Abstract
Deubiquitinating enzymes (DUBs) play an important role in ubiquitin-dependent processes as negative regulators of protein ubiquitination. Ubiquitin-specific protease 26 (USP26) is a member of this family. The expression of Usp26 in mammalian testis and in other tissues has yet to be fully elucidated. To study the expression of Usp26 mRNA and protein in various murine tissues, reverse transcription (RT)-PCR and immunohistochemistry analyses were carried out. The RT-PCR analysis showed that the Usp26 transcript was expressed in all of the tested tissues. USP26 protein localization was examined by immunohistochemistry, and it was shown that USP26 was not detectable at 20 days postpartum, with the expression restricted to the cytoplasm of condensing spermatids (steps 9–16), Leydig cells and nerve fibers in the brain. In addition, the USP26 protein was detected at moderate levels in myocardial cells, the corpus of epidydimis, epithelium of the renal tubules and the seminal gland of postnatal day 35 mice. Its spatial and temporal expression pattern suggests that Usp26 may play an important role in development or function of the testis and brain. Further research into these possibilities is in progress.
Keywords: ubiquitin-specific protease 26 (USP26); Usp26 gene; deubiquitination enzymes; protein degradation,; spermatogenesis; mouse
Introduction
Protein ubiquitination is a central regulator of numerous cellular processes, including cell cycle progression, transcriptional regulation, immune response and signal transduction 1, 2. Deubiquitinating enzymes (DUBs) are negative regulators of protein ubiquitination 3 that play an important role in regulating ubiquitin-dependent processes. Recent studies have found that diverse cellular mechanisms are employed to control the activity of DUBs, which fall into five distinct families: ubiquitin C-terminal hydrolases, ubiquitin-specific processing proteases (USPs or UBPs), the ovarian tumor (OTU)-domain ubiquitinaldehyde-binding proteins, Jab1/Pad1/MPN-domain-containing metalloenzymes and Ataxin-3/Josephin. Among these five families, UBPs represent the most evolutionarily conserved and well represented 4. Ubiquitin-specific protease 26 (USP26) belongs to the USPs or UBPs family.
Testis development and sperm production are highly specialized and complicated processes that form the basis of male fertility throughout the life of a man 5, 6. Mutual contributions of ubiquitin and DUBs play a role during these processes. For instance, ubiquitinated substrates can undergo either degradation due to the activity of the proteasome or stabilization through the activity of DUBs. Different phases of spermatogenesis probably also require different specialized ubiquitin and DUBs 7. It is anticipated that ubiquitination and deubiquitination activities will be found to play a role in mitotic cell cycle regulation, meiotic divisions and the later stages of spermatogenesis.
Our recent research showed that a mutation in the Usp26 gene was detected in 9 of 41 infertile Chinese men who suffered from idiopathic azoospermatism, oligozoospermatism and asthenozoospermatism 8. These results are in agreement with reports that mutations in the Usp26 gene may be associated with male infertility and could negatively affect testicular function 9, 10, 11, 12.
To gain insight into the roles of Usp26, we sought to examine the spatial and temporal expression of Usp26 in various mouse tissues, particularly in the testis. Mouse Usp26, a novel ubiquitin-specific protease gene, is located on the X chromosome and consists of 835 amino acids (Gene Bank: NM_031388.1). Recent reports 12 suggest that Usp26 may play an important role in spermatogenesis. However, the expression pattern of Usp26 in mammalian male reproductive tissues has not been reported.
Materials and methods
Animals
Male KM mice aged 20, 30, 35 and 45 days postpartum were obtained from the Experimental Animal Center of Xi'an Jiaotong University (Xi'an, China). All animals were maintained in a controlled environment (14 h:10 h light:dark cycle) and provided with a standard pellet diet and clean water ad libitum. All animal procedures were approved by the Animal Experimentation Committee Regulation.
To study the expression pattern of Usp26 during sexual maturation, the testes and epididymides of male mice aged 20, 30, 35 and 45 days were collected. The brains, hearts, lungs, livers, kidneys, adrenal glands and seminal vesicles of 35-day-old mice were also collected.
RNA extraction, reverse transcription PCR analysis and sequence analysis
To examine Usp26 mRNA expression patterns in mice, total RNA was extracted from snap-frozen tissues using Trizol following the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA).
