Abstract
Health-related quality-of-life (HRQOL) after a radical prostatectomy (RP) or external beam radiation therapy (EBRT) has not been studied in conjunction with oncological outcomes in relation to disease risk stratification. Moreover, the long-term outcomes of these treatment approaches have not been studied. We retrospectively analyzed oncological outcomes between consecutive patients receiving RP (n = 86) and EBRT (n = 76) for localized prostate cancer. HRQOL and functional outcomes could be assessed in 62 RP (79%) and 54 EBRT (79%) patients over a 3-year follow-up period (median: 41 months) using the Medical Outcomes Study Short Form-36 (SF-36) and the University of California Los Angeles Prostate Cancer Index (UCLA PCI). The 5-year biochemical progression-free survival did not differ between the RP and EBRT groups for low-risk (74.6% vs. 75.0%, P = 0.931) and intermediate-risk (61.3% vs. 71.1%, P = 0.691) patients. For high-risk patients, progression-free survival was lower in the RP group (45.1%) than in the EBRT group (79.7%) (P = 0.002). The general HRQOL was comparable between the two groups. Regarding functional outcomes, the RP group reported lower scores on urinary function and less urinary bother and sexual bother than the EBRT group (P < 0.001, P < 0.05 and P < 0.001, respectively). With risk stratification, the low- and intermediate-risk patients in the RP group reported poorer urinary function than patients in the EBRT group (P < 0.001 for each). The sexual function of the high-risk patients in the EBRT group was better than that of the same risk RP patients (P < 0.001). Biochemical recurrence was not associated with the UCLA PCI score in either group. In conclusion, low- to intermediate-risk patients treated with an RP may report relatively decreased urinary function during long-term follow-up. The patient's HRQOL after treatment did not depend on biochemical recurrence.
Keywords: long-term observation, quality-of-life, radiation therapy, radical prostatectomy, risk stratification
Introduction
Although the incidence of localized prostate cancer (PCa) has increased with prostate-specific antigen (PSA) screening, therapeutic options for treating this disease have been diversified 1. Currently, the prevailing management for localized PCa includes radical prostatectomy (RP), external beam radiation therapy (EBRT), brachytherapy, androgen deprivation therapy (ADT) and expectant management (watchful waiting or active surveillance) 2, 3. These options are occasionally used in combination, and patients diagnosed as having localized PCa face a bewildering number of choices among curative therapies. Owing to the lengthy disease history of patients with localized PCa and the possible clinical disadvantages of RP and EBRT, treatment for patients diagnosed with localized PCa is often selected with consideration for life expectancy, informed consent and statistics-based probability/risk of relapse 1, 4. The risk of relapse after primary treatment is generally estimated with combined clinical stage, biopsy Gleason score and serum PSA levels 4. Surgery and radiotherapy have an equivalent treatment efficacy when used for low-risk PCa 2, 3, 4. In clinical practice, however, patients' characteristics such as age frequently vary between those receiving surgery and radiotherapy, and combined options, represented by ADT, are utilized differently among the various risk categories 2, 4.
Although many studies have compared health-related quality-of-life (HRQOL) outcomes after an RP with outcomes using conventional low-dose EBRT 5, 6, 7, it has not been investigated whether post-treatment HRQOL and treatment-related functional outcomes depend on therapeutic options under disease-specific risk stratification. Additionally, despite the long disease history of localized prostate cancer patients, the long-term outcomes regarding QOL issues remain unknown. A more tailored approach to PCa therapy, considering disease risk together with QOL issues, is thought to play a significant role in the management of PCa patients. In addition, it remains unclear whether biochemical recurrence affects general HRQOL or functional outcomes in men treated for localized PCa. Despite the estimated risk of relapse, this theme has not been urged according to the criteria for risk stratification.
In this study, we first verified the demographic features and clinical differences between patients undergoing an RP and EBRT. The oncological outcomes after RP or EBRT were evaluated under risk stratification, which allowed for an analysis of long-term general HRQOL and functional HRQOL in both treatment groups. In addition, the influence of biochemical recurrence was examined in these patients.
