies were tested per day, with a total of five replicates being performed for each colony, giving a total of 50 replicates per experiment. The same protocol was used for both the temperature and viscosity experiments.
(e). Statistical analysis
The effects of temperature and viscosity on crop loads and total consumption were tested with Spearman rank order correlations. Linear regression analysis was used to determine the relationship between crop load and air temperature. Statistical comparisons between temperature and viscosity treatments were made using Kruskal–Wallis ANOVA with multiple comparisons of mean ranks for all groups. χ2-tests were used to determine the significance of changes in preference of marked bees between successive feeder visits, for both temperature and viscosity treatments. Separate-slopes models were used to test whether air temperature had differential effects on the treatment groups for both viscosity and temperature treatments. Correlation tests were used to investigate whether feeder temperatures were affected by ambient temperatures. Statistical analysis was done in STATISTICA v. 10. The level of significance was p < 0.05 for all tests; data are presented as means ± s.d.
3. Results
(a). Temperature experiment
Crop loads were positively correlated with nectar temperature (Spearman rank order correlation: R = 0.515, p < 0.01; figure 1a). Crop loads differed significantly between nectar temperatures (H3,1776 = 476.99, p < 0.0001), with all four temperatures differing from one another (p < 0.0001). The mean crop load of honeybees at the highest temperature of 35°C (42.4 ± 1.2 µl) was 1.4× that collected at 20°C (29.8±2.2 µl). The total volumes consumed by colonies were also positively correlated with nectar temperature (Spearman rank order correlation: R = 0.868, p < 0.05; figure 1b). The total volumes consumed differed significantly between nectar temperatures (H3,40 = 29.56, p < 0.0001). The volume collected at 30°C was higher than that at 20°C (p < 0.05), and the volume collected at 35°C was higher than those at 20°C (p < 0.0001) or 25°C (p < 0.02). The mean volume collected by colonies at 35°C (94.9±18.5 ml) was 3.3× that collected at 20°C (28.4±13.4 ml).
Figure 1.
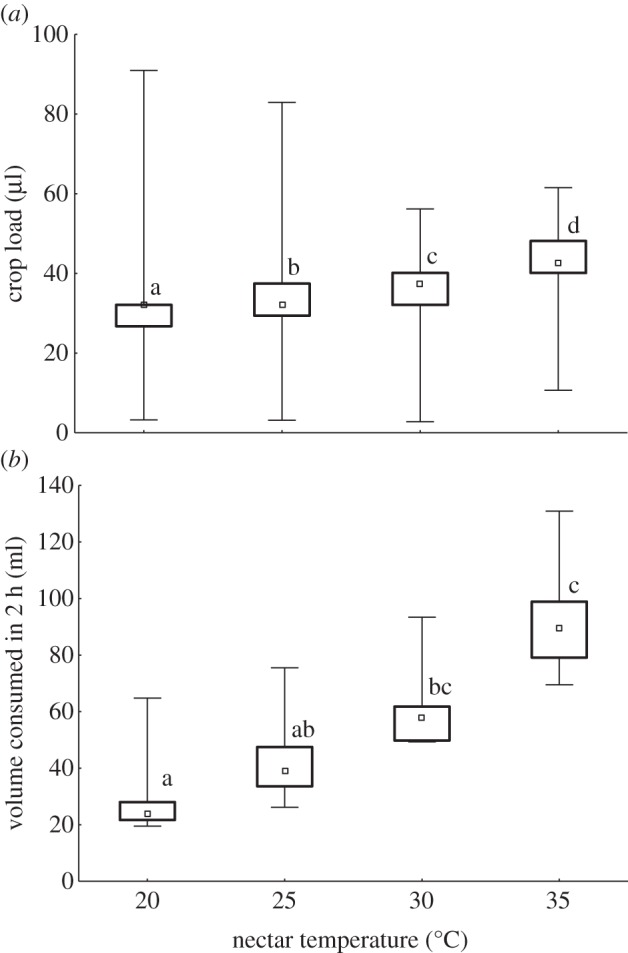
Effect of nectar temperature on feeding by honeybees. Bees ingested a 10% w/w sucrose solution maintained at 20°C, 25°C, 30°C or 35°C. (a) Crop loads (µl) of individual bees (n = 38–56 bees from each of 10 colonies). (b) Total volume (ml) consumed by colonies (n = 10) in 2 h. Small squares are median values, boxes indicate the interquartile range and whiskers indicate minimum and maximum values. Different letters indicate significant differences between nectar temperatures.
