Abstract
This study investigated the acute effects of green tea extract (GTE) and its polyphenol constituents, (−)-epigallocatechin-3-gallate (EGCG) and (−)-epicatechin (EC), on basal and stimulated testosterone production by rat Leydig cells in vitro. Leydig cells purified in a Percoll gradient were incubated for 3 h with GTE, EGCG or EC and the testosterone precursor androstenedione, in the presence or absence of either protein kinase A (PKA) or protein kinase C (PKC) activators. The reversibility of the effect was studied by pretreating cells for 15 min with GTE or EGCG, allowing them to recover for 1 h and challenging them for 2 h with human chorionic gonadotropin (hCG), luteinizing hormone releasing hormone (LHRH), 22(R)-hydroxycholesterol or androstenedione. GTE and EGCG, but not EC, inhibited both basal and kinase-stimulated testosterone production. Under the pretreatment conditions, the inhibitory effect of the higher concentration of GTE/EGCG on hCG/LHRH-stimulated or 22(R)-hydroxycholesterol-induced testosterone production was maintained, whereas androstenedione-supported testosterone production returned to control levels. At the lower concentration of GTE/EGCG, the inhibitory effect of these polyphenols on 22(R)-hydroxycholesterol-supported testosterone production was reversed. The inhibitory effects of GTE may be explained by the action of its principal component, EGCG, and the presence of a gallate group in its structure seems important for its high efficacy in inhibiting testosterone production. The mechanisms underlying the effects of GTE and EGCG involve the inhibition of the PKA/PKC signalling pathways, as well as the inhibition of P450 side-chain cleavage enzyme and 17β-hydroxysteroid dehydrogenase function.
Keywords: green tea polyphenols, Leydig cells, protein kinase A, protein kinase C, testosterone
Introduction
Green tea (Camellia sinensis) is one of the most commonly consumed beverages worldwide. Its active components are reported to have several biological properties, including cancer chemoprevention, inhibition of tumour cell growth, antiviral and anti-inflammatory activities 1, antioxidant activity 2, 3 and inhibitory effects on several enzymes, such as aromatase 4, 5, angiotensin converting enzyme 6 and thyroid peroxidase 7. Dried leaves of C. sinensis contain polyphenols (30%–36%), principally flavanols, more commonly known as catechins 8. The predominant catechins are epigallocatechin-3-gallate (EGCG), epicatechin-3 gallate (ECG), epigallocatechin (EGC) and epicatechin (EC).
The effects of catechins on the male reproductive system have been described. Epidemiological and laboratory studies suggest an association between diet and androgens that can alter prostate cancer risk 9, 10, 11. It has been shown that parenteral injection of EGCG can suppress human prostate and breast tumour growth in athymic mice 12 and reduce the weight of testes and accessory reproductive organs, as well the circulating level of luteinizing hormone (LH) and testosterone in the intact rat 13. Although the antigonadotropic effect of catechins is explained as a secondary effect of EGCG on food intake 13 or on aromatase activity 4, 5, a modulatory function could be present even at the gonadal level.
Currently, there is no evidence for a direct effect of green tea catechins on testicular steroidogenesis or on the enzymes involved in androgen production. Although the involvement of the protein kinase A (PKA) and protein kinase C (PKC) signalling pathways in testicular androgen production is well known 14, 15, it is unclear whether green tea catechins modulate these pathways in Leydig cells. There is evidence that EGCG and other flavonoids can modulate the PKC 16, 17 and PKA signalling pathways in other animal models 16, 18. The aim of this study was to investigate the direct in vitro effects of green tea extract (GTE) and its purified catechins on the basal and the PKA- and PKC-stimulated testosterone production by rat Leydig cells.
Materials and methods
Materials
Hank's balanced salt solution (HBSS) and Medium 199 were obtained from Gibco (Grand Island, NY, USA). Collagenase (Type I), soybean trypsin inhibitor, leupeptin, phorbol 12′,13-dibutyrate (PDBu), human chorionic gonadotropin (hCG), N6,2′-O-dibutyryladenosine3′,5′-cyclic monophosphate (dbcAMP), EC, EGCG from green tea, 3-(4′,5-dimethylthiazol-2-yl)-2′,5-diphenyltetrazolium bromide (MTT), dimethylsulphoxide (DMSO), 22(R)-hydroxycholesterol and 4-androstene-3′,17-dione were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Bovine serum albumin (BSA, fraction V) was obtained from Miles (Naperville, IL, USA). LH releasing hormone (LHRH) was purchased from Peninsula (San Carlos, CA, USA). Percoll was purchased from Pharmacia (Uppsala, Sweden).
