Abstract
Asthenozoospermia (AS) is a common cause of human male infertility. In one study, more than 80% of the samples from infertile men had reduced sperm motility. Seminal plasma is a mixture of secretions from the testis, epididymis and several male accessory glands, including the prostate, seminal vesicles and Cowper's gland. Studies have shown that seminal plasma contains proteins that are important for sperm motility. To further explore the pathophysiological character of AS, we separated the seminal plasma proteins from AS patients and healthy donors using sodium dodecyl sulfate polyacrylamide gel electrophoresis and in-gel digestion, and then subjected the proteins to liquid chromatography–mass spectrometry (LC-MS/MS) analysis. A total of 741 proteins were identified in the seminal plasma, with a false discovery rate of 3.3%. Using spectral counting, we found that 45 proteins were threefold upregulated and 56 proteins were threefold downregulated in the AS group when compared with the control. Most of these proteins originated from the epididymis and prostate. This study identified a rich source of biomarker candidates for male infertility and indicates that functional abnormalities of the epididymis and prostate can contribute to AS. We identified DJ-1—a protein that has been shown elsewhere to be involved in the control of oxidative stress (OS)—as a downregulated protein in AS seminal plasma. The levels of DJ-1 in AS seminal plasma were about half of those in the control samples. In addition, the levels of reactive oxygen species were 3.3-fold higher in the AS samples than in the controls. Taken together, these data suggest that downregulation of DJ-1 is involved in OS in semen, and therefore affects the quality of the semen.
Keywords: asthenozoospermia, comparative proteomics, DJ-1, seminal plasma
Introduction
Accumulating evidence has suggested that the quality of human semen may be deteriorating 1, 2. Explanations for this phenomenon include increased stress, infection, modern lifestyles and a variety of endocrine-altering chemicals in the environment that may result in a decrease in male reproductive capability. Asthenozoospermia (AS), or low sperm motility, is a common cause of human male infertility. In one study, more than 80% of the samples from infertile men had low sperm motility 3. Although proteomic studies recently showed that multiple spermatozoa proteins affect sperm motility 4, 5, our knowledge about the impact of post-testicular processes on sperm motility and male infertility is very limited.
Seminal plasma contains secretions that are derived from the testis, epididymis and male accessory glands, including the prostate, seminal vesicles and Cowper's gland. A high concentration of fructose in the seminal plasma serves as a major nutriment and energy source for spermatozoa. In addition, an increasing number of seminal plasma proteins, such as insulin-like growth factor-I, alpha2-macroglobulin and the enkephalin-degrading enzymes, have been shown to be associated with sperm motility 6, 7, 8. Researchers have employed high-throughput techniques to investigate the seminal plasma proteome. Two-dimensional gels and mass spectrometry (MS) were applied to study the role of seminal plasma proteins in impaired spermatogenesis, as early as in 2001, and about 750 spots were detected in the two-dimensional map of seminal plasma from a fertile man 9. Starita-Geribaldi et al. 10 have reported the identification of 61 differentially expressed proteins based on tandem MS analysis of seminal plasma. Pilch and Mann 11 successfully catalogued 932 proteins in seminal plasma using Fourier transform MS and two consecutive stages of MS fragmentation. In an earlier study, we identified 115 proteins from prostatic secretions 12, which are a part of the seminal plasma. However, comparative proteomic analysis of male infertility-associated seminal plasma has not been well documented. An in-depth understanding of the seminal plasma proteome would contribute greatly to the elucidation of the roles of seminal plasma proteins in the regulation of motility and to the establishment of biomarkers for male infertility.
Recent advances in proteomic technology and MS have made them valuable tools for studying seminal plasma. In this study, we identified with high accuracy 741 proteins in AS and control samples, with a false discovery rate (FDR) of 3.3%; 327 of these proteins were not found in Pilch and Mann's dataset 11, which suggests that our list will contribute to the seminal plasma proteome database. Using spectral counting, we found that 45 proteins were threefold upregulated and 56 proteins were threefold downregulated in the AS group compared with the control. Gene ontology (GO) enrichment analysis revealed that the most highly expressed proteins in the AS sample are enriched for metabolic enzymes, particularly those that are involved in proteolysis. Our work sheds light on the interaction of seminal plasma with spermatozoa in the regulation of motility and reveals a rich source of biomarkers for male infertility.
