Abstract
Androgens play a central role in prostate cancer pathogenesis, and hence most of the patients respond to androgen deprivation therapies. However, patients tend to relapse with aggressive prostate cancer, which has been termed as hormone refractory. To identify the proteins that mediate progression to the hormone-refractory state, we used protein-chip technology for mass profiling of patients' sera. This study included 16 patients with metastatic hormone-refractory prostate cancer who were initially treated with androgen deprivation therapy. Serum samples were collected from each patient at five time points: point A, pre-treatment; point B, at the nadir of the prostate-specific antigen (PSA) level; point C, PSA failure; point D, the early hormone-refractory phase; and point E, the late hormone-refractory phase. Using surface-enhanced laser desorption/ionization time-of-flight mass spectrometry, we performed protein mass profiling of the patients' sera and identified a 6 640-Da peak that increased with disease progression. Target proteins were partially purified, and by amino acid sequencing the peak was identified as a fragment of apolipoprotein C-I (ApoC-I). Serum ApoC-I protein levels increased with disease progression. On immunohistochemical analysis, the ApoC-I protein was found localized to the cytoplasm of the hormone-refractory cancer cells. In this study, we showed an increase in serum ApoC-I protein levels in prostate cancer patients during their progression to the hormone-refractory state, which suggests that ApoC-I protein is related to progression of prostate cancer. However, as the exact role of ApoC-I in prostate cancer pathogenesis is unclear, further research is required.
Keywords: apolipoprotein C-I, hormonal therapy, prognosis, prostate cancer, surface-enhanced laser desorption/ionization time-of-flight mass spectrometry
Introduction
Prostate cancer is one of the most common malignancies in western countries, and its incidence is increasing in Asia. Since 1941, when Huggins and Hodges 1 first published their findings, androgen deprivation therapy has been the initial treatment of choice for men with metastatic prostate cancer. Androgen deprivation therapy can be performed by orchiectomy or by administering oestrogen, anti-androgens, or, more recently, a luteinizing hormone-releasing hormone (LH-RH) agonist. Prostate cancer is initially androgen-dependent, but after androgen deprivation it often relapses to an androgen-independent state. Once the disease has progressed to this stage during hormone therapy, the mean survival is approximately 6–12 months 2. If the cancer does not acquire androgen independence, patient survival and quality of life would likely improve dramatically. Although several mechanisms, including mutations in the androgen receptor, have been suggested to explain the cancer's acquisition of androgen independence, the precise details are unclear 3, 4.
Cancer proteomics is expected to be useful for identifying new biomarkers and drug targets that are present in the serum and other biological tissues 5. The ability to identify such entities with the use of new biomarkers should eventually allow cancers to be diagnosed at an earlier stage and also aid in the discovery of novel drugs. With the improvement of existing technologies, such as 2D electrophoresis, and the recent introduction of high-throughput mass spectrometry instrumentation, the proteomics applications for cancer research have increased. Surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS) has recently been introduced to proteomic technology. It shows great potential to contribute to research dealing with the early detection of several cancers using serum and other biological materials 6. This approach has been useful for identifying specific and sensitive molecular markers in patients with ovarian, liver, pancreatic and prostatic malignancies 7, 8, 9, 10. However, there have been no reports of proteomics technology being used to identify the unique proteins that are up-regulated during the progression of prostate cancer to an androgen-independent state. If such proteins could be isolated, they would be promising biomarkers, as well as targets for an innovative therapy for androgen-independent prostate cancer. Thus, the purpose of this study was to identify proteins that mediate the progression of prostate cancer to an androgen-independent state using SELDI-TOF-MS technology for mass profiling of sera. The samples used for this study were obtained at different time points during the treatment of metastatic prostate cancer patients with androgen deprivation therapies.
Materials and methods
Patients
This study included 16 patients, aged 65.3 ± 6.6 years, who at the time of initial diagnosis had metastatic prostate cancer and had undergone hormone therapy, and then relapsed to a hormone-refractory state. The patients were treated at Chiba University Hospital (Chiba, Japan) between 1997 and 2001. Written informed consent to use the frozen sera for analysis was obtained from all patients, and this study was approved by the Internal Review Board of our medical institution. The patients' mean pre-treatment prostate-specific antigen (PSA) level was 640 ± 588 ng mL−1. The patients' clinical and histological characteristics are summarized in Table 1.
