Abstract
In vitro supplementation with date seed oil (DSO) can protect spermatozoa against hydrogen peroxide (H2O2)-mediated damage and can improve sperm function, possibly owing to antioxidant properties. We tested the antioxidant effects of DSO on human sperm motility, sperm viability, reacted acrosome and lipid peroxidation assessed in vitro after H2O2-mediated oxidative damage in spermatozoa. Sixteen patients (mean age: 35 years; range: 25–45 years) referred to the Histology–Embryology Laboratory of the Medicine Faculty of Sfax for semen analysis after 12–24 months of sexual intercourse without conception were selected. After spermiogram, sperm selection by two-interface discontinuous Sill Select gradient was performed, and selected spermatozoa were used in four experimental assays: control; incubation with 100 μm H2O2; incubation with 0.1% DSO; and co-incubation with 0.1% DSO and 100 μm H2O2. Motility and viability were determined using World Health Organization criteria. Acrosome reaction and lipid peroxidation were assessed by staining with fluorescein isothiocyanate-Pisum sativum and spectrophotometric measurement of malondialdehyde, respectively. Results showed that incubation with H2O2 alone led to a significant increase in lipid peroxidation (57.83%, P < 0.05) associated with a significant decrease in sperm motility, sperm viability (after 30 min and 24 h) and percentage of reacted acrosome (P < 0.05). Date seed oil improved sperm motility after 24 h of incubation (P < 0.05) and protected spermatozoa against the deleterious effects of H2O2 on motility, viability, acrosome reaction and lipid peroxidation. We conclude that supplementation with DSO may have a function in antioxidant protection against male infertility.
Keywords: acrosome reaction, date seed oil, in vitro, lipid peroxidation, sperm motility, sperm viability
Introduction
Although many studies have shown improvements in sperm quality with antioxidant treatment, recent attention has been directed toward extracts of plants rich in natural antioxidant compounds. In fact, there are many medicinal plants known to control human fertility; a large number of such plants have been used by indigenous people as part of folk medicine, especially in India 1. In recent years, extracts of plants have been the source of many new drug therapies for pathologies such as infertility 2. Fruits of the date palm (Phoenix dactylifera L. Arecaceae, also called ‘deglet nour') are popular food in many countries and the date palm is a vital component of diets in arid and semi-arid regions of the world. It has been found that date seed oil (DSO) has better oxidative stability than most vegetables and also has a high antioxidant capacity owing to its richness in polyphenol and tocopherol compounds 3, 4. Recently, Dammak et al. 5 showed that DSO has a protective effect against hydrogen peroxide (H2O2)-induced oxidative stress (OS) in human skin organ culture and suggested that the use of DSO as a dietary supplement may have beneficial effects in protecting against skin disorders in humans. Many pharmacological compounds have been shown to enhance sperm motility and, potentially, sperm-fertilizing capacity 6, 7. Spermatozoa are particularly susceptible to OS because their plasma membranes contain large amounts of polyunsaturated fatty acids 8. OS stress induces deleterious effects on sperm viability and function and may lead to persistent infertility 9, 10. Vitamin E, a major lipid-soluble antioxidant belonging to the tocopherols and the most effective antioxidant for chain-breaking within the cell membrane, is one of the vitamins that contribute to the detoxification process from reactive oxygen species (ROS). Vitamin E is able to repair oxidizing radicals directly, preventing the chain propagation step during lipid peroxidation 11.
In addition, many studies have shown that protective effects against ROS damage and an improvement of the overall functional parameters of spermatozoa can be provided by supplementing sperm preparation media with antioxidants such as vitamin E, catalase and ethylene diamine tetraacetic acid (EDTA) 12.
This study was carried out to test the antioxidant effects of DSO on human sperm motility, sperm viability, reacted acrosome and lipid peroxidation assessed in vitro after H2O2-mediated oxidative damage in spermatozoa.