Materials and methods
Patients and their characteristics
We retrospectively analyzed all the patients diagnosed with localized PCa and treated with an RP or EBRT in the Department of Urology and Radiology, Niigata University Hospital (Niigata, Japan) between January 1998 and December 2004. During this period, a total of 85 patients underwent EBRT (EBRT group) and 86 patients received an RP (RP group). Nine patients in the EBRT group were excluded because of probable bladder involvement or rectal invasion on imaging studies. All patients were staged according to the International Union Against Cancer guidelines (UICC 2002) 8, and the Gleason system was used for histological grading. Participants were stratified into three risk subgroups, delineated by the following National Comprehensive Cancer Network definitions: low-risk: clinical stage T1 or T2b, preoperative PSA ≤ 10 ng mL−1 and Gleason score ≤ 6; intermediate-risk: clinical stage T2c or preoperative PSA > 10, but ≤ 20 ng mL−1 or Gleason score 7; and high-risk: clinical stage T3 or preoperative PSA 20 ng mL−1 or Gleason score ≥ 8 4.
Treatment and oncological end point
All patients in the EBRT group were treated using three-dimensional conformal radiation therapy (3D-CRT). The total dose was 70.0–71.0 Gy. The RP surgeries were performed by different surgeons using standardized techniques 9. On the basis of evidence from randomized trials showing no difference in biochemical/PSA outcomes between patients receiving an RP alone and those treated with ADT before the RP 10, 11, we included 36 patients who underwent short-term (median: 8 months) ADT before surgery. Most of these patients were treated before 2002, when the role of neoadjuvant ADT in patients undergoing RP was under investigation. Sixteen of 36 patients with low-risk disease in the RP group and 4 of 26 with intermediate-risk selected unilateral nerve-sparing techniques.
Post-treatment assessments, including physical examination and serum PSA measurement, were conducted quarterly. In this study, we regarded biochemical recurrence as the primary end point. For RP patients, biochemical recurrence was defined as PSA levels of ≥ 0.2 ng mL−1 on two occasions. The period to recurrence was considered the time of the initial detectable level 12. For EBRT patients, the American Society of Therapeutic Radiology and Oncology consensus definition for biochemical failure, three consecutive rising PSA levels after a nadir, was used. The time to failure was calculated as midway between the time of nadir and the first PSA increase 13.
Assessment of HRQOL
HRQOL after RP or EBRT was evaluated using an HRQOL survey with a mail-in response. At analysis of the HRQOL, the follow-up period was longer than 36 months (median: 41 months) in all patients. Patients who had already died or did not return questionnaires were excluded from the following analysis. One-hundred forty-six patients in this study received questionnaires of the Medical Outcomes Study Short Form-36 (SF-36) and the University of California Los Angeles PCa Index (UCLA PCI) to assess general and functional HRQOL, respectively 14, 15. The SF-36 is an internationally validated generic HRQOL questionnaire. It consists of 36 items organized into eight scales, including physical function (PF), role-physical (RP), role-emotional (RE), bodily pain (BP), general health (GH), vitality (VT), social function (SF) and mental health (MH). The UCLA PCI quantifies functional outcomes specific to PCa treatments in six separate domains of urinary function, urinary bother, bowel function, bowel bother, sexual function and sexual bother 16. Generic and functional HRQOL subscale raw scores were converted to a scale of 0–100, with a higher score indicating a better QOL. We used the Japanese version of the SF-36 and UCLA PCI 16, 17.
Statistical analysis
Survival curves were generated using the method of Kaplan and Meier, and differences between curves were evaluated with the log-rank test. The Welch corrected t-test, the χ2 test and Tukey's honestly significant difference (HSD) test were used to compare demographic and clinical variables between the treatment groups. All analyses were performed using SPSS (version 11.0J: SPSS Inc., Chicago, IL, USA), on a Windows-based computer, with P < 0.05 considered statistically significant.
Results
Variables in patients' background
Patients' demographics are shown in Table 1. Patients in the EBRT group were older than those who underwent an RP (P < 0.001). The initial PSA level and clinical disease stage were higher in the EBRT group (P = 0.02 and P = 0.013, respectively), and the EBRT group included fewer low-risk and more high-risk patients than the RP group (P < 0.001). For the low-risk patients, seven (19.4%) from the RP group and eight (66.7%) from the EBRT group received neoadjuvant ADT (P = 0.002). For intermediate-risk patients, nine (34.6%) from the RP and 24 (88.9%) from the EBRT group received this additional treatment (P < 0.001). In the high-risk category, the fraction of patients who underwent neoadjuvant ADT did not differ between the RP (83.3%) and EBRT (91.9%) groups (P = 0.306). Other backgrounds were also similar between the two treatment groups (Table 1).
