Abstract
We investigated the potential value of prostate-specific antigen half-life (PSAHL) and decreasing velocity (PSAVd) to predict progression-free survival (PFS) and overall survival (OS) in Chinese patients with prostate cancer. A total of 153 patients treated with hormonal therapy were included in the study. Of these, 78 patients progressed to hormone-refractory prostate cancer (HRPC) and 24 patients died by the end of follow-up. PSAHL was defined as the time during which prostate-specific antigen (PSA) concentration became half of the initial value during the first hormonal therapy. PSAVd reflected the decreasing velocity of PSA during the first hormonal therapy. PFS was defined as the interval from the beginning of hormonal therapy to HRPC. Cox proportional hazards regression analysis was used to evaluate whether PSAHL and PSAVd were significantly associated with PFS and OS. The median PSAHL and PSAVd were 0.50 months and 33.8 ng mL−1 per month. The median PFS and OS were 22.7 months (95% confidence interval [CI], 22.0–29.6 months) and 43.5 months (95% CI, 37.9–48.4 months), respectively. On univariate and multivariate analysis, long PSAHL (> 0.5 months), metastatic disease, high biopsy Gleason scores (≥ 8) and high nadir PSA (> 0.4 ng mL−1) were all found to be significantly associated with short PFS. Long PSAHL, high nadir PSA and short PSA doubling time (PSADT ≤ 2.0 months) were significantly associated with short OS. There were no significant relationships between PSAVd and either PFS or OS. Thus, PSAHL is a promising new independent predictor of survival. Patients with long PSAHL were identified as those at high risk for a relatively short PFS and OS.
Keywords: predictor, prognosis, prostate cancer, prostate-specific antigen decreasing velocity, prostate-specific antigen half-life
Introduction
Prostate cancer is one of the most common cancers in the world. In China, the incidence of prostate cancer has been increasing sharply for a decade, and more than 50% of patients with new diagnoses have advanced stages of prostate cancer. Although various palliative therapies 1, 2, 3 are available, hormonal therapy is the most important therapy. Although almost all prostate cancers are initially hormone-sensitive, the duration of responsiveness to hormonal therapy is heterogeneous, with some patients progressing to hormone-refractory prostate cancer (HRPC) after more than 3 years of therapy, whereas others progress to HRPC within a few months. After the progression to HRPC stage, the disease quickly spreads. Reliable treatment strategies available are limited. An effective treatment option for patients with HRPC is docetaxel with prednisone every three weeks; this treatment has been shown to improve the quality of life (QoL) and to prolong the survival rates 4. However, the overall survival (OS) is still poor for most of the patients.
Thus, predictors of disease progression and survival are important for urologists in making prognoses and in selecting the therapy that would provide the greatest benefit to the patient. In earlier studies, metastatic disease has been identified as a predictor of prostate cancer progression 3, and the kinetic parameters of serum prostate-specific antigen (PSA), including PSA doubling time (PSADT) and PSA increasing velocity (PSAV), have also become reliable surrogate markers for prognosis 5, 6. Schulman et al. 5 reported that the PSADT could predict the duration of responsiveness to deferred anti-androgen therapy. In advanced prostate cancer, a PSAV of > 1.5 ng mL−1 per year was associated with significant increase in prostate cancer-specific mortality 6. Both of these reflect increase of PSA and indicate the progression of the disease. Recently, a new kinetic parameter, 'PSA half-life (PSAHL)', which represents the decrease of PSA levels, was reported to be predictive of prognoses. A PSAHL of 1 month was considered a surrogate marker for responsiveness to chemotherapy 7. But the value of this kinetic parameter in predicting progression-free survival (PFS) and OS was not clear. In this study, we investigated whether PSAHL and PSA decreasing velocity (PSAVd) were predictors of survival outcomes in Chinese prostate cancer patients.