GTE preparation
GTE was prepared according to Wang et al. 19 with slight modifications. Five grams of dry green tea leaves were infused for 5 min in 100 mL of boiling saline (90°C), allowed to cool to room temperature and then filtered. The resulting clear solution is similar to tea brews consumed by humans. The amount of solid matter present in this infusion was determined by drying samples (10 mL) in an oven overnight at 100°C and weighing the dry residue. The dry weight was determined to be 69.2 mg for 5 g of green tea leaves, making the concentration of this infusion 0.692% (w/v). The extracts were freshly prepared on a daily basis. This preparation is comprised of approximately 27% catechins, 8.0% caffeine and 0.4% theobromine, and EGCG represents ≥ 50% of total catechins 19.
Animals and Leydig cell-enriched preparation
Adult (70–80 days old) male Wistar rats weighing 200–300 g were kept in a controlled environment (temperature 25–29°C; lights on from 05:00 to 18:00) with free access to standard laboratory chow and tap water. Isolation and purification of rat Leydig cell-enriched preparations were performed as described by Hedger and Eddy 15, 20. The rats were killed by ether anaesthesia and the testes were quickly removed and decapsulated. The decapsulated testes were incubated in an enzyme solution of 0.5 mg mL−1 collagenase, 0.2 mg mL−1 soybean trypsin inhibitor and 5 μg mL−1 leupeptin in Hank's balanced salt solution containing 0.1% BSA (HBSS/BSA), pH 7.4, in a shaking water bath (20 min, 90 Hz, 34°C). The dispersed testes were suspended in a final volume of 50 mL HBSS/BSA and the dissociated tubules were allowed to settle (5 min). The supernatant was filtered and washed with 5 mL HBSS/BSA. The filtered cell suspension was centrifuged (150 × g, 15 min, 20°C), and the pellet was re-suspended in 5 mL HBSS/BSA, loaded onto the top of a discontinuous Percoll density gradient (20%, 35%, 43%, 68% and 90%) and centrifuged at 800 × g for 30 min at 20°C. Cells in the 43%–68% interface (specific gravity: 1.0640–1.0960 g mL−1) were collected, washed twice with M199 containing 0.1% BSA, re-suspended in M199/0.1% BSA and used immediately for the experiments.
Cell viability
To assess the effects of GTE and EGCG on cell viability, the Trypan blue exclusion and MTT reduction assays were used. These assays are widely used screening methods to measure plasma membrane integrity and active mitochondrial function, respectively. The Trypan blue assay was performed after incubating cells for 3 h with the different doses of GTE or EGCG. Cells were incubated with Trypan blue (0.5%) for 20 min and the resulting percentage of blue cells, indicating a capture of the colourant due to plasma membrane rupture, were counted. Normal cell viability was considered to be 90%–95% colourless cells. The MTT assay is based on the reduction of a soluble pale yellow tetrazolium dye to water-insoluble purple formazan crystals in living cells 21. Cells (0.3 × 106 per mL) were incubated in polypropylene tubes with GTE (69.2 μg mL−1) or EGCG (10, 50 and 100 μg mL−1) for 2 h at 34°C under 95% O2 and 5% CO2. After this time, the cells were washed twice with M199 to remove the GTE/EGCG and then resuspended in 100 μL of fresh M199. Subsequently, 25 μL of MTT in PBS (5 mg mL−1) was added to the cell suspensions followed by 3 h of incubation at 37°C. This procedure avoids a direct reaction between the polyphenols and the MTT. It is known that polyphenols can reduce MTT in lieu of living cells, so results obtained may not reflect the true cell viability and instead mask the effects of polyphenols 22. After this incubation period, the cell suspensions were centrifuged at 400 × g for 5 min. The supernatant was removed and 100 μL DMSO was added to each tube to dissolve formazan crystals overnight. The dissolved pellet was transferred to microplates and the absorbance was measured at 630 nm in an ELISA reader. Appropriate controls were included (i.e., medium plus MTT without cells or cells treated with the toxic reagent saponin 0.1%). The animal care committee of the Federal University of Pernambuco (Recife, PE, Brazil) approved all treatments.
In vitro testosterone secretion
Cells (0.3 × 106 cells per 0.5 mL) were treated for 3 h with M199, GTE (6.92–692.0 μg mL−1), EGCG (5–200 μg mL−1) or EC (200 μg mL−1) in the absence or presence of hCG (1 mIU mL−1), dbcAMP (1 mmol L−1), LHRH (10−7 mol L−1), PDBu (200 nmol L−1) or androstenedione (1–100 μmol L−1) in a shaking water bath (60 Hz, 34°C) under an atmosphere of 95% O2 and 5% CO2. It is noteworthy that a cup of GTE (100 mL) usually contains 50–150 mg mL−1 of tea polyphenols 23. To study the reversibility of the inhibitory effect of GTE and EGCG, cells were preincubated for 15 min with M199 GTE (69.2 μg mL−1) or EGCG (100 μg mL−1), washed with fresh medium, allowed to recuperate for 60 min, and then incubated for 2 h with 0.5 mUI mL−1 hCG, 10−7 mol L−1 LHRH, 20 μmol L−1 22-hydroxycholesterol or 10 μmol L−1 androstenedione. This 15-min preincubation period was chosen because it was sufficient to detect an inhibitory effect of GTE or EGCG on testosterone production similar to that seen after 3 h incubation (data not shown). At the end of the second incubation, the cells were centrifuged and the supernatant was collected and stored at −20°C until used for testosterone measurement by radioimmunological analysis (RIA).
