Abstract
Background
The purpose of this paper is to report the long-term results of surgery without bowel resection in patients suffering from deep infiltrating endometriosis with rectovaginal or colorectal involvement.
Methods
This retrospective observational study identified 42 patients suffering with deep infiltrating endometriosis who underwent surgery. Conservative surgery was performed in 23 women (only one of them with bowel resection), and 19 women underwent a hysterectomy and bilateral salpingo-oophorectomy (HBSO). In the conservative surgery group, a later HBSO was performed in eight patients as a second operation. Pregnancies, recurrences, reoperations, use of hormone replacement therapy, and outcomes during long-term follow-up were analyzed.
Results
The average follow-up duration was 7 ± 5.7 years in conservative surgery cases. Only one patient was treated with sigmoid bowel resection in 1997 and had complications. In this conservative surgery group, 13 patients (56%) received medical treatment after surgery, 10 patients wanted to get pregnant (of whom seven [70%] were successful), and eight patients underwent a subsequent HBSO because of recurrent symptoms and/or endometrioma. Therefore, HBSO was performed in 27 patients, of whom 14 (51.8%) used hormone replacement therapy for 5.6 ± 3.6 years. No recurrences or complications were observed in patients after HBSO with or without hormone replacement therapy.
Conclusion
Good clinical results can be obtained by performing only conservative surgery and/or HBSO without bowel resection, an alternative that could reduce the number of colorectal resections that are performed very frequently nowadays. After HBSO, patients may use hormone replacement therapy for several years with total satisfaction and well-being.
Keywords: deep infiltrating endometriosis, rectovaginal septum, bowel resection, colorectal, endometriosis
Introduction
Despite the relatively high rate of morbidity, surgical excision of deep infiltrating bowel endometriosis (disc and segmental bowel resection) has become a popular treatment modality due to improved operative laparoscopy techniques.1 An increasing number of studies have reported numerous cases in which laparoscopic segmental bowel resection has been performed,2 but the clinical reason or indication is often poorly documented. Some authors have analyzed quality of life in patients who underwent laparoscopic colorectal resection3,4 without comparing these patients with others who did not undergo bowel resection. Although quality of life is improved in most of patients managed by colorectal resection, it is unclear whether a greater or similar health improvement can be achieved with less aggressive surgery, with medical treatments only, or with both.5
We have previously noticed that surgery for endometriosis involving a bowel resection is rarely justified.6 In many cases with rectovaginal septum or intestinal involvement, endometriosis can be managed without excising these lesions, and these women may experience improvement merely by taking a low-dose contraceptive pill, an antiprostaglandin agent, and/or an aromatase inhibitor after conservative surgery. In addition, in cases of recurrent endometriosis and severe pelvic blockade or when a hysterectomy and bilateral salpingo-oophorectomy (HBSO) is necessary, this procedure could provide good results without bowel resection or radical excision of deep infiltrating endometriosis. The outcomes are most likely quite similar to those of more radical surgery, but carry less risk and morbidity. Moreover, no clear clinical benefit has been demonstrated to date after a bowel resection compared with treatment without intestinal surgery, particularly when an HBSO is performed, as already noted by Sampson in 1922.7 Therefore, the efficacy of colorectal surgery for endometriosis remains unclear.1
However, it is essential that patients have accurate information about the benefits and risks associated with the procedure versus treatment without intestinal surgery. Patients should be advised that, although the treatment can be performed using a minimally invasive technique, the evidence for improvement of quality of life is scarce. To our knowledge, no clinical trial has compared bowel resection versus a control group treated without bowel resection for endometriosis.3
The objectives of this study were to report the long-term clinical results in all our patients suffering from deep infiltrating endometriosis with rectovaginal or colorectal involvement (many of them with intraluminal tumors) who were operated on without bowel surgery (n = 42), and to determine if patients with deep infiltrating endometriosis, even those with intestinal involvement, could be appropriately treated by HBSO followed by hormone replacement therapy.