Materials and methods
Participants and sample collection
The study was approved by the institutional review board of the Shanghai Institute of Planned Parenthood Research. All the patients provided written informed consent. The seminal samples were obtained by masturbation into specific sterile containers after 3–5 days of sexual abstinence. After liquefaction of the semen, the sperm parameters (volume, sperm count, percentages of motility and motion characteristics) were evaluated according to WHO guidelines 13 using a computer-assisted semen analyser. Seminal samples in which the liquefaction was not complete within 30 min after masturbation were not selected for this study. The percentage of motile sperm (grades A and B) was 8.7% ± 2.4% from AS patients and 57.4% ± 9.7% from the fertile donors. The density of sperm was higher than 2 × 107 sperm mL−1 in all the samples. No samples from AS patients were selected for this study if the number of sperm with abnormal morphology was higher than 10% of the total. We prepared 38 AS samples and 20 normal control samples. All the patients and control donors were from Shanghai.
Preparation of seminal plasma
Soon after liquefaction, 300 μL of the ejaculate was taken from every sample and vortexed with 3 μL of protease-inhibitor cocktail (Sigma-Aldrich, Saint Louis, MO, USA). The mixture was centrifuged at 2000 × g for 10 min at room temperature. The resulting supernatant was collected and centrifuged for 20 min at 10 000 × g at 4°C. The seminal plasma (supernatant) was collected and stored at −80°C until analysis.
Chemiluminescence measurement of ROS
Chemiluminescence of reactive oxygen species (ROS) was measured using luminol (5-amino-2', 3-dihydro-1',4-phthalazinedione; Sigma) as the probe, according to the World Health Organization (WHO) guidelines 13. First, 4 μL of 25 mmol L−1 luminol in dimethyl sulphoxide and 8 μL horseradish peroxidase (HRP) (total 12.4 units) were added to 400 μL neat semen (freshly provided by donors). The samples were mixed briefly by shaking the tubes. The mixtures were then maintained at 25°C for 5 min, after which the levels of ROS were measured by determining chemiluminescence with a Berthold luminometer (Sirius C2, Berthold Detections Systems, Pforzheim, Germany).
Western blotting assay
Seminal plasma was diluted 1:1 with pre-chilled phosphate-buffered solution containing 1.0% (v/v) protease-inhibitor cocktail (Sigma-Aldrich). The concentration of diluted seminal proteins was measured using the bicinchoninic acid method (Bio-Rad, Hemel Hempstead, UK) using bovine serum albumin as a standard. A total of 50 μg of protein from each sample was loaded on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels. The samples were electrotransferred to nitrocellulose membranes, and nonspecific binding was blocked with 5% non-fat milk in TBST buffer (0.5 mmol L−1 Tris-HCl, 45 mmol L−1 NaCl, 0.05% Tween20 [pH 7.4]). The membranes were incubated at 4°C overnight with DJ-1 antibody (clone 3E8; Stressgen, San Diego, CA, USA) at a 1:3 000 dilution. The membranes were washed with tris-buffered saline tween-20 (TBST) buffer and incubated with HRP-labelled rabbit anti-mouse IgG antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at room temperature for 2 h. The antigen–antibody reaction was visualized using an ECL assay kit and exposed to ECL film (Fijifilm, Tokyo, Japan). Densitometric analysis of the DJ-1 bands on film was carried out using the Gel Image Scan System (Tianren, Shanghai, China).
