Abstract
The aim of this study was to investigate whether a relationship exists between the presence of low numbers of leukocytes in normal ovulatory cervical mucus and sperm quality and lipid content after migration. The percentages of live, motile and morphologically normal spermatozoa, movement parameters assessed by computer-aided sperm analysis (CASA), and ionophore-induced acrosome reaction measured by flow cytometry were determined before and after migration. High-performance liquid chromatography with ultraviolet detection was used to measure the sperm lipid content, including the various diacyl subspecies. The number of leukocytes found in solubilized mucus samples was counted using a haemocytometric method. Overall, the presence of leukocytes in the cervical mucus samples did not significantly influence sperm motility and morphology, sperm kinematic parameters, or the sperm content in sphingomyelin or cholesterol. In contrast, after migration, the decrease in various sperm diacyls and the level of induced acrosome reaction was significantly less pronounced in mucus samples containing ≥ 104 leukocytes than in mucus samples with no or rare leukocytes whereas the level of induced acrosome reaction was higher. The present data suggest that the low level of leukocytes found in normal ovulatory cervical mucus could influence the process of sperm lipid remodelling/capacitation.
Keywords: cervical mucus, leukocyte, lipid, spermatozoa
Introduction
Several studies, which were essentially in vitro studies, have shown that both the microenvironment of the female genital tract and the physiological status of the uterine cervix play a role in the acquisition of the fertilizing ability of sperm 1, 2. However, we still know very little about how precisely–and by which molecular mechanisms–the female genital tract participates in sperm capacitation. Moreover, in vivo data in the field are sparse. Using a method allowing the spermatozoa to swim in and out of human cervical mucus samples, we recently reported that human spermatozoa lose noticeable amounts of several lipid species after in vitro migration through ovulatory cervical mucus 3. We currently do not know the possible mode of action, the exact role of the mucus composition or the putative consequences of the sperm-mucus interaction on sperm-fertilizing ability. Nevertheless, it can be speculated that cervical mucus may contribute to the sperm capacitation process through a significant remodelling of the plasma membrane 4.
In addition to the pathological condition of endo- cervicitis, in which huge numbers of leukocytes are found in the cervical mucus, a physiological infiltration of the ovulatory cervical mucus by low numbers of leukocytes after sperm entry into the female genital tract, a process termed as the ‘leukocytic reaction', has been described, and it has been shown that neutrophils are the major leukocytes involved 5, 6, 7. However, whether the ‘leukocytic reaction' has a role in fertilization and how it could eventually affect sperm structures and functions is not currently known. Despite the described leukocyte infiltration of the normal cervical mucus and the fact that routine microscopic examination of the normal mucus collected for in vivo or in vitro tests of the sperm-mucus interaction often shows the presence of low numbers of cells, which may include leukocytes, the normal ovulatory cervical mucus should ideally contain a minimum number of cells; maximal cellularity scores should be 3 or 2 using the cervical mucus World Health Organization (WHO) scoring method 8. Polymorphonuclear leukocytes (PMN or neutrophils) are the major producers of reactive oxygen species (ROS) and strong generators of the superoxide anion 9. A leukocyte-induced oxidative attack has been repeatedly shown to severely damage the spermatozoa, either in vivo or in vitro, and particularly when the enzymatic and non-enzymatic antioxidative defences of the milieu and the sperm cell itself are overwhelmed 10. The highly significant decrease in the vitamin E (α-tocopherol) content of sperm after migration, which was reported earlier in the study of Feki et al. 3, may at least partly lower the resistance of sperm to an oxidative process in the female genital tract. In contrast, it has now been established that low amounts of ROS are necessary for sperm capacitation, acrosome reaction or hyperactivated motility, and it has been shown that inhibitors of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, the enzyme responsible for the neutrophil's respiratory burst, decrease sperm capacitation in vitro 11, 12. All together, these observations raise the following questions: Do the low levels of leukocytes observed in normal ovulatory cervical mucus play a direct/indirect role in the first steps of the sperm capacitation process or, more generally, do they have any putative contribution in the acquisition of the fertilizing ability of sperm?
In this preliminary study, we investigated whether the presence of leukocytes in normal ovulatory cervical mucus samples from healthy women modulates routine semen characteristics, sperm kinematic parameters, the acrosome reaction and the lipid profiles of human spermatozoa from healthy donors.