RIA and statistical analysis
Testosterone was measured directly (without extraction) in the incubation medium by a charcoal–dextran RIA method 24 that employs [3H]-testosterone as tracer and a primary antiserum raised in rabbits in our laboratory against testosterone-3-(0-carboxymethyl)oxime:BSA. Intra- and inter-assay coefficients of variation were 8.1% and 15.1%, respectively. The testosterone antibody showed < 0.1% cross-reactivity with androstenedione, dehydroepiandrosterone, androsterone, 17α-hydroxyprogesterone, β-estradiol and estrone. None of the substances tested interfered with the assays. The data from the different analyses were reported as the mean ± SEM. of triplicate determinations and were representative of results obtained in at least two similar experiments. One-way ANOVA and Dunnet tests were used to examine differences among control and GTE/EGCG/EC-treated cells. P-values less than 0.05 were considered to be statistically significant.
Results
GTE and EGCG inhibit basal and hCG-stimulated testosterone production
The modulatory effects of GTE and its pure constituents (EGCG and EC) were tested by incubating Leydig cell-enriched preparations with GTE or EGCG followed by determination of testosterone levels in the incubation medium. Cells were incubated with various concentrations (three different orders of magnitude) of GTE or EGCG. In Figures 1 and 2, Leydig cells were treated with and without GTE or the catechins EGCG and EC, respectively, in the absence or presence of maximum stimulation by hCG (indirect PKA activator). GTE and EGCG both produced an inhibitory effect on basal and stimulated testosterone production. At 6.92, 69.2 and 692 μg mL−1, GTE inhibited basal testosterone levels by 27.5% ± 4.8%, 60.5% ± 3.9% and 93.1% ± 0.2%, respectively, whereas EGCG at 5, 50 and 200 μg mL−1 produced 24.0% ± 6.7%, 37.9% ± 1.4% and 58.2% ± 1.4% inhibition, respectively. The inhibitory effects of 6.92, 69.2 and 692 μg mL−1 GTE on hCG-stimulated testosterone production were 11.2% ± 6.0%, 56.8% ± 1.5% and 99.4% ± 0.03%, respectively, whereas the inhibitory effect of 50 and 200 μg mL−1 EGCG on stimulation with hCG was 46.5% ± 7.8% and 98.8% ± 0.06%, respectively (the 5 μg mL−1 dose showed no significant inhibitory effect). Exposure to EC at 200 μg mL−1 did not induce a significant inhibitory effect on basal or stimulated testosterone production. To evaluate whether the effect of GTE or EGCG on testosterone production was due to a decrease in cell viability, the Trypan blue exclusion and MTT reduction assays were used. The Trypan blue exclusion test for cell viability showed that the inhibition was not due to GTE-induced toxicity at the concentrations of 6.92 μg mL−1 or 69.2 μg mL−1 or EGCG toxicity at any concentration used. This test showed that at the above-cited concentrations, the percentages of viable cells obtained after 3 h of incubation were comparable between the GTE- or EGCG-treated (91%) cells and the controls (95%). At the maximum concentration of GTE used (692 μg mL−1), cell viability was 80% of control. This difference (10%–15% fewer viable cells compared with the control group) could have contributed to the observed decrease in testosterone production. For this reason, this concentration was not used in further experiments in this study. When measured by MTT reduction, cell viability was not modified by any concentration of GTE or EGCG used. The two assays for cell viability showed that inhibition of testosterone production was not due to the toxicity of GTE (6.92 μg mL−1 or 69.2 μg mL−1) or EGCG (10–100 μg mL−1).
Figure 1.
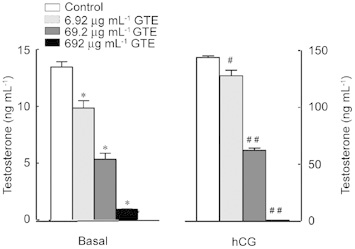
Treatment of Leydig cells with different concentrations of green tea extract (GTE) affects basal and hCG-induced testosterone production. Leydig cells (0.3 × 106 per 0.5 mL) were incubated for 3 h with hCG (1 mIU mL−1) in the presence or absence of 6.92, 69.2 or 692 μg mL−1 GTE. Results are the mean ± SEM of three determinations repeated in two independent experiments. *P <0.0001, #P < 0.01, ##P < 0.0001, compared with the control.
Figure 2.