Materials and methods
We reviewed all cases of severe endometriosis confirmed histopathologically that were operated on at our institution within the last 20 years (n = 450). We selected cases with surgical findings of pelvic adhesions blockade with deep infiltrating endometriosis and existence of endometriotic nodules in the rectovaginal septum, sigmoid wall, and/or intraluminal nodular inclusions in the rectosigmoid (n = 34). Eight other cases with similar characteristics were also selected and included from the 50 that were operated on by the same team at the Institute of Gynecology PAA within the last 23 years. Therefore, our study group was composed of 42 patients with deep infiltrating endometriosis with rectosigmoid involvement and, in some cases, universal adenomyosis over the uterine corpus. The diagnosis was made by previous symptoms, vaginal and rectal explorations, transvaginal ultrasound, barium enema, magnetic resonance in some cases, rectosigmoidoscopy, and surgical findings during laparotomy (see Figures 1 and 2). This study included clinical cases treated following standard medical practice and with written informed consent, so ethical approval was not necessary in Spain. The report of the ethical committee is available on request.
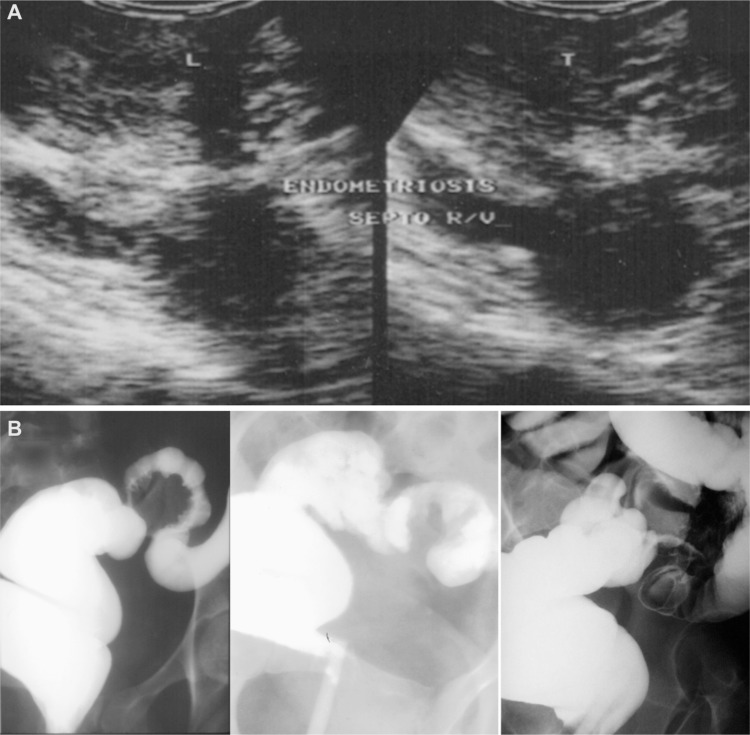
Figure 1.
(A) Transvaginal ultrasound image showing endometriosis of the rectovaginal septum. (B) Radiologic images of a barium enema in patients with deep infiltrating endometriosis and intraluminal sigmoid tumor.
Figure 2.
