Abstract
We report a rare case of a left ejaculatory duct that allotropically protrudes towards or invades the left vesicle triangular area with its dead end. The patient simultaneously exhibited multiple congenital malformations of the homolateral urogenital system, such as absence of the left kidney, dysplasia and allotopia of the left seminal vesicle, absence of the left ureterostoma, separation between the left testis and the epididymis tail, and maldevelopment of the left testis. According to all clinical and laboratory evidence, the case represented a new syndrome, which we named Wuyang's syndrome. It involved a rare phenomenon in embryonic development; the dysplastic proximal vas precursor, having intruded into a common mesonephric duct and accidentally encroaching on the ureteric bud position, resulted in the absence or dysplasia of the homolateral urinary tract and ectopic invasion of the bladder by the homolateral seminal tract.
Keywords: common mesonephric duct, proximal vas precursor, ureteric bud, urogenital system
Case history
A 33-year-old man, married, with two daughters, complained about having had a sensation of wandering foreign matter in the anus for 4 years and repeated dysuresia for the past 3 months. He was hospitalized in December 2007.
Four years ago, the patient felt as though there were wandering foreign matter in the anus, with a deviation to the left, and a fallen and distended feeling in the anal orifice and left testis, without obvious causative factors. There was no history of rectal tenesmus, bloody purulent stool, dyschesia or redness and swelling of the scrotum skin, and several therapies against internal haemorrhoid were ineffective. In the past 3 months, symptoms of urinary frequency, odynuria and dysuresia occurred frequently, and could be partly or completely relieved by abdominal hot fomentation or by changing the emiction posture. In addition, there was one episode of urinary retention, but no stomachache, urinary urgency or haematuria occurred during the course of the disease.
Preoperative examinations
Physical inspection showed that the patient was not sensitive to percussion at both renal areas, the left deferens was coarser than the right one and the left testis was separated from the epididymis tail. Digital rectal examination determined that the prostate was of normal size but had a soft cystic mass in the upper left that was not tender.
Ultrasonography of the urogenital system showed an absence of the left kidney in the renal region, and an elliptically aqueous dark space at the left of the vesicle triangle, which was 6.5 × 3.9 × 4.0 cm3 and extended towards the upper-left posterior position of the bladder. Moreover, the left testis and epididymis were smaller than their respective contralateral parts.
A plain abdominal radiograph displayed no radiopaque calculus image, and an intravenous urogram (IVU) revealed a normal upper-right urinary tract and a filling defect with a smooth surface on the left side of the bladder. However, there was no imaging of the left-upper urinary tract in the IVU picture (Figure 1A).
Figure 1.

Imaging studies of the patient showed the absence of a left upper urinary tract with a normal right upper urinary tract, and an aqueous mass on the left and below the bladder with a high signal in both T1- and T2-weighted images (T1WI and T2WI) of magnetic resonance imaging (MRI). (A): 90-min IVU image; (B) and (C): Computerized tomography (CT) plain and enhancement scanning of pelvic cavity; (D): MRI T1WI; (E): reconstituted image of MRI T1WI; (F): MRI T2WI; (G): reconstituted image of MRI T2WI.
Computerized tomography (CT) showed a fusiform cyst with 10 HU CT-value and about 7 × 4 × 5 cm3 in size protruding towards the urocyst from the left rear. It also revealed the absence of the left kidney and a normal prostate (Figures 1B and 1C).
Magnetic resonance imaging (MRI) displayed an absence of the left upper urinary tract and an oval high-signal change with a slick boundary towards the left part of an urocyst in both T1- and T2-weighted images (T1WI and T2WI). Furthermore, MRI urography showed a bending tubular structure with different calibres at the top of the cystic mass (Figures 1D–1G).
Cystoscopy exhibited a normal urethra without stenosis, an absence of the left ureterostoma and a smooth mass with a surface of normal urocystic mucous membrane protruding towards the left of the vesicle triangle.
Operative findings
The operation began with a successful epidural anaesthesia. After we explored the patient's cavitas pelvis, a cyst was found at the lower right side of the left external iliac vessels, protruding towards the bladder. We dissociated it outward, along the cyst's surface, and found its tail gradually becoming cordlike. At 4 cm above the cyst, there was a seminal vesicle-like lump, about 4.0 × 2.2 × 1.5 cm3 and connected to the cyst tail by the cordlike tissue. After successive blunt dissections along the cordlike pattern, we found the ventricose ampulla of the vas deferens, and so the cordlike tissue should be the left deferent duct (Figure 2).
Figure 2.