Materials and methods
Date seed oil extract, preservation and emulsification
The seeds of the date palm were separated from 50 kg of waste date fruit that was collected from the National Institute of Arid Zone (Degach, South of Tunisia) at the ‘Tamr stage' (full ripeness). The seeds were soaked in water, washed to free them of any adhering date flesh, air-dried and then dried (12 h) at about 50°C. Date pits were separately milled in a heavy-duty grinder to pass 1–2 mm screens and then kept at −20°C. Lipid extraction was carried out with an SER 148 Solvent Extractor (Velp Scientifica, Milan, Italy) equipped with six Soxhlet posts. The extraction was carried out over a 30-min period with thimbles immersed in boiling petroleum ether followed by 60 min of reflux washing. The solvents from seed oils were removed under a stream of nitrogen and then stored in a freezer (−20°C) until use 13. This oil was emulsified with 10% ‘gum arabic' (10 g of gum arabic/100 mL of physiological water: extemporarily prepared by dissolving 9 g of sodium chloride in 1 L of distilled water). Gum arabic did not show any negative effect on sperm parameters (motility, viability) at different times of incubation.
Collection and analysis of semen samples
Semen specimens were provided by 16 men aged from 25 to 45 years (mean age 35 years) and attending the Laboratory of Histology–Embryology for routine semen analysis after 12–24 months of sexual intercourse without conception. Azoospermic men and patients having a history of endocrine or anatomical disorders were excluded from this study. All semen samples were collected by masturbation after 3–5 days of abstinence. After liquefaction, semen volume, sperm concentration, motility, viability and morphology were determined according to World Health Organization (WHO) guidelines 14. Motility was assessed at room temperature; sperm count was determined by diluting (1/20) a semen sample in immobilizing solution (9 g NaCl and 100 mL formol 40% made up to 1 L with distilled water, pH 7.4). After that, some of the diluted sample was transferred to a hemocytometer counting chamber, and the spermatozoa were counted under a microscope. Sperm viability was assessed by eosine–nigrosine test. Only specimens with sperm concentrations ≥ 20 × 106 mL−1 and total motility < 50% were included in the study. We excluded samples with high leukocyte concentrations (leukocytes > 1 × 106 cell mL−1).
Preparation of samples and experimental design
After semen analysis, samples were prepared using a two-interface discontinuous Sill Select gradient (95%–45%) (FertiPro NV, Beernem, Belgium) and Ferticult culture medium (FertiPro NV) 15. Immediately after semen selection, sperm concentration, motility and viability were determined and, to optimize the experimental groups, the final sperm concentration was adjusted to 107 mL−1 by addition of an adequate volume of Ferticult medium to each preparation.
Four experimental samples were prepared: (1) spermatozoa without H2O2 or DSO (control); (2) spermatozoa with 100 μmol L−1 H2O2 (H2O2); (3) spermatozoa with 0.1% DSO (DSO); and (4) spermatozoa incubated successively with 0.1% DSO for 30 min, then with 100 μmol L−1 H2O2 (H2O2 + DSO). In each experimental situation, sperm motility and viability were determined 30 min and 24 h after addition of H2O2 and/or DSO.
To choose the optimal concentration of H2O2 to give a significant reduction of semen parameters, we tested a range of concentrations (20, 40, 60 and 100 μmol L−1). We found that significant changes in sperm motility and viability were observed only at 100 μmol L−1and after 30 min of incubation. We also tested two concentrations of DSO (0.01% and 0.1%) and found that semen parameters were improved only at the highest concentration.
Measurement of lipid peroxidation
Lipid peroxidation was assessed by a specific spectrophotometry method that measures thiobarbituric acid reacted substances, such as malondialdehyde (MDA), according to Yagi 16. After 30 min of incubation, 2 mL of TBA reagent (15% [v/v] trichloroacetic acid and 0.25 μmol L−1 HCl) was added to 1 mL of each experimental sample. The mixture was treated in a boiling water bath for 15 min. After cooling, it was centrifuged at 1 000 × g for 10 min. The supernatant was then removed and absorbance of the pellet was measured by a spectrophotometer at 535 nm. The MDA concentration was determined from a calibration curve, and the results were expressed as nmol MDA 10−7 cells.