Table 1. Patients' histological characteristics and clinical features.
| Number of patients | |
|---|---|
| Stage | |
| D1 (TxN1M0) | 1 |
| D2 (TxNxM1) | 15 |
| Gleason score | |
| 5–7 | 9 |
| 8–10 | 7 |
| Histological grade | |
| Moderately differentiated | 6 |
| Poorly differentiated | 10 |
Before starting the first line of hormonal therapy, a transrectal needle biopsy was performed, and the diagnosis of prostate adenocarcinoma was confirmed on histological analysis. Radioisotopic bone imaging with 99mTc-methylene-diphosphonate, magnetic resonance imaging and computer tomography helped determine the clinical stage. All patients were initially treated with androgen deprivation therapy, LH-RH analogue injection or surgical castration combined with oral anti-androgens. After the first-line therapy was completed, all patients showed a complete response to hormonal therapy based on their PSA levels. However, the patients invariably relapsed with a more aggressive, androgen-independent prostate cancer.
On the basis of the changes in patients' PSA levels, we retrospectively examined serum samples at five time points: point A, pre-treatment; point B, at the nadir of the PSA level; point C, at the time of PSA failure, which occurred during hormonal therapy after three consecutive increases in PSA; point D, during the early hormone-refractory phase; and point E, during the late hormone-refractory phase. The early hormone-refractory phase was defined as relapse after an initial androgen blockade, and the late hormone-refractory phase referred to the state of the cancer just before death. All samples were stored at −80°C until analysis.
SELDI-TOF-MS analysis
An aliquot of stored sera was used for SELDI-TOF-MS analysis with a nitrogen laser. The SELDI-TOF-MS technology (Ciphergen Biosystems, Fremont, CA, USA) consists of three major components: the ProteinChip® array, the reader and the software. The ProteinChip array is a 10-mm-wide × 80-mm-long chip with eight 2-mm spots that have a specific chromatographic surface. Each surface is designed to select proteins from crude extracts, according to general or specific protein properties. Each spot contains either a chemically treated (e.g., anionic, cationic, hydrophobic or metal) or a biochemically treated surface. In our experiments, a cationic exchanger (WCX2) was used. These chips were chosen because the type of ion exchange resin and the buffer conditions needed for purification of the recognized peaks could be based on ProteinChip affinity conditions during SELDI analysis.
To 10 μL of each serum sample, 10 μL of a solution containing 8 mol L−1 urea in phosphate-buffered saline was added. The mixture was vortexed for 10 min and diluted 5-fold with binding or washing buffer. The binding or washing buffer for the cationic arrays (WCX2) contained 50 mmol L−1 ammonium acetate (pH 6.5). On the same day, the diluted samples (100 μL) were applied to each spot on the ProteinChip array using an eight-well bioprocessor (Ciphergen Biosystems). After the samples were allowed to bind at room temperature for 20 min on a platform shaker in a wet condition, the array was washed three times with 125 μL of binding or washing buffer for 5 min, followed by two quick rinses with 400 μL of distilled water. The arrays were allowed to air-dry, and a saturated solution of sinapinic acid (2.5 μL) in 50% acetonitrile (250 μL) and 0.5% trifluoroacetic acid (247.5 μL) was added twice to each spot. We calibrated the ProteinChip reader daily using peptide standards (Arg8-vasopressin, somatostatin, and Hirudin BHVK) on the array. TOF-MS were generated in a Ciphergen Protein Biology System II by averaging 60 laser shots with an intensity of 255 and a detector sensitivity of 10. To stabilize the intensity, the system software was used to automatically calculate the average of 20 randomized spots. All spectra were compiled, and qualified mass peaks (signal-to-noise ratio of 5) with mass-to-charge ratios (m/z) between 3 000 and 30 000 were autodetected. Peak clusters were completed using a second pass-peak section (signal-to-noise ratio of 2, within a 0.3% mass window), and the estimated peaks were added. The relative peak intensities, normalized to a total ion current of m/z between 2 000 and 30 000, were expressed as arbitrary units. All of these analyses were performed using the Biomarker Wizard® 3.1 software (Ciphergen Biosystems).