Table 1. Patients' demographics.
| RP group (n = 86) | EBRT group (n = 76) | P-value | |
|---|---|---|---|
| Age (mean, years) | 64.9 ± 6.4 | 71.1 ± 5.3 | <0.001 |
| PSA (median, ng mL−1 [range]) | |||
| 8.5 (2.3–268.2) | 14.0 (3.3–205) | 0.020 | |
| Gleason score (n [%]) | 0.067 | ||
| ≤ 6 | 47 (54.6) | 26 (34.2) | |
| 7 | 24 (27.9) | 25 (32.9) | |
| ≥ 8 | 14 (16.3) | 19 (25.0) | |
| Unknown | 1 (1.2) | 6 (7.9) | |
| Clinical T stage (n [%]) | 0.013 | ||
| CT2 | 81 (94.2) | 62 (91.6) | |
| CT3 | 5 (5.8) | 14 (18.4) | |
| Risk subgroup (n [%]) | < 0.001 | ||
| Low | 36 (41.9) | 12 (15.8) | |
| Intermediate | 26 (30.2) | 27 (35.5) | |
| High | 24 (27.9) | 37 (48.7) | |
Abbreviations: RP, radical prostatectomy; EBRT, external beam radiation therapy; PSA, prostate-specific antigen.
Oncological outcomes
At median postoperative follow-up of 5 years (range: 19–130 months), 31 patients (36.0%) in the RP group showed a biochemical recurrence. In the RP group, four patients had disease-specific mortality and four died of other causes. Thirty-six (42%), 26 (30%) and 24 (28%) patients were diagnosed as having low-, intermediate- and high-risk disease, respectively. In this group, nine (25%) low-risk patients had a biochemical recurrence, and 35% and 54% of the intermediate- and high-risk patients, respectively, were regarded as having a biochemical recurrence. There was no treatment-related mortality in either group, and there was no malignancy secondary to EBRT.
In the EBRT group, 17 patients had a biochemical recurrence at a median follow-up of 3.9 years (range: 5–104 months). Three patients died of PCa, and there was one mortality from another cause. In the EBRT group, 12 (16%), 27 (35%) and 37 (49%) patients were categorized as having low-, intermediate- and high-risk disease, respectively. Three low-risk patients (25%) had a biochemical recurrence, and in the intermediate-risk and high-risk patients 26% and 19%, respectively, showed a biochemical recurrence.
For the entire group of patients, the biochemical recurrence-free survival did not differ between the RP and EBRT groups (P = 0.057) (Figure 1A). The 5-year biochemical recurrence-free survival rate was equivalent between the RP and EBRT groups for low-risk (74.6% and 75.0%, respectively, P = 0.931) (Figure 1B) and intermediate-risk patients (61.3% and 71.1%, respectively, P = 0.691) (Figure 1C). For high-risk patients, the biochemical recurrence-free survival rate was lower in the RP group than in the EBRT group (45.1% and 79.7%, respectively, P = 0.002) (Figure 1D).
Figure 1.
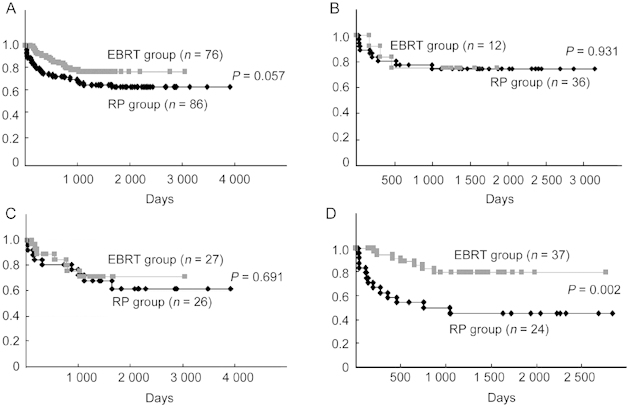
(A): Biochemical, recurrence-free survival in all patients. The 5-year biochemical recurrence-free survival rates in the radical prostatectomy (RP) and external beam radiation therapy (EBRT) groups were 62.3% and 76.2%, respectively. (B)–(D): Biochemical, recurrence-free survival in low, intermediate and high-risk patients, repectively.