Treatment of Leydig cells with different concentrations of epigallocatechin-3-gallate (EGCG) affects basal and hCG-induced testosterone production. Leydig cells (0.3 × 106 per 0.5 mL) were incubated for 3 h with hCG (1 mIU mL−1) in the presence or absence of 5, 50 or 200 μg mL−1 EGCG or 200 μg mL−1 EC. Results are the mean ± SEM of three determinations repeated in two independent experiments. *P < 0.05, **P < 0.01, #P < 0.001, compared with the control.
GTE and EGCG inhibit testosterone production elicited by activation of PKA and PKC
The action of GTE and EGCG was further investigated in the presence of a direct PKA activator, dbcAMP, and both an indirect and direct PKC activator, LHRH and PDBu, respectively. Table 1 shows testosterone production in the presence of each maximum stimulus alone or with GTE or EGCG. Testosterone production was reduced completely in all groups (to < 98%), regardless of the nature of the stimulus. These results indicate a probable interaction of GTE/EGCG with the signal-transduction process somewhere downstream of PKA and PKC.
Table 1. Effect of GTE or EGCG added together with dbcAMP, LHRH or PDBu on testosterone production in rat Leydig cells preparation.
| Additions | Testosterone (ng mL−1) |
|||
|---|---|---|---|---|
| Basal | dbcAMP (1 mmol L−1) | LHRH (0.1 mmol L−1) | PDBu (400 nmol L−1) | |
| M199 | 2.57 ± 0.03 | 191.70 ± 11.60 | 5.40 ± 0.40 | 54.00 ± 5.00 |
| GTE (69.2 μg mL−1) | 1.03 ± 0.03* | 4.20 ± 0.20* | 0.44 ± 0.02* | 1.47 ± 0.09* |
| EGCG (100 μg mL−1) | 1.20 ± 0.06* | 2.40 ± 0.06* | 0.27 ± 0.05* | 1.03 ± 0.03* |
Abbreviations: dbcAMP, dibutyryladenosine3′,5′-cyclic monophosphate; EGCG, (–)-epigallocatechin-3-gallate; GTE, green tea extract; LHRH, luteinizing hormone releasing hormone; PDBu, phorbol 12′,13-dibutyrate.
P < 0.0001, compared with respective control (M199).
Cells (0.3 × 106 per 0.5 mL) were incubated for 3 h. The data represent the mean ± SEM of triplicate determinations.
EGCG inhibits the stimulatory effect of androstenedione on testosterone production
To further evaluate the effect of EGCG, the steroidogenic precursor androstenedione was used to support testosterone production (a measure of 17β-hydroxysteroid dehydrogenase [17β-HSD] activity). Androstenedione crosses the cell membrane and moves to the smooth endoplasmic reticulum of Leydig cells in which, after binding to 17β-HSD, it is converted to testosterone. Several doses of androstenedione (1, 5, 10 and 20 μmol L−1) were used. These high concentrations were used to obviate the interference of any endogenous precursor. As shown in Figure 3, EGCG decreased testosterone production by approximately 50% at all concentrations of the precursor used. No significant inhibitory effect was observed in cells treated with EC.
Figure 3.
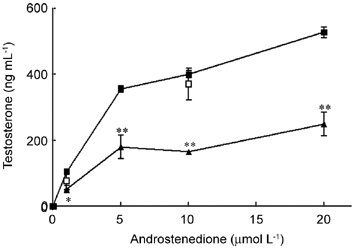
Effects of epigallocatechin-3-gallate (EGCG) on androstenedione-stimulated testosterone production. Leydig cells (0.3 × 106 per 0.5 mL) were incubated for 3 h with androstenedione (1–20 μmol L−1) in the absence (black square) or presence of 100 μg mL−1 EGCG (black triangle) or EC (white square). Results are the mean ± SEM of three determinations repeated in two independent experiments. *P < 0.001, **P < 0.0001, compared with respective androstenedione alone.
Effect of pretreatment with GTE or EGCG on hCG- or LHRH-stimulated and androstenedione-supported testosterone production
The aim of this experiment was to examine the reversibility of the inhibitory effect exerted by GTE or EGCG on hCG- or LHRH-stimulated and on androstenedione-supported testosterone production. The results shown in Figure 4 reveal that pretreatment with GTE (69.2 μg mL−1) followed by a 1-h recuperation period inhibited the responsiveness to subsequent incubation with M199 or stimulation with hCG or LHRH by 65.3%, 76.1% and 57.7%, respectively. Pretreatment with EGCG (100 μg mL−1) followed by a similar recuperation period decreased the responsiveness to subsequent incubation with M199 or stimulation with hCG or LHRH by 58.4%, 58.7% and 66.6%, respectively. In contrast to the effects observed with hCG and LHRH, pretreatment with GTE or EGCG did not reduce the responsiveness of Leydig cells to androstenedione. Similar results were observed with a 15-min recuperation period (data not shown). These results indicate that the inhibitory effects of GTE or EGCG on the steroidogenic process were reversible only in the androstenedione to testosterone enzymatic step.
