Abstract
This study was organized to see whether vitamin E, as a strong antioxidant, could affect the abnormalities of testis structure caused by para-nonylphenol (p-NP) during its development. A total of 32 female Wistar rats after mating were divided into four groups (n = 8): control, vitamin E (100 mg kg−1 per day), p-NP (250 mg kg−1 per day) and p-NP + vitamin E. The rats were treated from the seventh day of pregnancy till the twenty-first day. After weaning, the male pups were divided into the same groups and were treated orally for 90 days. Finally, the right testis was fixed, processed, stained and studied using stereological methods. The weight and volume of testis, volume of seminiferous tubules and its diameter, thickness of the basement membrane, height of the germinal epithelium, total number of types A and B spermatogonia, spermatocyte, spermatid and Sertoli cells were significantly reduced in p-NP group when compared with other groups. Co-administration of vitamin E and p-NP compensated for the adverse effects of p-NP on the above parameters. In addition, treatment with only vitamin E caused a significant increase in diameter, basement membrane thickness and height of germinal epithelium, number of spermatogonia and spermatocytes. Co-administration of vitamin E with p-NP could prevent the adverse effects of p-NP on the testis structure during its development.
Keywords: development, para-nonylphenol, stereology, testis, vitamin E
Introduction
Man-made environmental pollutants, especially those with estrogenic activity, have increased public concern regarding their effects on the health of reproductive system, because of their disrupting property on endocrine function in human as well as in wildlife populations. Alkylphenols as estrogenic compounds are manufactured for several commercial purposes, such as plastics, detergents, shampoos, paints, pesticides, cosmetics and other formulated products 1, 2. Among alkylphenols, para-nonylphenol (p-NP) is able to impair the endocrine system by inhibiting estrogen or androgen receptors, and causes dysfunction in male and female reproductive systems 3, 4, 5, 6, 7, 8. Investigations suggest that p-NP can cause oxidative stress and reduce activity of scavenging enzymes, such as superoxide dismutase and glutathione peroxidase, in the testis tissue 9, 10. After discovery of toxicity, estrogenic and oxidative properties of p-NP, some investigations have focused on the adverse effects of p-NP on the morphological changes in male reproductive system. In this case, the reduction in volume and weight of testis, diameter of seminiferous tubules, thickness of germinal epithelium, number of sperm, spermatogenesis and the induction of apoptosis in the germ cells are some of the changes that have been reported as the consequences of p-NP exposure 11, 12, 13, 14, 15, 16. On the other hand, antioxidants, such as vitamin E, can inhibit reactive oxygen species (ROS) 9, 17, 18, and thus terminate lipid peroxidation and stabilize the molecular composition of cellular membranes 19; therefore, it prevents the harmful effects of ROS. In addition, investigations have also shown that the vitamin E prevents toxic damages to testis and sperm after exposure to other chemicals and to γ-radiation 19, 20, 21. As vitamin E is a dietary factor for animal nutrition, which is important for normal reproduction 22, and also its requirement for testicular function is well established 23, therefore, this study was organized to investigate the preventing role of vitamin E as a strong antioxidant in ameliorating the undesired effects of p-NP on the structure of testis in rats treated with p-NP from prenatal to maturation for a period of 90 days.
Materials and methods
Animals and treatments
Male and female Wistar rats with the average weight of 200 ± 10 g were purchased from Pasteur Institute, Iran, and kept in the animal house of Arak University under standard conditions of 21 ± 2ºC, 12hrs light-12hrs dark and enough food and water. After mating, based on vaginal plug observation, female pregnant rats were divided into four groups (n = 8), including control, p-NP (250 mg kg−1 per day) (Acrose company, New Jersey, USA), vitamin E (100 mg kg−1 per day) (Sigma-Aldrich, Steinheim, USA) and p-NP + vitamin E. The treatment was carried out from day 7 of pregnancy to day 21 of postnatal, through mother's milk. After weaning, the male pups (F1 generation) were divided into the same groups (n = 8) as their parents, and the same treatments were continued orally till maturation (90 days) 24. Considering the high viscosity of p-NP and vitamin E, we used corn oil as a carrier 4, 25 with the concentration of 50 mg p-NP per 0.1 mL corn oil 26 and the same formulation was carried out for vitamin E.