Comparison of the SF-36 scores between the RP and EBRT patients
Of the 146 patients who received questionnaires about their HRQOL, 62 RP (79%) and 54 EBRT (79%) patients returned assessable surveys and proceeded to the following stage of analysis. The patients' ages were higher in the EBRT group than in the RP group (75.8 ± 2.8 and 70.7 ± 7.2, P = 0.047 for low-risk; 75.7 ± 5.3 and 70.5 ± 5.5, P = 0.003 for intermediate-risk; 74.3 ± 5.1; 67.8 ± 9.7, P = 0.010 for high-risk patients). Comparisons of data from the SF-36 for the RP and EBRT patients are presented in Table 2. For the entire patient population, the RP group showed higher scores than the EBRT group on physical function, based on the SF-36 analysis (P = 0.011). However, the two groups were comparable in terms of RP, RE, BP, GH, VT, SF and MH. On the basis of risk stratification, there were no noteworthy differences between the two groups, although the physical function score of the intermediate-risk patients was higher in the RP group (Table 2).
Table 2. Comparison of the Medical Outcomes Study Short Form-36 (SF-36) scores for the radical prostatectomy (RP) and external beam radiation therapy (EBRT) groups.
| RP group, mean index |
EBRT group, mean index |
P-value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 62) | Low-risk (n = 27) | Intermediate-risk (n = 22) | High-risk (n = 13) | Total (n = 54) | Low-risk (n = 9) | Intermediate-risk (n = 20) | High-risk (n = 25) | Total | Low-risk | Intermediate-risk | High-risk | |
| PF | 87.3 ± 14.2 | 88.2 ± 11.5 | 88.8 ± 11.9 | 83.2 ± 21.3 | 78.6 ± 20.8 | 75.9 ± 25.8 | 70.7 ± 24.4 | 85.8 ± 12.8 | 0.011 | 0.228 | 0.006 | 0.644 |
| RP | 82.3 ± 33.5 | 86.5 ± 27.6 | 77.5 ± 38.0 | 81.3 ± 38.6 | 70.3 ± 38.0 | 69.4 ± 42.9 | 63.2 ± 41.1 | 76.0 ± 34.2 | 0.079 | 0.289 | 0.265 | 0.678 |
| BP | 84.8 ± 20.9 | 86.3 ± 17.6 | 85.9 ± 20.8 | 79.8 ± 27.4 | 80.8 ± 23.1 | 81.7 ± 23.4 | 74.2 ± 23.8 | 85.5 ± 22.0 | 0.327 | 0.530 | 0.101 | 0.496 |
| SF | 91.3 ± 15.1 | 94.0 ± 10.6 | 89.8 ± 17.5 | 88.5 ± 18.7 | 90.7 ± 18.4 | 86.1 ± 25.3 | 87.5 ± 21.5 | 95.0 ± 11.4 | 0.850 | 0.389 | 0.708 | 0.189 |
| GH | 61.5 ± 20.4 | 58.2 ± 19.8 | 66.9 ± 20.2 | 59.4 ± 22.1 | 60.1 ± 16.3 | 51.4 ± 13.5 | 57.0 ± 16.2 | 65.0 ± 15.7 | 0.678 | 0.371 | 0.107 | 0.384 |
| VT | 75.3 ± 19.5 | 74.4 ± 18.5 | 76.7 ± 18.2 | 75.0 ± 25.0 | 74.6 ± 19.5 | 69.4 ± 30.2 | 72.2 ± 17.5 | 77.9 ± 16.6 | 0.843 | 0.665 | 0.456 | 0.679 |
| RE | 78.5 ± 39.0 | 84.6 ± 31.6 | 68.3 ± 46.5 | 83.3 ± 38.9 | 77.4 ± 38.5 | 70.4 ± 45.5 | 76.7 ± 40.6 | 80.6 ± 35.3 | 0.873 | 0.403 | 0.542 | 0.831 |
| MH | 77.4 ± 18.3 | 76.2 ± 19.9 | 78.5 ± 18.9 | 78.3 ± 14.6 | 81.8 ± 17.8 | 70.0 ± 28.5 | 81.0 ± 14.3 | 86.8 ± 12.8 | 0.211 | 0.480 | 0.659 | 0.083 |
Abbreviations: PF, physical function; RP, role-physical; BP, bodily pain; SF, social function; GH, general health; VT, vitality; RE, role-emotional; MH, mental health.