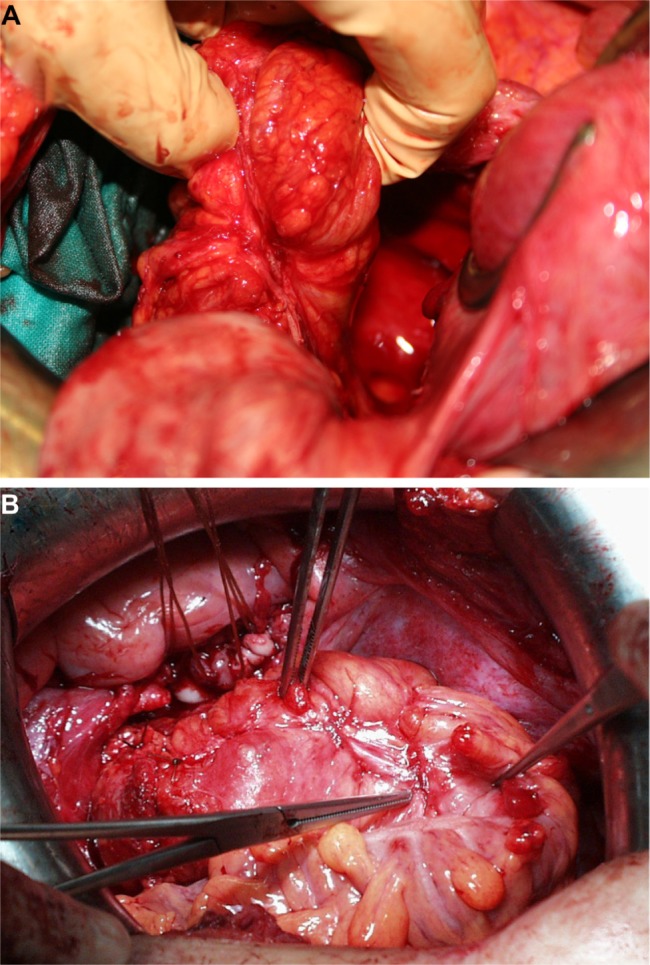
Deep infiltrating endometriosis showing location of intraluminal sigmoid tumor after hysterectomy and bilateral salpingo-oophorectomy.
Although the first author (PA) has been personally in charge of a weekly endometriosis outpatient clinic since the opening of the hospital and has control and follow-up records of all the cases attended and/or operated on at the service, medical records were reviewed in all cases, paying particular attention to the surgical reports and follow-up data, which were updated by a phone call to the patient when necessary. After HBSO, we made an appointment with many of the patients and they were followed in the menopause outpatient clinic, which one of the other authors of this paper (FQ) is in charge of. Current clinical status (including symptoms, transvaginal echography, and blood analysis) and follow-up time of each patient were considered at the time of last contact with us.
HBSO was performed initially in 19 patients (45.2%), and five cases (26.3%) had previously undergone surgery for endometriosis at another institution. The remaining 23 patients underwent conservative surgery for endometriosis, usually via laparotomy, practicing adhesiolysis, cystectomy of endometriomas, implant coagulation and eventual shaving of rectovaginal septum, myomectomy, or other additional surgery on pelvic organs, always preserving the uterus and ovaries. Later (on average, 6.3 years), a subsequent HBSO was performed in eight of these 23 cases. Reoperation was indicated due to recurrence of endometriosis, severity of symptoms, age, parity, and the patient’s wishes. Some young women without children asked for an HBSO because of severe and recurrent symptoms and no plans to have a child.
Clinical evaluation, included intensity of symptoms (using a visual analog scale for endometriosis), pelvic examination, transvaginal ultrasound (by the same gynecologist) and blood analysis (sedimentation rate, carcinoembryonic antigen, and cancer antigen CA-125 markers) were evaluated during follow-up. We considered recurrence of endometriosis in the presence of endometriomas, severe symptoms, and high tumor marker levels. The monitoring protocol was similar in the patients treated by HBSO, especially in those receiving hormone replacement therapy, paying attention to symptoms, clinical examination, and tumor markers.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences version 15.0 software (SPSS Inc, Chicago, IL, USA), RSigma (Systat Software, San Jose, CA, USA) and PEDro (Physiotherapy Evidence Database, Sydney, Australia) software. Relative risk, odds ratios, 95% confidence intervals, and the chi-squared test were used to compare groups. Data are expressed as percentages, means ± standard deviations, and minimum and maximum values. We considered data to be statistically significant at P < 0.05.
Results
Table 1 shows clinical characteristics observed in patients with deep endometriosis and rectovaginal or colorectal involvement separated according to the initial surgery performed (conservative or HBSO). The average age of patients undergoing hysterectomy was 41 years. Only 10 (52.6%) patients had had previous deliveries and the other five had undergone surgeries at other clinics. The mean age of the patients treated by conservative surgery was 30.7 years. Only two women (8.7%) in this group had had previous deliveries, and this procedure was the second surgery in one case.
Table 1.