Crop loads were negatively correlated with air temperature, with higher crop loads recorded for a particular nectar temperature at lower air temperatures (see table 2 for regression statistics). The regression slopes for the four temperatures were significantly different (d.f. = 3, F = 28.78, p < 0.0001), with 35°C having the steepest slope and 20°C the most gentle (table 2), showing that feeder temperature affects the choice of the workers. Post hoc comparisons showed that all four slopes were different (p < 0.0001 for all comparisons). Mean air temperatures during the nectar temperature trials ranged from 14.3°C to 22.0°C. The temperatures of the sucrose solutions were also monitored and varied within a narrow range, indicating that the feeder temperature was not governed by the ambient temperature (table 3; electronic supplementary material, figure S1).
Table 2.
The relationship between crop load and air temperature. Results of linear regressions describing the relationship between crop load of bees after the third feeder visit (y) and air temperature (x), for the four nectar temperatures. The intercepts are proportional to nectar temperature, with the highest intercept for nectar at 35°C. The regression slopes differed significantly, increasing from 20°C to 35°C.
| nectar temperature (°C) | R2 | equation | intercept at 14°C | p-value |
|---|---|---|---|---|
| 20 | 0.009 | y = 39.81 − 0.557x | 32.0 | <0.05 |
| 25 | 0.026 | y = 44.95 − 0.654x | 35.8 | <0.001 |
| 30 | 0.045 | y = 52.78 − 0.870x | 40.6 | <0.001 |
| 35 | 0.078 | y = 63.77 − 1.217x | 46.7 | <0.001 |
Table 3.
Measured temperatures of sucrose solutions in feeders. Values are means ± s.d. for 10 colonies (five replicates each).
| nectar temperature (°C) | measured temperature (°C) (mean ± s.d.) | range (°C) |
|---|---|---|
| 20 | 20.98 ± 0.88 | 18.9–23.3 |
| 25 | 25.31 ± 0.52 | 24.0–26.7 |
| 30 | 30.32 ± 0.61 | 28.9–32.1 |
| 35 | 35.25 ± 0.67 | 33.0–36.7 |
The preferences of individual honeybees for nectar of different temperatures during three successive feeder visits are shown in figure 2. The choices among solutions maintained at 20°C, 25°C, 30°C and 35°C differed significantly from random at the first (χ2 = 11.4, d.f. = 3, p = 0.01) and at the third feeder visits (χ2 = 42.2, d.f. = 3, p < 0.0001), but not at the second visit. If we consider all bees that visited at least once, the choices no longer differ from random at the first visit (see electronic supplementary material, figure S2): the subset of bees making three visits may have been more motivated to find warmer nectar after encountering cooler nectar during their first visit. When nectar temperatures were compared for the three feeder visits, the choice of the 20°C and 25°C solutions declined with successive visits (20°C: χ2 = 15.1, d.f. = 2, p < 0.001 and 25°C: χ2 = 6.00, d.f. = 2, p < 0.05), while the choice of the 35°C solution increased over time (χ2 = 19.7, d.f. = 2, p < 0.0001). There was no change over time for the 30°C solution.
Figure 2.
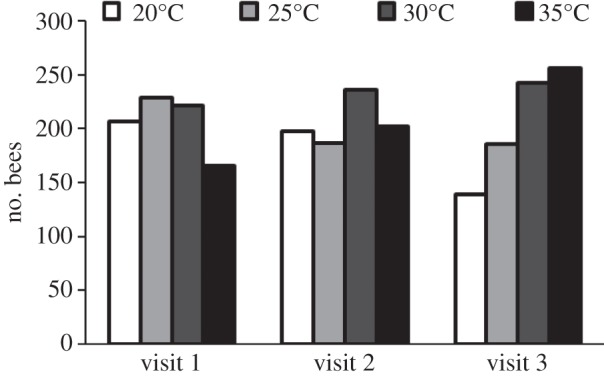
Preferences of marked honeybees for nectar of different temperatures. Choices made by bees offered 10% w/w sucrose solutions maintained at 20°C, 25°C, 30°C and 35°C: only data for individual bees observed during three successive feeder visits are included.