Figure 4.
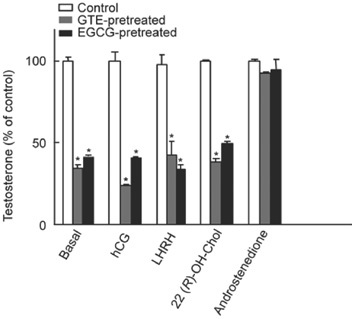
Effect of 15-min pretreatment with green tea extract (GTE) (69.2 μg mL−1) or epigallocatechin-3-gallate (EGCG) (100 μg mL−1) on testosterone production following a 1-h recovery period in Leydig cells (0.3 × 106 per 0.5 mL). To determine reversibility, after pretreatment, cells were rinsed with fresh M199 and incubated in M199 for 1 h before further challenged with hCG (0.5 mIU mL−1), LHRH (0.1 μmol L−1), 22(R)-hydroxycholesterol (20 μmol L−1) or androstenedione (10 μmol L−1) for 2 h. The data represent the mean ± SEM of triplicate determinations.
The 22(R)-hydroxycholesterol-induced production of testosterone was also examined to provide further evidence for the involvement of the earlier steps (before and after mitochondrial P450 side-chain cleavage enzyme (P450scc)-catalysed conversion of cholesterol to pregnenolone) of the steroidogenic pathway in the GTE/EGCG effect. 22(R)-hydroxycholesterol bypasses all signal-transduction pathways and has direct access to mitochondria in which it is a substrate for P450scc enzyme 25. The results shown in Figure 4 show that pretreatment with GTE or EGCG followed by a 1-h recuperation period inhibited the responsiveness of Leydig cells to subsequent incubation with 22(R)-hydroxycholesterol by 38.4% and 49.2%, respectively. These results show that GTE and EGCG also reduce the function of the P450scc enzyme. However, when we pretreated the cells with lower concentrations of GTE (13.8 μg mL−1) or EGCG (20 μg mL−1), a complete reversion of the inhibitory effect of the polyphenols on 22(R)-hydroxycholesterol-induced testosterone production was observed, and might even be classified as a stimulatory effect (Table 2).
Table 2. Inhibitory effect of GTE (13.8 μg mL−1) or EGCG (20 μg mL−1) on basal or 22(R)-hydroxycholesterol-induced testosterone production and its reversion after a 1-h recovery in Leydig cells (0.3 × 106 per 0.5 mL).
| Testosterone (ng mL−1) |
||||||
|---|---|---|---|---|---|---|
| Treated (no recovery) |
Pretreated (after 1-h recovery) |
|||||
| M199 | GTE | EGCG | M199 | GTE | EGCG | |
| Basal | 0.85 ± 0.05 | 0.78 ± 0.06 | 0.68 ± 0.03* | 1.07 ± 0.03 | 1.09 ± 0.03 | 1.13 ± 0.03 |
| 22-OH-Chol | 97.50 ± 0.10 | 70.30 ± 2.25** | 65.30 ± 1.25 ** | 75.83 ± 0.83 | 116.00 ± 4.00 ** | 100.00 ± 5.00 ** |
Abbreviations: EGCG, (−)-epigallocatechin-3-gallate; GTE, green tea extract.
P < 0.05,
P < 0.001, cpmopared with respective control (M199).
To determine reversibility, cells were rinsed with fresh M199 after a 15-min pretreatment with GTE/EGCG and incubated in M199 for 1 h before further challenge with 22(R)-hydroxycholesterol (22-OH-Chol: 20 μmol L−1) during 2 h. The data represent the mean ± SEM of triplicate determinations.
Discussion
In this study, the ability of GTE or individual catechins to inhibit steroidogenesis by rat Leydig cells in vitro was examined by quantifying effects on testosterone production. It was found that GTE and EGCG inhibited basal and stimulated testosterone production. This result indicates that GTE and the pure EGCG catechin have direct effects on the testis that are independent of any effects of the drug on the secretion of gonadotropin. The concentrations of GTE used in this study (6.92 and 69.2 μg mL−1) are within the range of those of pure EGCG (5-100 μg mL−1). These EGCG concentrations are similar to those used in most of the published in vitro tumour cell cytotoxicity studies (4.6–458.4 μg mL−1) in which the efficacy of inhibition depended on the cell type used (IC50 = 10–60 μg mL−1) 1, 26. Whereas concentrations higher than 4.6 μg mL−1 have been reported to reduce cell survival 1, 26, 27, the cytotoxicity assays used to assess our experimental conditions showed that the reduced testosterone production was not a consequence of a decline in Leydig cell viability.
