Abstract
Fragments of testis tissue from immature animals grow and develop spermatogenesis when grafted onto subcutaneous areas of immunodeficient mice. The same results are obtained when dissociated cells from immature testes of rodents are injected into the subcutis of nude mice. Those cells reconstitute seminiferous tubules and facilitate spermatogenesis. We compared these two methods, tissue grafting and cell-injection methods, in terms of the efficiency of spermatogenesis in the backs of three strains of immunodeficient mice, using neonatal porcine testicular tissues and cells as donor material. Nude, severe combined immunodeficient (SCID) and NOD/Shi-SCID, IL-2Rγcnull (NOG) mice were used as recipients. At 10 months after surgery, the transplants were examined histologically. Both grafting and cell-injection methods resulted in porcine spermatogenesis on the backs of recipient mice; the percentage of spermatids present in the transplants was 67% and 22%, respectively. Using the grafting method, all three strains of mice supported the same extent of spermatogenesis. As for the cell-injection method, although SCID mice were the best host for supporting reconstitution and spermatogenesis, any difference from the other strains was not significant. As NOG mice did not show any better results, the severity of immunodeficiency seemed to be irrelevant for supporting xeno-ectopic spermatogenesis. Our results confirmed that tubular reconstitution is applicable to porcine testicular cells. This method as well as the grafting method would be useful for studying spermatogenesis in different kinds of animals.
Keywords: pigs, seminiferous tubule, spermatogenesis, xeno-transplantation
Introduction
Spermatogenesis is a complex and long-lasting cell-differentiation process whereby spermatogonial stem cells develop into spermatozoa. This process takes 35 days in mice and boars 1. To elucidate the mechanism of spermatogenesis and the conditions required for this process, researchers have long attempted to recapitulate the process in vitro 2, 3. Several reports have claimed to have achieved the completion of meiosis leading to the production of spermatids in vitro 4, 5. Those studies, however, have used the testicular cells of immature animals as experimental material, which include not only spermatogonial stem cells but also spermatocytes from the meiotic stage. Therefore, no reliable methods have been developed yet to promote meiosis from spermatogonial stem cells to produce haploid gametes in vitro.
Since its technical development in 1994, transplantation into the lumen of seminiferous tubules has become the standard method of inducing complete spermatogenesis from spermatogonial stem cells 6, 7. This technique has been successful in various animal species, including rats, bovines and monkeys 8. However, the procedure has to be modified for individual animal species depending on the histo-anatomical structure of their testicles, and the efficiency in other species is quite low compared with that in mice. Xenogenic transplantation of testicular cells from different donor species into recipient immunodeficient mice has been explored but has proven to be successful only for cells from rats and hamsters 9, 10. In addition, to achieve satisfactory colonization of donor cells, the recipient animals have to be pretreated with the cytoablasive drug busulfan or with irradiation, which can be physically detrimental to the recipients 11. The use of larger animals as recipients for cell transplantation is not practical in most research situations because of the high costs involved and the need for more space.
Since 2002, studies have shown that a small fragment of testicular tissue of neonatal animals, including mice, pigs, goats and primates, grows larger and exhibits complete spermatogenesis after being grafted under the back skin of nude mice 12, 13, 14. This finding indicates that full spermatogenesis from spermatogonial stem cells is possible in ectopic sites when the micro-architecture of seminiferous tubules can be established. This ectopic spermatogenesis has several advantages over ordinary testicular spermatogenesis. For instance, it can advance the initiation of spermatogenesis in primates, which otherwise have long pre-pubertal periods. The grafted primate testis tissue fragments showed accelerated maturation, and fertilization-competent sperms were produced upon xenograft in host mice 15. In addition, the use of small experimental animals should facilitate any experimental manipulations like local or systemic administration of hormones or factors to the recipients.
However, if one intends to manipulate particular cell types of the testes, to perform transfection of germ cells for instance, the grafting method has very little superiority over natural spermatogenesis in the testes because the histological structure of the graft must be undisturbed throughout the experiment, while manipulation of a specific cell type often requires dissociation of tissue. This fact would limit the application of this grafting method as an experimental technique for the study of spermatogenesis. In contrast, if seminiferous tubules could be regenerated de novo from dissociated cells, a specific cell type could be manipulated, and its biological function could be examined in the reconstituted tubules.