Materials and methods
Patient selection
We included 153 patients who had prostate adenocarcinomas confirmed by prostate biopsy using the 10-core strategy 8 at one center in Shanghai, China, from May 2002 to December 2007. When prostate cancer was diagnosed, computed tomography (CT), magnetic resonance imaging (MRI) and bone scans were performed. The tumor–node–metastasis (TNM) clinical staging, according to AJCC guidelines, was performed on the basis of biopsy, clinical or radiological evidence. In these patients, the maximal androgen blockade (MAB) was the only treatment strategy used from the time of primary diagnosis until the disease progressed to HRPC stage. Patients taking other palliative therapies (such as radiotherapy, isotope treatment) were excluded. MAB included the use of luteinizing hormone-releasing hormone agonist (LH-RHa) or surgical castration combined with anti-androgen therapy (flutamide). The usage and dosage were prescribed on the basis of the European Association of Urology (EAU) guidelines of 2007. After the first hormonal therapy failed, the disease had become an anti-androgen-independent prostate cancer, and a secondary hormonal therapy, including ketoconazole, estrogen or any another anti-androgen (usually bicalutamide), was given. The anti-androgen was withdrawn for 4–6 weeks if the PSA continued to increase. Serum testosterone was < 50 ng dL−1.
All patients received serum PSA measurements every 1–3 months. Radiological examinations were carried out if needed. After an average follow-up of 34 months (range 2–101 months), 51% (78/153) of patients had progressed to HRPC (The definition of HRPC was based on the EAU guidelines of 2007). Chemotherapy with docetaxel or mitoxantrone and prednisone every 3 weeks were used for HRPC, according to the literature 4.
Definitions and calculations of PSAHL, PSAVd, PSADT and PSAV
PSAHL was defined as the time required for the PSA concentration to decline by exactly one-half during the first hormonal therapy, and was calculated from the slope of the linear regression of the natural logarithm values of PSA versus time. The equation used was PSAHL = −ΔT × log(2)/[log(PSAnadir)−log(PSAinitial)] 7, in which 'PSA'initial and 'PSA'nadir are the initial PSA and nadir PSA during the first hormonal therapy, ΔT is the time interval between them, and 'log' refers to the natural logarithm. If the nadir PSA value was zero or undetected, the nearest measurement of a non-zero value before it was used. If patients could not reach a nadir of PSA, the last follow-up PSA was used.
The PSAVd reflected the decreasing velocity during the first hormonal therapy and was calculated from the change in serum PSA over time, using the equation: PSAVd = −(PSAnadir−PSAinitial)/ΔT. PSAinitial, PSAnadir and ΔT were defined in the same way as in PSAHL's equation. It was similar to the formula for PSAV 9, 10, but the PSAV reflected an increasing tendency whereas the PSAVd represented a decreasing tendency. Two or more determinations between the PSAinitial and the PSAnadir were required to capture a sufficiently long time interval 11.
PSADT and PSAV were defined as factors indicating increasing PSA at the beginning of HRPC. The PSADT was calculated as the natural log of 2 divided by the slope of the relationship between the log of PSA and time of PSA measurement 12. The PSAV was calculated from the slope of the linear regression of PSA values over time 13. For PSADT and PSAV, the beginning point was the first PSA value in HRPC stage, and the end point was the last measurement of PSA during follow-up or before death.
The end point of this study
The first end point of this study was the interval from the first hormonal therapy to HRPC (defined as PFS). The second end point was the OS, which was the duration from the first hormonal therapy to death.
Statistical analysis
The data collected included age, biopsy Gleason score of primary tumor, TNM clinical stage, method of hormonal therapy, chemotherapy and all available PSA values during the routine follow-up visits. All of the information was gathered from patients' clinical records.
The relationships between patients' characteristics and their PSAHL and PSAVd were analyzed using Spearman's rank correlations. In survival analysis, PSAHL was categorized using different cutoff points. The cutoff points ranged from 0.4 to 0.7 month (which corresponded to the 25th percentile and 75th percentile, respectively, of the PSAHL range), and various cutoff points that differed by 0.05 month were used. A PSAHL of 0.5 month was considered the optimal cutoff point, because it had the greatest prognostic value. Using a similar method, the cutoff values of 20 ng mL−1 per month, 0.4 ng mL−1, 2 months and 5.0 ng mL−1 per month were chosen for PSAVd, nadir PSA, PSADT and PSAV, respectively. PFS and OS were estimated using the Kaplan–Meier method. Prognostic factors were assessed using Cox proportional hazards models. The computations were performed using both SPSS 12.0 (SPSS Inc., Chicago, IL, USA) and Stata 8.0 (Stata Corp., College Station, TX, USA). A two-sided P-value of < 0.05 was considered statistically significant.