Before reverse transcription (RT)-PCR, DNA-free RNA was prepared using DNase I (Fermentas, Lithuania). Reverse transcription of RNA was carried out using the RevertAid first-strand cDNA synthesis kit (Fermentas, Lithuania) according to the manufacturer's instructions. To amplify Usp26 from cDNA, the following set of primers was used: 5′-CACATCAAACAGGGAGTC-3′ (forward primer) and 5′-TAGGGAAATCTGCTTATC-3′ (reverse primer). The PCR was carried out in 13-μL reaction volumes containing a mixture of 10 × Taq Buffer with (NH4)2SO4, 0.2 mmol L-1 dNTP, 1.5 mmol L-1 MgCl2, 0.5 μmol L-1 of each primer, 2.5 U Taq DNA polymerase (Fermentas, Lithuania) and approximately 50 ng DNA template. Thermocycling conditions consisted of an initial denaturation step for 3 min at 95 °C, 35 cycles of 30 s at 94 °C, 60 s at 52 °C, 60 s at 70 °C and a final extension of 10 min at 70 °C. The PCR products were analyzed on a 2% agarose gel. The PCR reactions were carried out in triplicates to ensure consistent results.
Negative controls, in which water was used in the place of template, were run in parallel to samples for each primer to exclude amplification of contaminating DNA. The amount of Actb (also known as beta-actin) mRNA (accession no. NM-007393; forward primer: 5′N-aggctgtgctgtccctgtat-3′N; reverse primer: 5′N-aaggaaggctggaaaagagc-3′N) was measured in each tissue sample in parallel to normalized measurements between samples (data not shown). Identical results were obtained when at least three independent tissue samples were examined.
The PCR products were purified and sequenced by Shanghai Sunbiotechnology using an ABI3730 DNA Analyzer (ABI, Foster, CA, USA). For each sample, sequencing reactions were carried out using both forward and reverse primers.
Immunohistochemistry
Testes, epididymides, seminal vesicles and brains were fixed in freshly prepared Bouin's fixation solution; and hearts, lungs, livers, kidneys and adrenal glands were fixed in 4% (w/v) paraformaldehyde overnight. The tissues were embedded in paraffin wax and cut into 5-μm sections. The sections were exposed to 3% hydrogen peroxide in methanol for 20 min to block any endogenous peroxidase activity in the tissue. To increase the penetration of antibody into tissues and to reduce the background staining, 1% Triton X-100 was used. The nonspecific binding sites were blocked with normal rabbit serum (Boster, Wuhan, China) for 30 min. The sections were incubated overnight at 4 °C with primary antibody, a polyclonal goat antibody against USP26 (1:150, Santa Cruz, CA, USA), and then for 40 min at 37 °C with a secondary antibody, biotinylated rabbit anti-goat IgG (1:350, Vector Laboratories, Burlingame, CA, USA). Streptavidin–avidin–horseradish peroxidase complex (S–A/HRP, ZSGB, Beijing, China) was then applied for 25 min. Diaminobenzidine (DAB) was used to visualize the immunohistochemical reaction. The sections were then counterstained with Mayer's hematoxylin. After each step, the slides were rinsed thrice with phosphate-buffered saline (PBS, 0.01mol L-1, pH 7.4–7.6). Finally, sections were examined using a light microscope (Olympus BX 51, Tokyo, Japan).
For immunofluorescence experiments, all steps preceding the addition of the secondary antibody were the same as those described above. At this point, the sections were incubated in a dark container with a secondary antibody, fluorescein isothiocyanate (FITC)-conjugated affiniPure rabbit anti-goat IgG (1:50, Jackson ImmunoResearch Lab, West Grove, PA, USA), for 60 min at 37°C. After each step, sections were rinsed thrice with PBS (0.01mol L-1, pH 7.4–7.6), away from light. Finally, sections were examined with a fluorescence microscope (Olympus BX 51, Tokyo, Japan). Negative controls with no primary antibody added were set up for each slide (data not shown). Immunohistochemistry experiments were repeated at least twice on two different samples, yielding qualitatively identical results.
Results
Expression analysis of Usp26 by RT-PCR
To investigate the expression of Usp26 mRNA in various murine tissues, RT-PCR analysis was carried out on mouse brains, hearts, lungs, livers, kidneys, adrenal glands, testes, epididymides and seminal vesicles. Expression of the Usp26 gene was detected in all of the above tissues, and BLAST (Basic Local Alignment Search Tool) analysis of the sequencing results showed that these sequences were nearly identical (99%) to the known sequence of mouse Usp26 mRNA (Figure 1).
Figure 1.