Materials and methods
Cervical mucus: donor inclusion, collection, examination and selection
Cervical mucus samples were collected from volunteers claiming not to have had coitus for a period of at least three days before mucus collection. In addition, we included only the volunteers who had not received any medications having a potentially negative effect on mucus properties–for example, oestrogen-containing or progesterone-containing contraceptive pills–in the 3 months before the study. Informed consent was obtained from all donors. Only ovulatory cervical mucus samples were collected between the ninth and fourteenth days of the menstrual cycle. The cervix was exposed with a sterile speculum. Excess debris was removed with a large cotton swab and the mucus was carefully aspirated from the endocervix using a special capillary device (Aspiglaire; CCD, Paris, France). To guarantee that the mucus samples studied were devoid of spermatozoa before the experiments, each collected sample was carefully examined microscopically, and we excluded the rare cases in which spermatozoa were observed, as well as those contaminated with blood or vaginal secretions. The mucus samples were scored using the WHO scoring method and only normal samples according to the WHO criteria (score in the range of 10–15) were used for the experiments 8. The leukocyte concentration (× 106 mL−1) in the cervical mucus was assessed after mucus solubilization with 5 mg mL−1) bromelain (Sigma-Aldrich, Saint-Quentin Fallavier, France) and peroxidase staining using a haemocytometric method 8. Rough leukocyte counts in the mucus were estimated by multiplying the concentration with the volume of the mucus sample collected.
Semen collection
Semen samples were collected at the laboratory from healthy donors by masturbation after 3–5 days of sexual abstinence. Informed consent was obtained from all donors.
Experimental design of the study
The procedure used to allow sperm to migrate into and out of the cervical mucus has been reported earlier 3. In this study and for each experiment (see below), we pooled three semen samples from three donors. A 1-mL aliquot of each freshly prepared semen pool was washed twice in Earle's medium (Eurobio, Les Ulis, France) and centrifuged for 10 min at 600 × g to remove extracellular lipids. The pellet was resuspended in a final concentration of 107 spermatozoa mL−1) in Earle's medium. Part of this preparation was kept for the lipid assessment (see below) and the remainder was incubated at 37°C for 1 h before the acrosome reaction assessment. In all, 2 mL of pooled semen samples was placed in a Nunc tube (dimensions 100 × 14 mm with push-on cap), and the cervical mucus was then carefully placed on top of the semen suspension and incubated at 37°C in 5% CO2 in air for 25 min to allow the sperm to penetrate into the cervical mucus.
The mucus samples were then rinsed twice with Earle's solution to remove spermatozoa that had not entered the mucus and incubated in 3 mL of Earle's solution at 37°C in a 5% CO2 atmosphere for 30 min. The spermatozoa that had migrated out of the mucus were recovered, washed and prepared using the same procedure as that used for the spermatozoa from the semen pool. Similarly, their concentration was adjusted to be 107 mL−1). Figure 1 illustrates the general design of the study with the main tests carried out before and after migration: routine semen analysis, kinematic parameters assessment by computer-aided sperm analysis (CASA), acrosome reaction and measurement of sperm lipid content.
Figure 1.
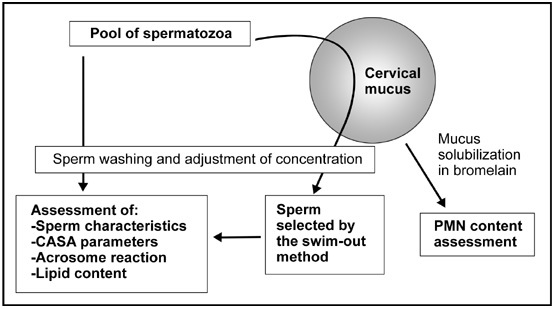
Flow chart of the experiments. Details of the modified swim-out method are reported in Feki et al. 3. CASA, computer-aided sperm analysis; PMN, polymorphonuclear leukocytes.