Chemicals and dosage
All the chemicals in this study were purchased from Merck Company, Hohenbrunn, Germany unless it is mentioned elsewhere. The p-NP dose was selected on the basis of the doses that have been used in the previous investigations with pathological consequences on male reproductive system of rats 4, 13, 14 and the same logic was also considered for vitamin E dose 19, 27. The duration of exposure to p-NP was 90 days, which is the required time for completion of maturation, and vitamin E treatment was also done along with p-NP treatment.
Tissue preparation
At the end of the treatments, the rats were weighed and anesthetized using diethyl ether, then killed, and their right testes were taken out and weighed. The volume of testis was estimated using the immersion method 28 and then fixed in the modified Davidson's fluid fixative 29.
Stereological study
The orientator method was used to obtain Isotropic Uniform Random (IUR) sections 30, 31. For this purpose, testis was randomly placed on the φ clock, which is divided into nine equal parts. By choosing random number from one to nine, an appropriate cut was made along the selected number, which resulted in two pieces of testis. The first piece was then placed on the θ clock—which is divided into nine unequal parts—along with its previous cut surface on the 0-0 axis, then a random number was selected again and a parallel cut was made along the selected number. The other piece that resulted from the cut made on the φ clock was placed on the θ clock vertically so that its cut surface overlapped the 0-0 axis. This piece was also cut parallel along a randomly selected number 30, 31; after tissue processing, sections of 5 and 20-μm thickness were cut using the microtome and stained using Heidenhain's Azan method.
Estimating the shrinkage and the total volume of testis
To estimate the shrinkage, three random segments were prepared using a trocar from the IUR sections. For each segment, the two vertical diameters were measured and their mean radius was estimated and considered as pre-fixing radius (rbefore). After fixation, tissue processing and sectioning, staining was carried out and the same measurements were performed as above, and the obtained mean radius was considered as the post-fixing radius (rafter). Using the following equation, the amount of shrinkage in each testis was estimated 30.
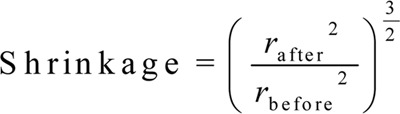 |
To obtain the true volume of testis, the amount of shrinkage was subtracted from the volume estimated by the immersion method.
Estimating the volume of seminiferous tubules and interstitial tissue
To estimate the volume of seminiferous tubules and interstitial tissue, using the systematic random sampling method, an average of five to seven fields per each 5-μm section was evaluated by randomly placing the point probe on each field.
The total points superimposed on the whole field (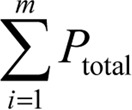 ), along with the total points superimposed on the seminiferous tubules (
), along with the total points superimposed on the seminiferous tubules (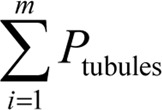 ) and the total points superimposed on the interstitial tissue (
) and the total points superimposed on the interstitial tissue (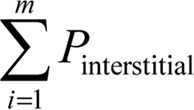 ) were counted, and the volume density of each was estimated using the following equation:
) were counted, and the volume density of each was estimated using the following equation: 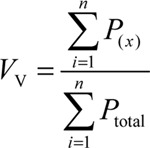 , where x is the interstitial tissue or seminiferous tubules. The total volume of each compartment was also calculated by multiplying its volume density (Vv) by the total volume of the testis 30.
, where x is the interstitial tissue or seminiferous tubules. The total volume of each compartment was also calculated by multiplying its volume density (Vv) by the total volume of the testis 30.