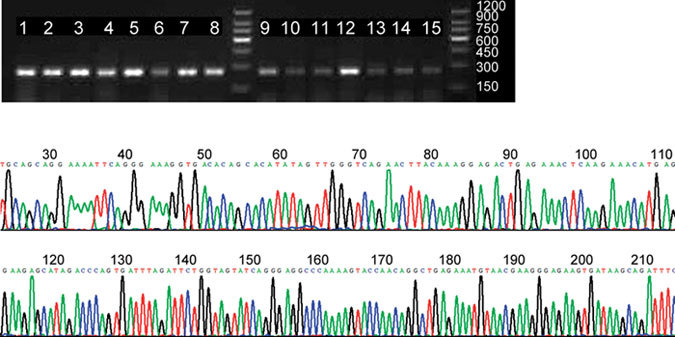
(A) Reserve-transcription polymerase chain reaction (RT-PCR) in multiple mouse tissues. 1, 3, 5 and 7: testes of postnatal days 20, 30, 35 and 45, respectively; 2, 4, 6 and 8: epididymides of postnatal days 20, 30, 35 and 45, respectively; 9–15: mouse brain, lung, heart, adrenal gland, liver, kidney and seminal gland, respectively, on postnatal day 35. (B) Sequencing results showed that these sequences agreed very well (99%) with Usp26 mRNA of mouse.
Examination of USP26 expression in mouse testis and epididymis during sexual maturation by immunohistochemistry
The USP26 expression was differentially detected in mouse testes during sexual maturation. On postnatal day 20, USP26 immunoreactivity was not observed in testis. However, by postnatal day 30, USP26 immunostaining was found in cytoplasm of condensing spermatids (steps 9–16) and Leydig cells, and by days 35 and 45, the staining in these cell types had become very strong (Figure 2).
Figure 2.
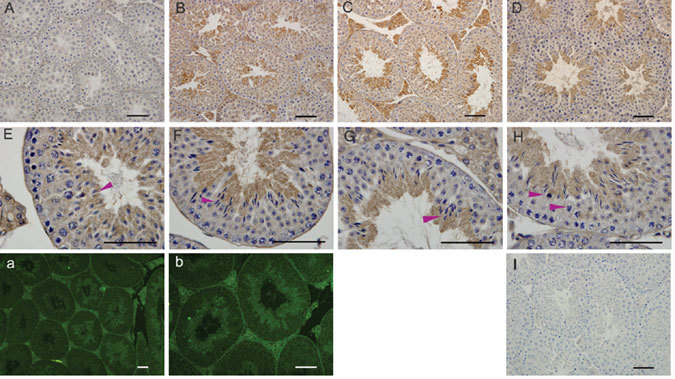
Immunolocalization of ubiquitin-specific protease 26 (USP26) protein in the developing mouse testis. USP26-positive cells are characterized by brown staining as a result of the DAB colorimetric reaction in (A–H) and in green as a result of FITC immunofluorescence staining in (a) and (b). USP26 immunostaining is absent in 20 day (A), weak in 30 day (B) and intense in 35 and 45-day mouse testes, in which the staining is strong in the cytoplasm of condensing spermatids (steps 9–16) (C and D). (E-G) show seminiferous epithelium at late(E) and early stages(F, G) respectively, and the arrowheads in (H) point to cells undergoing meiotic metaphase. Immunofluorescence staining in panels (a) and (b) also shows USP26 expression in spermatids and in Leydig cells in testes. I: Control section. Scale bars = 50μm.
Similarly, USP26 expression was not detected in the caput, corpus and cauda epididymis on day 20. On day 30, the signal was moderate in the corpus region, but lower in the caput and cauda regions. By day 35, the staining was stronger in the tubular epithelium of all three regions, particularly in corpus (Figure 3).
Figure 3.
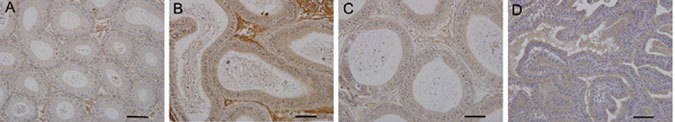
Immunohistochemical localization of USP26 in epididymis (A–C) and seminal gland (D) of a 35-day-old mouse. USP26 is expressed in the epithelium of the two tissues. The staining is stronger in the corpus region (B) than in the caput and cauda regions of epididymis (A and C). Scale bars = 50μm.
USP26 expression in mouse brain, heart, lung, liver, kidney, adrenal gland and seminal gland
In addition, USP26 immunostaining was observed in the nerve fibers of the cerebra and cerebellum of postnatal day 35 mice, with the most intense staining in the cingulum, the cerebral external capsules and in the white matter of the cerebellum.