Sperm tests
Each semen pool was analysed following the WHO recommendations (1999) 8 except for sperm morphology, which was routinely assessed using a modified version of the method described by Auger et al. 13. The same characteristics were assessed for the sperm preparations after migration. Various sperm kinematic parameters were assessed using CASA with the Hamilton-Thorne Version 10 HTM-IVOS analyzer (Hamilton-Thorne BioSciences, Beverly, MA, USA) before and after migration. We followed the CASA guidelines of the European Society of Human Reproduction and Embryology and measured the following parameters: straight line velocity (VSL, μm s−1), average path velocity (VAP, μm s−1), curvilinear velocity (VCL, μm s−1), beat cross frequency (BCF, Hz), amplitude of lateral head displacement (ALH, μm), straightness of trajectory (STR, %) and linearity of trajectory (LIN, %). The analyzer was set up to assess standard human sperm motility: frame rate, 60 Hz; frames acquired, 30; STR threshold, 80.0% medium VAP cut-off, 25.0 μm s−1; and temperature, 37°C 14. A minimum of 200 motile sperms from at least four different fields were analysed from each sample. Spontaneous and induced acrosome reactions were assessed in washed sperm preparations before and after migration into the cervical mucus. An aliquot of each sperm sample studied was incubated with calcium ionophore A23187 (Sigma-Aldrich) in a final concentration of 10 μmol L−1 for 30 min at 37°C to artificially induce the acrosome reaction. After being washed in phosphate-buffered saline (PBS), both aliquots were resuspended in a 20-μL anti-human CD46 FITC-conjugated monoclonal antibody (Beckman Coulter, Miami, FL, USA) and mixed well. Test tubes were incubated at 37°C for 30 min in the dark, followed by centrifugation with 1 mL PBS. Final pellets were resuspended in 1 mL PBS and then analysed with a flow cytometer (Beckmann Coulter) as described earlier 15. According to the WHO guidelines (1999) 8, results were expressed as the percentage difference between induced and spontaneous acrosome reactions.
Lipid extraction and assessment
Aliquots (1 mL) of the washed sperm pools or sperm preparations recovered after migration, both adjusted to the same concentration, were mixed with 3 mL of chloroform-methanol (2:1, v/v). The mixtures were vortexed and centrifuged immediately at 600 × g for 10 min. The chloroform layer (under the phase containing lipids) was evaporated under nitrogen. The dried lipid residue was dissolved in 125 μL of methanol just before being injected into the high-performance liquid chromatography (HPLC) system. The HPLC equipment included an automatic injector with a 200-μL sample loop and an ultraviolet-visible light detector (Thermo Finnigan, Villebon sur Yvette, France). Molecular species belonging to different phospholipid classes (1-alkenyl-2-acyl, called plasmalogen or plasmenyl; 1-alkyl-2-acyl, termed plasmanyl; and diacyl), vitamin E, cholesterol and sphingomyelin were separated using two serial analytical columns: a 250 × 4.6 mm C18 and a 150 × 4.6 mm C8 Kromasil 5 μm, respectively (AIT, Houille, France). Each phospholipid peak separated by HPLC was collected and the acyl and alkenyl groups were identified by selective hydrolysis 3. Each peak of interest on a chromatographic profile was identified by comparing its retention time with that of commercial standards. The concentration of each component was determined by comparing the surface of the peaks with that of the standards.
Statistical analysis
All statistical analyses were carried out using the BMDP statistical software (Los Angeles, CA, USA) 16. The non-parametric Wilcoxon signed-rank test for paired data was used to test the difference for each variable measured in the pool and the sample after migration. The Mann-Whitney non-parametric test was used for all other unpaired two-sample comparisons.
Results
It was possible to report a leukocyte (PMN) count in the mucus for four samples: 3 × 104, 5 × 104, 8 × 104 and 8 × 104, whereas for the remaining seven samples no leukocytes (or only rare leukocytes not sufficient in number to be counted) were observed. Therefore, an arbitrary threshold of 104 leukocytes in the mucus was used in the following experiments to distinguish ‘PMN-free' mucus samples when the leukocyte count was ≤ 104, roughly corresponding to 3 or 2 WHO cellularity scores, and ‘PMN+' mucus samples were defined as having a leukocyte count > 104, roughly corresponding to 1 or 0 WHO cellularity scores.
Overall, the proportion of motile, live and normal spermatozoa was significantly improved after migration into cervical mucus, whereas the kinematic parameters remained unchanged (Table 1). The number of PMNs in the cervical mucus did not significantly change these results. The percentage of induced acrosome reaction was significantly increased after migration into the cervical mucus (15.9 ± 10.9 vs. 5.7 ± 6.4, respectively; P < 0.005). The percentage of induced acrosome reaction in sperm samples after migration into PMN-free cervical mucus was significantly lower than that of the sperm samples that migrated into PMN+ mucus (9.2 ± 2.0 vs. 18.4 ± 3.5, respectively; P < 0.05).