In contrast to GTE and EGCG, pure EC (200 μg mL−1) failed to produce an inhibitory effect on testosterone production. This result suggests a structure–activity relationship. As the only structural difference between EGCG and EC is the presence of a gallate group at the 3′ position in EGCG, we expect that this group is critical for the potent ability of EGCG to inhibit testosterone production. This structure–activity relationship has been observed by other authors 28, 29, 30. As the inhibition was observed with EGCG, but not with EC, the effect cannot be attributed to the antioxidant properties characteristic of both of these catechins.
To investigate the mechanisms of the inhibitory effects of GTE and EGCG on stimulated testosterone production, an established in vitro model was used. This model is based on the ability of hCG/dbcAMP or LHRH/PDBu to stimulate testosterone production through mechanisms involving PKA or PKC pathway activation, respectively 14, 15, 31. These data show that both GTE and EGCG inhibited hCG-, dbcAMP-, LHRH- and PDBu-stimulated testosterone production. These results suggest that GTE and EGCG act directly on Leydig cells to regulate testosterone secretion at the PKA/PKC level and/or at a point downstream of the activation of PKA and PKC.
To further study the effects of EGCG, the enzymatic function of the steroidogenic enzyme that converts androstenedione to testosterone was also examined. The production of testosterone in Leydig cells involves several enzymatic steps 32. First, the side chain of cholesterol is cleaved to yield pregnenolone by mitochondrial P450scc, the rate-limiting enzyme. A series of steroidogenic enzymes that are located in the smooth endoplasmic reticulum then convert pregnenolone to testosterone, the principal secreted hormone. Pregnenolone is oxidized to progesterone by 3β-hydroxysteroid dehydrogenase (3β-HSD), progesterone is hydroxylated to 17α-hydroxyprogesterone by 17α-hydroxylase and 17α-hydroxyprogesterone is converted to androstenedione by C17-20 lyase. Androstenedione is then reduced to testosterone by 17β-HSD. As we were measuring the concentration of testosterone, the end product of the steroidogenic process, the activity of the final enzyme, 17β-HSD, was specifically examined. To that end, Leydig cells were incubated with the direct precursor of the last metabolic step, androstenedione. The inhibitory effect of EGCG was detected at all concentrations of androstenedione, indicating that the regulation of 17β-HSD is affected by EGCG and contributes to the reduction in the cell's steroidogenic capacity.
This effect of GTE or EGCG on the function of 17β-HSD seems to be independent of the effects on the PKA/PKC signalling pathways and the transfer of cholesterol from the outer to the inner mitochondrial membrane. This was shown by the reversibility of the inhibitory effects of GTE or EGCG on androstenedione-supported testosterone production. In contrast, hCG/LHRH-stimulated and 22(R)-hydroxycholesterol-supported testosterone production continued to be inhibited after a recuperation period after pretreatment with a high concentration of GTE (69.2 μg mL−1) or EGCG (100 μg mL−1). It is possible that the reversibility of androstenedione-supported testosterone production is related to the constitutively high expression levels of 17β-HSD 33. On the other hand, the results also indicate that the steroidogenic step in the mitochondrial compartment (P450scc function) remained inhibited in these experimental conditions. However, after pretreatment with a lower concentration of GTE (13.8 μg mL−1) or EGCG (20 μg mL−1), the inhibitory effects on P450scc function were indeed reversed after a recuperation period, suggesting that inhibition/reversion might be concentration-dependent.
Several studies have addressed the ability of other subclasses of phytochemicals to decrease the activity of a variety of steroidogenic enzymes, including P450scc, P450c17, P450c21, P45011β, 3β-HSD 34, 35, 36, 37, 38, aromatase and 17β-HSD 35, 38, and affect testosterone production 39, 40. Additionally, EGCG has been shown to inhibit ovarian aromatase activity 5 and estradiol and progesterone production by swine granulosa cells 41.
Taken together, these results show that the GTE and its major constituent EGCG inhibit testosterone production by rat Leydig cells in vitro and that the inhibitory effects observed with GTE may be explained, at least in part, by the EGCG present in the extract. The results also indicate that the mechanisms underlying these effects involve inhibition of the PKA/PKC signalling pathways, as well as direct or indirect inhibition of both P450scc and 17β-HSD, which are required for hormone synthesis. The inhibition of P450scc activity could explain the observed inhibitory effects of GTE and EGCG on basal testosterone production, which is maintained after a recuperation period of 1 h. Finally, the data presented here might help to explain the decrease in testosterone plasma levels observed in in vivo studies with green tea catechins 13.