Clinical characteristics observed in patients with deep endometriosis and rectovaginal or colorectal involvement, separated according to the first surgery performed at our center
| Conservative surgery | HBSO | All study patients | |
|---|---|---|---|
| Cases (n) | 23 (54.8) | 19 (45.2) | 42 |
| Age (years) | 30.7 ± 4.7 (22–44) | 40.9 ± 5.1 (30–49)a | 35.3 ± 7.04 (22–49) |
| Parity | 2 (8.7) | 10 (52.6)b | 12 (28.6) |
| Previous conservative surgery for endometriosis | 1 (4.3) | 5 (26.3) | 6 (14.3) |
| Associated leiomyoma | 4 (17.4) | 9 (47.4)c | 13 (30.9) |
| Associated adenomyosis | 3 (13.0) | 4 (21.1) | 7 (16.3) |
| Intraluminal tumor | 12 (52.2) | 11 (57.9) | 23 (54.8) |
| Atypical endometriosis | 2 (8.7) | 1 (5.3) | 3 (7.1) |
| Follow-up (years) | 7.0 ± 5.7 (1–23) | 4.3 ± 4.5 (1–18) | 5.6 ± 5.3 (1–23) |
Notes: Data are expressed as the number and percentage within parentheses, except for age and follow-up, for which the interval values are indicated in parentheses.
Difference between two means, CI 7.14–13.26, P < 0.001
comparison of two proportions, RR 6.05 (CI 1.5–24.3), P < 0.05
comparison of two proportions, RR 2.72 (CI 0.99–7.47), NS.
Abbreviations: HBSO, hysterectomy and bilateral salpingo-oophorectomy; NS, not significant; RR, relative risk; CI, confidence interval.
Nine patients (47.4%) from the hysterectomy group and four (17.4%) from the conservative surgery group had associated leiomyomas, so abdominal myomectomy was also performed in the conservative surgery group. Adenomyosis was found in four patients from the hysterectomy group (21.1%) and three from the conservative surgery group (13%). Atypical endometriosis (with overexpression of p53 protein) was diagnosed in one (5.3%) and two (8.7%) cases, respectively. Over half of the patients studied, ie, 11 in the hysterectomy group (57.9%) and 12 in the conservative surgery group (50.2%), had intraluminal colorectal tumors. The mean follow-up duration was 4.3 ± 4.5 (1–18) years in HBSO cases and 7 ± 5.7 (1–23) years in the conservative surgery cases. Eight patients in the latter group later underwent HBSO.
Table 2 shows the additional surgery performed during the initial operation, and post-surgical treatments, pregnancies, recurrences, and reoperations for all study patients. During hysterectomy, we performed a nonaggressive resection of rectosigmoid nodules with the shaving technique8 in two patients, and an appendectomy was also performed in the other four cases. In the conservative surgery group, we performed a shaving technique in one patient and an appendectomy in another. In addition, in the conservative surgery group (helped by a digestive surgeon) we performed a sigmoid bowel resection (with end-to-end anastomosis) in one patient having an intraluminal tumor. This intervention was complicated by anastomotic leak and peritonitis, and a colostomy and subsequent surgery were necessary to restore intestinal continuity. We decided to perform no other bowel resection in the future if possible. In the other four conservative surgery women (17.4%), a myomectomy was made.
Table 2.
Data on additional surgery performed during the first CS or HBSO, post-surgical treatments, pregnancies, recurrences, and reoperations of all study patients
| HBSO (n = 19) | Conservative surgery (n = 23) | |
|---|---|---|
| Additional surgery | Shaving: 2 (10.5) | Myomectomy: 4 (17.4) |
| Appendectomy: 4 (21.0) | Shaving: 1 (4.3) Appendectomy: 1 (4.3) Bowel resection: 1 (4.3) |
|
| Post-surgical treatment | Expectation: 8 (42.1) | Expectation: 10 (43.4) |
| HRT: 11 (57.9) | GnRH analogs: 10 (43.5) Danazol: 1 (4.3) Contraceptive pill: 2 (8.7) |
|
| Post-surgery term pregnancy | No | Not desired: 13 (56.5)a |
| Spontaneous: 6 (26.1) With AR: 1 (4.3) Infertility, adoption: 3 (13.0) |
||
| Recurrences | No: 0 (0.0) | No: 10 (43.5) Symptoms: 8 (34.8) Endometriomas: 5 (21.7) |
| Reoperations | No: 0 (0.0) | No: 14 (60.9) CS again: 1 (4.3) HBSO: 8 (34.8) |
Notes: Data are expressed as the number and percentage within parentheses.