(b). Viscosity experiment
Crop loads were negatively correlated with nectar viscosity (Spearman rank order correlation: R = −0.635, p < 0.01; figure 3a). Crop loads differed significantly between nectar viscosities (H3,1776 = 721.19, p < 0.0001), with all four viscosities differing from one another (p < 0.001). Mean crop loads were 40.3 ± 1.2 µl for bees feeding on the 20% sucrose solution without Tylose, decreasing to 31.0±0.5 µl for bees feeding on the solution with the highest viscosity. The total volumes consumed by colonies were also negatively correlated with nectar viscosity (Spearman rank order correlation: R = −0.468, p < 0.05; figure 3b). The total volumes consumed differed significantly between nectar viscosities (H3,40 = 8.88, p < 0.05): the volume collected at a viscosity equivalent to 34.5% sucrose was lower than that collected from a 20% sucrose solution (p < 0.05), but no other differences between nectar viscosities were significant. The mean volume collected by colonies at the 20% sucrose feeder (44.0±10.9 ml) was 1.5× higher than that collected at the feeder with a viscosity equivalent to 34.5% sucrose (29.5±10.0 ml).
Figure 3.
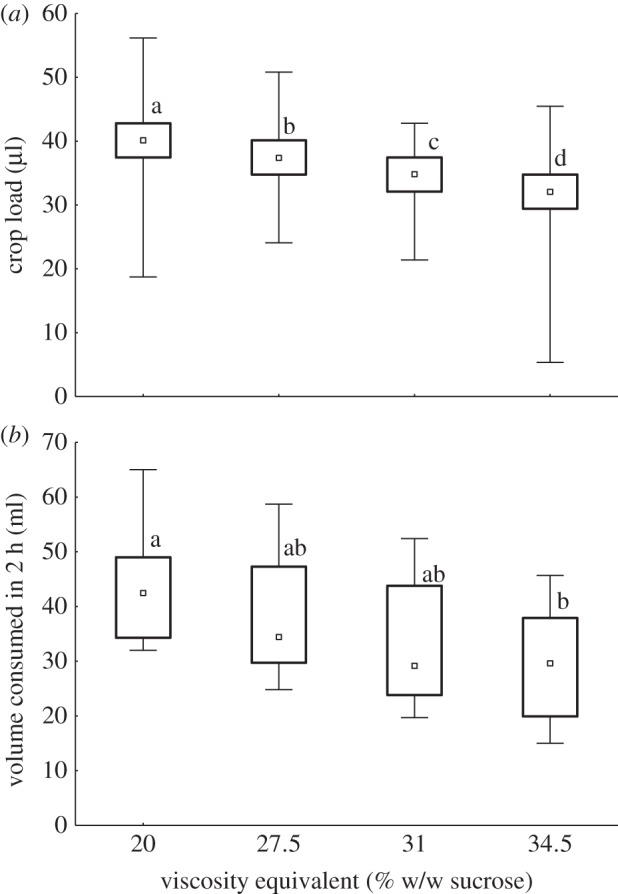
Effect of nectar viscosity on feeding by honeybees. Bees ingested a 20% w/w sucrose solution with Tylose used to increase its viscosity to those of 27.5%, 31% and 34.5% w/w sucrose solutions. (a) Crop loads (µl) of individual bees (n = 35–52 bees from each of 10 colonies). (b) Total volume (ml) consumed by colonies (n = 10) in 2 h. Small squares are median values, boxes indicate the interquartile range and whiskers indicate minimum and maximum values. Different letters indicate significant differences between nectar viscosities.
There was no significant correlation found between the crop loads and the associated air temperatures (p > 0.05, n = 1776); mean air temperatures during the nectar viscosity trials ranged from 17.3 to 24.1°C. The interaction between viscosity treatment and air temperature was not significant (d.f. = 3, F = 0.855, p = 0.49), indicating that the slopes do not differ significantly among the four levels of nectar viscosity.