Data analysis and protein profiling
SELDI-TOF-MS peak labelling and clustering were performed using Ciphergen's Biomarker Wizard tool, and the data were transferred onto a spreadsheet. The intensity values for each patient's peak were averaged. We retained a pool of peaks that were best able to discriminate between point A (pre-treatment) samples and point E (late hormone-refractory phase) samples, as well as between point B (at the nadir PSA level) samples and point E (late hormone-refractory phase) samples. The statistical significance of mean differences in the height of discriminating peaks between point A and point E, as well as between point B and point E, was assessed by t-test using the Biomarker Wizard 3.1 software. P < 0.05 was considered statistically significant. A 6 640-Da protein peak of interest was increased in the time course from the first-line hormonal therapy to the hormone-refractory stage (points A vs. E and points B vs. E, P < 0.05). We referred to the peak intensity of point C (at the time of PSA failure) samples and point D (early hormone-refractory phase) samples for distinguishing between earlier and later time points.
Protein isolation and identification
To purify and identify the proteins of interest, serum samples obtained at point E (the late hormone-refractory phase) were used to isolate the protein that corresponded to the 6 640-Da peak, which was overexpressed in the progressive cancer state.
The serum was subjected to ion-exchange fractionation by fast protein liquid chromatography (FPLC Pharmacia LKB; Amersham Pharmacia Biotech, Uppsala, Sweden) with a linear gradient of 0–1 000 mmol L−1 NaCl. The buffer condition was based on the ProteinChip affinity determined by SELDI analysis. FPLC fractions were monitored on a hydrophilic NP20 ProteinChip array (1 μL sample per spot) with an SPA matrix.
The FPLC fractions that were rich in the specific protein of interest were collected, concentrated by SpeedVac (Holbrook, NY, USA) and subjected twice to high-performance liquid chromatography (HPLC CCPM/PX-8010, TOSOH, Tokyo, Japan). First, HPLC was performed with a sephasil protein C18 column (Aquapore OD-300, Perkin-Elmer, MA, USA). Then, after passage through a C4 column (Cadenza CD-C4, Intakt, Kyoto, Japan) to remove albumin, a second purification with HPLC was performed using the C18 column (Cadenza CD-C18, Intakt), followed by elution with a linear gradient of 0.1%–80% acetonitrile at a flow rate of 200 μL min−1. HPLC fractions that contained pure target protein were monitored using a SELDI-TOF-MS GoldChip array (1 μL sample per spot) with an SPA matrix. The fractions that contained the protein peak of interest were used for N-terminal amino-acid sequence analysis. The N-terminal amino acids of the purified protein samples were determined using an amino-acid sequencer (Procise 494 cLC Protein Sequencing System, APLO Life Science Institute, Inc., Tokushima, Japan). The sensitive analysis of the N-terminal amino acid sequence (protein sequencing) uses a method called Edman degradation, in which amino acids are excised one at a time from the N-terminus of a protein or peptide. These amino acids are then isolated using HPLC.
Western blotting
The patients' sera were subjected to HPLC purification (Ajilent Technology, Tokyo, Japan) and protein extracts from the sera were separated by electrophoresis on 10%–20% gradient gels (Bio-Rad, Hercules, CA, USA). The proteins were then transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA) in a tank-transfer apparatus (Bio-Rad) and the membranes were blocked with 0.3% skimmed milk in phosphate-buffered saline (PBS). An anti-apolipoprotein C-I (ApoC-I) antibody diluted 1:1 000 was used as the primary antibody. Goat anti-mouse IgG horseradish peroxidase (Bio-Rad) diluted 1:1 000 was used as the secondary antibody. Antigens on the membrane were detected with enhanced chemiluminescence detection reagents.
Cell culture and reverse transcription-polymerase chain reaction (RT-PCR)
LNCaP, PC-3 and DU145 human prostate cancer cells were maintained in RPMI 1640 medium with 1% PBS and 10% fetal bovine serum. These cell lines were kept in a humidified incubator at 37°C with 5% CO2, and they were subcultured when 75-mm flasks were 90% confluent.