In one case, the partner had had a vasectomy. Ten of the patients treated with primary CS wanted to become pregnant, of whom seven (70%) did so.
Abbreviations: GnRH, gonadotropin-releasing hormone; HBSO, hysterectomy and bilateral salpingo-oophorectomy; HRT, hormone replacement therapy; AR, assisted reproduction; CS, conservative surgery.
Among the 19 patients who underwent initial HBSO, 11 (57.9%) were treated with estrogen-progesterone hormone replacement therapy for 1–2 years and then with low-dose estrogen alone or with tibolone indefinitely. The other eight women who did not want to use hormone replacement therapy remained under annual periodic clinical review with or without an alternative treatment. No recurrences of symptoms or endometriosis were observed, and all patients showed good clinical evolution.
The post-surgical treatment recommended in the conservative surgery group included gonadotropin-releasing hormone analogs for 6 months (n = 10, 43.5%), danazol (n = 1), contraceptive pills (n = 2), and no treatment or an anti-inflammatory or similar drug during menstruation. Thirteen women (56.5%) did not intend to become pregnant (in one case, the partner had had a vasectomy). The other 10 women did desire pregnancy; six of these women became pregnant spontaneously, and one became pregnant after in vitro fertilization (70%). Three women were unable to become pregnant (30%) despite assisted reproduction, and two chose adoption. Symptoms or recurrence of endometriomas were observed in 13 patients (56.5%), and nine were reoperated on (one underwent conservative surgery and eight (34.8%) underwent HBSO).
No differences were found with regard to recurrences and reoperations according to post-surgical treatment in the patients who underwent initial conservative surgery. However, as shown in Table 3, the rates of recurrence and reoperation were lower (albeit not significantly) among women who became pregnant compared with those who did not.
Table 3.
Recurrences and reoperations according to subsequent pregnancy in the cases treated with conservative uterine-ovarian surgery
| Subsequent pregnancy | Recurrences | Reoperations |
|---|---|---|
| Not desired (13) | 9 (69.2) | 5 HBSO + 1 CS (46.2) |
| Yes (7) | 2 (28.6)a | 1 HBSO (14.3)b |
| Infertility (3) | 2 (66.7) | 2 HBSO (66.7) |
| Total (23) | 13 (56.5) | 9 (39.1) |
Notes: Data are expressed as the number and percentage within parentheses. Comparison of two proportions in recurrences and reoperations between “yes” versus “no” to pregnancy
relative risk = 2.4 (CI 0.71–8.12); odds ratio = 5.5 (CI 0.78–38.70)
relative risk = 3.5 (CI 0.53–22.92); odds ratio = 6.0 (CI 0.58–61.84).
Abbreviations: CI, confidence interval; HBSO, hysterectomy and bilateral salpingo-oophorectomy; CS, conservative surgery.
Patient age at the time of the last HBSO was 37 ± 4.4 years, ie, the reoperations were performed an average of more than 6 years after the first conservative surgery. Three of these patients were then treated with hormone replacement therapy (see Table 4). Thus, of the 42 patients with deep infiltrating endometriosis, 27 finally underwent HBSO (64.3%). These 27 women were followed up for 5.6 ± 4.3 years after hysterectomy, and 14 of them (51.9%) continued with hormone replacement therapy for 5.6 ± 3.6 years. They have been periodically evaluated to exclude reactivation (symptoms, clinical examination, and CA-125 levels) and no recurrence, reactivation, or problems related to endometriosis were observed, with all patients showing greater satisfaction and subjective perception of quality of life than before surgery.
Table 4.