The preferences of individual honeybees for nectar of different viscosities during three successive feeder visits are shown in figure 4. The choices made by bees among solutions of different viscosity differed significantly from random choices at the first (χ2 = 11.9, d.f. = 3, p < 0.01) and second feeder visits (χ2 = 13.2, d.f. = 3, p < 0.005). As in the case of different nectar temperatures (see electronic supplementary material, figure S2), the bees making three successive feeder visits were a subset of those making at least one visit. At the second visit, it is possible that they perceived higher viscosity as indicating higher sucrose concentration. At the third visit there were significantly fewer bees choosing the highest viscosity (χ2 = 15.7, d.f. = 3, p < 0.005), perhaps because they had more experience of the lack of reward due to higher viscosity. When nectar viscosities were compared for the three feeder visits, the only significant change with successive visits was for the highest viscosity (χ2 = 16.0, d.f. = 2, p < 0.001).
Figure 4.
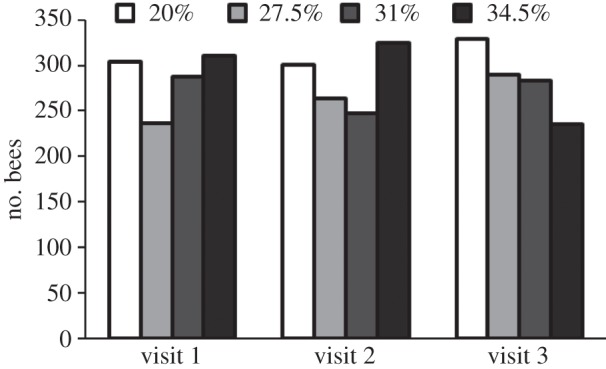
Preferences of marked honeybees for nectar of different viscosities. Choices made by bees offered 20% w/w sucrose solutions with Tylose used to increase the viscosity to that of 27.5%, 31% and 34.5% w/w sucrose solutions: only data for individual bees observed during three successive feeder visits are included.
4. Discussion
The preference of honeybees for drinking warm water was reported a century ago by Gendot [24], based on simple experiments and on observations of bees drinking at a manure heap on cold mornings and at thermal springs. We have shown that honeybees foraging on cold mornings prefer warmer artificial nectar, and also less viscous artificial nectar; the preference for warmer nectar may be partly owing to its lower viscosity.
The crop load that a honeybee obtains from nectar sources while foraging is dependent on nectar composition and concentration, nectar flow rate, distance to the hive and air temperature [12,25,26]. Our data demonstrate convincingly that individual crop loads and colony food consumption of honeybees increase when they forage on warmer sucrose solutions. At the colony level, food consumption is the product of crop load and the number of foragers visiting the feeder, and the preference is multiplied as bees are recruited to the warm solutions and as individual bees are motivated to make successive visits, shown by the steeper relationship in figure 1b than in figure 1a. Kovac et al. [5] found that crop loads of water foragers increased with air temperature, and Afik & Shafir [12] showed that honeybees collected larger crop loads at higher air temperatures (with a maximum load at 32°C), presumably as a result of increased nectar temperature in the temperature-controlled flight room. In both these studies, air and water/food temperatures were the same. In our experiments, warming the nectar had a greater influence on crop loads at lower air temperatures, but crop load did not increase with air temperature: in fact it decreased. The explanation for these opposite trends is that for our bees the thermal benefit of warmed nectar was highest at low air temperature, whereas in the other two studies [5,12] low air temperature offered no advantage. Using thermal imaging, Norgate et al. [13] showed a clear effect of nectar temperature on body temperatures of stingless bees T. carbonaria, but did not measure crop loads except to show for a small number of bees that crop loads averaged 2.8 µl on both cool and warm feeders.
Bumblebees are able to perceive floral warmth as an additional reward, and learn to use the colours of flowers to predict the floral temperature before feeding [11,27]. That they process warmth and sucrose concentration independently has nicely been demonstrated by their ability to use lower temperature as a cue to higher rewards [27]. Using the proboscis extension reflex, Hammer et al. [28] showed that honeybees learn to associate warmth with food rewards when their antennae are touched with a warm surface (mimicking contact with a warm flower). Since all feeders in our experiments were identical, there were no visual cues and thus the honeybees had to learn which feeder would give them the greatest benefits (see electronic supplementary material, figure S2).