Results
Patients' characteristics
The clinical and pathological characteristics of the 153 patients are shown in Table 1. At the end of the follow-up, 100 patients had been treated with secondary hormonal therapy, of which 78 patients had progressed to HRPC and 64 patients were treated with chemotherapy (33 with docetaxel and 31 with mitoxantrone, with prednisone). Of the patients with HRPC, 30.8% (24/78) died. The remaining patients were still responsive to the first hormonal therapy. For the clinical stage, 124 patients (81%) were in stage IV, three patients (2%) in stage III and 26 patients (17%) in stage II. The medians and ranges of PSA kinetic parameters, including PSAHL, PSAVd, PSADT and PSAV, are listed in Table 1.
Table 1. Clinical characteristics and PSA kinetic parameters of the patients.
| No. of patients | Median (range) | |
|---|---|---|
| Age at diagnosis(years) | 68.0 (44.7–85.8) | |
| Initial PSA (ng mL−1) | 100.0 (7.0–5570.0) | |
| T stage | ||
| T2 | 85 (56) | |
| T3 | 3 (2) | |
| T4 | 20 (13) | |
| Tx | 45 (29) | |
| N stage | ||
| N0 | 68 (44) | |
| N1 | 29 (19) | |
| Nx | 56 (37) | |
| M stage | ||
| M0 | 40 (26) | |
| M1 | 106 (69) | |
| Mx | 7 (5) | |
| Biopsy Gleason score | ||
| ≤ 6 | 22 (14) | |
| 7 | 37 (24) | |
| 8 | 30 (20) | |
| 9 | 14 (9) | |
| 10 | 5 (3) | |
| Unknown | 45 (29) | |
| Castration method | ||
| LH-RHa | 80 (52) | |
| Surgical | 73 (48) | |
| PSAVd (ng mL−1 per month) | 33.8 (1.3–2518.9) | |
| PSAHL (months) | 0.5 (0.1–34.2) | |
| Nadir PSA (ng mL−1) | 0.4 (0.0–69.4) | |
| First PSA at HRPC (ng mL−1) | 10.2 (0.0–268.0) | |
| PSADT (months) | 1.9 (0.2–27.5) | |
| PSAV (ng mL−1 per month) | 7.4 (0.1–341.5) | |
| Chemotherapy | ||
| Docetaxel | 33 (52) | |
| Mitoxantrone | 31 (48) | |
Abbreviations: HRPC, hormone-refractory prostate cancer; LH-RHa, luteinizing hormone-releasing hormone agonist; PSA, prostate-specific antigen; PSADT, prostate specific antigen doubling time; PSAHL, prostate-specific antigen half-life during the first hormonal therapy; PSAV, prostate-specific antigen increasing velocity at HRPC; PSAVd, prostate-specific antigen decreasing velocity during the first hormonal therapy.
Relationship between clinical characteristics and the PSAHL and PSAVd
PSAHL was correlated with the nadir PSA and the first PSA at HRPC (Spearman's coefficients were 0.491 and 0.367, respectively, P < 0.05, Table 2). There were no significant relationships between PSAHL and TNM stage, biopsy Gleason score, initial PSA, PSADT or PSAV. The PSAVd was correlated with T stage, N stage, initial PSA and nadir PSA (Spearman's coefficients were 0.298, 0.318, 0.622 and 0.295, respectively, P < 0.05, Table 2). We also found that there was no significant correlation between the PSAHL and the PSAVd (Spearman's coefficient was −0.060; P = 0.494).
Table 2. Relationship between the selected variables and PSAHL and PSAVd.
| PSAHL |
PSAVd |
|||
|---|---|---|---|---|
| Spearman's coefficient | P-value | Spearman's coefficient | P-value | |
| T stage | −0.027 | 0.792 | 0.298 | 0.003 |
| N stage | −0.131 | 0.212 | 0.318 | 0.002 |
| M stage | 0.146 | 0.096 | 0.138 | 0.115 |
| Biopsy Gleason score | −0.165 | 0.107 | 0.115 | 0.264 |
| Initial PSA | −0.016 | 0.857 | 0.822 | < 0.001 |
| Nadir PSA | 0.491 | < 0.001 | 0.295 | 0.001 |
| First PSA at HRPC | 0.367 | 0.002 | −0.187 | 0.130 |
| PSADT | −0.046 | 0.718 | −0.141 | 0.263 |
| PSAV | −0.324 | 0.216 | 0.342 | 0.347 |
Abbreviations: HRPC, hormone-refractory prostate cancer; PSA, prostate-specific antigen; PSADT, prostate-specific antigen doubling time; PSAHL, prostate-specific antigen half-life during the first hormonal therapy; PSAV, prostate-specific antigen increasing velocity at HRPC; PSAVd, prostate-specific antigen decreasing velocity during the first hormonal therapy.