In our earlier report, we showed that reconstitution of seminiferous tubules from dissociated neonatal testicular cells of rodents is possible in the subcutis of nude mice. The mouse germ-line stem (GS) cells 16, 17, which are cultured spermatogonial stem cells, were integrated in reconstituted tubules and were shown to undergo spermatogenesis up to the spermatid stage. The resultant spermatids were fertility-competent, and progeny were produced by microinsemination 18. We also tested whether such reconstitution of seminiferous tubules is possible in other animal species by using porcine testicular cells. The dissociated neonatal porcine cells formed reorganized tubular structures in nude mice 18.
In this study, to extend our earlier results, we examine whether porcine spermatogenesis will proceed in those reconstituted tubules. In addition, we hypothesize that immunodeficiency of the host mice influences the success rate of ectopic spermatogenesis of inter-species transplantation, because NOD-NOD/Shi-SCID, IL-2Rγcnull(NOG), severe combined immunodeficient (SCID) or NOG mice, which are more severely immunocompromised than nude and SCID mice, are more suitable hosts for accepting and sustaining human hematopoietic cells 19. Therefore, we compare the efficiency of spermatogenesis between cell-injection and tissue grafting methods in three different types of immunodeficient mice (nude, SCID and NOG) to examine the effect of immunological conditions on the de novo formation of seminiferous tubules and spermatogenesis in graft tissues.
Materials and methods
Animals
Male nude mice (BALB/cAJcl-nu/nu) and SCID mice (C.B-17/lcr-scid/scidJcl), 6 weeks old, were purchased from Clea (Tokyo, Japan). NOG mice (NOD/Shi-SCID, IL-2Rγcnull) were created at the Central Institute of Experimental Animals (Kawasaki, Japan) by backcrossing γcnull null mice to NOD/Shi-SCID mice, as reported earlier 19. Four NOG mice, 6 weeks old, were shipped to the animal facility of Yokohama City University Graduate School of Medicine (Yokohama, Japan). All mice were kept under specific pathogen-free conditions. Neonatal pig testes were donated by a breeding farm. All of the experiments with animals conformed to the Guide for Care and Use of Laboratory Animals and were approved by the Institutional Committee of Laboratory Animal Experimentation (Research Institute for Yokohama City University, Yokohama, Japan).
Transplantation of testicular cell suspension
Donor cells for transplantation were prepared from six testes of neonatal pigs within a day after delivery (Figure 1A). The testes were decapsulated, and tissue fragments 1 mm3 in size were dissected out for the tissue grafting experiment. The remaining tissues were digested using a two-step enzymatic treatment 20. Briefly, testicular tissue was treated with digestion solution I, containing collagenase type II (2 mg mL−1) and DNAse (10 μg mL−1) in phosphate buffered saline at 37°C for 20 min. The specimens were centrifuged and then treated with digestion solution II, which contained collagenase type II (2 mg mL−1), DNAse (10 μg mL−1) and hyaluronidase (2 mg mL−1) in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) at 37°C for 5 min. The cell suspension was washed twice with phosphate buffered saline and filtered through a mesh with a pore size of 40 μm. In total, 30 × 106 cells were harvested. The cells were suspended in Dulbecco's modified Eagle's medium and mixed with the same volume of growth factor-reduced Matrigel Matrix (MGM; Becton Dickinson Labware, Bedford, MA, USA) on ice, with a final concentration of 20 × 106 cells mL−1. Implantation was performed in mice at the age of 7 weeks. Mice were anesthetized using an intraperitoneal injection of a ketamine-xylazine cocktail. The MGM cell suspension (0.05 mL) was injected subcutaneously using a 26-G needle into the dorsal region of the mice. Each animal was injected at two sites on the left side of the back. At two sites on the right side of the back, porcine testis tissue fragments of 1 mm3 in size were grafted through a small skin incision. In all, 1 mm3 of neonatal porcine testis tissues contained approximately 5 × 104 cells, which is an estimate based on counting every single cell in the histological sections. The mice were then castrated using a lower midline abdominal incision. Four mice in each immunodeficient strain were used as recipients. Hence, 12 mice in total were used.