Total ribonucleic acid (RNA) was extracted from these cell lines using the RNeasy Mini Kit (Qiagen KK, Tokyo, Japan). Complementary DNA (cDNA) was synthesized from the total RNA using the first-strand cDNA synthesis kit for RT-PCR (Qiagen KK, Tokto, Japan). Using the cDNA as a template, the ApoC-I was amplified with suitable primers. The forward primer was 5′-CTCCAGTGCCTTGGATAAGC-3′ and reverse primer was 5′-TTGAGTTTCTCCTTCACTTTCTGA-3′. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. Reagents for the iQ SYBR Green Supermix (Bio-Rad) were used according to the manufacturer's protocol. PCR was performed in a total volume of 25 μL, including 7.5 pmol of each primer and 1.0 μL of cDNA synthesized with random hexamers. PCR conditions were 3 min at 95°C for pre-heating, then 1 min at 94°C, 1 min at 57°C and 1 min at 72°C, for 37 cycles. All specific quantities were divided by the control, GAPDH.
Immunohistochemistry
Paraffin-embedded tissues taken from seven untreated, early-stage prostate cancer patients at surgery and three hormone-refractory prostate cancer patients at autopsy, as well as normal prostate and liver tissues, were used for the immunohistochemical study. In brief, immunohistochemistry was performed in the following manner. First, 5-μm sections were cut from formalin-fixed, paraffin-embedded tissues. The tissue sections were attached to slides by heating at 60°C for 3 h. They were then deparaffinized and rehydrated with three baths of xylene, followed by graded ethanol baths ranging from absolute to 70%, and then by a wash with a citric acid (pH 6.0) buffer. To retrieve the antigens, the slides were boiled three times in a microwave oven for 5 min each. The slides were then soaked for 10 min with 10% H2O2 in methanol at room temperature. After washing thrice with phosphate buffered saline (PBS), the non-specific binding of antibodies was blocked using a blocking buffer (1% bovine serum albumin/PBS) for 10 min.
The tissues were then incubated for 30 min at room temperature with an anti-ApoC-I antibody diluted 1:200 (Chemicon International, Inc., Billerica, MA, USA). After washing with PBS, biotinylated link and streptavidin-HRP from the DAKO LSAB2 Kit® (DAKO Japan, Kyoto, Japan) were used to visualize the tissue antigens, according to the manufacturer's instructions. Tissue sections were counterstained with haematoxylin for 30 s and dehydrated with 100% ethanol and xylene, and the coverslips were mounted with Malinol (Mito Pure Chemicals, Tokyo, Japan).
Results
SELDI-TOF-MS data analysis
SELDI-TOF-MS analysis identified a 6 640-Da peak, which had a significantly increased intensity associated with disease progression (from point A to point E, as well as from point B to point E). A representative case (case 2) is shown in Figure 1. This increase in the 6 640-Da peak was found in 11 of the 16 cases (69%) (Figure 2). On the other hand, no increase in the 6 640-Da peak was identified in sera from four other metastatic prostate cancer patients who continued to respond well to hormonal therapy for more than 2 years. These findings suggested that the peak intensity could be clinically useful as a prognostic biomarker.
Figure 1.
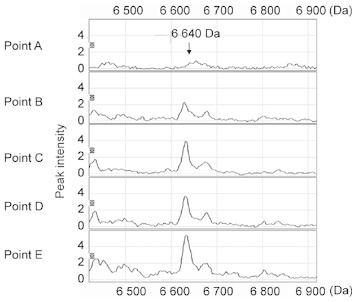
Surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS) data analysis and protein profiling of a representative case (case 2). The 6 640-Da SELDI-TOF-MS peak increased significantly from point A to E.
Figure 2.
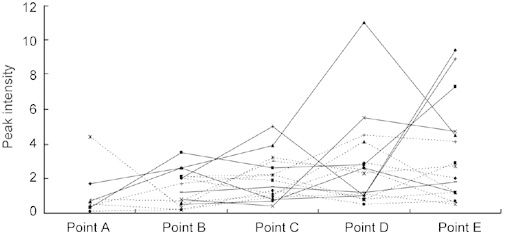
Changes in the relative intensities of the 6 640-Da peak in the Surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS) analysis of all the 16 cases. In eight cases, sera were collected at five time points (from point A to point E). In the remaining eight cases, sera were collected at four time points (from point B to point E). SELDI-TOF-MS mean peak intensity was 1.12 ± 1.41 at point A, 1.45 ± 1.01 at point B and 3.36 ± 2.92 at point E. There was a significant difference (P = 0.035) between point A and point E, as well as between point B and point E.