Analysis of the predictors for PFS
The median PFS was 22.7 months (mean, 25.8 months; 95% confidence interval [CI], 22.0–29.6 months; range, 4.0–82.8 months). In a univariate analysis, a PSAHL of > 0.5 months was significantly associated with shorter PFS compared with a PSAHL of ≤ 0.5 months; the median PFS was 17.2 months (95% CI, 16.4–24.0 months) and 24.6 months (95% CI, 22.0–36.9 months), respectively (P < 0.001, curves for PFS are illustrated in Figure 1). Metastatic disease, high biopsy Gleason score and high nadir PSA were also associated with poor PFS. However, PSAVd was not positively correlated with PFS (P = 0.163). On the multivariate Cox analysis, the four factors listed above were found to be significant predictors of a short PFS (Table 3).
Figure 1.
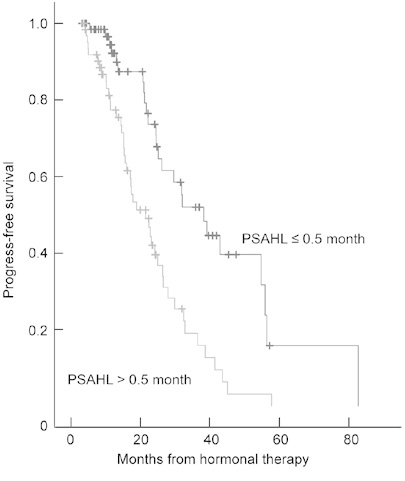
Progression-free survival (PFS, which was defined as the time interval from hormonal therapy to hormone-refractory prostate cancer) according to prostate-specific antigen half-life (PSAHL) during the first hormonal therapy. Differences in survival were tested by the log-rank test (P = 0.0001).
Table 3. Cox proportional hazards analysis of various clinical and pathological characteristics and PSA kinetic parameters in predicting PFS.
| Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| P-value | HR (95% CI) | P-value | HR (95% CI) | ||
| Age at diagnosis | ≤ 70 vs. > 70 | 0.297 | 1.28 (0.80–2.06) | ||
| Initial PSA | ≤ 100 vs. > 100 | 0.848 | 1.05 (0.66–1.67) | ||
| T stage | T1+T2 vs. T3+T4 | 0.617 | 0.82 (0.38–1.77) | ||
| N stage | N0 vs. N1 | 0.813 | 1.09 (0.55–2.16) | ||
| M stage | M0 vs. M1 | 0.002 | 2.53 (1.41–4.56) | 0.005 | 3.84 (1.49–9.91) |
| Biopsy Gleason score | < 8 vs. ≥ 8 | 0.036 | 1.89 (1.04–3.42) | 0.009 | 2.51 (1.26-4.98) |
| Castration method | LH-RHa vs. surgical | 0.206 | 0.69 (0.39–1.22) | ||
| PSAVd | ≤ 20 vs. > 20 | 0.163 | 1.47 (0.86−2.52) | ||
| PSAHL | ≤ 0.5 vs. > 0.5 | < 0.001 | 2.71 (1.63–4.51) | 0.014 | 2.47 (1.20–5.06) |
| Nadir PSA | ≤ 0.4 vs. > 0.4 | 0.008 | 2.11 (1.22–3.66) | 0.002 | 3.06 (1.51–6.22) |
Abbreviations: CI, confidence interval; HR, hazard ratio; LH-RHa, luteinizing hormone-releasing hormone agonist; PFS, progression-free survival; PSA, prostate-specific antigen; PSAHL, prostate-specific antigen half-life during the first hormonal therapy; PSAVd, prostate-specific antigen decreasing velocity during the first hormonal therapy.
Metastatic disease, high biopsy Gleason score, long PSAHL (> 0.5 month) and high nadir PSA (> 0.4 ng mL−1) were positively correlated with short PFS, P < 0.05.