Figure 1.
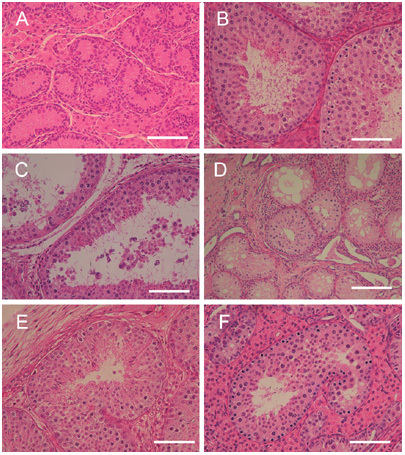
Reconstitution of seminiferous tubules from dissociated neonatal testicular cells. Histology of neonatal testes used in this study. (A): Porcine spermatogenesis developed in a tissue graft, 10 months after grafting. (B): Seminiferous tubules of graft experiments showing dilatation of the tubule with fluid retention. Some epithelial cells were sloughed off. (C): The transplants of isolated cells showing reconstitution of seminiferous tubules, most of which were composed only of Sertoli cells. Some tubules, however, contained germ cells and showed spermatogenic differentiation on the backs of SCID mice. (D): Foci showing complete spermatogenesis in the reconstituted tubules of SCID (E) and NOG mice (F). Bars = 50 μm (A–C, E–F) and 100 μm (D).
Gross and histological examination
Each sample was analyzed at 10 months after transplantation. After the mice were killed, subcutaneous specimens were dissected out, and their diameters and weights were measured. The specimens were then fixed in Bouin's solution at room temperature overnight. After being rinsed in 70% ethanol for 1–2 days, the tissues were cut into half using a scalpel to make a large cut surface on each tissue. The tissues were then embedded in paraffin. Five-micrometer-thick histological sections were made and stained with hematoxylin and eosin. The seminiferous tubules were counted, and the extent of spermatogenesis was evaluated. We used elongated spermatids as a hallmark of complete spermatogenesis. The presence of round spermatids was considered as semi-complete spermatogenesis.
Statistical analyses
Data were expressed as mean ± SD. One-way analysis of variance, the non-parametric Mann-Whitney U test and/or the Kruskal-Wallis test were carried out to detect differences between methods and among groups of mice.
Results
General observations
The sites of grafting and cell injections both gradually bulged and became grossly noticeable by around 4 weeks. The size of transplants reached approximately 15 mm in diameter and 4 mm in height during the subsequent observation period. One NOG mouse died within a week of surgery for unknown reasons. Two SCID mice died 5 and 7 months after the surgery, respectively, for unknown reasons. The remaining four nude, two SCID and three NOG mice were analyzed 10 months after the transplantation.
Tissue grafting method
The diameters of the transplants achieved by the tissue grafting method were 11.3 ± 4.4, 11.8 ± 2.1 and 8.5 ± 1.8 mm in nude, SCID and NOG mice, respectively. Their weights were 292.8 ± 288.5, 885.0 ± 267.5 and 149.3 ± 115.7 mg in nude, SCID and NOG mice, respectively.
Histological examination of tissue grafting samples revealed complete spermatogenesis at high frequencies in cross-sections of seminiferous tubules (Figure 1B). The frequencies were 74.0%, 88.1% and 70.7% in nude, SCID and NOG mice, respectively (Table 1). There was a tendency for seminiferous tubules located in the central area of the grafts to show poor development of spermatogenesis, probably as a result of paucity in the blood supply. In some cases, central degeneration of the tissue was found. It can be noted that we occasionally found seminiferous tubules that were dilated and showed a cyst-like structure that contained some sperm in the lumen (Figure 1C). This could be interpreted as indicating that luminal fluid produced by Sertoli cells was retained because of the lack of a drainage route like that in the normal testes, where the fluid flows out into the epididymis, which carries sperm. In 12 out of 18 grafts (66.7%) spermatogenesis progressed to completion, with sperm production in the lumen (Table 1).