Moderate USP26 staining was detected in other tissues at this age, including the cytoplasm of the epithelium of renal tubules and seminal gland, as well as in myocardial cells (Figure 3), and weaker expression was found in the tissues of lung, liver and adrenal gland (Figure 4).
Figure 4.
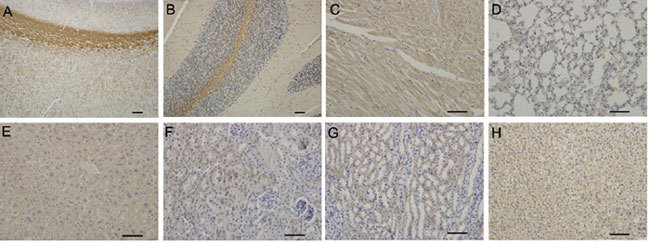
Immunohistochemical staining of USP26 in brain, heart, lung, liver, kidney and adrenal gland of 35-day-old mice. Nerve fibers of the cerebra and cerebellum (A and B) show intense immunoreactive staining, especially in cingulum and external capsule of the cerebra and medulla of the cerebellum. USP26-positive staining is detected at a moderate level in myocardial cells and the epithelium of renal tubule (C, F, G), and at a lower level in lung, liver and adrenal gland, respectively (D, E, H). Scale bars = 50μm.
Discussion
USP26 is a member of the DUB family of enzymes. Recent studies have shown that not only ubiquitination but also activity. According to some analyses, DUB enzymes have several possible functions. First, these enzymes process the products of ubiquitin genes. Second, DUBs can remove esters and amides from ubiquitin to produce free monomeric ubiquitin in the cell. Third, ubiquitin-dependent protein degradation requires attachment of at least one ubiquitin to a target protein through an isopeptide bond between the carboxy-terminal glycine of ubiquitin and the ε-amino group of the side chain of a lysine residue on the target protein. Finally, DUBs might counteract the effects of ubiquitin-conjugating enzymes and ubiquitin protein ligase-mediated conjugation by competitively removing the polyubiquitin chain from the conjugated protein 13.
In this report, we studied the mRNA and protein expression of Usp26, a gene known to encode a DUB in various mouse tissues, particularly in mouse testis and brain.
The expression of Usp26 mRNA was investigated by RT-PCR in a broad sampling of tissues, and the expression of USP26 protein was confirmed by immunohistochemistry in testis and in other tissues. Our results showed that both the Usp26 transcript and protein were detectable in all tissues tested, with particularly strong expression of the protein evident in the testis and in the brain. The expression level of the USP26 protein gradually increased starting at 30 days postpartum, which is notably the appearance stage of condensing spermatid (steps 9–16), and then decreased in stage γ of the seminiferous epithelium, coinciding with spermatids' emission. The changes in expression of this gene during the different stages of spermatogenesis indicate that Usp26 may play a role in the cellular processes involved in mouse germ cell development and spermatogenesis in the later stages of meiosis. We suggest that the gene could be essential for meiosis, together with others, including Stra8, Uba6 and Tex11, all of which could act together to regulate the complex process of meiotic cell division.
According to the prevailing view, mammalian X chromosomes are deficient in spermatogenesis genes expressed after meiosis. This has been interpreted as a consequence of meiotic sex chromosome inactivation (MSCI), the process of transcriptional silencing of the X and Y chromosomes during meiotic prophase. However, USP26 Protein expression was apparent in spermatids in our study. Thus, there seems to be an obvious contradiction between MSCI and the expression of X chromosome genes at postmeiotic spermatids. However, Wang et al. 16 observed the reactivation of selected single-copy X-linked genes in the postmeiotic phase, and Mueller et al. 17 reported that postmeiotic X repression is incomplete and X-linked multicopy genes exhibited a degree of expression similar to that of autosomal genes. This apparent conflict thus presents a reasonable interpretation. It is possible that some important mechanisms may be counteracting postmeiotic repression and causing X chromosome reactivation after the completion of MSCI.
In conclusion, this study suggests that Usp26 may participate in the process of transcriptional regulation or be involved in signal transduction pathways during postmeiotic spermiogenesis and may also play an important role in the normal functioning of the brain.
Acknowledgments
We thank the laboratory, clinical and paramedical staff of the center of Reproductive Medicine, and the Department of Pathology for their assistance. This study was supported by the National Natural Science Foundation of China (30471735 and 30700654) and the Sci-Technical Development Project of Shanxi Province, China (2006K15-G4).