Follow-up in the study patients with deep endometriosis and rectovaginal or colorectal involvement after hysterectomy and bilateral salpingo-oophorectomy
| n | % | Mean ± SD (range) | |
|---|---|---|---|
| Patients with HBSO | 27/42 | 64.3 | – |
| Age at second operation (years) | 8 | – | 37 ± 4.4 (31–44) |
| Follow-up in all patients after HBSO (years) | 27 | – | 5.6 ± 4.34 (1–18) |
| Patients with HRT (n) | 14/27 | 51.9 | – |
| Follow up with HRT (years) | 14 | – | 5.6 ± 3.6 (1–12) |
| Recurrences and reoperations | 0 | 0.0 | – |
Abbreviations: HBSO, hysterectomy and bilateral salpingo-oophorectomy; HRT, hormonal replacement therapy; n, number; SD, standard deviation.
Discussion
As already mentioned, we performed a bowel resection in only one patient with deep infiltrating endometriosis and a sigmoid intraluminal tumor (in 1997), and she had serious postoperative complications that were most likely due to inadequate preoperative bowel preparation. Since then, we have declined to perform bowel resection in cases with deep infiltrating endometriosis. Although we observed a recurrence rate of 56% in the patients treated with conservative surgery for endometriosis, in most of the cases, it was not due to colorectal involvement but to ovarian endometriomas, pelvic adhesion blockade, and adenomyosis. Ten of the 23 patients treated initially with conservative surgery wished to become pregnant, seven (70%) of whom were successful. The rates of recurrence and reoperation were reduced, although not significantly, for the women who became pregnant compared with those who did not.
In these recurrent cases, we performed HBSO for older women and those not wanting to have more children. By performing this operation alone without bowel resection, all symptoms were resolved, and the patients did not have any subsequent problems. The eight patients who required a reoperation of HBSO for clinical recurrence or persistence and the 19 patients who initially underwent HBSO showed good clinical evolution after a follow-up period of 1–18 years, in spite of the fact that more than 50% of the women were receiving hormone replacement therapy indefinitely. All these patients reported total satisfaction and well-being, and many of them had forgotten they had had deep infiltrating endometriosis with rectovaginal or colorectal involvement. These results call into question whether aggressive rectovaginal or intestinal surgery is appropriate for these cases, due to the risk of serious complications.
Therefore, with the increasing popularity of laparoscopic surgical excision of deep infiltrating endometriosis with a bowel resection in the absence of clear indications,2 patients should be informed of the risks and complications of this type of surgery (with a reported incidence of up to 20%) and the high rates of endometriomas and clinical recurrence if conservative uterine-ovarian surgery is performed. Moreover, patients should be made aware of the normal clinical evolution that can follow HBSO alone, particularly older women with completed fertility, and the possibility of being treated with hormone replacement therapy. Naturally we must consider that maintenance of the intestinal tumor requires ruling out other types of tumor (tumor markers and other blood analysis, magnetic resonance imaging, and colonoscopy).
No randomized studies have compared these interventions, but our small series shows that HBSO without bowel resection was sufficient in all cases. Moreover, patients treated by primary conservative surgery showed good clinical evolution, although 35% of them subsequently underwent HBSO. In some symptomatic cases, the disease may present only as an intraluminal colorectal tumor in young women without evidence of ovarian endometriosis; in these cases, disc bowel or segmental resection would be more appropriate. However, such cases are infrequent. Therefore, our data strongly suggest that bowel resection is not routinely necessary and that HBSO and subsequent hormone replacement therapy may be considered as a valid alternative (and perhaps be more suitable) to intestinal surgery for women near menopause or for those who do not wish to have children.
These considerations are in agreement with those reported by Vercellini et al9 who performed a meta-analysis of studies describing surgery for rectovaginal lesions. They concluded that excision of these lesions is of doubtful value and associated with severe morbidity. Besides, it does not improve the likelihood of pregnancy or reduce time to conception in women with infertility associated with endometriosis. Likewise, they noted that the possibility of treating peritoneal and ovarian endometriomas without excising the rectovaginal plaques should be considered, particularly in women with limited pain. However, a growing body of literature describes colorectal resection for severe endometriosis,10–15 particularly procedures involving a laparoscopically assisted technique. Meanwhile, others16 suggest that a laparoscopic resection of rectovaginal endometriosis may be associated with a higher incidence of complications than resection performed for other diagnoses.