There are two potential benefits that nectarivores may derive from drinking warm nectar. The first is energetic, as demonstrated by differences in the metabolic rate of hummingbirds drinking cool and warm nectar [29]. In temperate climates honeybees have to invest energy to elevate and maintain their thoracic temperature for flight [3,4,30]. The availability of warmer nectars at lower air temperatures will aid in the maintenance of body temperature while feeding: this has been confirmed by the use of thermal imaging techniques to show that stingless bees feeding on warmer nectars are better able to maintain their body temperatures while on flowers [13]. In honeybees, Afik & Shafir [12] found that the time from stopping imbibing to flying off the artificial flower was shortest at high air temperatures, but here the effect of higher nectar temperatures cannot be separated from that of higher air temperatures. Ingestion of warm nectar should reduce the cost of intermittent warm-up during honeybee foraging, necessary to counteract convective heat losses in flight [1]. Crop loads measured in this study, especially at the higher nectar temperatures, are a high proportion of the abdominal volume in A. m. scutellata. Abdominal temperatures of honeybees tend to be low and unregulated [1], and there is little evidence for shunting of heat between thorax and abdomen, but this has been tested during thoracic overheating [31], not in conditions relevant to foraging at low air temperatures.
The second potential benefit from drinking warm nectar concerns food viscosity. We found that an increase in viscosity of 20% sucrose solutions resulted in a decrease in crop loads and total consumption, even though the range of viscosities used was narrow in terms of natural nectar concentrations. Viscosity has been assumed to have little effect on the intake rates of bees drinking sugar solutions below 35–40% in concentration [17,18] and was not considered in the experiments of Norgate et al. [13] using stingless bees. However, the model of optimal nectar intake constructed by Heyneman [16] showed benefits of feeding at higher temperatures for nectar concentrations as low at 10%. Tezze & Farina [20] measured rates of trophallaxis between honeybees and found that the transfer rate of a 30% sucrose solution decreased with increasing viscosity. Suction-feeding orchid bees show declining rates of nectar intake when Tylose is used to increase the food viscosity [21]. The lower viscosity of warmer nectars enables honeybees to drink more quickly: imbibing time of bees feeding on 30% sucrose decreases with an increase in air temperature [12]. In our viscosity trials, individual honeybees demonstrated no preference for viscosity in their initial choice and only over time did they show a greater attraction to the less viscous solutions. For experiments on both nectar temperature and viscosity, we had to use dilute artificial nectar in order to avoid overcrowding at the feeders, so the viscosity benefits from warming would be small. According to empirical data [14], the viscosity of the 10% sucrose used in our temperature experiment would decrease from 1.26 mPa s at 20°C to 0.89 mPa s at 35°C. However, we found significant effects on intake and preferences in the viscosity experiment, even at the relatively low viscosities used: these, assuming that air temperature averaged 20°C, would have ranged from 1.96 mPa s for the 20% sucrose solution to approximately 4.34 mPa s for the 34.5% equivalent [14,32]. The temperature effect on viscosity will be much more pronounced at the higher concentrations occurring in natural nectars, e.g. 15.04 mPa s for a 50% sucrose solution at 20°C [14].
Even small differences between air and flower temperatures can be beneficial for small ectothermic insects visiting or sheltering in flowers, as well as for plant reproduction [8,9,33,34]. Floral attributes such as colour and shape lead to passive heating in direct solar radiation, and the intrafloral temperature can increase by several degrees above ambient [8]. In addition, it has recently been shown that yeasts in nectar can increase its temperature, although this may be a mixed blessing for pollinators because fermentation also reduces the sugar content [35]. The microclimate in some endothermic flowers that do not produce nectar offers a significant energetic benefit to insect pollinators, often beetles [36]. Given the results that we obtained, a bee drinking nectar that is above air temperature will not only benefit in terms of thermoregulation during foraging but will also ingest the nectar more easily and carry a greater crop load. Moreover, floral warming increases nectar production, as demonstrated, for example, in the inflorescences of Grevillea robusta [37], and may also increase nectar concentration if post-secretory evaporation rates increase [38]. That the preference of bees for flowers in sunshine might be due to improved nectar rewards was also acknowledged by Kovac & Stabentheiner [4]. Floral warmth may act as a cue for pollinators (signalling improved nectar rewards) as well as a reward [39]. Other cues recently shown to be used by hawkmoths are CO2 [40,41] and relative humidity [42]: like floral warmth, these will be effective only under certain environmental conditions.