Table 1. Sperm characteristics and kinematic parameters (mean ± SD) before and after migration through the cervical mucus with rare or absent leukocytes (PMN-free) or with noticeable amounts of leukocytes (PMN+).
| PMN-free (n = 7) | PMN+ (n = 4) | |||
|---|---|---|---|---|
| |
Before migration |
After migration |
Before migration |
After migration |
| % of live sperms | 76 ± 6 | 85 ± 8a | 80 ± 4 | 83 ± 16a |
| % of normal sperms | 39 ± 8 | 50 ± 15a | 43 ± 8 | 53 ± 12a |
| Total motility (%) | 53 ± 9 | 76 ± 11a | 57 ± 15 | 76 ± 20a |
| Progressive motility (%) | 36 ± 14 | 52 ± 12a | 35 ± 16 | 52 ± 25a |
| VSL (μm s−1) | 54 ± 16 | 55 ± 8 | 50 ± 7 | 50 ± 13 |
| VAP (μm s−1) | 62 ± 14 | 64 ± 10 | 58 ± 5 | 58 ± 13 |
| VCL (μm s−1) | 91 ± 20 | 106 ± 25 | 86 ± 5 | 94 ± 16 |
| ALH (μm) | 3.6 ± 0.6 | 4.3 ± 1.1 | 3.5 ± 0.2 | 4.1 ± 0.4 |
| BCF (Hz) | 28 ± 1 | 28 ± 3 | 30 ± 3 | 28 ± 1 |
| STR (%) | 84 ± 6 | 83 ± 5 | 83 ± 6 | 83 ± 6 |
| LIN (%) | 59 ± 6 | 53 ± 6a | 57 ± 7 | 53 ± 8a |
Abbreviations: ALH, amplitude of lateral head displacement; BCF, beat cross-frequency; LIN, linearity of trajectory; PMN, polymorphonuclear leukocytes; STR, straightness of trajectory; VAP, average path velocity; VCL, curvilinear velocity; VSL, straight line velocity.
P < 0.01, in the comparison of the washed pooled spermatozoa and the washed spermatozoa after migration into the mucus sample.
The level of most sperm lipids after migration into either PMN+ or PMN-free cervical mucus was significantly decreased (Table 2). However, the difference between pre- and post-migration values was significantly lower in the presence of PMN than in the absence of PMN for all of the assessed phospholipid molecular species, except for cholesterol and sphingomyelin (Figure 2). The spermatozoa that migrated into the mucus containing leukocytes had a non-significantly decreased cholesterol/total diacyls ratio compared with the spermatozoa in the pool before migration (mean ± SEM: 1.12 ± 0.13 vs. 1.44 ± 0.18, respectively; P = 0.25), whereas those that migrated into the PMN-free mucus had a non-significantly increased ratio compared with the spermatozoa in the pool used (1.80 ± 0.24 vs. 1.39 ± 0.08, respectively; P = 0.16). The sperm cholesterol/total diacyl ratios for the migrated spermatozoa were significantly different between the PMN+ and PMN-free groups (1.12 ± 0.13 vs. 1.80 ± 0.24, respectively; P = 0.04), whereas the ratio values for the spermatozoa of the two pools were similar (1.39 ± 0.08 vs. 1.44 ± 0.18, respectively; P = 0.85).
Table 2. Lipid and phospholipid molecular species content (mean ± SD) in spermatozoa before and after migration through cervical mucus with rare or absent leukocytes (PMN-free) or with noticeable amounts of leukocytes (PMN+).
| Lipid and phospholipid species (nmoL per 108 sperms) | PMN-free (n = 7) |
PMN+ (n = 4) |
||
|---|---|---|---|---|
| Before migration | After migration | Before migration | After migration | |
| D*16:0/22:6 | 33.3 ± 5.8 | 19.3 ± 7.0a | 33.9 ± 4.2 | 29.2 ± 4.3b |
| D16:0/20:4 | 5.6 ± 0.2 | 2.3 ± 1.5a | 5.2 ± 0.3 | 4.0 ± 0.5 |
| D16:0/18:2 | 11.5 ± 1.8 | 2.8 ± 2.3a | 11.0 ± 3.2 | 6.2 ± 2.2b |
| D18:0/22:6 | 8.9 ± 1.9 | 3.0 ± 1.5a | 9.5 ± 2.2 | 7.0 ± 1.6b |
| D18:0/20:4 | 5.0 ± 0.9 | 1.1 ± 1.3a | 4.5 ± 0.8 | 3.8 ± 0.4b |
| Total diacyls | 68.0 ± 13.1 | 28.8 ± 10.1a | 65.6 ± 8.3 | 51.9 ± 9.2b |
| Plasmalogen | 20.9 ± 10.8 | 7.2 ± 2.4a | 16.4 ± 3.4 | 12.6 ± 1.8b |
| Sphingomyelin | 37.9 ± 7.0 | 16.1 ± 7.2a | 41.0 ± 17.0 | 23.9 ± 3.9 |
| Cholesterol | 94.3 ± 6.7 | 51.6 ± 13.4a | 90.6 ± 25.3 | 50.2 ± 6.4 |
Abbreviation: PMN, polymorphonuclear leukocytes.