Protein isolation and identification
The target protein was purified. The protein of interest at 6 640-Da could be eluted at around the 510-mmol L−1 NaCl fraction by FPLC and then collected at around 28% ACN using HPLC. Adequate separation of the target protein from the high-molecular-weight proteins, including albumin, was achieved. The HPLC fraction containing the apparently pure target protein was confirmed by SELDI-TOF-MS (Figure 3A). The N-terminal sequence of the 15 amino-acid residues was determined and is shown in Figure 3B. This sequence is the same as the N-terminal sequence of ApoC-I.
Figure 3.
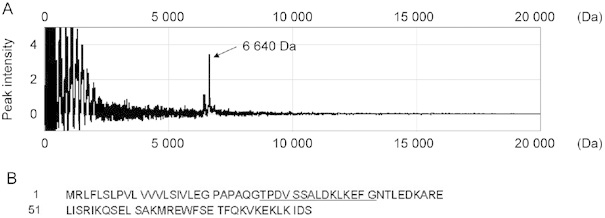
(A): Isolation and purification of the protein corresponding to the 6 640-Da peak using FPLC and HPLC. (B): The N-terminal amino-acid sequences of the 6 640-Da purified protein samples by FPLC and HPLC. Fifteen amino-acid residues (underlined) were determined. The 6 640-Da protein was identified as a fragment of ApoC-I.
Western blotting of ApoC-I in sera obtained from patients
As the technique to measure serum ApoC-I has not yet been developed, we used immunoblotting with a monoclonal anti-ApoC-I antibody to confirm the presence of ApoC-I in the patients' sera. Of the 16 patients, three patients' sera were available for western blot analysis with anti-ApoC-I antibodies. Western blotting of ApoC-I in the sera revealed two bands: a 9.3-kDa precursor and a 6.6-kDa mature fragment (Figure 4). These two bands of ApoC-I protein increased as the disease progressed, which supported the data from the SELDI-TOF-MS analysis.
Figure 4.
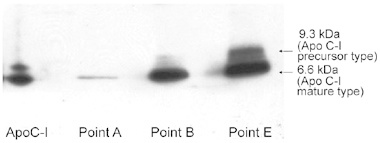
ApoC-I Western blotting of the serum fractions by HPLC purification. A representative case (case 1) of Western blotting of patient serum that shows two bands of ApoC-I (a 9.3-kDa precursor and a 6.6-kDa mature protein). The intensity of these two ApoC-I bands increased as the disease progressed, similar to what was observed with the SELDI-TOF-MS analysis (shown at points A, B and E).
ApoC-I expression in human prostate cancer cell lines
ApoC-I messenger RNA (mRNA) expression was examined in three human prostate cancer cell lines (LNCaP, PC3 and DU145). ApoC-I mRNA was detected by RT-PCR in both androgen-dependent (LNCaP) and androgen-independent (PC3 and DU145) lines (Figure 5). In addition, ApoC-I protein was present at a low level, as observed by western blot analysis (data not shown).
Figure 5.
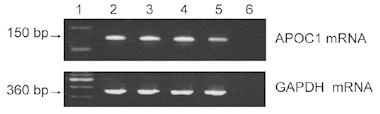
mRNA expression of ApoC-I in human prostate cancer cells. Lane 1: Molecular weight marker; Lane 2: LNCaP; Lane 3: PC-3; Lane 4: DU145; Lane 5: MIAPaC II (positive control); Lane 6: DDW (negative control).