LC-MS/MS analysis
A total of 100 μg of each sample was separated on 12% SDS polyacrylamide gels. Peptides were extracted with the Pierce In-Gel Trypsin Digestion Kit according to the protocol provided by the manufacturer (Pierce, Rockford, IL, USA). Separation of the resulting tryptic peptide mixtures was carried out with the Ettan MDLC nanoflow/capillary LC system (GE Healthcare, Pittsburgh, PA, USA). The separated peptides were sequenced using LTQ-Orbitrap (Thermo Finnigan, Bremen, Germany) with a nanospray configuration. The precursor ion scan MS spectra (m/z 300– 1 600) was acquired in the Orbitrap with a resolution of R = 60 000 at m/z 400; the number of accumulated ions was 1×106. The five most intense ions were isolated and fragmented in a linear ion trap (number of accumulated ions: 3×104). The resulting fragment ions were recorded in the Orbitrap, with a resolution of R = 15 000 at m/z 400. The spectra were searched against the ipi.HUMAN.v3.29.fasta protein database (70757 entries) using the BioWorks program V3.2 (Thermo Electron Inc., Waltham, MA, USA). The MS/MS data from the AS and control samples were analysed by the PeptideProphet and ProteinProphet program for statistical validation 14. To prioritize the protein expression list, we treated the non-expression protein spectrum as 1. The expression ratio was calculated by dividing the spectral count. Spectral counts are the average results of duplicate LC-MS/MS (liquid chromatography–mass spectrometry) analyses. Enrichment analysis in the GO categories of the differentially expressed genes was carried out using GoMiner 15.
Statistical analysis
All values were represented as the mean ± SD unless otherwise indicated. The unpaired t-test with a P-value of < 0.05 was considered as statistical significance.
Results
We identified 741 proteins with ProteinProphet P-values > 0.5 in the seminal plasma of AS patients and healthy donors (see online supplementary Tables 1 and 2). Using the random database of the ipi.HUMAN.v3.29.fasta as a decoy database, we calculated an FDR of 3.4% for these 741 proteins. We used the spectral counting method 16 for semi-quantitative comparative analysis of the proteome between the AS group and the control group. Spectral counting was shown to be a valid method for quantitative proteomic analysis. Zybailov et al. 17 showed a strong positive correlation between relative abundance ratios derived from spectral counting and from reconstructed peptide ion chromatograms using a 1:1 mix of N14 and N15-labelled proteins. They also showed that spectral counting had the advantage of a larger dynamic range for quantification 17. Using this method for quantification, we identified 45 proteins as overexpressed (≥ threefold) and 56 proteins as underexpressed (≥ threefold) in the AS samples (Supplementary Table 3). Some of these proteins (Table 1) seem to play roles in semen quality, but the biological significance of most of these 101 proteins with respect to human reproduction has not been reported. All of the identified proteins from both the AS and control samples, which have an FDR < 0.05 and > 10 matched proteins, have been grouped into GO categories (Figure 1). On the basis of the GO molecular function categories, there was ≥ 40% enrichment in the catalytic activity (GO:0003824), hydrolase activity (GO:0016787) and enzyme regulator activity (GO:0030234) in the AS seminal plasma.
Table 1. Selected differentially expressed proteins identified in the seminal plasma of AS patients and healthy donors.
| ID | Description | Spectral counts AS/control | Origin | Reference |
|---|---|---|---|---|
| IPI00465439 | Fructose-bisphosphate aldolase A | 64/1 | Prostate | 12 |
| IPI00219018 | Glyceraldehyde-3-phosphate dehydrogenase | 45/1 | Prostate | 12, 36 |
| IPI00293303 | Legumain precursor | 26/1 | Unreported | |
| IPI00291488 | Epididymal secretary protein E4 | 25/1 | Epididymis | 21 |
| IPI00291737 | Intelectin-1 precursor | 14/1 | Unreported | |
| IPI00010314 | Delta-aminolevulinic acid dehydratase | 11/1 | Unreported | |
| IPI00220271 | Alcohol dehydrogenase | 22/4 | Prostate | 12, 36 |
| IPI00022429 | Alpha-1-acid glycoprotein 1 precursor | 34/10 | Prostate | 36 |
| IPI00301579 | Epididymal secretory protein E1 precursor | 71/21 | Epididymis | 21 |
| IPI00291262 | Clusterin precursor | 443/125 | Epididymis, prostate | 5, 12, 24, 36 |
| IPI00478003 | Alpha-2-macroglobulin precursor | 1/12 | Prostate | 6, 36 |
| IPI00298547 | Protein DJ-1 | 1/16 | Testis, epididymis | 12, 35, 36 |
| IPI00026216 | Puromycin-sensitive aminopeptidase | 4/27 | Prostate | 12, 36 |
| IPI00018953 | Dipeptidyl peptidase 4 | 12/72 | Prostate | 12, 22, 36 |
| IPI00221224 | Aminopeptidase N | 37/142 | Prostate | 12, 36 |
Abbreviation: AS, asthenozoospermia/asthenozoospermic
Figure 1.