P < 0.05 in the comparison of the washed pooled spermatozoa and the washed spermatozoa after migration into the mucus sample;
P < 0.05 in the comparison of the washed spermatozoa that migrated into PMN+ and those that migrated into PMN-free mucus samples.
D*: diacyls (D16:0/22:6 (1-palmitoyl-2-docosahexaenoyl-sn-glycero-3-phospholipid); D16:0/20:4 (1-palmitoyl-2-arachidonoyl-sn-glycero-3-phospholipid); D18:0/22:6 (1-stearoyl-2-docosahexaenoyl-sn-glycero-3-phospholipid).
Figure 2.
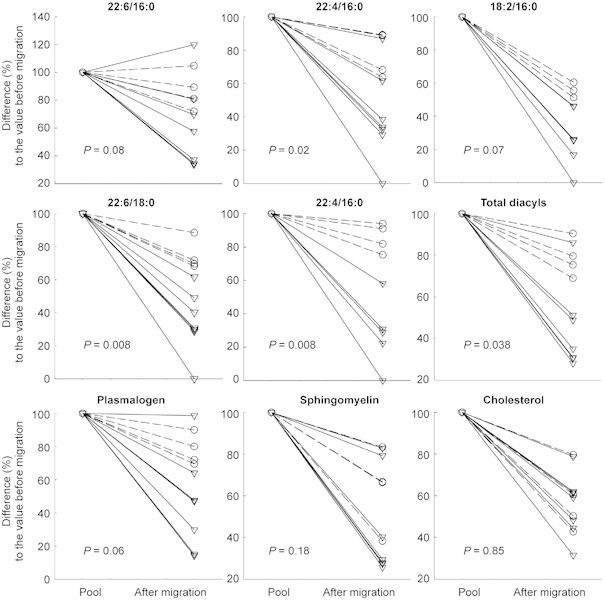
Individual difference (%) from the initial value (100%) for several sperm lipids after migration into PMN-free (strait lines) and PMN+ (dotted lines) mucus samples. The percentage difference from the initial value was significantly smaller in the presence of PMN than in the absence of PMN for the majority of assessed phospholipid molecular species (P = 0.02 for 22:4/16:0, P = 0.007 for18:2/16:0 and 20:4/18:0, P = 0.008 for 22:6/18:0 and P = 0.03 for total diacyls), but not for cholesterol, sphingomyelin or plasmalogen (P = 0.85, P = 0.18 and P = 0.06, respectively). PMN, polymorphonuclear leukocytes. Diacyls: D16:0/22:6 (1-palmitoyl-2-docosahexaenoyl-sn-glycero-3-phospholipid); D16:0/20:4 (1-palmitoyl-2-arachidonoyl-sn-glycero-3-phospholipid); D18:0/22:6 (1-stearoyl-2-docosahexaenoyl-sn-glycero-3-phospholipid).
Discussion
The interaction of spermatozoa with the secretions of the female reproductive tract is of critical importance for the survival and functional ability of spermatozoa 2, 17. The cervical mucus, especially at or just before ovulation, plays a pivotal role through its receptivity to the spermatozoa and its filtering property, based on differential sperm motility and morphology. Our study, which was conducted on normal human semen samples and normal ovulatory mucus samples, clearly shows that the presence of low numbers of leukocytes in the cervical mucus had no impact on the filtering function of mucus towards sperm motility or morphology, whereas it significantly mitigated the process of the decrease in sperm lipids after migration, as reported earlier 3. In addition, the extent of inducible acrosome reactions and that of the decrease in lipid levels also differed, depending on the presence of leukocytes in the mucus samples studied.