Table 1. Summary of results.
| |
Tissue grafting method |
Cell-injection method |
||||||
|---|---|---|---|---|---|---|---|---|
| Mice (n) | Grafts (n) | Grafts containing spermatids (n, [%]) | Spermatid-containing tubules (n) / seminiferous tubules (n) | Transplants (n) | Transplants containing reconstituted tubules (n) | Transplants containing spermatids (n, [%]) | Spermatid-containing tubules (%)/reconstituted tubules (%) | |
| Nude | 4 | 8 | 6 (75)* | 1 382/1 867 | 8 | 8 | 0 (0)* | 0/354 (0.0)** |
| SCID | 2 | 4 | 2 (50) | 570/645 | 4 | 3 | 3 (75) | 89/1 010 (8.8)*** |
| NOG | 3 | 6 | 4 (67) | 1 633/2 311 | 6 | 6 | 1 (17) | 1/613 (0.2)**** |
| Total | 9 | 18 | 12 (67) | 18 | 17 | 4(22) | ||
Abbreviation: SCID, severe combined immunodeficient.
P = 0.01;
P < 0.001;
P < 0.001;
P = 0.026, compared with tissue grafting method.
Cell-injection method
The diameters of transplants achieved by the cell-injection method were 7.3 ± 1.8, 9.8 ± 1.7 and 8.5 ± 2.6 mm in nude, SCID and NOG mice, respectively. Their weights were 71.9 ± 54.1, 179.0 ± 96.1 and 165.1 ± 107.0 mg in nude, SCID and NOG mice, respectively. Of 18 transplants in nine recipient mice, we observed the reorganization of tubules in 17 transplants (Table 1). The number of seminiferous tubules reconstituted was counted in each histological section and found to be 44.3 ± 31.4, 336.7 ± 137.9 and 102.2 ± 43.2 in nude, SCID and NOG mice, respectively. Most of these reconstituted tubules were Sertoli-only and did not contain germ cells (Figure 1D). However, in total, four out of 18 transplants showed complete spermatogenesis with elongated spermatids (22.2%), of which three were found in SCID recipients and one was found in an NOG recipient. Two more transplants in NOG recipients showed spermatogenesis up until round-spermatid production. In the four samples showing complete spermatogenesis, the frequency of tubules with elongated spermatids among all sections of seminiferous tubules was 16.3%, 3.4%, 1.2% and 0.7%, respectively, with a total average value of 7.8% (Figure 2).
Figure 2.
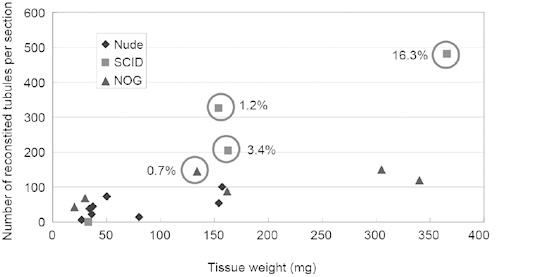
Relationship of spermatogenesis development to tissue weight, the number of seminiferous tubules in a tissue section and the type of recipient mouse as presented in a dot graph. The x axis indicates the weight of the reconstituted transplants. The y axis indicates the number of tubules counted in a section of the transplants. A circle indicates the presence of complete spermatogenesis. Percentage figures beside the circle indicate the frequency of seminiferous tubules having elongated spermatids among all sections of tubules counted. This graph suggests that the chance of spermatogenesis development increased with the number of reconstituted tubules in a tissue derived from transplanted cells. NOG, NOD/Shi-SCID, IL-2Rγcnull; SCID, severe combined immunodeficient.