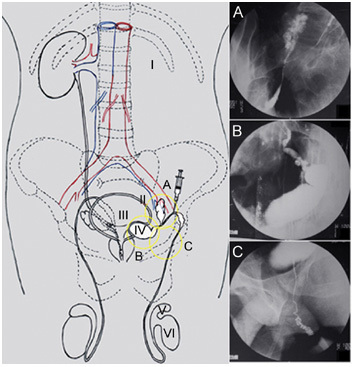
The conceptual diagrams of dysplasia in the genitourinary system and X-ray graphs of deferent duct visualization. I: the congenital absence of left kidney; II: connatural dysplasia and allotopia of left seminal vesicle verified; III: the absence of left ureterostoma; IV: left scrotiform-expansive ejaculatory duct, which allotropically protruded or ‘debouched' towards the bladder with its dead end; V: the separation of the left testis and epididymis tail; VI: the maldevelopment of left testis. After 50% cardiografin was infused by paracentesis of the vas deferens during the operation: (A): visualization of the left ectopic seminal vesicle; (B): visualization of the left scrotiform-expansive ejaculatory duct; (C): visualization of the left ectatic deferent duct and ductus epididymidis.
After 50% cardiografin was infused by paracentesis of the left vas deferens, the left deferent duct, the left ectopic seminal vesicle, the left diverticula ampullae ductus deferentis (Figure 2B), the cyst (Figure 2C) and the left epididymis (Figure 2D) were visualized, in order, under the X-ray monitoring during operation. We cut open the serous membrane and tried to separate the cyst from the bladder, but we could not find an anatomical boundary between the inward surface of the cyst and the left posterior wall of the bladder. In fact, the cyst wall may have invaded, or grown into, the tunica muscularis vesicae urinariae. Some cyst fluid, a thick oyster-white liquid without an abnormal flavour, was drawn out by a 10-mL injector for biochemical examination.
It was difficult to resect the cyst completely; so we mutilated the cyst paries from the bladder wall and excised the conjunct wall of the bladder and the cyst, and we then resected the left ectopic seminal vesicle and ligated the proximal-testis end of the left vas deferens. The nick of the bladder wall was sewn up and repaired with a 3-0 Vicryl.
Postoperative investigations
The patient left the hospital on the sixth postoperative day without any of the symptoms that he had had earlier, except for the fallen and distended feeling in the left scrotum, which might be due to a ligation of the proximal-testis end of the left vas deferens.
The cyst fluid collected during the operation was first smeared and tested under a microscope, and we found some dead sperm. Then we did a comparative biochemical analysis of the patient's cyst fluid and semen (partial results in Table 1). In addition, a chromosomal test of peripheral blood showed nothing abnormal, and the karyotype of the patient was 46,XY.
Table 1. Comparison of partial biochemical-analysis results between the patient's cyst fluid and semen.
| Cyst fluid | Semen | Reference values of normal semen 1 | |
|---|---|---|---|
| pH value | 8.5 | 7.7 | 7.2–8.0 |
| Fructose (mg per 100 mL) | 198 | 335 | 152–360 |
| Protein (mg per 100 mL) | 8.7 | 3.2 | 2.8–4.4 |
| Citric acid (mg per 100 mL) | 0.6 | 7.0 | 3.6–7.6 |
| Acid phosphatase (U mL−1) | 33 | 866 | 470–1 294 |
| Zinc (μg mL−1) | 28.4 | 134.0 | 124–136 |
A pathologic examination of the operative specimen found that the cystic wall was 1.5–3.5 mm thick and coated with pseudostratified columnar epithelium inwards (Figure 3A, a), which contained abundant yellow-pigment granules. The lamina propria of the cyst wall had plentiful elastic fibres and many granulomas infiltrated with abundant inflammatory cells, and it was encircled by smooth muscles connected close to the bladder wall. However, we failed to find any serous membrane layers at the junction of the bladder and the cyst under the microscope, and so we concluded that there was no obvious anatomical boundary between the cyst and the bladder. Furthermore, the outward surface of the conjunct wall (that is, the surface protruding towards the intracavitary side of the urinary bladder) was coated with the normal mucous membrane of a urinary bladder and a typical transitional epithelium of the urinary tract, and the thinnest part of the junction was only about 1.5 mm thick without smooth muscle (Figure 3I).
Figure 3.

Pathological and immunohistochemical examinations of the cystic wall. I: the pathological-dissection schematic diagram of the excisional specimen; (A) and (a): HE staining of the cystic wall sections; (B) and (b): negative result of PSA immunohistochemical staining with the cystic wall sections; (C) and (c): negative result of PAP immunohistochemical staining with the cystic wall sections. (A), (B), (C): bars = 100 μm; (a), (b), (c): bars = 10 μm.