Estimating the length, diameter, height of the germinal epithelium and the basement membrane thickness of seminiferous tubules
To estimate the length of seminiferous tubules, five to seven random fields from 5-μm-thick sections, using the objective of 10 ×, were selected and the number of the selected seminiferous tubule profiles was counted using an unbiased counting frame 30, 31, 32; thus, an average of 110–130 seminiferous tubules per testis was estimated. The length density of seminiferous tubules was also estimated using the following formula:
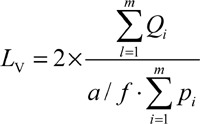 |
,
where ∑Qi is the total number of the selected tubules, a / f is the frame area in tissue scale and ∑Pi is the total points superimposed on the testis tissue. To obtain the absolute length of the seminiferous tubules, the lengths density (Lv) was multiplied by the total volume of the testis 30.
To estimate the mean diameter of seminiferous tubules, we used the Olympus DP12 microscope equipped with a camera. The diameter of the tubules was measured on the sampled tubules in the counting frame used for estimating the length of the seminiferous tubules. The diameter was measured perpendicularly to the long axis of the tubules where the tubules were widest 32. Approximately 110–130 tubules were measured.
To estimate the height of the germinal epithelium, the following equation was used 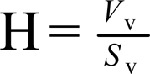 , in which Vv is the volume density of the germinal epithelium and Sv is the surface density of the germinal epithelium. For this purpose, an average of 8–10 fields, using the objective of 10 ×, from all of the 5-μm-thick sections of rat testis were studied using the systematic random sampling method.
, in which Vv is the volume density of the germinal epithelium and Sv is the surface density of the germinal epithelium. For this purpose, an average of 8–10 fields, using the objective of 10 ×, from all of the 5-μm-thick sections of rat testis were studied using the systematic random sampling method.
To obtain the volume density of the germinal epithelium, the total number of points of the point probe superimposed on each image of the testis was counted, and then the total number of the points superimposed on the germinal epithelium was also counted and divided into the total number of points counted for the testis.
To estimate the surface density (Sv) of the germinal epithelium following equation was used: 30:
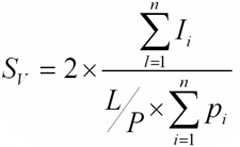 |
Where, (∑Pi) is the total number of points superimposed on the germinal epithelium of the seminiferous tubule,(L / P) is the length of the linear test probe in actual tissue scale and (∑Ii) is the total number of intersections of linear test probe with the inner surface of the germinal epithelium.
To estimate the mean basement membrane thickness of seminiferous tubules, the harmonic mean of the basement membrane thickness was estimated 33. A number of the fields were selected from all of the 5-μm-thick sections using the objective of 100 ×, then a probe consisting of isotropic lines was superimposed on the images and the distance between the inner and outer surfaces of the basement membrane was measured by drawing a line from the outer surface to the touch point of the isotropic line with the inner surface of the membrane. The distance of the drawn line was considered as the thickness of basement membrane. An average of 110–120 intercepts was estimated, and the mean basement membrane thickness was calculated using the following equation:
Harmonic mean layer thickness = 8 / 3π × harmonic mean of orthogonal intercepts,
where harmonic mean = number of measurements/sum of the reciprocal of orthogonal intercepts (oi)
 |
Estimating the number of spermatogonia (A and B), spermatocyte, spermatid and Sertoli cells
To estimate the number of cells, the optical disector method and an unbiased counting frame were used 30, 34, 35.
A number of fields, using the objective of × 100, from all of the 20-μm-thick sections were selected and a microcator (ND 221 B, Heidenhain, Germany) was used for counting. Using the following equation, the number density (Nv) of different types of cells was estimated.
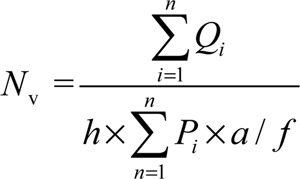 , where ∑Qi is the total number of the counted cells, h is the tissue thickness considered for counting, a / f is the frame area in the true tissue scale and ΣPi is the total number of the points superimposed on the selected fields. The result of the equation was then multiplied by the total volume of the testis to obtain the total number of cells (Ntotal = Nv × Vtotal).