These intriguing new data bring the unanswered question of the role of the leukocytic reaction of ovulatory mucus up to date 5. A dual role for this leukocyte infiltration has been speculated: the non-specific protection of the upper reproductive tract against bacterial intrusion and the phagocytosis of the nonviable/non-fertilizing sperm populations left at the cervix 7. Indeed, leukocytes in the mucus induce recognition and removal of apoptotic cells during inflammation of the cervix 18, but the unequivocal inflammatory condition of the endocervicitis, in which high numbers of activated leukocytes are involved, is not similar to the physiological situation of the leukocytic infiltration of the normal ovulatory cervical mucus by low numbers of leukocytes. Unfortunately, only a sparse amount of data on the magnitude of cells and/or leukocytes in normal pre-ovulatory/ovulatory mucus has been reported. Thompson et al. 7 reported concentrations of leukocytes in the pre-ovulatory cervical mucus of potentially fertile women that are slightly higher than the values found in this study. This could be explained by the fact that these authors used a more sensitive method–an immunobead cell selection assay–for detecting all of the leukocytes present in the mucus, whereas our method detected only the inactivated PMN. Interestingly, Thompson et al. 7 have shown that the leukocyte concentration in cervical mucus increased after an insemination and that this ‘leukocytic reaction' varied in terms of onset, duration and intensity, according to the initial levels of leukocytes detected in the baseline period. This may explain, at least in part, the variable leukocyte content observed in this study. It should be pointed out that although all the women included in our study had at least 3 days of sexual abstinence (and no spermatozoa were observed in their mucus), measurable numbers of leukocytes were detected for some, but not for others. This raises questions about the relationship between a sperm's entry into the female genital tract during the pre-ovulatory period and the presence of leukocytes in the mucus. However, we cannot rule out the possibility that some of the volunteers may not have strictly followed the 3-day abstinence period or that some may have had coitus 4–5 days before the test.
Several investigators have shown evidence suggesting an apparent priming effect of cervical mucus on capacitation 19, 20. The present findings of a significantly decreased amount of sperm lipids and a significantly increased level of inducible acrosome reaction after sperm migration into mucus samples support this view. This effect is similar to the influence of capacitating media, but sperms require a much longer exposure incubation time (24 h) before they show similar acrosome reactivity 2.
We found that the levels of phospholipid molecular species and plasmalogens were less reduced after migration through leukocyte-infiltrated mucus samples than through mucus samples free of leukocytes, whereas cholesterol levels (or sphingomyelin levels) were not significantly modified. This resulted in lower sperm cholesterol/phospholipid ratios after migration into mucus samples containing leukocytes than in mucus samples with no detected leukocytes. As there is a relationship among the cholesterol/phospholipid ratio, sperm membrane fluidity and the capacitation process 21, 22, this could suggest that the spermatozoa that migrated into mucus samples containing leukocytes reached a more advanced capacitation step. On the other hand, this explanation may be incorrect, because the kinematic parameters were similar between the sperm samples that migrated into mucus samples that contained leukocytes and those that did not. On the contrary, the percentage of acrosome reaction was lower for the spermatozoa that migrated into the PMN+ mucus samples. Therefore, our data instead suggest that the low leukocyte levels found in the pre-ovulatory normal mucus samples decrease the ability of the cervical mucus to promote capacitation. This may play a favourable role by delaying capacitation and decreasing precocious acrosome reactions. This assumption warrants further experiments.
It has been established that the lipid molecular species of the sperm membrane, which have a high polyunsaturated fatty acid content, are the major target for lipid peroxidation 23. In this study, we found that the levels of such species, for example, 1-palmitoyl-2-docosahexaenoyl-sn-glycero-3-phospholipid (16:0/22:6) and 1-stearoyl-2-docosahexaenoyl-sn-glycero-3-phospholipid (18:0/22:6), were less reduced in the sperm after migration. Owing to the prelimi- nary nature of our study, it is not known whether the leukocytes are directly or indirectly involved in the sperm lipid removal or what the mode of action is. It can be hypothesized that this qualitatively and quantitatively controlled lipid removal could result in a sperm oxidative status that allows the physiological oxidative reaction involved in the sperm capacitation process to occur 24. In addition, it has been shown that a controlled incubation of sperm with low numbers of activated PMNs resulted in an increase in sperm capacitation 25. Thus, the low levels of neutrophils present in the mucus could be one of the main direct and/or indirect factors of this physiological reaction (our unpublished data). However, this presupposed favourable role of the leukocytes should plausibly depend on the oxidative balance in the ovulatory cervical mucus and, therefore, on the level of pro- and anti-oxidant system activity in the cervical mucus in the light of the fact that leukocyte levels as low as those found here in the mucus are able to exert a toxic effect on sperm in vitro when added in a milieu not containing scavenging compounds 26, 27.