The cross section of the seminal vesicle-like lump above the cyst showed a honeycomb-like structure, which was composed of glandular cavities with unequal sizes and irregular arrays, coated with monostratal or pseudostratified columnar epithelium, and was diagnosed as a depauperate and dystopic seminal vesicle.
The immunohistochemical examinations of the cystic-wall sections showed negative results for both tests of prostate specific antigen (PSA) and prostatic acid phosphatase (PAP) (Figure 3B, b and C, c).
Discussion
The patient, who complained of the sensation of a wandering foreign body in the anus and repeated dysuresia, exhibited multiple developmental malformations on physical examination, imaging studies and pathology, and operative exploration (Figures 1 and 2).
The normal ejaculatory duct is about 2 cm long, enters into the bottom of the prostate and opens at the prostatic urethra. The end epidermis of the ejaculatory duct is a pseudostratified columnar epithelium containing abundant yellow-pigment granules. Its lamina propria has plentiful elastic fibres, and its smooth muscle layer is joined with the prostatic muscle fibres 1. According to the results of left deferent duct visualization (Figure 2) and operative anatomy, the cyst should be diagnosed as a scrotiform-dilated ejaculatory duct, which was confirmed by pathology. We failed to find an anatomical boundary at the junction of the cyst and the bladder, and pathological examination showed that the bladder side of the conjunct wall was coated with a typical transitional epithelium and that the thinnest part of the junction was only about 1.5 mm thick without smooth muscle (Figure 3I). Therefore, we concluded with confidence that the cyst should be the left ejaculatory duct with saccular dilatation, whose dead end allotropically protruded towards or invaded the left vesicle triangular area.
Cases with seminal passages allotropically invading the urinary tract are very rare. Although this reported case only showed the dead end of the left ejaculatory duct allotropically protruding towards or invading the bladder, we were not able to find similar case reports in a search of the literature. Moreover, this patient had multiple congenital malformations of the genitourinary system; so we presumed that it might be a new malformation syndrome due to embryonic dysplasia and reported it here.
The patient was married and had two daughters, and the preoperative biochemical analysis of his semen did not show any abnormal results (Table 1), which attested to the fact that he had a normal right seminal tract and testis. The biochemical analysis of the cystic fluid showed a remarkably high protein level of 8.7 mg per 100 mL (reference value: 2.8–4.4 mg per 100 mL), which could be explained by the chronic seminal accumulation and concentration from the lack of a normal debouchement of the left ejaculatory duct. Citric acid, zinc and acid phosphatase are mainly secreted by the prostate in normal semen, and their levels in the cystic fluid were all much lower than in the autologous semen sample or the reference values of normal semen 2, 3. In addition, the pH value of the cyst fluid was 8.5, higher than the value for the patient's semen (7.7) and the range of normal values (7.2–8.0) 2, 3. These data could indirectly prove that the cyst had nothing to do with the prostate and that it must be situated upstream of the prostate along the seminal passage.
The epithelium of prostatic tubes and gland alveoli expresses PAP and PSA proteins, as do the columnar epithelium and umbrella cells of the utriculus, but the epithelium of the ejaculatory duct does not 4, 5, 6. The immunohistochemical inspections showed that the cystic-wall epithelium did not express either the PSA or the PAP protein (Figure 3B, b and C, c), which further proved that the cyst was a dilated ejaculatory duct instead of an ampliate utriculus or a Mullerian duct cyst.
The karyotype of the patient was 46,XY and showed nothing abnormal; so we presumed that the multiple malformations in the patient's urogenital system were due to an anomaly in the embryonic development and not due to chromosomal abnormality. Mackie et al. 7, 8 in 1975 proposed an embryological theory that an extremital Wolffian duct abnormality would lead to ureteral dysplasia, and this theory could explain why the ureteric bud (UB) shifting towards the Wolffian duct head end would result in an allotopic ureteral orifice with homolateral renal agenesis. In the same year, a case report by Tanagho 9 also supported Mackie's theory. Subsequently, on the basis of Mackie and coworkers 8 and Tanagho's 10 theory, Gibbons et al. 11 put forward the concept of a deferent duct precursor to illuminate the embryological principle for seminal passage allotropically invading the urinary tract.
The current view in embryology is that the dysplastic proximal vas precursor (PVP) of the Wolffian duct results in the allotropic opening of the seminal passage. Normally, when the common mesonephric duct (CMD) forms the bladder trigone, PVP enters the vesicourethral canal at the place of the seminal crest and continues to develop into the ejaculatory duct, the seminal vesicle and the proximal part of the deferent duct (Figure 4A) 11. Gibbons et al. 12, 13 hypothesized that the ejaculatory duct would ectopically invade or open towards the urinary tract if the PVP intruded into the CMD, and that the intruded position of PVP decided the site of the ectopic ejaculatory duct orifice. This orifice could then emerge at any position from the posterior urethra to the ureter, even at the renal calyx. If the PVP invaded the inferior segment of the CMD, the ejaculatory duct would ectopically open to or invade the bladder.