, where ∑Qi is the total number of the counted cells, h is the tissue thickness considered for counting, a / f is the frame area in the true tissue scale and ΣPi is the total number of the points superimposed on the selected fields. The result of the equation was then multiplied by the total volume of the testis to obtain the total number of cells (Ntotal = Nv × Vtotal).
Data analysis
The results were analyzed by one-way analysis of variance (ANOVA) and Tukey's test using the SPSS V11/0 software. The means were considered significantly different at P < 0.05.
Results
The total volume of testis, seminiferous tubules and interstitial tissue
A highly significant reduction in the mean total volume of testis and seminiferous tubules in the p-NP group compared with the other groups was seen (P < 0.01), whereas no reduction was found in the above-mentioned parameters in the vitamin E + p-NP group when compared with control and vitamin E groups. No significant difference was found in the mean volume of the interstitial tissue between the groups (Table 1).
Table 1. Comparing the mean total volume of testis (mm3), seminiferous tubules and interstitial tissue (mm3) in different groups of rats after 90 days of treatment. Values are means ± s.d.
| Groups | Testis volume | Seminiferous tubules volume | Interstitial tissue volume |
|---|---|---|---|
| Control | 1 125.7 ± 124.8a | 890.0 ± 96.43a | 232.8 ± 43.4a |
| Vitamin E | 1 145.7 ± 103.7a | 928.5 ± 97.1a | 210.0 ± 25.1a |
| p-NP | 875.0 ± 119.1b | 690.0 ± 103.3b | 185.0 ± 23.4a |
| p-NP+vitamin E | 1 275.0 ± 190.7a | 1 053.3 ± 150.9a | 223.3 ± 55.0a |
The mean with the same letter code does not differ significantly (ANOVA, Tukey's test, P > 0.05).
The length (m), diameter, basement membrane thickness and germinal epithelium height of the seminiferous tubules (μm)
On comparing the mean diameter and basement membrane thickness of the seminiferous tubules, a highly significant reduction was seen in the p-NP group compared with the other groups (P < 0.01), whereas in the vitamin E + p-NP group, no reduction was found regarding the above-mentioned parameters when compared with the control group. On the other hand, the vitamin E group showed a highly significant increase in the mean diameter, basement membrane thickness of seminiferous tubules and also in the height of the germinal epithelium of seminiferous tubules compared with other groups (P < 0.01). The mean height of the germinal epithelium in p-NP group showed a highly significant reduction compared with the p-NP + vitamin E and vitamin E groups (P < 0.001), whereas a highly significant increase was found in the vitamin E group compared with the remaining groups (P < 0.001). Meanwhile, a comparison of the mean length of the seminiferous tubules in all four groups showed no significant difference (Table 2).
Table 2. Comparing the mean length, diameter, basement membrane thickness and germinal epithelium height of the seminiferous tubules in different groups of rats after 90 days of treatment. Values are means ± s.d.
| Groups | Length of seminiferous tubules (m) | Diameter of seminiferous tubules (μm) | Thickness of basement membrane (μm) | Height of germinal epithelium (μm) |
|---|---|---|---|---|
| Control | 17.7 ± 4.8a | 340.8 ± 7.9a | 8.5 ± 0.2a | 71.3 ± 8.5a |
| Vitamin E | 16.5 ± 2.1a | 354.8 ± 12.2b | 9.3 ± 0.4b | 96.9 ± 4.1b |
| p-NP | 13.7 ± 2.0a | 226.6 ± 2.8c | 6.7 ± 0.5c | 63.1 ± 3.3ac |
| p-NP+vitamin E | 19.0 ± 6.0a | 241.0 ± 0.8a | 8.4 ± 0.3 | 77.3 ± 6.2ad |
The mean with the same letter code does not differ significantly (ANOVA, Tukey's test, P > 0.05).