Comparison of the UCLA PCI scores between the RP and EBRT patients
Regarding the UCLA PCI, the RP group reported lower scores than the EBRT group on urinary function, urinary bother and sexual bother (P < 0.001, P = 0.036 and P < 0.001, respectively) (Table 3). Table 3 shows the UCLA PCI scores for each risk subgroup. With risk stratification, patients in the RP group reported poorer urinary function than patients in the EBRT group for any risk subgroup, although the difference had borderline significance for the high-risk patients (P < 0.001, P < 0.001 and P = 0.050, respectively). With regard to sexual function, high-risk patients in the RP group had lower scores than patients in the EBRT group (P < 0.001), but, conversely, low-risk patients in the RP group had better scores than patients in the EBRT group (P < 0.001). The scores of other domains were similarly distributed between the RP and EBRT subgroups, except for sexual bother. In this case, the intermediate-risk patients in the RP group had lower scores than patients in the EBRT group (P = 0.004). Biochemical recurrence was not associated with the UCLA PCI score in either treatment group, regardless of risk stratification (Table 4).
Table 3. Comparison of the University of California Los Angeles Prostate Cancer Index (UCLA PCI) scores from the total patient population and from the risk subgroups for the radical prostatectomy (RP) and external beam radiation therapy (EBRT) groups.
| RP group (mean index) |
EBRT group (mean index) |
P-value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 62) | Low-risk (n = 27) | Intermediate-risk (n = 22) | High-risk (n = 13) | Total (n = 54) | Low-risk (n = 9) | Intermediate-risk (n = 20) | High-risk (n = 25) | Total | Low-risk | Intermediate-risk | High-risk | |
| Urinary function | 71.6 ± 35.7 | 71.6 ± 36.4 | 63.6 ± 35.2 | 85.7 ± 30.7 | 93.6 ± 17.1 | 90.7 ± 19.5 | 93.9 ± 19.8 | 93.9 ± 14.7 | < 0.001 | < 0.001 | < 0.001 | 0.050 |
| Urinary bother | 80.3 ± 30.0 | 78.7 ± 33.8 | 77.3 ± 28.8 | 89.6 ± 22.5 | 90.1 ± 19.3 | 80.6 ± 20.8 | 83.8 ± 26.0 | 96.9 ± 8.4 | 0.036 | 0.878 | 0.450 | 0.298 |
| Bowel function | 91.5 ± 19.6 | 92.4 ± 18.4 | 91.3 ± 20.2 | 90.2 ± 21.5 | 88.8 ± 22.4 | 83.3 ± 27.5 | 86.8 ± 27.5 | 92.9 ± 19.2 | 0.161 | 0.073 | 0.122 | 0.443 |
| Bowel bother | 89.8 ± 20.1 | 89.8 ± 21.1 | 90.5 ± 16.7 | 88.5 ± 24.2 | 89.8 ± 18.1 | 80.6 ± 24.3 | 88.8 ± 19.0 | 95.0 ± 12.5 | 0.983 | 0.280 | 0.759 | 0.375 |
| Sexual function | 11.1 ± 21.8 | 11.9 ± 22.8 | 14.5 ± 24.7 | 4.0 ± 10.2 | 9.1 ± 18.7 | 3.4 ± 9.1 | 10.2 ± 19.8 | 12.2 ± 21.3 | 0.116 | < 0.001 | 0.082 | < 0.001 |
| Sexual bother | 57.5 ± 34.8 | 57.7 ± 33.7 | 47.6 ± 34.4 | 73.1 ± 34.6 | 81.6 ± 24.1 | 78.6 ± 26.7 | 79.2 ± 28.8 | 80.4 ± 21.3 | < 0.001 | 0.142 | 0.004 | 0.433 |
Table 4. Comparison of the University of California Los Angeles Prostate Cancer Index (UCLA PCI) scores for patients in the radical prostatectomy (RP) or external beam radiation therapy (EBRT) groups, with and without biochemical recurrence.