Analysis of the predictors for OS
PSADT, PSAV, first PSA at HRPC and chemothe-rapy were included for the analysis of OS. The median OS was 43.5 months (mean, 43.2 months; 95% CI, 37.9–48.4 months; range, 19.0–63.0 months). A PSAHL of > 0.5 months, a nadir PSA of > 0.5 ng mL−1 and a PSADT of ≤ 2.0 months had significant associations with poor OS on univariate and multivariate analysis (P < 0.05, Table 4). The OS for patients with a PSAHL of > 0.5 months and ≤ 0.5 months was 43.0 months (95% CI, 35.1–47.2 months) and 48.0 months (95% CI, 39.6–62.0 months), respectively. OS curves are shown in Figure 2. The PSAVd was not a significant predictor of OS (P = 0.980).
Table 4. Cox proportional hazards analysis of various clinical and pathological characteristics and PSA kinetic parameters in predicting OS.
| Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| P-value | HR (95% CI) | P-value | HR (95% CI) | ||
| Age at diagnosis | ≤ 70 vs. > 70 | 0.269 | 0.61 (0.25–1.47) | ||
| Initial PSA | ≤ 100 vs. > 100 | 0.567 | 1.27 (0.56–2.88) | ||
| T stage | T1+T2 vs. T3+T4 | 0.394 | 1.74 (0.49–6.25) | ||
| N stage | N0 vs. N1 | 0.220 | 1.96 (0.67–5.76) | ||
| M stage | M0 vs. M1 | 0.149 | 2.47 (0.72–8.40) | ||
| Biopsy Gleason score | < 8 vs. ≥ 8 | 0.072 | 2.62 (0.92–7.48) | ||
| Castration method | LH-RHa vs. surgical | 0.526 | 0.73 (0.27–1.95) | ||
| PSAVd | ≤ 20 vs. > 20 | 0.980 | 1.01 (0.42–2.44) | ||
| PSAHL | ≤ 0.5 vs. > 0.5 | 0.026 | 3.10 (1.15–8.37) | 0.026 | 3.41 (1.16–10.06) |
| Nadir PSA | ≤ 0.4 vs. > 0.4 | 0.041 | 8.06 (1.09–59.8) | 0.024 | 10.20 (1.37–76.17) |
| First PSA at HRPC | ≤ 10 vs. > 10 | 0.229 | 1.65 (0.73–3.72) | ||
| Chemotherapy | Docetaxel vs. mitoxantrone | 0.092 | 0.48 (0.20–1.13) | ||
| PSAV | ≤ 5.0 vs. > 5.0 | 0.143 | 1.52 (0.81–3.73) | ||
| PSADT | ≤ 2.0 vs. > 2.0 | 0.001 | 5.70 (2.01–15.56) | < 0.001 | 8.12 (2.73–24.16) |
Abbreviations: CI, confidence interval; HR, hazard ratio; HRPC, hormone-refractory prostate cancer; LH-RHa, luteinizing hormone-releasing hormone agonist; OS: overall survival; PSA, prostate-specific antigen; PSADT, prostate specific antigen doubling time; PSAHL, prostate-specific antigen half-life during the first hormonal therapy; PSAV, prostate-specific antigen increasing velocity; PSAVd, prostate-specific antigen decreasing velocity during the first hormonal therapy.
Long PSAHL (> 0.5 months), high nadir PSA (> 0.4 ng mL−1) and short PSADT (≤ 2.0 months) were positively correlated with short OS, P < 0.05.
Figure 2.
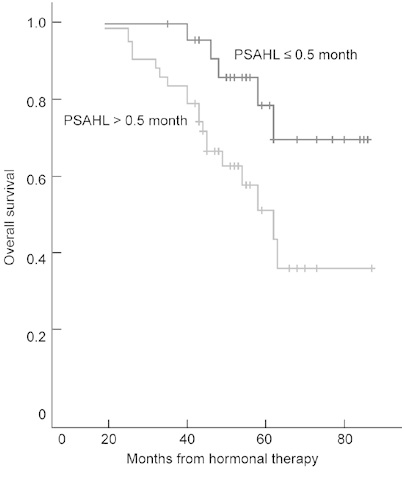
Overall survival (OS) according to prostate-specific antigen half-life (PSAHL) during the first hormonal therapy. Differences in survival were tested by the log-rank test (P = 0.026).