Number of the spermatogonia (A and B), spermatocyte, spermatid and Sertoli cells
The mean number of type A spermatogonia showed a highly significant reduction in the p-NP group compared with the other groups (P < 0.01), whereas the mean number of type B spermatogonia showed a highly significant reduction in the p-NP group in comparison with the vitamin E and P-NP + vitamin E groups (P < 0.001). The reduction in the mean number of spermatocyte, spermatid and Sertoli cells in the p-NP was highly significant when compared with the other groups (P < 0.001). In the group of vitamin E + p-NP, numbers of all the above-mentioned germ cells did not show reduction when compared with the control group. A highly significant increase (P < 0.001) was found in the mean number of type B spermatogonia and spermatid in the vitamin E group compared with the control and p-NP groups, and the same finding (P < 0.001) was observed in the mean number of type A spermatogonia, spermatocytes and Sertoli cells in vitamin E group when compared with p-NP group only (Table 3).
Table 3. Comparing the number of the spermatogonia (A and B), spermatocyte, spermatid and Sertoli cells in different groups of rats after 90 days of treatment. Values are means ± s.d.
| Groups | Spermatogonia A × 106 | Spermatogonia B × 106 | Spermatocyte × 106 | Spermatid × 106 | Sertoli × 106 |
|---|---|---|---|---|---|
| Control | 8.3 ± 10.0a | 1.8 ± 6.0a | 122.0 ± 20.0a | 363.0 ± 58.0a | 27.0 ± 6.0a |
| Vitamin E | 10.1 ± 14.0a | 2.8 ± 8.0b | 147.0 ± 28.0a | 482.0 ± 63.0b | 35.0 ± 8.0a |
| p-NP | 5.6 ± 7.0b | 1.4 ± 2.0ad | 84.0 ± 10.0b | 228.0 ± 33.0c | 17.0 ± 3.0b |
| p-NP+vitamin E | 10.2 ± 21.0a | 2.8 ± 7.0ab | 135.0 ± 27.0a | 349.0 ± 57.0a | 28.0 ± 9.0a |
The mean with the same letter code does not differ significantly (ANOVA, Tukey's test, P > 0.05).
Histopathological findings
The histopathological study in the p-NP-treated group showed that germinal epithelium was irregular and vacuolated with reduction in height. The amount of sperm also seemed to be less in this group, which is conformed by sperm counts (data not shown) Figure 1.
Figure 1.
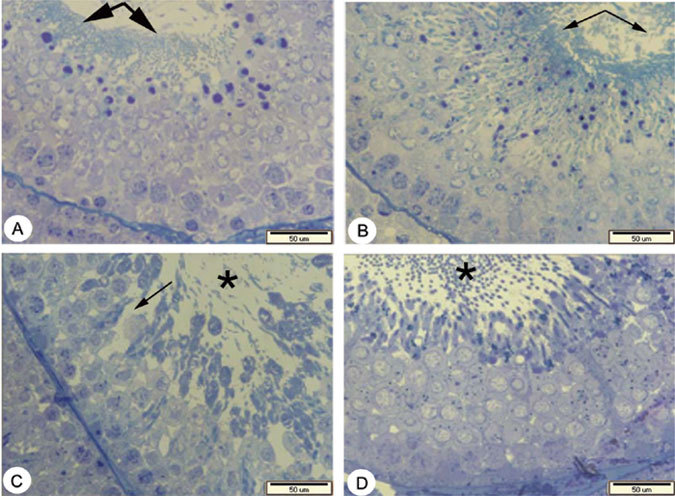
A to D: Micrographs of testis tissue in different groups of rats, consisting of A) control, B) vitamin E (100mg / kg / day), C) p-NP (250mg / kg / day), and D) p-NP + vitamin E. sections with 500 nm thickness and Toluiden blue staining. A) Showing the normal amount of sperm (thick arrows) and normal height with regular arrangement of germinal epithelium in the control group. B) Showing normal amount of sperm (thin arrows) and an increase in the height of germinal epithelium in the vitamin E treated group compared with the other groups. C) Showing reduction in the amount of sperm (star) with an irregular and vacuolated germinal epithelium in p-NP group (thin arrow). D) Showing an increase in the amount of sperm (star) with regular arrangement of germinal epithelium in p-NP + vitamin E group compared with p-NP group.