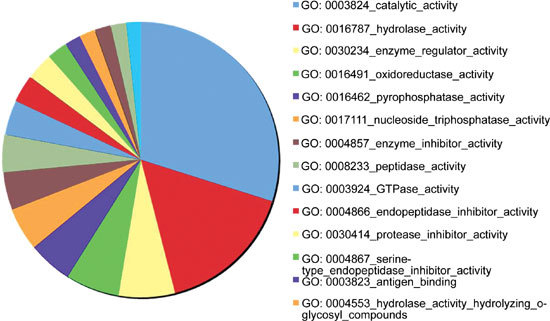
GoMiner analysis of the enriched GO Molecular Function terms. Genes only with a false discovery rate < 0.05 and with > 10 matched proteins are shown in the graph.
The 10 most overexpressed proteins in the AS samples were fructose-bisphosphate aldolase A (ALDOA), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), the IGKC protein, isoform M2 of the pyruvate kinase isozymes M1/M2, the putative uncharacterized protein DKFZp686C11235, cathepsin H, L-lactate dehydrogenase B chain, legumain precursor and epididymal-secretory protein E4 (Table 1 and online supplementary Table 1). The 10 most underexpressed proteins in the AS samples were the four laminin subunit alpha-5 precursor, type I cytoskeletal 9, rab GDP dissociation inhibitor beta, annexin VI isoform 2, attractin precursor isoform 2, alpha-actinin-4, nesprin-2 isoform 1, transitional endoplasmic reticulum ATPase, the SNC66 protein and the alpha-N-acetylglucosaminidase precursor. The data suggest that multiple seminal proteins are associated with AS.
These abnormally expressed proteins are potential biomarkers for AS, and thus need to be further validated. In our dataset, DJ-1 ranks as the most downregulated protein in the AS samples (Table 1). We evaluated the expression difference of DJ-1 between AS patients and healthy donors using immunoblot analysis. The levels of DJ-1 varied significantly among individuals (data not shown). Therefore, we randomly selected eight AS samples and eight control samples, and mixed two samples of the same group to create a new 'pooled' sample with equal amounts of proteins before loading them for western blot analysis. As shown in Figure 2, seminal plasma DJ-1 was detected as a single band that had a molecular weight at 20 kD. The levels of DJ-1 in all four pooled control samples were obviously higher than those in the three pooled AS samples. We next measured the intensity of the bands using densitometric analysis. The average intensity of DJ-1 in these eight AS samples was 56% lesser than that in the eight control samples. In addition, the average intensity of DJ-1 in all 38 AS samples was 49.1% lesser than that in all 20 control samples. These data indicated that the levels of DJ-1 were significantly lower in AS seminal plasma than in the control seminal plasma.
Figure 2.
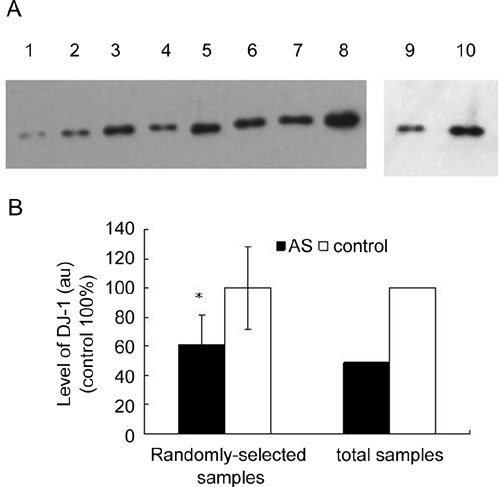
Levels of DJ-1 in seminal plasma, as analysed by immunoblot. (A): 50 μg of seminal plasma protein from asthenozoospermia(AS) patients and healthy donors was loaded onto each lane. Each sample loaded onto lanes 1–4 and lanes 5–8 was a mixture of two randomly selected seminal samples with the same amount of protein from AS patients and healthy donors, respectively. The mixtures of seminal plasma with the same amounts of protein from 38 AS patients and 20 normal controls were loaded onto lanes 9 and 10, respectively. These samples were subjected to immunoblot analysis with the primary antibody against DJ-1 after being separated in SDS-PAGE. (B): The above band intensity was measured using the Gel Imagine Scan System. The density was expressed as a percentage of that of the control group. *P < 0.05, compared with the corrresponding control. au: arbitrary units.