Immunohistochemical analysis of ApoC-I for benign and malignant prostate tissues
Immunohistochemical analysis of ApoC-I was performed in various prostate cancer tissues (Figure 6). On the basis of the literature, the cytoplasm of normal hepatocytes has been reported to produce ApoC-I. Thus, in this study, we used normal liver tissue as a positive control (Figure 6A) and detected ApoC-I protein expression in the cytoplasm of normal hepatocytes. Normal prostatic tissues have been reported not to express the ApoC-I protein, and we found no ApoC-I protein expression in three normal prostate tissues (Figure 6B). Among seven untreated early-stage prostate cancer cases, ApoC-I expression was recognized in none of two well-differentiated (Figure 6C), one of two moderately differentiated and two of three poorly differentiated (Figure 6D) prostate cancer tissues. Two of three hormone-refractory prostate cancer tissues showed ApoC-I protein expression (Figure 6E). These results indicated that ApoC-I was expressed in the cytoplasm of hormone-refractory prostate cancer tissues or in localized cancer tissues before treatment, but was not found in normal prostate tissue.
Figure 6.
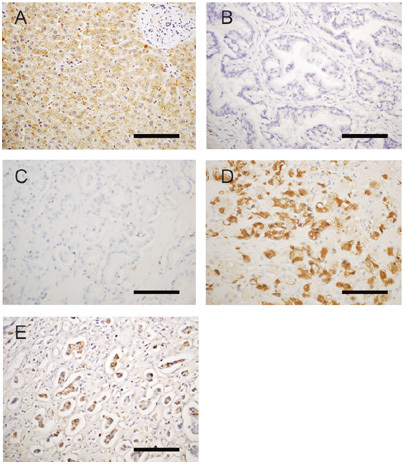
Expression of ApoC-I using immunohistochemical analysis of paraffin sections. (A): Normal liver (positive control); (B): normal prostate; (C): well-differentiated prostate cancer tissue; (D): poorly differentiated prostate cancer tissue; (E): hormone-refractory prostate cancer lymph node metastasis; (A–E) were stained with an anti-ApoC-I antibody (A–D magnification, × 400). Bars=100 μm
Relationship between ApoC-I level and patients' clinical outcome
We investigated the relationship between ApoC-I levels and several clinical factors, such as performance status (PS), extent of disease (EOD) on bone scan, alkaline phosphatase (ALP) level, serum PSA and haemoglobin (HGB) levels, and patients' survival, but failed to find any relationship.
Discussion
It has been observed that patients with metastatic prostate cancer may have significantly different prognoses. The ability to predict patient prognosis and to stratify patients by their risk of progression is important for providing personalized treatment and determining follow-up strategies. To date, various clinical and biochemical parameters, as well as tumour characteristics, have been reported to be useful for predicting the survival of patients with metastatic prostate cancer 11. Clinical and biochemical factors, such as lower PS, pain score, EOD on bone scan, lower pretreatment serum testosterone levels, higher serum ALP and acid phosphatase levels, higher PSA levels, and lower HGB levels, have all been reported to be associated with treatment response or patient survival 12. Pathological and immunohistochemical studies have also shown that nuclear texture, oligosaccharide sialyl Lewis (x), c-erbB-2 (Her2/neu) and tissue factor could be predictors of survival 13. In this study, ApoC-I levels were found to be increased in the sera of metastatic prostate cancer patients whose disease progressed from an androgen-dependent state to an androgen-independent phase.
ApoC-I is synthesized primarily in the liver and to a minor degree in the small intestine. It is involved mainly in lipoprotein metabolism. ApoC-I is originally formed as a 9 332-Da pro-peptide that has 83 amino acids. The mature protein is generated from this proform upon cleavage during translation. ApoC-I appears to modulate the interaction of apolipoprotein E (ApoE) with β-migrating very low-density lipoprotein (VLDL) and to inhibit the binding of β-VLDL to the low-density lipoprotein receptor (LDLr)-related protein. ApoC-I constitutes about 10% of VLDL protein and 2% of high-density lipoprotein protein 14. It is of note that the incidence of clinically significant prostate cancer and disease-specific mortality rates are highest in Western populations, which also have a high fat intake and a high prevalence of obesity. In fact, some epidemiological studies have suggested that there is an association between prostate cancer risk and obesity 15, although other reports have shown contradictory results 16. Earlier studies have shown that arachidonic acid is primarily delivered by LDL via LDLr. Hughes-Fulford et al. 17 examined the role of LDLr in up-regulating the growth of PC-3 prostate cancer cells. The analysis of LDLr mRNA expression and LDLr function showed that human PC-3 prostate cancer cells lack normal feedback regulation. On the basis of these results, a relationship between fatty acid synthesis and prostate cancer pathogenesis has been suggested 17. Stanbrough et al. 18 identified genes that are highly expressed in androgen-independent prostate cancer bone marrow metastases and found the ApoE gene to be significantly highly expressed in metastatic androgen-independent prostate cancer. Also, some reports have suggested clinical potential for apolipoprotein A-II and apolipoprotein-D as biomarkers for prostate cancer 19, 20.