References
- Yang CS, Chung JY, Yang G, Chhabra SK, Lee MJ. Tea and tea polyphenols in cancer prevention. J Nutr. 2000;130:472S–8S. doi: 10.1093/jn/130.2.472S. [DOI] [PubMed] [Google Scholar]
- Morel I, Lescoat G, Cogrel P, Sergent O, Pasdeloup N, et al. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem Pharmacol. 1993;45:13–9. doi: 10.1016/0006-2952(93)90371-3. [DOI] [PubMed] [Google Scholar]
- Guo Q, Zhao B, Li M, Shen S, Xin W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim Biophys Acta. 1996. 1304. pp. 210–22. [DOI] [PubMed]
- Satoh K, Sakamoto Y, Ogata A, Nagai F, Mikuriya H, et al. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem Toxicol. 2002;40:925–33. doi: 10.1016/s0278-6915(02)00066-2. [DOI] [PubMed] [Google Scholar]
- Goodin MG, Rosegren RJ. Epigallocatechin gallate modulates CYP450 isoforms in the female Swiss-Webster mouse. Toxicol Sci. 2003;76:262–70. doi: 10.1093/toxsci/kfh001. [DOI] [PubMed] [Google Scholar]
- Actis-Goretta L, Ottaviani JI, Fraga CG. Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. J Agirc Food Chem. 2006;54:229–34. doi: 10.1021/jf052263o. [DOI] [PubMed] [Google Scholar]
- Divi RL, Doerge DR. Inhibition of thyroid peroxidase by dietary flavonoids. Chem Res Toxicol. 1996;9:16–23. doi: 10.1021/tx950076m. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Mukhtar H. Green tea polyphenols and cancer: biologic mechanisms and practical implications. Nutr Rev. 1999;57:78–83. doi: 10.1111/j.1753-4887.1999.tb06927.x. [DOI] [PubMed] [Google Scholar]
- Ripple MO, Henry WF, Rago RP, Wilding G. Prooxidant-antioxidant shift induced by androgen treatment of human prostate carcinoma cells. J Natl Cancer Inst. 1997;89:40–8. doi: 10.1093/jnci/89.1.40. [DOI] [PubMed] [Google Scholar]
- Clinton SK, Giovannucci E. Diet, nutrition, and prostate cancer. Ann Rev Nutr. 1998;18:413–40. doi: 10.1146/annurev.nutr.18.1.413. [DOI] [PubMed] [Google Scholar]
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–43. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- Liao S, Umekita Y, Guo J, Kokontis JM, Hiipakka RA. Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Lett. 1995;96:239–43. doi: 10.1016/0304-3835(95)03948-v. [DOI] [PubMed] [Google Scholar]
- Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000;141:980–7. doi: 10.1210/endo.141.3.7368. [DOI] [PubMed] [Google Scholar]
- Dehejia A, Nozu K, Catt KJ, Dufau ML. Luteinizing hormone receptors and gonadotropic activation of purified rat Leydig cells. J Biol Chem. 1982;257:13781–6. [PubMed] [Google Scholar]
- Wanderley MI, Negro-Vilar A. Pretreatment with phorbol ester and an LHRH agonist reduces testosterone production and protein kinase C activity in rat Leydig cells challenged with PDBu and LHRH. Braz J Med Biol Res. 1996;29:1557–65. [PubMed] [Google Scholar]
- Lin JK. Cancer chemoprevention by tea polyphenols through modulating signal transduction pathways. Arch Pharm Res. 2002;25:561–71. doi: 10.1007/BF02976924. [DOI] [PubMed] [Google Scholar]
- Levites Y, Amit T, Mandel S, Youdim MB. Neuroprotection and neurorescue against Abeta toxicity and PKC-dependent release of nonamyloidogenic soluble precursor protein by green tea polyphenol (−)-epigallocatechin-3-gallate. FASEB J. 2003;17:952–4. doi: 10.1096/fj.02-0881fje. [DOI] [PubMed] [Google Scholar]
- Lorenz M, Wessler S, Follmann E, Michaelis W, Dusterhoft T, et al. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J Biol Chem. 2004;279:6190–5. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Huang MT, Ferraro T, Wong CQ, Lou YR, et al. Inhibitory effect of green tea in the drinking water on tumorigenesis by ultraviolet light and 12-O-tetradecanoylphorbol-13-acetate in the skin of SKH-1 mice. Cancer Res. 1992;52:1162–70. [PubMed] [Google Scholar]
- Hedger MP, Eddy EM. Monoclonal antibodies against rat Leydig cell surface antigens. Biol Reprod. 1986;35:1309–19. doi: 10.1095/biolreprod35.5.1309. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Peng L, Wang B, Ren P. Reduction of MTT by flavonoids in the absence of cells. Colloids Surf B Biointerfaces. 2005;45:108–11. doi: 10.1016/j.colsurfb.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Sakanaka S, Okada Y. Inhibitory effects of green tea polyphenols on the production of a virulence factor of the periodontal-disease-causing anaerobic bacterium Porphyromonas gingivalis. J Agric Food Chem. 2004;52:1688–92. doi: 10.1021/jf0302815. [DOI] [PubMed] [Google Scholar]