Discussion
Many predictors have been identified for prostate cancer in earlier studies. The serum PSA was thought to be correlated with cancer emergence, progression and efficacy of treatment 14, 15, and some scholars proclaimed that the 'PSA era' had begun. However, many studies have found that serum PSA as an end point was unstable and heterogeneous for evaluating prognoses 16, 17, 18. To improve the value of prediction, kinetic parameters based on PSA were developed that could more precisely reflect biological characteristics 7, 13, 19, 20. PSADT and PSAV, as kinetic parameters, have been shown to be predictors of prognosis in many retrospective studies 21, 22, 23. For patients with radical prostatectomy, a PSAV of > 3.4 ng mL−1 per year was positively correlated with increased disease progression and death from prostate cancer 21. A PSADT of < 3 months has also been shown to predict the OS 22. PSADT also predicted whether the recurrence was local or systemic; men with a long PSADT (≥ 6 months) were more likely to have a local recurrence, whereas men with a short PSADT (< 6 months) were more likely to have a systemic recurrence 23.
These parameters focused on increase in serum PSA, which indicated disease progression. After specific therapies, serum PSA tends to decrease. Many studies have shown that PSA decline of > 50% is associated with survival for HRPC, and thus it became the end point for responses to therapies 24, 25. In particular, the PSAHL was shown to be a significant predictor of disease recurrence and survival 7, 26. Banu et al. 7 showed that PSAHL dynamics predicted OS. They defined three response categories according to PSAHL dynamics: responders (patients in whom PSAHL decreased initially and then increased), late progressors (patients in whom PSAHL continued to increase) and initial progressors (patients in whom PSAHL was decreased initially and then continued to decrease), and the OS was 27, 19.7 and 12.3 months, respectively (P = 0.0001). Patients who were responders exhibited longer survival than those in other categories. However, most reports focused on HRPC, and few studies focused on the decrease in serum PSA during the hormonal sensitive stage. Recently, Park et al. 27 suggested that PSAHL was a prognostic factor for response to androgen deprivation therapy. But the value of the decrease in predicting the outcome was still not clear. In this study, we added 'time' as a factor along with the decline amplitude of PSA during the hormonal therapy and defined two kinetic parameters—PSAHL and PSAVd. We tried to find the relationship between the decreasing status of PSA and the prognosis of prostate cancer.
Serum PSA of all patients in our study decreased after hormonal therapy and the response patterns were different; in some patients serum PSA could be decreased quickly, whereas in others the nadir PSA was reached over a long period of time. Thus, PSAHL was calculated according to the equation given above and the cutoff point of 0.5 month provided the greatest discrimination between the two groups defined by the outcome. The median PFS for patients with short PSAHL (which meant ≤ 0.5 month) was 7 months longer than that for patients with long PSAHL (> 0.5 month). This difference was significant in univariate and multivariate analyses, and the risk of progression for patients with long PSAHL was two times greater than that for patients with short PSAHL, which suggested that long PSAHL during hormonal therapy predicted a short duration of hormonal sensitiveness. For the analysis of OS, the long PSAHL was still a predictor of shorter OS. The survival of patients with long PSAHL was 5 months shorter than that of patients with short PSAHL. The hazard ratio for death for the two groups was 3.41 (95% CI, 1.16–10.06). One possible explanation was that serum PSA fluctuations reflected the change of tumor burden; thus, a short PSAHL might mean a higher percentage of hormonal sensitive cancer cells and a better efficacy of hormonal therapy for these patients.
The positive correlation between the PSAHL and the nadir PSA indicated that the shorter the PSAHL during hormonal therapy, the lower the nadir PSA. The nadir PSA has been considered as a prognostic factor in earlier reports, as well as in our study; therefore, the relationship between the PSAHL and the nadir PSA provided more evidence that the PSAHL was a new predictor of PFS and OS. More importantly, the PSAHL might predict the outcome earlier than the nadir PSA, especially for patients who could not reach the nadir PSA. The PSAHL had a tendency to be associated with metastatic disease, but it was not significant. There was no correlation between the PSAHL and initial PSA, even though the latter was used to calculate the PSAHL.
The PSAVd reflected the absolute decrease in PSA over time. In our study, it was another factor related to the decline in serum PSA; however, univariate and multivariate analyses did not show that it was a significant predictor. Although some studies have reported that PSAV in HRPC was positively correlated with prognosis, PSAVd during hormonal therapy was not as predictive.