| Patients without recurrence (mean index) |
Patients with recurrence (mean index) |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 86) | RP group (n = 41) | EBRT group (n = 45) | Total (n = 30) | RP group (n = 21) | EBRT group (n = 9) | Total | RP group | EBRT group | |
| Urinary function | 83.0 ± 29.5 | 71.8 ± 35.0 | 93.3 ± 18.0 | 77.9 ± 33.8 | 71.2 ± 37.2 | 93.5 ± 15.3 | 0.100 | 0.880 | 0.949 |
| Urinary bother | 84.7 ± 25.6 | 79.9 ± 29.7 | 89.2 ± 20.5 | 83.6 ± 27.8 | 81.3 ± 31.3 | 88.9 ± 18.2 | 0.847 | 0.868 | 0.966 |
| Bowel function | 90.6 ± 20.9 | 91.7 ± 20.0 | 89.7 ± 21.7 | 89.5 ± 20.8 | 91.2 ± 19.1 | 85.7 ± 24.1 | 0.621 | 0.851 | 0.320 |
| Bowel bother | 90.9 ± 18.4 | 90.6 ± 20.2 | 91.1 ± 17.0 | 87.5 ± 20.5 | 88.1 ± 20.3 | 86.1 ± 22.0 | 0.403 | 0.644 | 0.447 |
| Sexual function | 11.3 ± 20.8 | 11.9 ± 22.3 | 10.7 ± 19.3 | 8.7 ± 20.5 | 9.68 ± 20.9 | 6.31 ± 19.4 | 0.099 | 0.288 | 0.090 |
| Sexual bother | 67.6 ± 30.2 | 57.7 ± 31.5 | 77.6 ± 25.5 | 66.7 ± 38.5 | 57.1 ± 41.2 | 88.9 ± 18.2 | 0.891 | 0.958 | 0.216 |
Discussion
According to one of the largest studies comparing the biochemical outcome in men with clinically localized prostate cancer, the 7-year biochemical recurrence-free rates for RP and EBRT were 79% and 77%, respectively 2. Oncological outcomes in this study were similar to those of the representative earlier reports. The long disease history of low- to intermediate-risk patients makes it difficult to execute conclusive, well-controlled or randomized studies comparing survival outcomes after RP and EBRT. On the other hand, better oncological outcomes, focused on overall survival after EBRT for locally advanced or high-risk prostate cancers, have been reported 18. However, earlier studies suggesting this type of outcome had several limitations. The results of this study may support these opinions, but our study also had some limitations. The most obvious limitation is the use of retrospective analysis. Additionally, the definition of biochemical recurrence according to the currently accepted criteria differs between RP and EBRT 12, 13. The patients' characteristics and the significance of hormone therapy are not comparable between the RP and EBRT groups 3. Thus, we should focus our attention not only on oncological assessment, but also on functional and physical/mental issues, represented by the general and functional HRQOL, under risk stratification.
We compared general HRQOL for the two treatment groups using the SF-36 survey over a 3-year follow-up period. There were no significant differences between the RP and EBRT groups, except for physical function in the intermediate-risk patients. Earlier studies have also shown only a small difference between generic HRQOL after an RP and EBRT 19, 20. Penson 21 reported that treatments differentially affect general HRQOL during the early period just after diagnosis, but the study concluded that these differences seem to dissipate within 3–6 months after therapy. A longitudinal, population-based study that followed cohorts for 5 years showed no differences in general HRQOL between men who received an RP and EBRT during the 2–5 years after diagnosis 20. Our current study further shows that generic HRQOL is equivalent between the two treatment groups in any risk category.