References
- Taya S, Yamamoto T, Kanai-Azuma M, Wood SA, Kaibuchi K. The deubiquitinating enzyme Fam interacts with and stabilizes beta-catenin. Genes Cells. 1999;4:757–67. doi: 10.1046/j.1365-2443.1999.00297.x. [DOI] [PubMed] [Google Scholar]
- Baarends WM, Roest HP, Grootegoed JA. The ubiquitin system in gametogenesis. Mol Cell Endocrinol. 1999;151:5–16. doi: 10.1016/s0303-7207(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Cohen M, Stutz F, Dargemont C. Deubiquitination, a new player in golgi to endoplasmic reticulum retrograde transport. J Biol Chem. 2003;278:21989–92. doi: 10.1074/jbc.C300451200. [DOI] [PubMed] [Google Scholar]
- Kin YK, Kim YS, Yoo KJ, Lee HJ, Lee DR, et al. The expression of Usp42 during embryogenesis and spermatogenesis in mouse. Gene Expr Patterns. 2007;7:143–8. doi: 10.1016/j.modgep.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Toshimori K. Biology of spermatozoa maturation: an overview with an introduction to this issue. Microsc Res Tech. 2003;61:1–6. doi: 10.1002/jemt.10311. [DOI] [PubMed] [Google Scholar]
- Tokuhiro K, Miyagawa Y, Tanaka H. Characterizing mouse male germ cell-specific actin capping protein alpha3 (CPalpha3): dynamic patterns of expression in testicular and epididymal sperm. Asian J androl. 2008;10:711–8. doi: 10.1111/j.1745-7262.2008.00426.x. [DOI] [PubMed] [Google Scholar]
- Baarends WM, van der Laan R, Grootegoed JA. Specific aspects of the ubiquitin system in spermatogenesis. J Endocrinol Invest. 2000;23:597–604. doi: 10.1007/BF03343782. [DOI] [PubMed] [Google Scholar]
- Zhang J, Qiu SD, Li SB, Zhou DX, Tian H, et al. Novel mutations in ubiquitin-specific protease 26 gene might cause spermatogenesis impairment and male infertility. Asian J Androl. 2007;9:809–14. doi: 10.1111/j.1745-7262.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- Christensen GL, Griffin J, Carrell DT. Sequence analysis of the X-linked USP26 gene in severe male factor infertility patients and fertile controls. Fertil Steril. 2008;90:851–2. doi: 10.1016/j.fertnstert.2007.06.096. [DOI] [PubMed] [Google Scholar]
- Stouffs K, Lissens W, Tournaye H, Van Steirteghem A, Liebaers J. Alterations of the USP26 gene in Caucasian men. Int J Androl. 2006;29:614–7. doi: 10.1111/j.1365-2605.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- Stouffs K, Lissens W, Tournaye H, Van Steirteghem A, Liebaers I. Possible role of USP26 in patients with severely impaired spermatogenesis. Eur J Hum Genet. 2005;13:336–40. doi: 10.1038/sj.ejhg.5201335. [DOI] [PubMed] [Google Scholar]
- Paduch PA, Mielnik A, Schligel PN. Novel mutations in testis-specific ubiquitin protease 26 gene may cause male infertility and hypogonadism. Reprod Biomed Online. 2005;10:747–54. doi: 10.1016/s1472-6483(10)61119-4. [DOI] [PubMed] [Google Scholar]
- Lin H, Keriel A, Morales CR, Bedard N, Zhao Q, et al. Divergent N-terminal sequences target an inducible testis deubiquitinating enzyme to distinct subcellular structures. Mol Cell Biol. 2000;20:6568–78. doi: 10.1128/mcb.20.17.6568-6578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Zhang J, Gasana V, Fu W, Liu Y, et al. Differential expression of protein kinase C alpha and delta in testes of mouse at various stages of development. Cell Biochem Funct. 2005;23:415–20. doi: 10.1002/cbf.1167. [DOI] [PubMed] [Google Scholar]
- Jeong JH, Jin JS, Kim HN, Kang SM, Liu JC, et al. Expression of Runx2 transcription factor in non-skeletal tissues, sperm and brain. J Cell Physiol. 2008;217:511–7. doi: 10.1002/jcp.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PJ, Page DC, McCarrey JR. Differential expression of ex-linked and autosomal germ-cell-specific genes during spermatogenesis in the mouse. Hum Mol Genet. 2005;14:2911–8. doi: 10.1093/hmg/ddi322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JL, Mahadevaiah SK, Park PJ, Warburton PE, Page DC, et al. The mouse X chromosome is enriched for multicopy testis genes showing postmeiotic expression. Nat Genet. 2008;40:794–9. doi: 10.1038/ng.126. [DOI] [PMC free article] [PubMed] [Google Scholar]