Donnez and Squifflet8 have defended the shaving technique for deep rectovaginal endometriotic nodules and presented a prospective series of 500 patients who were operated on with this type of surgery. Similarly, Meuleman et al17 conducted a systematic review of surgical treatment of deep infiltrating endometriosis with colorectal involvement and concluded that “prospective studies reporting standardized and well-defined clinical outcomes after surgical treatment of this pathology with long-term follow-up are needed”. Further, it may be possible to obtain similar or better results using medical treatments, such as aromatase inhibitors, which should be considered in the future.18–21 Roman et al5 state that instead of choosing between medical and surgical management for treatment of rectal deep infiltrating endometriosis, it is most likely that the two therapies should be associated.
Our conclusion is similar to the comment of Wright and Ballard, ie, “although the surgical treatment of bowel endometriosis appears to have become an established practice, it is worth bearing in mind that its efficacy may be lower than that of medical treatment”1 and that HBSO alone with or without subsequent hormone replacement therapy may be a good treatment option. Only randomized studies of medical versus surgical treatments and/or conservative surgery or HBSO alone versus addition of bowel resection performed in centers serving a large number of women with deep infiltrating endometriosis (or multicenter studies) will allow us to determine which option provides the most patient satisfaction. Meanwhile, our good results without performing bowel resection or making a nonaggressive shaving to treat deep infiltrating endometriosis may contribute to restraining the current trend of excessive use of laparoscopically assisted colorectal resections.
Acknowledgments
This study was supported by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III, and FEDER, Madrid, Spain (PI07/0417, PI10/01815) within the Plan Nacional de I + D + I 2008–2011. The authors acknowledge American Journal Experts for editing the manuscript for English language.
Author contributions
PA conceived and designed the study, analyzed the data, interpreted the results, and wrote the manuscript. CN and FQ collaborated in the study design and together with MV, contributed in the acquisition, analysis and interpretation of data and revised critically the intellectual content of the manuscript. IV and VV helped with the bibliographic search, contributed with the analysis and the interpretation of the data and the critical revision of the manuscript. All authors approved the final version of the manuscript to be published. PA had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of data analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wright J, Ballard K. The surgical management of rectovaginal endometriosis: plus ç change? BJOG. 2011;118:274–277. doi: 10.1111/j.1471-0528.2010.02818.x. [DOI] [PubMed] [Google Scholar]
- 2.De Cicco C, Corona R, Schonman R, Mailova K, Ussia A, Koninckx P. Bowel resection for deep endometriosis: a systematic review. BJOG. 2011;118:285–291. doi: 10.1111/j.1471-0528.2010.02744.x. [DOI] [PubMed] [Google Scholar]
- 3.Dubernard G, Piketty M, Rouzier R, Houry S, Bazot M, Darai E. Quality of life after laparoscopic colorectal resection for endometriosis. Hum Reprod. 2006;21:1243–1247. doi: 10.1093/humrep/dei491. [DOI] [PubMed] [Google Scholar]
- 4.Dubernard G, Rouzier R, David-Montefiore E, Bazot M, Darai E. Use of the SF-36 questionnaire to predict quality-of-life improvement after laparoscopic colorectal resection for endometriosis. Hum Reprod. 2008;23:846–851. doi: 10.1093/humrep/den026. [DOI] [PubMed] [Google Scholar]
- 5.Roman H, Vassilieff M, Gourcerol G, et al. Surgical management of deep infiltrating endometriosis of the rectum: pleading for a symptom-guided approach. Hum Reprod. 2011;26:274–281. doi: 10.1093/humrep/deq332. [DOI] [PubMed] [Google Scholar]