Using the UCLA PCI in this study, the RP group of patients also reported decreased urinary function compared with patients in the EBRT group. Disease-specific/treatment-related functional HRQOL in PCa after RP or EBRT has also been a matter of general concern 5, 6, 21, 22, 23, 24, 25; the conclusion was similar in earlier studies. RP patients had a tendency to report poorer urinary function than EBRT patients. However, no study has compared functional HRQOL between patients undergoing radical surgery and radiotherapy under risk stratification. Interestingly, urinary function was poorer in the RP group, especially in the low- and intermediate-risk patients. Many low- to intermediate-risk patients in the RP group, whose age was lower than that of the EBRT patients, potentially had few urinary tract symptoms preoperatively and might be more aware of having their urinary function reduced by treatment. On the other hand, sexual function of the high-risk patients was better in the EBRT group than in the RP group, and this result is possible, assuming equal ADT in this risk category. In the current study, however, sexual function in the RP group seemed to be better for patients with low-risk disease. Sexual function of the present low-risk EBRT patients, who were older, might be more affected by irradiation. Additionally, the fraction of patients receiving prior ADT was higher in the EBRT group, suggesting an adverse impact brought about by ADT 26. These tendencies are thought to be applicable to the general population. We further compared HRQOL between the RP and EBRT patients treated with or without ADT, and similar tendencies were noted under the ADT and non-ADT conditions (data not shown), although the number of patients in the non-ADT groups was too small to draw a definite conclusion. A large population-based study for the significance/impact of ADT on physiological or functional outcomes after radical treatments is currently underway.
Few investigations published to date have focused on HRQOL outcomes in men who experience biochemical recurrence after definitive therapy for localized prostate cancer 24. In our study, HRQOL was not affected by whether patients developed disease recurrence in both the RP and the EBRT groups. Despite a definite correlation between the disease-specific risk and biochemical recurrence rate, the biochemical recurrence had little influence on functional outcomes even with risk stratification.
This study has several limitations. Specifically, it was retrospectively designed for oncological assessment, and the HRQOL surveys were conducted in a cross-sectional manner. A longitudinal trial may have provided more comprehensive information on the post-treatment HRQOL of prostate cancer patients.
In conclusion, this study employed risk stratification and showed that low- to intermediate-risk patients may report decreased urinary function in the long-term when treated with an RP. After an RP, high-risk patients are likely associated with poorer sexual function. Even with risk stratification, functional HRQOL after radical surgery or radiotherapy did not depend on biochemical recurrence. Further assessment of generic and functional HRQOL after surgery, radiotherapy or hormone therapy with risk stratification is warranted.
References
- Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, et al. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148:435–48. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- Potters L, Klein EA, Kattan MW, Reddy CA, Ciezki JP, et al. Monotherapy for stage T1-T2 prostate cancer: radical prostatectomy, external beam radiotherapy, or permanent seed implantation. Radiother Oncol. 2004;71:29–33. doi: 10.1016/j.radonc.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Sommers BD, Beard CJ, D'Amico AV, Dahl D, Kaplan I, et al. Decision analysis using individual patient preferences to determine optimal treatment for localized prostate cancer. Cancer. 2007;110:2210–7. doi: 10.1002/cncr.23028. [DOI] [PubMed] [Google Scholar]
- Scherr D, Swindle PW, Scardino PT. National comprehensive cancer network. National comprehensive cancer network guidelines for the management of prostate cancer. Urology. 2003;61:14–24. doi: 10.1016/s0090-4295(02)02395-6. [DOI] [PubMed] [Google Scholar]
- Wei JT, Dunn RL, Sandler HM, McLaughlin PW, Montie JE, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20:557–66. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]