Evaluation of the acrosome status with fluorescein isothiocyanate-Pisum sativum (FITC-PSA)
After 30 min of incubation, samples were kept in the incubator with 5% CO2 in air at 37°C for spontaneous acrosome reaction. Staining with FITC-PSA was performed following Mendoza et al. 17. Briefly, aliquots (100–150 μL) of the sperm suspensions were smeared onto glass slides and air-dried. To permeabilize the sperm membranes, the slides were dipped in 100% methanol for 15 min at room temperature. Thereafter, the smears were mounted with 150 μL FITC-PSA (50 mg mL−1 phosphate-buffered saline, pH 7.4) and incubated for 30 min at room temperature in a humid chamber. The FITC-PSA solution was washed by dipping the slides 20 times in purified water. After air-drying, labelled spermatozoa were covered by 30 μL of an anti-fading medium (50% v/v glycerol, 50% v/v distilled water, 25 mg mL−1 1′,4-diazabicyclo[2, 2, 2]octane) to prevent photobleaching. By means of a fluorescence microscope (Nikon B 2A filter, excitation filter 450–490 nm, dichroic mirror 510 nm, barrier filter 520 nm), at least 200 spermatozoa were differentiated blindly according to the fluorescence pattern of their acrosomes (bright fluorescence: non-reacted acrosome; no fluorescence or only fluorescence of the equatorial segment: reacted acrosome) (Figure 1).
Figure 1.
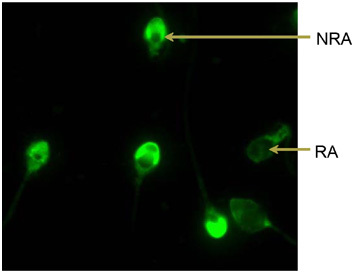
Evaluation of the acrosome status with fluorescein isothiocyanate-Pisum sativum (FITC-PSA) after 30 min of incubation. No reacted acrosome (NRA): bright fluorescence, acrosome intact; reacted acrosome (RA): no fluorescence or only fluorescence of the equatorial segment, loss of acrosomal membranes.
Statistics
Data were expressed as mean ± SD. The one-way analysis of variance and the Student–Newman–Keuls post hoc test were performed on the data for inter-group comparisons. Database management and statistical analysis were performed using the SPSS (Chicago, IL, USA) statistical software package.
Results
Sperm motility and viability
Data concerning sperm motility and viability are presented in Table 1. We found that after 30 min of incubation with 100 μmol L−1 H2O2, sperm motility and viability significantly decreased (P < 0.05) compared with the control. However, co-incubation with DSO and H2O2 improved motility and vitality after 30 min and 24 h compared with H2O2 alone (Table 1). Sperm motility was also significantly increased after 24h of incubation with DSO alone, in comparison with the control.
Table 1. Sperm motility and viability of the different experimental groups (n = 16) after 30 min and 24 h of incubation.
| Experimental groups |
||||
|---|---|---|---|---|
| Control | H2O2 | DSO | DSO + H2O2 | |
| After 30 min | ||||
| Motility (%) | 42.19 ± 2.56 | 21.56 ± 3.96* | 43.21 ± 5.12 | 30.72 ± 4.16*,** |
| Viability (%) | 65.38 ± 6.13 | 34.94 ± 11.54* | 60.00 ± 6.83 | 39.38 ± 8.92* |
| After 24 h | ||||
| Motility (%) | 33.75 ± 7.63 | 0* | 42.19 ± 8.36* | 27.81 ± 5.76** |
| Viability (%) | 38.75 ± 9.33 | 0* | 45.31 ± 7.29 | 31.75 ± 5.96*,** |
Abbreviation: DSO, date seed oil.