DJ-1 has been shown to have the capacity to reduce oxidative stress (OS) 18. The reduced levels of DJ-1 suggest the presence of OS in AS seminal samples. We next measured the levels of ROS in neat semen from AS and control samples in the presence of seminal antioxidant protection. As shown in Figure 3, levels of ROS in the AS samples were 3.3-fold higher than those in the control, indicating the presence of OS in the semen of the AS patients.
Figure 3.
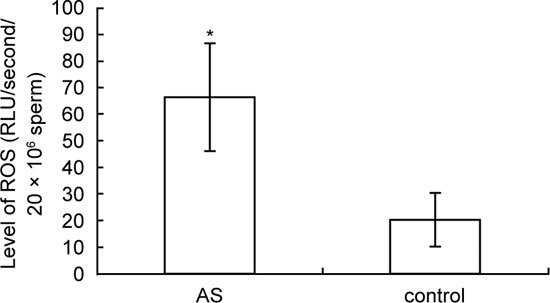
Levels of reactive oxygen species (ROS) in the semen of asthenozoospermia (AS) patients (n = 38) and healthy donors (n = 20). *P < 0.01.
Discussion
Proteome of AS seminal plasma
In this study, we reported the identification of 741 proteins with a ProteinProphet P value >0.5 in the seminal plasma of AS patients and healthy donors. Pilch and Mann 11 recently presented a dataset of 923 seminal plasma proteins. Although 56% of the proteins identified in our work were also present in their dataset (data not shown), 327 proteins (35.5%) in our dataset had not been reported earlier 11. In proteomics analyses, different database search algorithms can result in varied protein identification, including differences in quality and quantity 19. Combining search methods could enhance the confidence of peptide identification. Therefore, the discrepancy between our data and the published data 11 could be partly due to the use of different database search software (SEQUEST vs. Mascot). In addition, we used the Trans-Proteomic pipeline, which includes PeptideProphet and ProteinProphet 14, to statistically validate the data, thereby filtering out many low-probability proteins and enhancing the accuracy of the data.
Contribution of functional abnormality of prostate and epididymis to AS
Seminal plasma is a mixture of secretions from the testis, epididymis and the male accessory glands, including the prostate, seminal vesicles and Cowper's gland. The majority of seminal proteins, such as Serpin, protein C inhibitor, seminogelins I and II, and nitric oxide synthetase, are secreted from the seminal vesicles 20. Although in this study we failed to identify nitric oxide synthetase, the other four proteins were detectable at abundant levels in the seminal plasma. The quantity of these four known seminal proteins was not significantly different in the AS seminal plasma (data not shown). Mucin is accepted as the protein that is characteristic of Cowper's gland secretions 11, and its expression was also determined not to differ significantly between the AS and normal control seminal plasma (data not shown). These results suggest that the proteins secreted from the seminal vesicles and Cowper's gland do not seem to be closely related to the pathogenesis of AS. However, the quantities of two epididymis-secreted proteins—epididymal secretary protein E1 and epididymal secretary protein E4 21—were increased in AS seminal plasma (Table 1). These data could imply a relationship between AS and abnormal epididymal maturation of sperm. In addition, we found that the levels of a few prostate-secreted proteins 12, such as alpha 2-macroglobulin, ALDOA, GAPDH, clusterin and dipeptidyl peptidase IV, were reduced in the AS samples. In addition, most of the abnormally expressed proteins shown in Table 1 originate in the prostate. These findings show that functional abnormality of the prostate plays a key role in the pathogenesis of AS. Our proteomic data on AS indicate a profound impact of the epididymis and prostate on sperm quality during the post-testicular process.