- Davis JW, Kuban DA, Lynch DF, Schellhammer PF. Quality of life after treatment for localized prostate cancer: differences based on treatment modality. J Urol. 2001;166:947–52. [PubMed] [Google Scholar]
- Namiki S, Tochigi T, Kuwahara M, Ioritani N, Terai A, et al. Health related quality of life in Japanese men after radical prostatectomy or radiation therapy for localized prostate cancer. Int J Urol. 2004;11:619–27. doi: 10.1111/j.1442-2042.2004.00860.x. [DOI] [PubMed] [Google Scholar]
- Van Oort IM, Witjes JA, Kok DE, Kiemeney LA, Hulsbergen-Van De Kaa CA. The prognostic role of the pathological T2 subclassification for prostate cancer in the 2002 Tumour-Nodes-Metastasis staging system. BJU Int. 2008;102:438–41. doi: 10.1111/j.1464-410X.2008.07611.x. [DOI] [PubMed] [Google Scholar]
- Walsh PC. Radical prostatectomy for localized prostate cancer provides durable cancer control with excellent quality of life: a structured debate. J Urol. 2000;163:1802–7. [PubMed] [Google Scholar]
- Soloway MS, Pareek K, Sharifi R, Wajsman Z, McLeod D, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol. 2002;167:112–6. [PubMed] [Google Scholar]
- Hurtado-coll A, Goldenberg SL, Klotz L, Gleave ME. Preoperative neoadjuvant androgen withdrawal therapy in prostate cancer: the Canadian experience. Urology. 2002;60:45–51. doi: 10.1016/s0090-4295(02)01570-4. [DOI] [PubMed] [Google Scholar]
- Boccon-Gibod L, Djavan WB, Hammerer P, Hoeltl W, Kattan MW, et al. Management of prostate-specific antigen relapse in prostate cancer: a European Consensus. Int J Clin Pract. 2004;58:382–90. doi: 10.1111/j.1368-5031.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- Consensus statement: guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int J Radiat Oncol Biol Phys. 1997;37:1035–41. [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, et al. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002–12. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J Clin Epidemiol. 1998;51:1037–44. doi: 10.1016/s0895-4356(98)00095-x. [DOI] [PubMed] [Google Scholar]
- Kakehi Y, Kamoto T, Ogawa O, Arai Y, Litwin MS, et al. Development of Japanese version of the UCLA Prostate Cancer Index: a pilot validation study. Int J Clin Oncol. 2002;7:306–11. doi: 10.1007/s101470200045. [DOI] [PubMed] [Google Scholar]
- Saito T, Kitamura Y, Komatsubara S, Matsumoto Y, Sugita T, et al. Outcomes of locally advanced prostate cancer: a single institution study of 209 patients in Japan. Asian J Androl. 2006;8:555–61. doi: 10.1111/j.1745-7262.2006.00175.x. [DOI] [PubMed] [Google Scholar]
- Penson DF, Feng Z, Kuniyuki A, McClerran D, Albertsen PC, et al. General quality of life 2 years following treatment for prostate cancer: what influences outcomes? Results from the prostate cancer outcomes study. J Clin Oncol. 2003;21:1147–54. doi: 10.1200/JCO.2003.07.139. [DOI] [PubMed] [Google Scholar]
- Potosky AL, Davis WW, Hoffman RM, Stanford JL, Stephenson RA, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst. 2004;96:1358–67. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- Penson DF. Quality of life after therapy for localized prostate cancer. Cancer J. 2007;13:318–26. doi: 10.1097/PPO.0b013e3181570121. [DOI] [PubMed] [Google Scholar]
- Frank SJ, Pisters LL, Davis J, Lee AK, Bassett R, et al. An assessment of quality of life following radical prostatectomy, high dose external beam radiation therapy and brachytherapy iodine implantation as monotherapies for localized prostate cancer. J Urol. 2007;177:2151–6. doi: 10.1016/j.juro.2007.01.134. [DOI] [PubMed] [Google Scholar]
- McCammon KA, Kolm P, Main B, Schellhammer PF. Comparative quality-of-life analysis after radical prostatectomy or external beam radiation for localized prostate cancer. Urology. 1999;54:509–16. doi: 10.1016/s0090-4295(99)00163-6. [DOI] [PubMed] [Google Scholar]
- Litwin MS, Gore JL, Kwan L, Brandeis JM, Lee SP, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109:2239–47. doi: 10.1002/cncr.22676. [DOI] [PubMed] [Google Scholar]
- Shrader-Bogen CL, Kjellberg JL, McPherson CP, Murray CL. Quality of life and treatment outcomes: prostate carcinoma patients' perspectives after prostatectomy or radiation therapy. Cancer. 1997;79:1977–86. doi: 10.1002/(sici)1097-0142(19970515)79:10<1977::aid-cncr20>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Kang SH, Kim JW, Bae JH, Park HS, Moon DG, et al. Targeted-cryosurgical ablation of the prostate with androgen deprivation therapy: quality of life in high-risk prostate cancer patients. Asian J Androl. 2006;8:629–36. doi: 10.1111/j.1745-7262.2006.00176.x. [DOI] [PubMed] [Google Scholar]