Comparing the three strains of mice, we found that reconstitution of tubules and spermatogenesis progressed most successfully in the backs of SCID mice (Table 1, Figure 1D and E). Out of 1 010 tubule sections examined, 89 contained elongated spermatids in SCID recipients. Such sections were rarely found in nude and NOG recipients (Table 1, Figure 1F). Overall, the proportions of complete spermatogenesis among all reconstituted tubules were 8.8% and 0.16% in the SCID and NOG mouse groups, respectively (Table 1). In contrast to the tissue grafting experiment, we did not observe dilated seminiferous tubules in the reconstituted samples.
We examined the relationship between the size of cell transplants and the number of reconstituted tubules observed in histological sections. Sperm production was confirmed in larger transplants with a higher number of tubules in them (Figure 2). Although we injected the same number of cells in each subcutaneous site, the actual number of cells injected and their later survival might have been influenced by various factors, including technical variability and the subcutaneous microenvironment at the injection site in each animal.
Discussion
We showed earlier that spermatogenesis of mice and rats can take place in seminiferous tubules, which were reconstituted subcutaneously from dissociated embryonic or neonatal testicular cells 18. We found that, once dissociated into single cells, a few testicular germ cells were integrated again in the reconstituted seminiferous tubules. Those germ cells underwent differentiation up to the mid-meiotic phase. In very rare cases, even complete spermatogenesis was observed. To confirm this phenomenon of reintegration of germ cells into immature testicular somatic cells we used GS cells, and we found that they were also integrated in the reconstituted tubules. The integrated GS cells differentiated to round spermatids. Such spermatids were shown to be fertilization-competent by microinsemination into oocytes to deliver normal pups 18.
Practically, this reconstitution (cell-injection) method should be especially useful for animals other than experimental rodents. For mice and rats, germ cell transplantation into the seminiferous tubule of recipient animals is readily available as a standard method to induce spermatogenesis from a given sample of spermatogenic stem cells. However, the transplantation method is laborious, inefficient and expensive to perform in larger animals such as livestock. Therefore, producing a reconstituted mini-testis of large animals on the backs of immunodeficient mice should be an ideal alternative to study spermatogenesis of those animal species. In fact, it was reported recently that porcine testicular cells can rebuild testicular histo-architecture and give rise to complete spermatogenesis 21. This and our present result both indicate that the reconstitution method is useful for studying porcine spermatogenesis and for producing porcine sperm. However, there were several differences in experimental procedures between that report and our study. They used 1–2-week-old neonatal testes, whereas we used testes excised within 1 day of birth. They made cell pellets of 50 × 106 cells to surgically transplant them into the subcutis of immunodeficient mice. In contrast, we simply injected 1 × 106 cells mixed with MGM using a syringe with a 26-G needle. Their results showed that each graft of cell pellet produced spermatids in approximately 11% of reconstituted seminiferous tubules 21. This frequency of spermatogenic development in the reconstituted tubules was better than that found in our study. In that set of experiments, the best four samples yielding spermatids in 7.8% of tubules could be ascribed to the initial cell dosage, which was 50 times higher in their experiment than in ours. Conversely, because production of structurally stable cell pellets requires a greater number of cells, our cell-injection method should be easier to perform and more feasible, especially when small numbers of cells are available, while providing results comparable to the cell-pellet method.
Among the three different types of immunodeficient mice, SCID mice most successfully supported the development of spermatogenesis on their backs. How-ever, this study was too small in its sample number to verify the superiority of SCID mice over the other two strains. As shown in Figure 2, the most effective spermatogenesis was achieved when transplants became larger. This result suggests that a higher number of transplanted cells might increase the success rate of sperm production regardless of the strain of the recipient. At the same time, it is desirable for transplanted cells to have a microenvironment suitable for proliferation as well as tissue organization. This postulated hospitable microenvironment seemed to have little effect on the immunocompetency of the recipients because, contrary to our expectation, NOG mice, which are the most severely immunodeficient among the three mouse strains 19, did not show the most successful development of porcine spermatogenesis.