The motivational state of foraging honeybees influences their body temperatures, which increase in response to higher sucrose concentrations, higher nectar flow rates, and food sources closer to the hive (references in [4]). When rewards are not so great, bees reduce their thoracic temperature and save energy: for example, bees obtaining dilute food at feeders allow their thoracic temperature to drop while foraging [3,43]. In honeybees drinking warm nectar, it would be interesting to distinguish between passive heating due to crop filling (which would be expected to heat the abdomen) and ‘motivational’ heating, as occurs in bees provided with high-quality food (which would be expected to heat the thorax) [3,44]. Further research is needed to distinguish between the direct energetic benefit of warm nectar and the effects of lower viscosity. Depending on the thermal environment, elevated floral temperatures may be important for the foraging behaviour of many insect pollinators.
Acknowledgements
We thank Robin Crewe for the use of the honeybees on which the study was conducted, Luke Verburgt who assisted in the design of the heating system, Hannelie Human for advice and Jeri Wright for comments on the manuscript.
Funding statement
This work was supported by the University of Pretoria and the South African National Research Foundation.
References
- 1.Heinrich B. 1979. Thermoregulation of African and European honeybees during foraging, attack, and hive exits and returns. J. Exp. Biol. 80, 217–229 [Google Scholar]
- 2.Roberts SP, Harrison JF. 1999. Mechanisms of thermal stability during flight in the honeybee Apis mellifera. J. Exp. Biol. 202, 1523–1533 [DOI] [PubMed] [Google Scholar]
- 3.Waddington KD. 1990. Foraging profits and thoracic temperature of honey bees (Apis mellifera). J. Comp. Physiol. B 160, 325–329 (doi:10.1007/BF00302599) [Google Scholar]
- 4.Kovac H, Stabentheiner A. 2011. Thermoregulation of foraging honeybees on flowering plants: seasonal variability and influence of radiative heat gain. Ecol. Entomol. 36, 686–699 (doi:10.1111/j.1365-2311.2011.01313.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovac H, Stabentheiner A, Schmaranzer S. 2010. Thermoregulation of water foraging honeybees—balancing of endothermic activity with radiative heat gain and functional requirements. J. Insect Physiol. 56, 1834–1845 (doi:10.1016/j.jinsphys.2010.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison JF, Fewell JH, Roberts SP, Hall HG. 1996. Achievement of thermal stability by varying metabolic heat production in flying honeybees. Science 274, 88–90 (doi:10.1126/science.274.5284.88) [DOI] [PubMed] [Google Scholar]
- 7.Kevan PG, Baker HG. 1983. Insects as flower visitors and pollinators. Annu. Rev. Entomol. 28, 407–453 (doi:10.1146/annurev.en.28.010183.002203) [Google Scholar]
- 8.Herrera CM. 1995. Floral biology, microclimate, and pollination by ectothermic bees in an early-blooming herb. Ecology 76, 218–228 (doi:10.2307/1940644) [Google Scholar]
- 9.McKee J, Richards AJ. 1998. Effect of flower structure and flower colour on intrafloral warming and pollen germination and pollen-tube growth in winter flowering Crocus L. (Iridaceae). Bot. J. Linn. Soc. 128, 369–384 (doi:10.1111/j.1095-8339.1998.tb02127.x) [Google Scholar]
- 10.Corbet SA. 1990. Pollination and the weather. Israel J. Bot. 39, 13–30 [Google Scholar]
- 11.Dyer AG, Whitney HM, Arnold SEJ, Glover BJ, Chittka L. 2006. Bees associate warmth with floral colour. Nature 442, 525 (doi:10.1038/442525a) [DOI] [PubMed] [Google Scholar]