Prostasomes have been reported to be with fused human spermatozoa, and such a fusion can promote sperm motility and prevent premature acrosome reactions22. Dipeptidyl peptidase IV, a prostasome-bound protein, was observed to be transferred to the spermatozoa through this fusion 23. In addition, clusterin seems to be a biomarker of human sperm with low quality, as an increased quantity of clusterin was detected not only in AS spermatozoa 6 but also in the prostasomes of infertile men 24 and in AS seminal plasma (Table 1). It is reasonable to speculate, therefore, that overexpression of AS spermatozoa-bound clusterin may be a result of the increased shuttling of seminal plasma clusterin molecules to spermatozoa by fusion. Whether other prostasome-bound proteins, such as ALDOA and GAPDH, can affect the quality of sperm through similar mechanisms remains to be further studied.
Potential new markers for OS in seminal plasma
Seminal OS can damage sperm by different mechanisms, and is a common pathology seen in two-thirds of infertile cases 25, 26. Agarwal et al. 27 recently reported that high levels of ROS could be an independent marker of male-factor infertility. We also detected a 3.3-fold increase in the level of ROS in AS semen. Our AS seminal proteome has suggested several ways by which seminal OS could be produced. The first is microbial infection—infection of the genital tract has been shown to produce seminal OS 28. Given that intelectin-1 is an infection-induced antimicrobial protein 29, its overexpression in the AS seminal plasma (Table 1) indicates the presence of genital tract infection in the AS patients 29. The second way involves alcohol consumption. We detected a higher quantity of alcohol dehydrogenase, the key enzyme for human alcohol metabolism, in AS seminal plasma. Excessive alcohol consumption causes an increase in testicular OS, as ethanol stimulates the production of ROS 30. These data suggest that there is a link between alcohol intake and AS. Finally, environmental contamination of metals, such as lead, has been shown to raise seminal levels of ROS, induce the upregulation of delta-aminolevulinic acid dehydratase (ALAD) and cause poor semen parameters 31. ALAD catalyses the second step of haem synthesis; its presence in semen was first identified in a proteomic study of seminal plasma 11 and was further confirmed in this study. Seminal ALAD may originate from blood. Our data suggested that these AS patients had been exposed to reproductive toxicants in the environment. Consistent with earlier published data, our findings suggest that seminal OS is a common downstream event that can be caused by multiple pathogenic mechanisms.
The presence of OS in AS semen was partially the result of a functional abnormality of the ROS-degrading capability in the male genital tract 25, 26. DJ-1 has been shown to fight against OS caused by environmental pollutants, such as endocrine-disrupting chemicals 32, and to protect neurons against OS and cell death 33. SP221 or CAP1, rat homologues of human DJ-1, were identified as key proteins related to infertility in male rats that have been exposed to sperm toxicants, such as ornidazole and epichlorohydrin 34. Seminal plasma DJ-1is secreted from testis, epididymis 35 and prostate 12, 36. In this study, the levels of DJ-1 were significantly lower in AS seminal plasma than in that from healthy donors. The data, together with the observation that the levels of ROS were 3.3-fold higher in the AS patient samples, strongly suggested that downregulation of the DJ-1 protein could result in increased OS for spermatozoa, which would then affect their viability and/or motility, as is seen in AS.
In summary, we detected 101 differentially expressed proteins in the seminal plasma of AS patients. The association of these proteins with AS indicates a post-testicular regulatory mechanism for sperm motility, which is exerted mainly by the prostate and epididymis. In addition, our data reveal that the pathophysiology of AS includes some infectious and environmental factors. We believe that these data will help the development of new techniques for the clinical diagnosis and treatment of male infertility.
Supplementary Information accompanies the paper on Asian Journal of Andrology website (http://www.nature.com/aja).