In our study, PSADT was another independent predictor of OS for patients with HRPC. Patients with a PSADT of ≤ 2.0 months exhibited poor survival. Our findings were similar to those reported in the earlier literature 28. However, PSAV was not a significant predictor, although there was a tendency for differences in this measure by outcome. One possible reason for this may be the limited numbers of HRPCs (only 78 cases).
The predictive value of the biopsy Gleason score of the primary tumor is controversial. Some studies have reported that patients with high Gleason scores (≥ 8) had poor prognoses 29, but others found that the Gleason scores had limited prognostic value for prostate cancer patients. Our study suggested that the biopsy Gleason score could predict PFS, but not OS.
For patients with HRPC, docetaxel with prednisone every three weeks was the standard chemotherapy, and it prolonged survival 3. In our study, the OS for the docetaxel group was 6 months longer than that of patients in the mitoxantrone group, although this difference was not significant.
Although we were able to provide some important data on the predictive value of certain measures for the prediction of prostate cancer outcomes, this study had a few limitations. For example, for patients with HRPC, this study did not include measures of hemoglobin, which has been considered a prognostic factor, because during data collection, the data on hemoglobin were incomplete. The absence of this data could limit the application of this model in predicting OS, although it would not have influenced the value of the model for prediction of PFS.
In conclusion, for Chinese patients with prostate cancer, the PSAHL may be a new independent predictor of PFS and OS. A PSAHL of ≤ 0.5 months identified men who were at low risk for short PFS and OS. The PSAHL would aid urologists in making better treatment strategies and judging the prognosis of their patients.
Acknowledgments
We thank Dr Yi-Ke Li of Eye and ENT Hospital, Shanghai, China, who read this manuscript and gave some suggestions.
References
- Zacharakis E, Ahmed HU, Ishaq A, Scott R, Illing R, et al. The feasibility and safety of high-intensity focused ultrasound as salvage therapy for recurrent prostate cancer following external beam radiotherapy. BJU Int. 2008;102:786–92. doi: 10.1111/j.1464-410X.2008.07775.x. [DOI] [PubMed] [Google Scholar]
- Kang SH, Kim JW, Bae JH, Park HS, Moon DG, et al. Targeted-cryosurgical ablation of the prostate with androgen deprivation therapy: quality of life in high-risk prostate cancer patients. Asian J Androl. 2006;8:629–36. doi: 10.1111/j.1745-7262.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- Lam JS, Leppert JT, Vemulapalli SN, Shvarts O, Belldegrun AS. Secondary hormonal therapy for advanced prostate cancer. J Urol. 2006;175:27–34. doi: 10.1016/S0022-5347(05)00034-0. [DOI] [PubMed] [Google Scholar]
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- Schulman MJ, Karam JA, Benaim EA. Prostate-specific antigen doubling time predicts response to deferred antiandrogen therapy in men with androgen-independent prostate cancer. Urology. 2004;63:732–6. doi: 10.1016/j.urology.2003.11.016. [DOI] [PubMed] [Google Scholar]
- D'Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, et al. Surrogate end point for prostate cancer specific mortality in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;173:1572–6. doi: 10.1097/01.ju.0000157569.59229.72. [DOI] [PubMed] [Google Scholar]
- Banu E, Banu A, Medioni J, Levy E, Thiounn N, et al. Serum PSA half-life as a predictor of survival for hormone-refractory prostate cancer patients: modelization using a standardized set of response criteria. Prostate. 2007;67:1543–9. doi: 10.1002/pros.20627. [DOI] [PubMed] [Google Scholar]