- 12.Afik O, Shafir S. 2007. Effect of ambient temperature on crop loading in the honey bee, Apis mellifera (Hymenoptera: Apidae). Entomol. Gen. 29, 135–148 (doi:10.1127/entom.gen/29/2007/135) [Google Scholar]
- 13.Norgate M, Boyd-Gerny S, Simonov V, Rosa MGP, Heard TA, Dyer AG. 2010. Ambient temperature influences Australian native stingless bee (Trigona carbonaria) preference for warm nectar. PLoS ONE 5, e12000 (doi:10.1371/journal.pone.0012000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Telis VRN, Telis-Romero J, Mazzotti HB, Gabas AL. 2007. Viscosity of aqueous carbohydrate solutions at different temperatures and concentrations. Int. J. Food Prop. 10, 185–195 (doi:10.1080/10942910600673636) [Google Scholar]
- 15.Nicolson SW, Thornburg RW. 2007. Nectar chemistry. In Nectaries and nectar (eds Nicolson SW, Nepi M, Pacini E.), pp. 215–264 Dordrecht, The Netherlands: Springer [Google Scholar]
- 16.Heyneman AJ. 1983. Optimal sugar concentrations of floral nectars: dependence on sugar intake efficiency and foraging costs. Oecologia 60, 198–213 (doi:10.1007/BF00379522) [DOI] [PubMed] [Google Scholar]
- 17.Harder LD. 1986. Effects of nectar concentration and flower depth on flower handling efficiency of bumble bees. Oecologia 69, 309–315 (doi:10.1007/BF00377639) [DOI] [PubMed] [Google Scholar]
- 18.Roubik DW, Buchmann SL. 1984. Nectar selection by Melipona and Apis mellifera (Hymenoptera: Apidae) and the ecology of nectar intake by bee colonies in a tropical forest. Oecologia 61, 1–10 (doi:10.1007/BF00379082) [DOI] [PubMed] [Google Scholar]
- 19.Kim W, Gilet T, Bush JWM. 2012. Optimal concentrations in nectar feeding. Proc. Natl Acad. Sci. USA 108, 16 618–16 621 (doi:10.1073/pnas.1108642108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tezze AA, Farina WM. 1999. Trophallaxis in the honeybee, Apis mellifera: the interaction between viscosity and sucrose concentration of the transferred solution. Anim. Behav. 57, 1319–1326 (doi:10.1006/anbe.1999.1110) [DOI] [PubMed] [Google Scholar]
- 21.Borrell BJ. 2006. Mechanics of nectar feeding in the orchid bee Euglossa imperialis: pressure, viscosity and flow. J. Exp. Biol. 209, 4901–4907 (doi:10.1242/jeb.02593) [DOI] [PubMed] [Google Scholar]
- 22.Köhler A, Leseigneur CDC, Verburgt L, Nicolson SW. 2010. Dilute bird nectars: viscosity constrains food intake by licking in a sunbird. Amer. J. Physiol. 299, R1068–R1074 (doi:10.1152/ajpregu.00208.2010) [DOI] [PubMed] [Google Scholar]
- 23.Josens RB, Farina WM. 2001. Nectar feeding by the hovering hawkmoth Macroglossum stellatarum: intake rate as a function of viscosity and concentration of sucrose solutions. J. Comp. Physiol. A 187, 661–665 (doi:10.1007/s00359-001-0238-x) [DOI] [PubMed] [Google Scholar]
- 24.Gendot G. 1907. Eau nécessaire aux abeilles. Apiculteur 51, 164–168 [Google Scholar]
- 25.Waller GD, Bachman WW. 1981. Use of honey-sac load and dance characteristics of worker honeybees to determine their sugar preferences. J. Apicult. Res. 20, 23–27 [Google Scholar]
- 26.Moffatt L. 2000. Changes in the metabolic rate of the foraging honeybee: effect of the carried weight or of the reward rate? J. Comp. Physiol. A 186, 299–306 (doi:10.1007/s003590050430) [DOI] [PubMed] [Google Scholar]
- 27.Whitney HM, Dyer AG, Chittka L, Rands SA, Glover BJ. 2008. The interaction of temperature and sucrose concentration on foraging preferences in bumblebees. Naturwissenschaften 95, 845–850 (doi:10.1007/s00114-008-0393-9) [DOI] [PubMed] [Google Scholar]