With respect to the relationship between ApoC-I and cancer, Goncçalves et al. 21, using SELDI-TOF-MS, suggested that ApoC-I has the potential to clinically predict metastatic relapse in high-risk breast cancer patients receiving adjuvant chemotherapy. Engwegen et al. 22 identified a potential role for the ApoC-I protein in discriminating between colorectal cancer patients and healthy controls. Yasui et al. 23 found that ApoC-I was up-regulated in gastric cancer cells compared with normal gastric epithelial cells, on the basis of serial analysis of gene expression (SAGE). Christine et al. 24 performed in situ hybridization of pancreatic tissue to characterize the expression of ApoC-I genes identified by SAGE and found that ApoC-I was highly expressed in invasive pancreatic cancer tissues. On the basis of these results, ApoC-I expression seems to be regulated in several types of cancers. These results suggested that its potential involvement in prostate cancer pathogenesis was worth investigating.
Until now, the underlying mechanism of the relationship between ApoC-I and prostate cancer has been unclear. In this study, we showed an increase in serum protein levels of ApoC-I in prostate cancer patients during the progression of cancer to a hormone-refractory state. Our experimental observations suggest that ApoC-I protein was obviously expressed in hormone-refractory prostate cancer cells. Given these results, the ApoC-I protein seems to be related to the progression of prostate cancer. This suggests that ApoC-I could be a promising biomarker, as well as a target for an innovative therapy for hormone-refractory prostate cancer. However, as the exact role of ApoC-I in prostate cancer pathogenesis is not yet clear, further research is required.
Acknowledgments
This study was supported in part by a Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Technology and Culture, Japan (Grants 16591582, 16689026, and 19591834); the Ministry of Health, Labour and Welfare, Japan (Aid for Cancer Research); the Public Trust Haraguchi Memorial Cancer Research Fund (2003); and Grants-in-Aid from the Yamaguchi Endocrine Research Association (2005), the Japanese Foundation for Prostate Cancer (2005) and the Japanese Urological Association (2006).
References
- Huggins C, Hodges CV. The effect of castration of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–7. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- Schmidt JD. The patient, disease status, and treatment options for prostate cancer: stages D1 and D2. Prostate. 1983;4:493–501. doi: 10.1002/pros.2990040508. [DOI] [PubMed] [Google Scholar]
- Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59:2511–5. [PubMed] [Google Scholar]
- Suzuki H, Ueda T, Ichikawa T, Ito H. Androgen receptor involvement in progression of prostate cancer. Endocr Relat Cancer. 2003;10:209–16. doi: 10.1677/erc.0.0100209. [DOI] [PubMed] [Google Scholar]
- Nomura F, Tomonaga T, Sogawa K, Ohashi T, Nezu M, et al. Identification of novel and downregulated biomarkers for alcoholism by surface enhanced laser desorption/ionization-mass spectrometry. Proteomics. 2004;4:1187–94. doi: 10.1002/pmic.200300674. [DOI] [PubMed] [Google Scholar]
- Liu AY, Zhang H, Sorensen CM, Diamond DL. Analysis of prostate cancer by proteomics using tissue specimens. J Urol. 2005;173:73–8. doi: 10.1097/01.ju.0000146543.33543.a3. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Amneus MW, Pusey SM, Su F, Luong MN, et al. Identification of biomarkers for ovarian cancer using strong anion-exchange ProteinChips: potential use in diagnosis and prognosis. Proc Natl Acad Sci USA. 2003;100:12343–8. doi: 10.1073/pnas.2033602100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis V, Degoes F, Dagère D, Pham N, Belghiti J, et al. Identification of a new marker of hepatocellular carcinoma by serum protein profiling of patients with chronic liver diseases. Hepatology. 2005;41:40–7. doi: 10.1002/hep.20505. [DOI] [PubMed] [Google Scholar]