The significance of infiltrating leukocytes in impairing the functional capacity of spermatozoa was investigated by Zalata et al. 28. These authors reported impairment of the acrosine activity of sperm without impairment of the basic semen parameters. It is not known whether this effect is reversible. Anti-inflammatory and pro-inflammatory molecules such as cytokines could act directly on spermatozoa to enhance their level of lipid peroxidation, indicating a direct ROS production by the spermatozoa. The mechanism by which cytokines can stimulate ROS production by the spermatozoa is not clearly understood 29.
In conclusion, the present data provide evidence of a relationship between the PMN leukocytes contained in normal ovulatory mucus and the sperm lipid status after migration, suggesting a possible physiological role of leukocytes in the complex process of acquisition of the fertilizing ability of sperm in vivo. This process involves complex molecular changes, notably in the sperm plasma membrane composition and architecture, including lipid changes and exposure of receptors that initiate in the female genital tract. Our findings contribute to a better knowledge of sperm–mucus interaction and sperm capacitation in vivo, which remains a major step towards solving the many unanswered questions in reproductive biology and could allow the improvement of in vitro capacitation and, consequently, the outcome of in vitro fertilization. It should also help in the understanding of some aspects of male infertility and could contribute to the avoidance of assisted reproductive technologies in the future.
Further experiments are required to confirm the present results and decipher the molecular mechanisms possibly involved, as well as the exact role played by the leukocytes. In the light of the facts associating sperm capacitation with the production of oxidative anions, studies on the effect of cervical mucus composition and cellular content on the production of ROS are warranted.
Acknowledgments
We thank Dr C. Serres for her assistance with the acrosome reaction assessment and Mr R. Dolan for reviewing the English revision of the manuscript.
References
- Barratt CL, Cooke ID. Sperm transport in the human female reproductive tract—a dynamic interaction. Int J Androl. 1991;14:394–411. doi: 10.1111/j.1365-2605.1991.tb01268.x. [DOI] [PubMed] [Google Scholar]
- De Jonge C. Biological basis for human capacitation. Hum Reprod Update. 2005;11:205–14. doi: 10.1093/humupd/dmi010. [DOI] [PubMed] [Google Scholar]
- Feki NC, Thérond P, Couturier M, Liméa G, Legrand A, et al. Human sperm lipid content is modified after migration into human cervical mucus. Mol Hum Reprod. 2004;10:137–42. doi: 10.1093/molehr/gah018. [DOI] [PubMed] [Google Scholar]
- Baldi E, Luconi M, Bonaccorsi L, Muratori M, Forti G. Intracellular events and signaling pathways involved in sperm acquisition of fertilizing capacity and acrosome reaction. Front Biosci. 2000;5:E110–23. doi: 10.2741/baldi. [DOI] [PubMed] [Google Scholar]
- Thompson LA, Barratt CL, Bolton AE, Cooke ID. The leucocytic reaction of the human uterine cervix. Am J Reprod Immunol. 1992;28:85–9. doi: 10.1111/j.1600-0897.1992.tb00765.x. [DOI] [PubMed] [Google Scholar]
- Williams M, Thompson LA, Li TC, Mackenna A, Barratt CL, et al. Uterine flushing: a method to recover spermatozoa and leukocytes. Hum Reprod. 1993;8:925–8. doi: 10.1093/oxfordjournals.humrep.a138168. [DOI] [PubMed] [Google Scholar]
- Thompson LA, Tomlinson MJ, Barratt CL, Bolton AE, Cooke ID. Positive immunoselection—a method of iso-lating leukocytes from leukocytic-reacted human cervical mucus samples. Am J Reprod Immunol. 1991;26:58–61. doi: 10.1111/j.1600-0897.1991.tb00971.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction Cambridge: Cambridge University Press; 1999 [Google Scholar]
- Kobayashi T, Tsunawaki S, Seguchi H. Evaluation of the process for superoxide production by NADPH oxidase in human neutrophils: evidence for cytoplasmic origin of superoxide. Redox Rep. 2001;6:27–36. doi: 10.1179/135100001101536003. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol. 2008;59:2–11. doi: 10.1111/j.1600-0897.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. Reactive oxygen species and sperm physiology. Rev Reprod. 1997;2:48–54. doi: 10.1530/ror.0.0020048. [DOI] [PubMed] [Google Scholar]