- 28.Hammer TJ, Hata C, Nieh JC. 2009. Thermal learning in the honeybee, Apis mellifera. J. Exp. Biol. 212, 3928–3934 (doi:10.1242/jeb.034140) [DOI] [PubMed] [Google Scholar]
- 29.Lotz CN, Martínez del Rio C, Nicolson SW. 2003. Hummingbirds pay a high cost for a warm drink. J. Comp. Physiol. B 173, 455–462 (doi:10.1007/s00360-003-0346-8) [DOI] [PubMed] [Google Scholar]
- 30.Woods WA, Heinrich B, Stevenson RD. 2005. Honeybee flight metabolic rate: does it depend upon air temperature? J. Exp. Biol. 208, 1161–1173 (doi:10.1242/jeb.01510) [DOI] [PubMed] [Google Scholar]
- 31.Heinrich B. 1980. Mechanisms of body-temperature regulation in honeybees, Apis mellifera. II. Regulation of thoracic temperature at high air temperatures. J. Exp. Biol. 85, 73–87 [Google Scholar]
- 32.Weast R. 1985. CRC Handbook of Chemistry and Physics. Boca Raton, FL: CRC Press [Google Scholar]
- 33.Orueta D. 2002. Thermal relationships between Calendula arvensis inflorescences and Usia aurata bombyliid flies. Ecol. Lett. 83, 3073–3085 (doi:10.2307/3071843) [Google Scholar]
- 34.Sapir Y, Shmida A, Ne'eman G. 2006. Morning floral heat as a reward to the pollinators of the Oncocyclus irises. Oecologia 147, 53–59 (doi:10.1007/s00442-005-0246-6) [DOI] [PubMed] [Google Scholar]
- 35.Herrera CM, Pozo MI. 2010. Nectar yeasts warm the flowers of a winter-blooming plant. Proc. R. Soc. B 277, 1827–1834 (doi:10.1098/rspb.2009.2252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seymour RS, White CR, Gibernau M. 2003. Heat reward for insect pollinators. Nature 426, 243–244 (doi:10.1038/426243a) [DOI] [PubMed] [Google Scholar]
- 37.Nicolson SW. 1995. Direct demonstration of nectar reabsorption in the flowers of Grevillea robusta (Proteaceae). Funct. Ecol. 9, 584–588 (doi:10.2307/2390148) [Google Scholar]
- 38.Corbet SA. 1978. Bee visits and the nectar of Echium vulgare. In The pollination of flowers by insects (ed. Richards AJ.), pp. 21–30 London, UK: Academic Press [Google Scholar]
- 39.Rands SA, Whitney HM. 2008. Floral temperature and optimal foraging: is heat a feasible floral reward for pollinators? PLoS ONE 3, e2007 (doi:10.1371/journal.pone.0002007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goyret J, Markwell PM, Raguso RA. 2008. Context- and scale-dependent effects of floral CO2 on nectar foraging by Manduca sexta. Proc. Natl Acad. Sci. USA 105, 4565–4570 (doi:10.1073/pnas.0708629105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thom C, Guerenstein PG, Mechaber WL, Hildebrand JG. 2004. Floral CO2 reveals flower profitability to moths. J. Chem. Ecol. 30, 1285–1288 (doi:10.1023/B:JOEC.0000037887.33885.c0) [DOI] [PubMed] [Google Scholar]
- 42.von Arx M, Goyret J, Davidowitz G, Raguso RA. 2012. Floral humidity as a reliable sensory cue for profitability assessment by nectar-foraging hawkmoths. Proc. Natl Acad. Sci. USA 109, 9471–9476 (doi:10.1073/pnas.1121624109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmaranzer S, Stabentheiner A. 1988. Variability of the thermal behavior of honeybees on a feeding place. J. Comp. Physiol. B 158, 135–141 (doi:10.1007/BF01075826) [Google Scholar]
- 44.Nieh JC, Leon A, Cameron S, Vandame R. 2006. Hot bumble bees at good food: thoracic temperature of feeding Bombus wilmattae foragers is tuned to sugar concentration. J. Exp. Biol. 209, 4185–4192 (doi:10.1242/jeb.02528) [DOI] [PubMed] [Google Scholar]