Acknowledgments
This work was supported by grants from The National Basic Research Program of China (2006CB504005); the Shanghai Municipal Committee of Science and Technology (07119518 and 08140901700) (RS Li); the Ministry of Science and Technology, China (2006AA02Z4A2) (B Lin); The National Basic Research Program of China (2009CB941704) (D Ma)
Supplementary Information
List of proteins identified in seminal plasma of AS patients
List of proteins identified in seminal plasma of healthy donors
References
- Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995;332:281–5. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. Br Med J. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curi SM, Ariagno JI, Chenlo PH, Mendeluk GR, Pugliese MN, et al. Asthenozoospermia: analysis of a large population. Arch Androl. 2003;49:343–9. doi: 10.1080/01485010390219656. [DOI] [PubMed] [Google Scholar]
- Martinez-Heredia J, de Mateo S, Vidal-Taboada JM, Ballesca JL, Oliva R. Identification of proteomic differences in asthenozoospermic sperm samples. Human Reprod. 2008;23:783–91. doi: 10.1093/humrep/den024. [DOI] [PubMed] [Google Scholar]
- Zhao C, Huo R, Wang FQ, Lin M, Zhou ZM, et al. Identification of several proteins involved in regulation of sperm motility by proteomic analysis. Fert Steril. 2007;87:436–8. doi: 10.1016/j.fertnstert.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Glander HJ, Kratzsch J, Weisbrich C, Birkenmeier G. Insulin-like growth factor-I and alpha 2-macroglobulin in seminal plasma correlate with semen quality. Human Reprod. 1996;11:2454–60. doi: 10.1093/oxfordjournals.humrep.a019136. [DOI] [PubMed] [Google Scholar]
- Razusta J, Valdivia A, Fernandez D, Agirregoitia E, Ochoa C, et al. Enkephalin-degrading enzymes in normal and subfertile human semen. J Androl. 2004;25:733–9. doi: 10.1002/j.1939-4640.2004.tb02848.x. [DOI] [PubMed] [Google Scholar]
- Wennemuth G, Schiemann PJ, Krause W, Gressner AM, Aumüller G. Influence of fibronectin on the motility of human spermatozoa. Int J Androl. 1997;20:10–6. doi: 10.1046/j.1365-2605.1997.00005.x. [DOI] [PubMed] [Google Scholar]
- Starita-Geribaldi M, Poggioli S, Zucchini M, Garin J, Chevallier D, et al. Mapping of seminal plasma proteins by twodimensional gel electrophoresis in men with normal and impaired spermatogenesis. Mol Hum Reprod. 2001;7:715–22. doi: 10.1093/molehr/7.8.715. [DOI] [PubMed] [Google Scholar]
- Starita-Geribaldi M, Roux F, Garin J, Chevallier D, Fenichel P, et al. Development of narrow immobilized pH gradients covering one pH unit for human seminal plasma proteomic analysis. Proteomics. 2003;3:1611–9. doi: 10.1002/pmic.200300493. [DOI] [PubMed] [Google Scholar]
- Pilch B, Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006;7:R40. doi: 10.1186/gb-2006-7-5-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Guo Y, Han BM, Yan X, Utleg AG, et al. Proteomics cataloging analysis of human expressed prostatic secretions reveals rich source of biomarker candidates. Proteomics Clin Appl. 2008;2:543–55. doi: 10.1002/prca.200780159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for Examination of human semen and Semen–Cervical Mucus InteractionCambridge, UK: Cambridge University Press; 1999
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–58. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- Zeeberg BR, Feng W, Wang G, Wang MD, Fojo AT, et al. GoMiner: a resource for biological interpretation of genomic and proteomic data. Genome Biol. 2003;4:R28. doi: 10.1186/gb-2003-4-4-r28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Sadygov RG, Yates III JR. A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;76:4193–201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- Zybailov B, Coleman MK, Florens L, Washburn MP. Correlation of relative abundance ratios derived from peptide ion chromatograms and spectrum counting for quantitative proteomic analysis using stable isotope labeling. Anal Chem. 2005;77:6218–24. doi: 10.1021/ac050846r. [DOI] [PubMed] [Google Scholar]