- Leclerc P, de Lamirande E, Gagnon C. Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic Biol Med. 1997;22:643–56. doi: 10.1016/s0891-5849(96)00379-6. [DOI] [PubMed] [Google Scholar]
- Auger J, Eustache F, Andersen AG, Irvine DS, Jorgensen N, et al. Sperm morphological defects related to environment, lifestyle and medical history of 1001 male partners of pregnant women from four European cities. Hum Reprod. 2001;16:2710–7. doi: 10.1093/humrep/16.12.2710. [DOI] [PubMed] [Google Scholar]
- European Society of Human Reproduction and Embryology (ESHRE) Andrology Special Interest Group. Guidelines on the application of CASA technology in the analysis of spermatozoa Hum Reprod 199813142–5. [PubMed] [Google Scholar]
- Carver-Ward JA, Hollanders JM, Jaroudi KA, Einspenner M, Al-Sedairy ST, et al. Progesterone does not potentiate the acrosome reaction in human spermatozoa: flow cytometric analysis using CD46 antibody. Hum Reprod. 1996;11:121–6. doi: 10.1093/oxfordjournals.humrep.a019003. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. BMDP Statistical Software Manual. Berkeley: University of California Press; 1988. [Google Scholar]
- Suarez SS, Pacey AA. Sperm transport in the female reproductive tract. Hum Reprod Update. 2006;12:23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- Lessig J, Spalteholz H, Reibetanz U, Salavei P, Fischlechner M, et al. Myeloperoxidase binds to non-vital spermatozoa on phosphatidylserine epitopes. Apoptosis. 2007;12:1803–12. doi: 10.1007/s10495-007-0113-5. [DOI] [PubMed] [Google Scholar]
- Lambert H, Overstreet JW, Morales P, Hanson FW, Yanagimachi R. Sperm capacitation in the human female reproductive tract. Fertil Steril. 1985;43:325–7. [PubMed] [Google Scholar]
- Zinaman M, Drobnis EZ, Morales P, Brazil C, Kiel M, et al. The physiology of sperm recovered from the human cervix: acrosomal status and response to inducers of the acrosome reaction. Biol Reprod. 1989;41:790–7. doi: 10.1095/biolreprod41.5.790. [DOI] [PubMed] [Google Scholar]
- Martinez P, Morros A. Membrane lipid dynamics during human sperm capacitation. Front Biosci. 1996;1:d103–17. doi: 10.2741/a119. [DOI] [PubMed] [Google Scholar]
- Benoff S. Preliminaries to fertilization: the role of cholesterol during capacitation of human spermatozoa. Hum Reprod. 1993;8:2001–6. doi: 10.1093/oxfordjournals.humrep.a137971. [DOI] [PubMed] [Google Scholar]
- Alvarez JG, Storey BT. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol Reprod Dev. 1995;42:334–46. doi: 10.1002/mrd.1080420311. [DOI] [PubMed] [Google Scholar]
- O'Flaherty C, de Lamirande E, Gagnon C. Positive role of reactive oxygen species in mammalian sperm capacitation: triggering and modulation of phosphorylation events. Free Radic Biol Med. 2006;41:528–40. doi: 10.1016/j.freeradbiomed.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Villegas J, Kehr K, Soto L, Henkel R, Miska W, et al. Reactive oxygen species induce reversible capacitation in human spermatozoa. Andrologia. 2003;35:227–32. doi: 10.1046/j.1439-0272.2003.00564.x. [DOI] [PubMed] [Google Scholar]
- Sanocka D, Florek E, Klejewski A, Kurpisz M. Pro- and antioxidant system activity in cervical mucosa. Ginekol Pol. 2002;73:573–6. [PubMed] [Google Scholar]
- Aitken RJ, Buckingham DW, Brindle J, Gomez E, Baker HW, et al. Analysis of sperm movement in relation to the oxidative stress created by leukocytes in washed sperm preparations and seminal plasma. Hum Reprod. 1995;10:2061–71. doi: 10.1093/oxfordjournals.humrep.a136237. [DOI] [PubMed] [Google Scholar]
- Zalata AA, Ahmed AH, Allamaneni SS, Comhaire FH, Agarwal A. Relationship between acrosin activity of human spermatozoa and oxidative stress. Asian J Androl. 2004;6:313–8. [PubMed] [Google Scholar]
- Martínez P, Proverbio F, Camejo MI. Sperm lipid peroxidation and pro-inflammatory cytokines. Asian J Androl. 2007;9:102–7. doi: 10.1111/j.1745-7262.2007.00238.x. [DOI] [PubMed] [Google Scholar]