*P < 0.05, all groups are compared with control (spermatozoa without H2O2 and DSO).
**P < 0.05, comparison between group H2O2 (spermatozoa with 100 μmol L−1 H2O2) and group DSO + H2O2 (spermatozoa combined successively with 0.1% DSO and 100 μmol L−1 H2O2).
Lipid peroxidation
Compared with the control, the levels of lipid peroxide contents, as determined by MDA assay, were significantly increased after the exposure of spermatozoa to H2O2, and significantly decreased after incubation with DSO alone and with both DSO and H2O2 (P < 0.05) (Figure 2). In addition, a significant decrease in sperm MDA contents was observed after addition of DSO and H2O2 compared with incubation in H2O2 alone.
Figure 2.
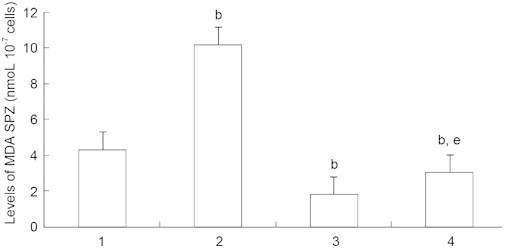
Malondialdehyde (MDA) levels in spermatozoa in each experimental group (n = 16) after 30 min of incubation. Values are presented as mean ± SD. Experimental groups include: (1) spermatozoa without H2O2 and date seed oil (DSO) (control); (2) spermatozoa with 100 μmol L−1 H2O2 (H2O2); (3) spermatozoa with 0.1% DSO (DSO); and (4) spermatozoa incubated successively with 0.1% DSO for 30 min, then with 100 μmol L−1H2O2 (H2O2+ DSO). bP < 0.05, compared with control. eP < 0.05, compared with group 2.
Acrosome reaction
Figure 3 reveals that the percentage of spermatozoa with reacted acrosome was significantly decreased after incubation with H2O2 in comparison with the control; it was significantly increased after incubation with DSO alone and with both DSO and H2O2. We also observed that the percentage of reacted acrosome was higher after incubation with DSO and H2O2 than with H2O2 alone (P < 0.05).
Figure 3.
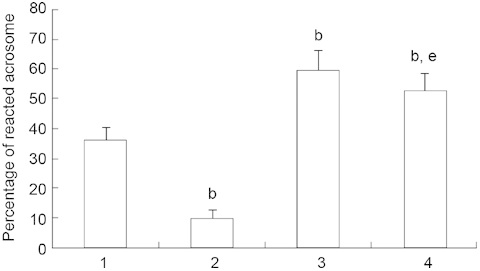
Evaluation of the acrosome reaction in different experimental groups (n = 16) after 30 min of incubation. Values are presented as mean ± SD. Experimental groups include: (1) spermatozoa without H2O2 and date seed oil (DSO) (control); (2) spermatozoa with 100 μmol L−1 H2O2 (H2O2); (3) spermatozoa with 0.1% DSO (DSO); and (4) spermatozoa incubated successively with 0.1% DSO for 30 min, then with 100 μmol L−1 H2O2 (H2O2 + DSO). bP < 0.05, compared with control. eP < 0.05, compared with group 2.
Discussion
The fruits of the date palm (Phoenix dactylifera L. Arecaceae) are popular in many countries. Recently, Dammak et al. 5 showed that DSO prevents H2O2-induced OS in human skin organ culture. Their data about DSO composition and physico-chemical properties have encouraged us to test it in human fertility and sperm quality. To our knowledge, this study is the first to test the effects of DSO on human sperm motility, sperm viability, acrosome reaction and lipid peroxidation after in vitro H2O2-induced oxidative damage. We found that DSO has a protective effect on sperm motility and viability against in vitro induced OS and can improve sperm function. This oil has been analyzed in terms of phenol and tocopherol profiles, and it was found to be a rich source of natural phenolic compounds in the Mediterranean diet (in DSO, total phenol content is 520.81 mg kg−1), which was one of the main reasons for the better oxidative stability of this oil. Alpha-tocopherol constituted about 24.97% of the total tocopherols for DSO 3.