- Tara T, Saito Y, Niki T, Iguchi-Agriga SM, Takahashi K, et al. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–8. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves G, Wu WW, Wang G, Shen RF, Yu YK. Enhancing peptide identification confidence by combining search methods. J Proteome Res. 2008;7:3102–13. doi: 10.1021/pr700798h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales GF. Function of seminal vesicles and their role on male fertility. Asian J Androl. 2001;3:251–8. [PubMed] [Google Scholar]
- Kirchhoff C. Molecular characterization of epididymal proteins. Rev Reprod. 1998;3:86–95. doi: 10.1530/ror.0.0030086. [DOI] [PubMed] [Google Scholar]
- Burden HP, Holmes CH, Persad R, Whittington K. Prostasomes--their effects on human male reproduction and fertility. Hum Reprod Update. 2006;12:283–92. doi: 10.1093/humupd/dmi052. [DOI] [PubMed] [Google Scholar]
- Arienti G, Polci A, Carlini E, Palmerini CA. Transfer of CD26/dipeptidyl peptidase IV from prostasomes to sperm. FEBS Lett. 1997;410:343–6. doi: 10.1016/s0014-5793(97)00655-8. [DOI] [PubMed] [Google Scholar]
- Carlsson L, Ronquist G, Nilsson BO, Larsson A. Dominant prostasome immunogens for sperm-agglutinating autoantibodies of infertile men. J Androl. 2004;25:699–705. doi: 10.1002/j.1939-4640.2004.tb02844.x. [DOI] [PubMed] [Google Scholar]
- Whittington K, Harrison SC, Williams KM, Day JL, McLaughlin EA, et al. Reactive oxygen species (ROS) production and the outcome of diagnostic tests of sperm Function. Int J Androl. 1999;22:236–42. doi: 10.1046/j.1365-2605.1999.00174.x. [DOI] [PubMed] [Google Scholar]
- Tremellen K. Oxidative stress and male infertility--a clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Sharma RK, Nallella KP, Thomas AJ, Jr, Alvarez JG, et al. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril. 2006;86:878–85. doi: 10.1016/j.fertnstert.2006.02.111. [DOI] [PubMed] [Google Scholar]
- Potts JM, Pasqualotto FF. Seminal oxidative stress in patients with chronic prostatitis. Andrologia. 2003;35:304–8. [PubMed] [Google Scholar]
- Tsuji S, Uehori J, Matsumoto M, Suzuki Y, Matsuhisa A, et al. Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J Biol Chem. 2001;276:23456–63. doi: 10.1074/jbc.M103162200. [DOI] [PubMed] [Google Scholar]
- Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27:277–84. [PMC free article] [PubMed] [Google Scholar]
- Telisman S, Colak B, Pizent A, Jurasović J, Cvitković P. Reproductive toxicity of low-level lead exposure in men. Environ Res. 2007;105:256–66. doi: 10.1016/j.envres.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Ooe H, Taira T, Iguchi-Ariga SM, Ariga H. Induction of reactive oxygen species by bisphenol A and abrogation of bisphenol A-induced cell injury by DJ-1. Toxicol Sci. 2005;88:114–26. doi: 10.1093/toxsci/kfi278. [DOI] [PubMed] [Google Scholar]
- Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, et al. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA. 2004;101:9103–8. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenfeld A, Gromoll J, Cooper TG. Molecular cloning and expression of rat contraception associated protein 1 (CAP1), a protein putatively involved in fertilization. Biochem Biophys Res Commun. 1998;251:545–9. doi: 10.1006/bbrc.1998.9512. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Sato Y, Yoshiike M, Nozawa S, Ariga H, et al. Immunocytochemical localization of DJ-1 in human male reproductive tissue. Mol Reprod Dev. 2003;66:391–7. doi: 10.1002/mrd.10360. [DOI] [PubMed] [Google Scholar]
- Utleg AG, Yi EC, Xie T, Shannon P, White JT, et al. Proteomic analysis of human prostasomes. Prostate. 2003;56:150–61. doi: 10.1002/pros.10255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of proteins identified in seminal plasma of AS patients
List of proteins identified in seminal plasma of healthy donors