The mechanism of reorganization of seminiferous tubules is not clear. In another study using mice and rats more than 2 days old as the source of testicular cells, we found that the reorganization of the seminiferous tubules was poor in the subcutis of nude mice 18. As the age of the animals increased, the efficiency of reconstitution and structural completeness of seminiferous tubules declined. On the basis of such observations, we speculated that one of the most important features of testicular cells for reorganizing tubular structures would be their proliferation potential. In fact, the proliferation of Sertoli cells of mice rapidly decreases after birth and ceases by 3 weeks of age. Another potentially important factor for reorganization would be the induction of neovascular formation. The reconstructed samples almost always received vascular supply from the host circulation. This observation indicates that immature tissue with more robust developmental potential also has better neovascularization ability, facilitating reconstitution of the testes.
It remains to be addressed whether this reconstitution method is applicable to other animal species, especially to primates and humans. As noted above, we speculated that the proliferation potential of testicular somatic cells, especially Sertoli cells, is the key factor for the success of reconstitution. It has been reported that human Sertoli cells maintain their proliferative state during their long pre-pubertal period 22. Therefore, it might be possible for testicular cells from children to reconstitute seminiferous tubules at ectopic locations. Such reconsti-tuted tubules might also support spermatogenesis, from which sperm could be retrieved for intracytoplasmic sperm injection. This scenario of ectopic regeneration of spermatogenesis could be applicable to pediatric cancer patients who do not benefit from semen cryopreservation 23, 24. The testicular cells of such young patients could be cryopreserved for later cell transplantation to their subcutis as auto-transplantation.
Recently, it has become possible to culture sper-matogonial stem cells of mice and rats 16, 17, 25, 26. These GS cells can expand in vitro 16. Manipulation of their genome has also become possible 27, 28. To extend this technique to other mammals such as livestock and primates, an experimental system to induce spermatogenesis from GS cells is necessary. The reconstitution method would be a convenient and reliable assay system for GS cells, when established, from a variety of mammalian species. As GS cells can be manipulated (for example, by transfection with transgenes) while they are expanded in vitro, the reconstitution method could allow the production of gametes from such manipulated GS cells. Therefore, the establishment of human GS cells should provide important tools for the study of human spermatogenesis in an experimental setting. The reconstitution method will allow us to improve our knowledge of human spermatogenesis.
Acknowledgments
We acknowledge the technical assistance of Ms Kumiko Katagiri. This work was supported by 2006 Strategic Research Project Grant K17002 from Yokohama City University, Japan, and Grant-in-Aid for Scientific Research 18591783 from the Ministry of Education, Science, Sports and Culture, Japan.
References
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum AL. Mammalian spermatogenesis in vivo and in vitro: a partnership of spermatogenic and somatic cell lineages. Endocr Rev. 1994;15:116–34. doi: 10.1210/edrv-15-1-116. [DOI] [PubMed] [Google Scholar]
- Staub C. A century of research on mammalian male germ cell meiotic differentiation in vitro. J Androl. 2001;22:911–26. doi: 10.1002/j.1939-4640.2001.tb03430.x. [DOI] [PubMed] [Google Scholar]
- Staub C, Hue D, Nicolle JC, Perrard-Sapori MH, Segretain D, et al. The whole meiotic process can occur in vitro in untransformed rat spermatogenic cells. Exp Cell Res. 2000;260:85–95. doi: 10.1006/excr.2000.4998. [DOI] [PubMed] [Google Scholar]