Recently, treatment of infertility by natural products has been a topic of interest to several research teams. In fact, Piomboni et al. 7 reported that treatment with natural supplements containing high concentrations of vitamins C and E can be considered a valid alternative to anti-inflammatory drugs in the treatment of asthenospermia with leukocytosis. Similarly, a positive effect of a natural potent antioxidant, astaxanthin, as complementary treatment was found to improve sperm parameters and fertility 18. In other studies, it has been reported that the benzene chromatographic fraction of Carica papaya seed extract showed contraceptive efficacy without adverse toxicity, mediated through inhibition of sperm motility 19.
Some reports have questioned the usefulness of antioxidants in the treatment of male infertility. Oral antioxidant treatment appears to improve intracytoplasmic sperm injection outcomes in patients with sperm DNA damage, and reduces the percentage of damaged spermatozoa 20. Conversely, Ménézo et al. 21 reported that oral administration of antioxidant vitamins associated with zinc and selenium induced a decrease in DNA fragmentation but an unexpected increase in sperm decondensation, probably owing to the ability of antioxidant vitamins to interfere with interchain disulfide bridges of sperm protamines.
Male infertility has been linked with the excessive generation of ROS by defective spermatozoa. When produced in excess, H2O2 activates the lipid peroxidation cascade, which in turn causes loss of membrane fluidity and decreases sperm quality. In this investigation, we studied the direct effect of lipid peroxidation induced by H2O2 on sperm quality. We showed that H2O2 increased the formation of lipid peroxidation products (MDA) in sperm, and we confirmed published reports regarding the deleterious effects of H2O2 on sperm motility and viability 10. In addition, we found that H2O2 decreased the percentage of reacted acrosomes. Furthermore, we showed for the first time a protective effect of DSO against in vitro H2O2-induced OS and its beneficial effect on sperm motility in patients with poor semen quality. Date seed oil improved the ability of sperms to perform the acrosome reaction and therefore their ability to fertilize oocytes. It is known that hyperactivation and acrosome reaction can be induced when spermatozoa are incubated in culture medium 22. In our view, the improvement in the percentage of spermatozoa with reacted acrosome seen in this study is not related to the influence of the culture medium but could be attributed to the beneficial effect of DSO, as the same culture medium was used in all experimental groups with and without DSO. Therefore, we suggest that natural antioxidants present in DSO protect against H2O2-induced oxidative damage.
Various in vitro and in vivo studies have indicated that supplementation by antioxidants attenuated the negative effects of ROS and improved sperm function, capacity for fertilization and sperm membrane fluidity 12, 23, 24. Chi et al. 12 reported that in vitro supplementation by antioxidants (EDTA, catalase) in sperm suspensions significantly reduced the ROS levels and DNA fragmentation rate of spermatozoa. Moreover, incubation with catalase increased the acrosome reaction ability of spermatozoa. In the same way, in a recent study testing the in vitro antioxidant effects of the isoflavones genistein, equol (plant compound), ascorbic acid and alpha-tocopherol on sperm DNA integrity after H2O2-induced damage, Sierens et al. 23 found that genistein was the most potent antioxidant, followed by equol, ascorbic acid and alpha-tocopherol. Genistein and equol added in combination were more protective than either added singly. Furthermore, Comhaire et al. 24. evaluated the effects of oral antioxidants (N-acetyl-cysteine or vitamins A plus E) and essential fatty acids on sperm biology and showed that treatment reduced ROS production and increased both acrosome reaction and sperm membrane fluidity. In contrast, another study indicates that live and acrosome-reacted spermatozoa, kinetic parameters and fertility rate were not modified by alpha-tocopheryl acetate and selenium in vitro supplementation of rabbit spermatozoa 25.