- Niswender GD, Akbar AM, Nett TM.Use of specific antibodies for quantification of steroid hormonesIn: O'Malley BW, Hardman JG, editors. Methods in Enzymology. New York: Academic Press Inc.1975. p16–34. [DOI] [PubMed] [Google Scholar]
- Papapadopoulos V, Guarneri P, Krueger KE, Guidotti A, Costa E. Pregnenolone biosynthesis in C6-2B glioma cell mitochondria: regulation by a mitochondrial diazepam binding inhibitor receptor. Proc Natl Acad Sci USA. 1992;89:5113–7. doi: 10.1073/pnas.89.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle DG, Ferreira D, Zhou YD. Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry. 2006;67:1849–55. doi: 10.1016/j.phytochem.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levites Y, Amit T, Youdim MB, Mandel S. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (−)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem. 2002;277:30574–80. doi: 10.1074/jbc.M202832200. [DOI] [PubMed] [Google Scholar]
- Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276:13322–30. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- Hiipakka RA, Zhang HZ, Dai W, Dai Q, Liao S. Structure-activity relationships for inhibition of human 5alpha-reductases by polyphenols. Biochem Pharmacol. 2002;63:1165–76. doi: 10.1016/s0006-2952(02)00848-1. [DOI] [PubMed] [Google Scholar]
- Doss MX, Potta SP, Hescheler J, Sachinidis A. Trapping of growth factors by catechins: a possible therapeutical target for prevention of proliferative diseases. J Nutr Biochem. 2005;16:259–66. doi: 10.1016/j.jnutbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lin YM, Liu MY, Poon SL, Leu SF, Huang BM. Gonadotrophin-releasing hormone-I and -II stimulate steroidogenesis in prepubertal murine Leydig cells in vitro. Asian J Androl. 2008;10:929–36. doi: 10.1111/j.1745-7262.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- Zirkin BR, Chen H. Regulation of Leydig cell steroidogenic function during aging. Biol Reprod. 2000;63:977–98. doi: 10.1095/biolreprod63.4.977. [DOI] [PubMed] [Google Scholar]
- Payne AH, O'Shaughnessy PJ.Structure, function and regulation of steroidogenic enzymes in the Leydig cellIn: Payne AH, Hardy MP, Russel LD, editors. The Leydig Cell. Vienna, IL: Cache River Press; 1996. p259–86. [Google Scholar]
- Wong CK, Keung WM. Bovine adrenal 3beta-hydroxysteroid dehydrogenase (E.C. 1.1.1.145)/5-ene-4-ene isomerase (E.C. 5.3.3.1): characterization and its inhibition by isoflavones. J Steroid Biochem Mol Biol. 1999;71:191–202. doi: 10.1016/s0960-0760(99)00135-1. [DOI] [PubMed] [Google Scholar]
- Le Bail JC, Champavier Y, Chulia AJ, Habrioux G. Effects of phytoestrogens on aromatase, 3beta and 17beta-hydroxysteroid dehydrogenase activities and human breast cancer cells. Life Sci. 2000;66:1281–91. doi: 10.1016/s0024-3205(00)00435-5. [DOI] [PubMed] [Google Scholar]
- Krazeisen A, Breitling R, Möller G, Adamski J. Phytoestrogens inhibit human 17beta-hydroxysteroid dehydrogenase type 5. Mol Cell Endocrinol. 2001;171:151–62. doi: 10.1016/s0303-7207(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Ohno S, Shinoda S, Toyoshima S, Nakazawa H, Makino T, et al. Effects of flavonoid phytochemicals on cortisol production and on activities of steroidogenic enzymes in human adrenocortical H295R cells. J Steroid Biochem Mol Biol. 2002;80:355–63. doi: 10.1016/s0960-0760(02)00021-3. [DOI] [PubMed] [Google Scholar]
- Lacey M, Bohday J, Fonseka SM, Ullah AI, Whitehead SA. Dose-response effects of phytoestrogens on the activity and expression of 3beta-hydroxysteroid dehydrogenase and aromatase in human granulosa-luteal cells. J Steroid Biochem Mol Biol. 2005;96:279–86. doi: 10.1016/j.jsbmb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Moundipa PF, Ngouela S, Kamtchouing P, Tsamo E, Tchouanguep FM, et al. Effects of extracts from Hibiscus macranthus and Basella alba mixture on testosterone production in vitro adult rat testes slices. Asian J Androl. 2006;8:111–4. doi: 10.1111/j.1745-7262.2006.00057.x. [DOI] [PubMed] [Google Scholar]
- Hammami I, Nahdi A, Mauduit C, Benahmed M, Amri M, et al. The inhibitory effects on adult male reproductive functions of crude garlic (Allium sativum) feeding. Asian J Androl. 2008;10:593–601. doi: 10.1111/j.1745-7262.2008.00358.x. [DOI] [PubMed] [Google Scholar]
- Basini G, Bianco F, Grasselli F. Epigallocatechin-3-gallate from green tea negatively affects swine granulosa cell function. Domest Anim Endocrinol. 2005;28:243–56. doi: 10.1016/j.domaniend.2004.10.002. [DOI] [PubMed] [Google Scholar]