- Marh J, Tres LL, Yamazaki Y, Yanagimachi R, Kierszenbaum AL. Mouse round spermatids developed in vitro from preexisting spermatocytes can produce normal offspring by nuclear injection into in vivo-developed mature oocytes. Biol Reprod. 2003;69:169–76. doi: 10.1095/biolreprod.102.015099. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA. 1994;91:11298–302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat Med. 2000;6:29–34. doi: 10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatt S, Rosiepen G, Weinbauer GF, Rolf C, Brook PF, et al. Germ cell transfer into rat, bovine, monkey and human testes. Hum Reprod. 1999;14:144–50. doi: 10.1093/humrep/14.1.144. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Avarbock MR, Maika SD, Hammer RE, Brinster RL. Rat spermatogenesis in mouse testis. Nature. 1996;381:418–21. doi: 10.1038/381418a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Xenogeneic spermatogenesis following transplantation of hamster germ cells to mouse testes. Biol Reprod. 1999;60:515–21. doi: 10.1095/biolreprod60.2.515. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Behboodi E, Hausler CL, Blash S, Ayres S, et al. Depletion of endogenous germ cells in male pigs and goats in preparation for germ cell transplantation. J Androl. 2005;26:698–705. doi: 10.2164/jandrol.05032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaramooz A, Snedaker A, Boiani M, Schöler H, Dobrinski I, et al. Sperm from neonatal mammalian testes grafted in mice. Nature. 2002;418:778–81. doi: 10.1038/nature00918. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Honaramooz A, Boiani M, Schöler HR, Dobrinski I. Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biol Reprod. 2003;68:2331–5. doi: 10.1095/biolreprod.102.014894. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Schlatt S. Testicular xenografts: a novel approach to study cytotoxic damage in juvenile primate testis. Cancer Res. 2006;66:3813–8. doi: 10.1158/0008-5472.CAN-05-3754. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Li MW, Penedo MC, Meyers S, Dobrinski I. Accelerated maturation of primate testis by xenografting into mice. Biol Reprod. 2004;70:1500–3. doi: 10.1095/biolreprod.103.025536. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–6. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Ohmura M, Tamura Y, Kita K, Ohbo K, et al. Derivation and morphological characterization of mouse spermatogonial stem cell lines. Arch Histol Cytol. 2004;67:297–306. doi: 10.1679/aohc.67.297. [DOI] [PubMed] [Google Scholar]
- Kita K, Watanabe T, Ohsaka K, Hayashi H, Kubota Y, et al. Production of functional spermatids from mouse germline stem cells in ectopically reconstituted seminiferous tubules. Biol Reprod. 2007;76:211–7. doi: 10.1095/biolreprod.106.056895. [DOI] [PubMed] [Google Scholar]
- Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–82. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- van der Wee K, Hofmann MC. An in vitro tubule assay identifies HGF as a morphogen for the formation of semi-niferous tubules in the postnatal mouse testis. Exp Cell Res. 1999;252:175–85. doi: 10.1006/excr.1999.4630. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Megee SO, Rathi R, Dobrinski I. Building a testis: formation of functional testis tissue after trans-plantation of isolated porcine (Sus scrofa) testis cells. Biol Reprod. 2007;76:43–7. doi: 10.1095/biolreprod.106.054999. [DOI] [PubMed] [Google Scholar]
- Cortes D, Müller J, Skakkebæk NE. Proliferation of Sertoli cells during development of the human testis assessed by stereological methods. Int J Androl. 1987;10:589–96. doi: 10.1111/j.1365-2605.1987.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Kubota H, Brinster RL. Technology insight: in vitro culture of spermatogonial stem cells and their potential therapeutic uses. Nat Clin Pract Endocrinol Metab. 2006;2:99–108. doi: 10.1038/ncpendmet0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Söder O, Schlatt S. Clinical potential and putative risks of fertility preservation in children utilizing gonadal tissue or germline stem cells. Pediatr Res. 2006;59:40R–7R. doi: 10.1203/01.pdr.0000205153.18494.3b. [DOI] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2004;101:16489–94. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu BY, Kubota H, Avarbock MR, Brinster RL. Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat. Proc Natl Acad Sci USA. 2005;102:14302–7. doi: 10.1073/pnas.0506970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Tokokuni S, Shinohara T. Genetic selection of mouse male germline stem cells in vitro: offspring from single stem cells. Biol Reprod. 2005;72:236–40. doi: 10.1095/biolreprod.104.035659. [DOI] [PubMed] [Google Scholar]
- Hamra FK, Chapman KM, Nguyen DM, Williams-Stephens AA, Hammer RE, et al. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc Natl Acad Sci USA. 2005;102:17430–5. doi: 10.1073/pnas.0508780102. [DOI] [PMC free article] [PubMed] [Google Scholar]