- 6.Acién P. Deeply infiltrating endometriosis and transvaginal ultrasonography. Letter to the editor. Hum Reprod. 2009;24:2385. doi: 10.1093/humrep/dep268. [DOI] [PubMed] [Google Scholar]
- 7.Sampson JA. Intestinal adenomas of endometrial type: their importance and their relation to ovarian hematomas of endometrial type. Arch Surg. 1922;5:217–280. [Google Scholar]
- 8.Donnez J, Squifflet J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum Reprod. 2010;25:1949–1958. doi: 10.1093/humrep/deq135. [DOI] [PubMed] [Google Scholar]
- 9.Vercellini P, Crosignani PG, Somigliana E, Berlanda N, Barbara G, Fedele L. Medical treatment for rectovaginal endometriosis: what is the evidence? Hum Reprod. 2009;24:2504–2514. doi: 10.1093/humrep/dep231. [DOI] [PubMed] [Google Scholar]
- 10.Wills HJ, Reid GD, Cooper MJ, Tsaltas J, Morgan M, Woods RJ. Bowel resection for severe endometriosis: an Australian series of 177 cases. Aust N Z J Obstet Gynaecol. 2009;49:415–418. doi: 10.1111/j.1479-828X.2009.01020.x. [DOI] [PubMed] [Google Scholar]
- 11.Darai E, Dubernard G, Coutant C, Frey C, Rouzier R, Ballester M. Randomized trial of laparoscopically assisted versus open colorectal resection for endometriosis: morbidity, symptoms, quality of life, and fertility. Ann Surg. 2010;251:1018–1023. doi: 10.1097/SLA.0b013e3181d9691d. [DOI] [PubMed] [Google Scholar]
- 12.Tarjanne S, Sjöberg J, Heikinheimo O. Radical excision of rectovaginal endometriosis results in high rate of pain relief – results of a long-term follow-up study. Acta Obstet Gynecol Scan. 2010;89:71–77. doi: 10.3109/00016340903362558. [DOI] [PubMed] [Google Scholar]
- 13.Roman JD. Surgical treatment of endometriosis in prívate practice: cohort study with mean follow-up of 3 years. J Minim Invasive Gynecol. 2010;17:42–46. doi: 10.1016/j.jmig.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Kondo W, Bourdel N, Jardon K, et al. Comparison between standard and reverse laparoscopic techniques for rectovaginal endometriosis. Surg Endosc. 2011;25:2711–2717. doi: 10.1007/s00464-011-1635-z. [DOI] [PubMed] [Google Scholar]
- 15.Kavallaris A, Chalvatzas N, Hornemann A, Banz C, Diedrich K, Agic A. 94 months follow-up after laparoscopic assisted vaginal resection of septum rectovaginale and rectosigmoid in women with deep infiltrating endometriosis. Arch Gynecol Obstet. 2011;283:1059–1064. doi: 10.1007/s00404-010-1499-9. [DOI] [PubMed] [Google Scholar]
- 16.Maythan GD, Dowson HM, Levy B, Kent A, Rockall TA. Laparoscopic excision of rectovaginal endometriosis: report of a prospective study and review of the literature. Colorectal Dis. 2010;12:1105–1112. doi: 10.1111/j.1463-1318.2009.01993.x. [DOI] [PubMed] [Google Scholar]
- 17.Meuleman C, Tomassetti C, D′Hoore A, Van Cleynenbreugel B, Penninckx F, D′Hooghe T. Surgical treatment of deeply infiltrating endometriosis with colorectal involvement. Hum Reprod Update. 2011;17:311–326. doi: 10.1093/humupd/dmq057. [DOI] [PubMed] [Google Scholar]
- 18.Hefer LA, Grimm C, van Trotsenburg M, Nagele F. Role of the vaginally administered aromatase inhibitor anastrozole in women with rectovaginal endometriosis: a pilot study. Fertil Steril. 2005;84:1033–1036. doi: 10.1016/j.fertnstert.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 19.Ferrero S, Camerini G, Ragni N, et al. Letrozole and norethisterone acetate in colorectal endometriosis. Eur J Obstet Gynecol Reprod Biol. 2010;150:199–202. doi: 10.1016/j.ejogrb.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Ferrero S, Gillott DJ, Venturini PL, Remorgida V. Use of aromatase inhibitors to treat endometriosis-related pain symptoms: a systematic review. Reprod Biol Endocrinol. 2011;9:89. doi: 10.1186/1477-7827-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol. 2011;9:87. doi: 10.1186/1477-7827-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]