- Rosty C, Christa L, Kuzdazal S, Baldwin WM, Zahurak ML, et al. Identification of hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein I as a biomarker for pancreatic ductal adenocarcinoma by protein biochip technology. Cancer Res. 2002;62:1868–75. [PubMed] [Google Scholar]
- Li J, White N, Zhang Z, Rosenzweig J, Mangold LA, et al. Detection of prostate cancer using serum proteomics pattern in a histologically confirmed population. J Urol. 2004;171:1782–7. doi: 10.1097/01.ju.0000119823.86393.49. [DOI] [PubMed] [Google Scholar]
- Gretzer MB, Partin AW.Prostate cancer tumor markersIn: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, et al. editors. Campbell–Walsh Urology9th edn. Philadelphia: Saunders; 2007p2896–911. [Google Scholar]
- Cho D, Di Blasio CJ, Rhee AC, Kattan MW. Prognostic factors for survival in patients with hormone-refractory prostate cancer (HRPC) after initial androgen deprivation. Urol Oncol. 2003;21:282–91. doi: 10.1016/s1078-1439(03)00057-7. [DOI] [PubMed] [Google Scholar]
- Nishio Y, Yamada Y, Kokubo H, Nakamura K, Aoki S, et al. Prognostic significance of immunohistochemical expression of the HER-2/neu oncoprotein in bone metastatic prostate cancer. Urology. 2006;68:110–5. doi: 10.1016/j.urology.2006.01.060. [DOI] [PubMed] [Google Scholar]
- Jong MC, Hofker MH, Havekes LM. Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler Thromb Vasc Biol. 1999;19:472–84. doi: 10.1161/01.atv.19.3.472. [DOI] [PubMed] [Google Scholar]
- Gong Z, Agalliu L, Lin DW, Stanford JL, Kristal AR. Obesity is associated with increased risk of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109:1192–202. doi: 10.1002/cncr.22534. [DOI] [PubMed] [Google Scholar]
- Kristal AR, Gong Z. Obesity and prostate cancer mortality. Future Oncol. 2007;3:557–67. doi: 10.2217/14796694.3.5.557. [DOI] [PubMed] [Google Scholar]
- Hughes-Fulford M, Chen Y, Tjandra Winata RR. Fatty acid regulates gene expression and growth of human prostate cancer PC-3 cells. Carcinogenesis. 2001;22:701–7. doi: 10.1093/carcin/22.5.701. [DOI] [PubMed] [Google Scholar]
- Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- Malik G, Ward MD, Gupta SK, Trosset MW, Grizzle WE, et al. Serum levels of an isoform of apolipoprotein A-II as a potential marker for prostate cancer. Clin Cancer Res. 2005;11:1073–85. [PubMed] [Google Scholar]
- Hall RE, Horsfall DJ, Stahl J, Vivekanandan S, Ricciardelli C, et al. Apolipoprotein-D: a novel cellular marker for HGPIN and prostate cancer. Prostate. 2004;58:103–8. doi: 10.1002/pros.10343. [DOI] [PubMed] [Google Scholar]
- Goncçalves A, Esterni B, Bertucci F, Sauvan R, Chabannon C, et al. Postoperative serum proteomic profiles may predict metastatic relapse in high-risk primary breast cancer patients receiving adjuvant chemotherapy. Oncogene. 2006;25:981–9. doi: 10.1038/sj.onc.1209131. [DOI] [PubMed] [Google Scholar]
- Engwegen JY, Helgi HH, Cats A, Harris N, Bonfrer JM, et al. Identification of serum proteins discriminating colorectal cancer patients and healthy controls using surface-enhanced laser desorpt ion ionisation-time of flight mass spectrometry. World J Gastroenterol. 2006;12:1536–44. doi: 10.3748/wjg.v12.i10.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui W, Oue N, Ito R, Kuraoka K, Nakayama H. Search for new biomarkers of gastric cancer through serial analysis of gene expression and its clinical implications. Cancer Sci. 2004;95:385–92. doi: 10.1111/j.1349-7006.2004.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobuzio-Donahue CA, Ryu B, Hruban RH, Kern SE. Exploring the host desmoplastic response to pancreatic carcinoma. Am J Pathol. 2002;160:91–9. doi: 10.1016/S0002-9440(10)64353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