On the basis of the promising reports here and in the literature, it seems that treatment with natural products containing high concentrations of alpha-tocopherol and phenols can be considered as a valid alternative to improve sperm parameters in the treatment of male infertility.
This original study proved that supplementation with DSO may have a function in antioxidant protection against male infertility. Further investigations testing the effects of DSO extract in vivo should be performed to confirm the beneficial effects on semen parameters and on the fertilization capacity of spermatozoa.
Acknowledgments
This work is dedicated to the memory of the late Mrs Hentati Basma. We will never forget you and you are always in our heart.
References
- de Lazlo H, Henshaw PS. Plant materials used by primitive people to affect fertility. Science. 1954;119:626–31. doi: 10.1126/science.119.3097.626. [DOI] [PubMed] [Google Scholar]
- Chen Z, Yin CP, Liu JH, Fang JG, Wang WQ, et al. [Extract of Acanthopanacis senticosus improves sperm motility of asthenospermia patients in vitro] Zhonghua Nan Ke Xue. 2007;13:21–3. [PubMed] [Google Scholar]
- Besbes S, Blecker C, Deroanne C, Bahloul N, Lognay G, et al. Date seed oil: phenolic, tocopherol and sterol profiles. J Food Lipids. 2004;11:251–65. [Google Scholar]
- Besbes S, Blecker C, Deroanne C, Lognay G, Drira NE, et al. Quality characteristics and oxidative stability of date seed oil during storage. Food Sci Tech Int. 2004;10:333–8. [Google Scholar]
- Dammak I, Ben Abdallah F, Boudaya S, Keskes L, Besbes S, et al. Effects of date seed oil on normal human skin in vitro. Eur J Dermatol. 2007;17:516–9. doi: 10.1684/ejd.2007.0267. [DOI] [PubMed] [Google Scholar]
- Taepongsorat L, Tangpraprutgul P, Kitana N, Malaivijitnond S. Stimulating effects of quercetin on sperm quality and reproductive organs in adult male rats. Asian J Androl. 2008;10:249–58. doi: 10.1111/j.1745-7262.2008.00306.x. [DOI] [PubMed] [Google Scholar]
- Piomboni P, Gambera L, Serafini F, Campanella G, Morgante G, et al. Sperm quality improvement after natural anti-oxidant treatment of asthenoteratospermic men with leukocytospermia. Asian J Androl. 2008;10:201–6. doi: 10.1111/j.1745-7262.2008.00356.x. [DOI] [PubMed] [Google Scholar]
- Alvarez JG, Storey BT. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol Reprod Dev. 1995;42:334–46. doi: 10.1002/mrd.1080420311. [DOI] [PubMed] [Google Scholar]
- Fraczek M, Szkutnik D, Sanocka D, Kurpisz M. [Peroxi-dation components of sperm lipid membranes in male infertility] Ginekol Pol. 2001;72:73–9. [PubMed] [Google Scholar]
- Oehninger S, Blackmore P, Mahony M, Hodgen G. Effects of hydrogen peroxide on human spermatozoa. J Assist Reprod Genet. 1995;12:41–7. doi: 10.1007/BF02214128. [DOI] [PubMed] [Google Scholar]
- Zhang JG, Nicholls-Grzemski FA, Tirmenstein MA, Fariss MW. Vitamin E succinate protects hepatocytes against the toxic effect of reactive oxygen species generated at mitochondrial complexes I and III by alkylating agents. Chem Biol Interact. 2001;138:267–84. doi: 10.1016/s0009-2797(01)00278-2. [DOI] [PubMed] [Google Scholar]