- Dai B, Ye DW, Kong YY, Shen YJ, Wang BH. Individualized prostate biopsy strategy for Chinese patients with different prostate-specific antigen levels. Asian J Androl. 2008;10:325–31. doi: 10.1111/j.1745-7262.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- Raaijmakers R, Wildhagen MF, Ito K, Pàez A, de Vries SH, et al. Prostate-specific antigen change in the European randomized study of screening for prostate cancer, section Rotterdam. Urology. 2004;63:316–20. doi: 10.1016/j.urology.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Schröder FH, Roobol MJ, van der Kwast TH, Kranse R, Bangma CH. Does PSA velocity predict prostate cancer in pre-screened populations. Eur Urol. 2006;49:460–5. doi: 10.1016/j.eururo.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Connolly D, Black A, Murray LJ, Napolitano G, Gavin A, et al. Methods of calculating prostate-specific antigen velocity. Eur Urol. 2007;52:1044–50. doi: 10.1016/j.eururo.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- Daskivich TJ, Regan MM, Oh WK. Distinct prognostic role of prostate specific antigen doubling time and velocity at emergence of androgen independence in patients treated with chemotherapy. Urology. 2007;70:527–31. doi: 10.1016/j.urology.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Kelly WK, Scher HI, Mazumdar M, Vlamis V, Schwartz M, et al. Prostate-specific antigen as a measure of disease outcome in metastatic hormone-refractory prostate cancer. J Clin Oncol. 1993;11:607–15. doi: 10.1200/JCO.1993.11.4.607. [DOI] [PubMed] [Google Scholar]
- Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- Oudard S, Banu E, Beuzeboc P, Voog E, Dourthe LM, et al. Multicenter randomized phase II study of two schedules of docetaxel, estramustine, and prednisone versus mitoxantrone plus prednisone in patients with metastatic hormone-refractory prostate cancer. J Clin Oncol. 2005;23:3343–51. doi: 10.1200/JCO.2005.12.187. [DOI] [PubMed] [Google Scholar]
- Carducci MA, DeWeese TL, Nelson JB. Prostate-specific antigen and other markers of therapeutic response. Urol Clin North Am. 1999;26:291–302. doi: 10.1016/s0094-0143(05)70069-0. [DOI] [PubMed] [Google Scholar]
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- D'Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125–35. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- D'Amico AV, Renshaw AA, Sussman B, Chen MH. Pretreatment PSA velocity and risk of death from prostate cancer following external beam radiation therapy. JAMA. 2005;294:440–7. doi: 10.1001/jama.294.4.440. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Myers RP, Slezak JM, Bergstralh EJ, Zincke H, et al. Preoperative prostate specific antigen doubling time and velocity are strong and independent predictors of outcomes following radical prostatectomy. J Urol. 2005;174:2191–6. doi: 10.1097/01.ju.0000181209.37013.99. [DOI] [PubMed] [Google Scholar]
- D'Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, et al. Prostate specific antigen doubling time as a surrogate end point for prostate cancer specific mortality following radical prostatectomy or radiation therapy. J Urol. 2004;172:S42–6. doi: 10.1097/01.ju.0000141845.99899.12. [DOI] [PubMed] [Google Scholar]
- Patel A, Dorey F, Franklin J, deKernion JB. Recurrence patterns after radical retropubic prostatectomy: clinical usefulness of prostate specific antigen doubling times and log slope prostate specific antigen. J Urol. 1997;158:1441–5. doi: 10.1016/s0022-5347(01)64238-1. [DOI] [PubMed] [Google Scholar]
- Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the prostate-specific antigen working group. J Clin Oncol. 1999;17:3461–7. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- Scher HI, Mazumdar M, Kelly WK. Clinical trials in relapsed prostate cancer: defining the target. J Natl Cancer Inst. 1996;88:1623–34. doi: 10.1093/jnci/88.22.1623. [DOI] [PubMed] [Google Scholar]
- Chautard D, Cellier P, Dalifard I, Pabot du Chatelard P, Chaussis F, et al. Biochemical monitoring of prostate cancer treated exclusively by radiotherapy: prognostic value of pretreatment PSA, PSA nadir and PSA half-life. Prog Urol. 2002;12:421–8. [PubMed] [Google Scholar]
- Park YH, Park DS, Jeong CW, Ku JH, Kwak C, et al. PSA half life and PSA doubling time as a predictor of response to androgen deprivation therapy for metastatic prostate cancer. J Urol. 2008;179:S185. doi: 10.1016/j.juro.2009.01.104. [DOI] [PubMed] [Google Scholar]
- Tomioka S, Shimbo M, Amiya Y, Nakatsu H, Murakami S, et al. Significance of prostate-specific antigen-doubling time on survival of patients with hormone refractory prostate cancer and bone metastasis: analysis on 56 cases of cancer-specific death. Int J Urol. 2007;14:123–7. doi: 10.1111/j.1442-2042.2007.01672.x. [DOI] [PubMed] [Google Scholar]
- Humphrey PA. Gleason grading and prognostic factors in carcinoma of the prostate. Mod Pathol. 2004;17:292–306. doi: 10.1038/modpathol.3800054. [DOI] [PubMed] [Google Scholar]