Figure 4.

The embryonic-development schematic diagrams of the urogenital system 11. (A): PVP enters the vesicourethral canal at the place of the seminal crest. (B): The ejaculatory duct ectopically opens towards the bladder, and the development of the homolateral vesicle triangle, ureter and kidney is interrupted because the PVP accidentally encroaches on the position of the UB emanating from the CMD. CMD, common mesonephric duct; ED, ejaculatory duct; MB, metanephrogenic blastema; PVP, proximal vas precursor; SC, seminal crest; Sem. ves., seminal vesicle; UB: ureteric bud; UGS: urogenital sinus.
Furthermore, the development of the homolateral vesicle triangle, the ureter and the kidney would be interrupted if PVP accidentally encroached on the position of the UB emanating from the CMD (Figure 4B) 11, 12.
Conclusion
On the basis of the evidence from clinical and laboratory tests, this case had a new syndrome, which we named Wuyang's syndrome. It arose from a rare phenomenon in embryonic development: dysplastic PVP intruded into the CMD and accidentally encroached on the UB position, which resulted in the absence or dysplasia of the homolateral urinary tract and in the homolateral seminal tract ectopically invading the bladder.
References
- Nguyen HT, Etzell J, Turek PJ. Normal human ejaculatory duct anatomy: a study of cadaveric and surgical specimens. J Urol. 1996;155:1639–42. doi: 10.1016/s0022-5347(01)66150-0. [DOI] [PubMed] [Google Scholar]
- Mandal A, Bhattacharyya AK. Phosphate, zinc, calcium, citric acid, and acid phosphatase in human ejaculates as related to coagulation/liquefaction. Arch Androl. 1987;19:275–83. doi: 10.3109/01485018708986828. [DOI] [PubMed] [Google Scholar]
- McNeal JE.Prostate Gland: Morphology and PathobiologyStamey TA, edtor. Monographs in Urology Princeton: Burroughs Welcome Company; 1988p36–54. [Google Scholar]
- Ma J, Shao Q. [Observation on normal prostatic anatomy and histology in adults] Chin J Anat. 2002. pp. 156–60.
- Grignon DJ, Ro JY, Ordonez NG, Ayala AG, Clearly KR. Basal cell hyperplasia, adenoid basal cell tumor, and adenoid cystic carcinoma of the prostate gland: an immunohistochemical study. Hum Pathol. 1988;19:1425–33. doi: 10.1016/s0046-8177(88)80235-1. [DOI] [PubMed] [Google Scholar]
- NcNeal JE.Architecture of the Glandular ProstateIn: Sternberg SS, editor. Histology for Pathologists New York: Raven Press; 1992p753–757. [Google Scholar]
- Mackie GG, Awang H, Stephens FD. The ureteric orifice: the embryologic key to radiologic status of duplex kidneys. J Pediatr Surg. 1975;10:473–81. doi: 10.1016/0022-3468(75)90187-6. [DOI] [PubMed] [Google Scholar]
- Mackie GG, Stephens FD. Duplex kidneys: a correlation of renal dysplasia with position of the ureteral orifice. J Urol. 1975;114:274–80. doi: 10.1016/s0022-5347(17)67007-1. [DOI] [PubMed] [Google Scholar]
- Tanagho EA. A case against incorporation of bowel segments into the closed urinary system. J Urol. 1975;113:796–802. doi: 10.1016/s0022-5347(17)59582-8. [DOI] [PubMed] [Google Scholar]
- Tanagho EA. Embryologic basis for lower ureteral anomalies: a hypothesis. Urology. 1976;7:451–64. doi: 10.1016/0090-4295(76)90179-5. [DOI] [PubMed] [Google Scholar]
- Gibbons MD, Cromie WJ, Duckett Ectopic vas deferens. J Urol. 1978;120:597–604. doi: 10.1016/s0022-5347(17)57294-8. [DOI] [PubMed] [Google Scholar]
- Gibbons MD, Duckett JW., Jr. Classification of ectopia of vas deferens. Urology. 1982;20:350. doi: 10.1016/0090-4295(82)90664-1. [DOI] [PubMed] [Google Scholar]
- Gibbons MD, Duckett JW., Jr. Single vaginal ectopic ureter: a case report. J Urol. 1978;120:493–5. doi: 10.1016/s0022-5347(17)57241-9. [DOI] [PubMed] [Google Scholar]