- Chi HJ, Kim JH, Ryu CS, Lee JY, Park JS, et al. Protective effect of antioxidant supplementation in sperm-preparation medium against oxidative stress in human spermatozoa. Hum Reprod. 2008;23:1023–8. doi: 10.1093/humrep/den060. [DOI] [PubMed] [Google Scholar]
- Besbes S, Blecker C, Deroanne C, Drira NE, Attia H, et al. Date seed: chemical composition and characteristic profile of the lipid fraction. Food Chem. 2004;84:577–84. [Google Scholar]
- World Health Organization WHO Laboratory Manual for the Examination of Human Semen and Semen–Cervical Mucus Interactions 4thedn. Cambridge: Cambridge University Press; 1999p4–23. [Google Scholar]
- Hyne RV, Stojanoff A, Clarke GN, Lopata A, Johnston WI. Pregnancy from in vitro fertilization of human eggs after separation of motile spermatozoa by density gradient centrifugation. Fertil Steril. 1986;45:93–6. doi: 10.1016/s0015-0282(16)49103-x. [DOI] [PubMed] [Google Scholar]
- Yagi K. Assay for blood plasma or serum. Methods Enzymol. 1984;105:328–31. doi: 10.1016/s0076-6879(84)05042-4. [DOI] [PubMed] [Google Scholar]
- Mendoza C, Carreras A, Moos J, Tesarik J. Distinction between true acrosome reaction and degenerative acrosome loss by a one-step staining method using Pisum sativum agglutinin. J Reprod Fertil. 1992;95:755–63. doi: 10.1530/jrf.0.0950755. [DOI] [PubMed] [Google Scholar]
- Comhaire FH, El Garem Y, Mahmoud A, Eertmans F, Schoonjans F. Combined conventional/antioxidant “Astaxanthin” treatment for male infertility: a double blind, randomized trial. Asian J Androl. 2005;7:257–62. doi: 10.1111/j.1745-7262.2005.00047.x. [DOI] [PubMed] [Google Scholar]
- Lohiya NK, Manivannan B, Goyal S, Ansari AS. Sperm motility inhibitory effect of the benzene chromatographic fraction of the chloroform extract of the seeds of Carica papaya in langur monkey, Presbytis entellus entellus. Asian J Androl. 2008;10:298–306. doi: 10.1111/j.1745-7262.2008.00331.x. [DOI] [PubMed] [Google Scholar]
- Greco E, Romano S, Iacobelli M, Ferrero S, Baroni E, et al. ICSI in cases of sperm DNA damage: beneficial effect of oral antioxidant treatment. Hum Reprod. 2005;20:2590–4. doi: 10.1093/humrep/dei091. [DOI] [PubMed] [Google Scholar]
- Ménézo YJ, Hazout A, Panteix G, Robert F, Rollet J, et al. Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod Biomed Online. 2007. pp. 418–21. [DOI] [PubMed]
- Henkel RR, Schill WB. Sperm preparation for ART. Reprod Biol Endocrinol. 2003;14:108. doi: 10.1186/1477-7827-1-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierens J, Hartley JA, Campbell MJ, Leathem AJ, Woodside JV. In vitro isoflavone supplementation reduces hydrogen peroxide-induced DNA damage in sperm. Teratog Carcinog Mutagen. 2002;22:227–34. doi: 10.1002/tcm.10015. [DOI] [PubMed] [Google Scholar]
- Comhaire FH, Christophe AB, Zalata AA, Dhooge WS, Mahmoud AM, et al. The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men. Prostaglandins Leukot Essent Fatty Acids. 2000;63:159–65. doi: 10.1054/plef.2000.0174. [DOI] [PubMed] [Google Scholar]
- Castellini C, Lattaioli P, Bosco AD, Beghelli D. Effect of supranutritional level of dietary alpha-tocopheryl acetate and selenium on rabbit semen. Theriogenology. 2002;58:1723–32. doi: 10.1016/s0093-691x(02)01083-x. [DOI] [PubMed] [Google Scholar]