Body and testis weight
At the end of the treatments, the mean body weight in p-NP and p-NP + vitamin E groups showed a highly significant reduction (P < 0.01) compared with the remaining groups. A highly significant reduction (P < 0.001) in the mean weight of testis was also seen in p-NP group compared with the other groups. In p-NP + vitamin E group no reduction in the weight of testis was found when compared with control group, but a significant reduction (P < 0.05) of body weight was observed (Table 4).
Table 4. Comparing the testis and body weight of rats (g) in different groups after 90 days of treatment. Values are means ± s.d.
| Groups | Testis weight | Body weight at the end of weaning | Body weight at the end of treatment |
|---|---|---|---|
| Control | 1.4 ± 0.1a | 46.6 ± 10.1a | 305.0 ± 19.3a |
| Vitamin E | 1.4 ± 0.1a | 43.9 ± 1.2a | 307.0 ± 23.9a |
| p-NP | 1.1 ± 0.2b | 39.0 ± 3.8a | 206.4 ± 29.3b |
| p-NP+vitamin E | 1.4 ± 0.1a | 42.6 ± 5.5a | 246.0 ± 32.5b |
The mean with the same letter code does not differ significantly (ANOVA, Tukey's test, P > 0.05).
Discussion
In the present study, the volume of seminiferous tubules in p-NP-treated group significantly reduced. According to Zhang et al. 15, treatment of Sprague–Dawley (SD) rats with 100 mg kg−1 of p-NP for 28 days, and 200 mg kg−1 of p-NP from the seventh day of pregnancy to the end of weaning 16 resulted in atrophied seminiferous tubules. In addition, the maldeveloped seminiferous tubules were reported by Qiu et al. 12, when SD rats were treated with 200 mg kg−1 of p-NP from day 7 of gestation to the weaning period, which confirms our findings. In addition, our results indicated that the diameter and thickness of basement membrane were also reduced following treatment with p-NP, which is in agreement with the published results by De Jager et al. 13, wherein the rats were treated with 100 and 250 mg kg−1 p-NP for 10 weeks. However, they reported a reduction in the germinal epithelium thickness, whereas in our results, no reduction in the germinal epithelium thickness was observed. This might be because of differences in the strain of the used animals and the duration of the treatment. In addition, our results revealed a significant reduction in the total volume of testis after treatment with p-NP. As the interstitial tissue of testis did not show any changes after p-NP treatment and seminiferous tubules are the main components of the testis, so the reduction in the volume of seminiferous tubules can be considered as the most important reason for the reduction in the volume of testis.
According to the present results, a significant reduction in the number of type A spermatogonia, spermatocyte, spermatid and Sertoli cells was found in the p-NP group. Other studies have also reported a reduction in the number and quality of sperm after treatment with 250 mg kg−1 of p-NP 13, 14. Qiu et al. 12 showed that the treatment of rats with 200 mg kg−1 of NP from day 7 of gestation to the weaning period, can cause a significant reduction in Sertoli cell numbers, sperm count and motility, as well as impairment of spermatogenesis. Even the administration of very low concentrations of p-NP (50–500 μg L−1 of drinking water) for a period of 4 weeks causes sertoli cell damage in mice 5. Therefore, damage to Sertoli cells due to p-NP may be another reason for the reduction in the number of germinal cells 36. These observations show that the germ cells and spermatogenesis are very susceptible to p-NP toxicity.
Many investigations have referred to the estrogenic property of alkylphenols (such as p-NP and octylphenol) 1, 2, 37, responsible for degeneration of endocrine glands 6, which leads to changes in the sexual hormone levels 38 and thereby affects spermatogenesis. It is notable that treatment with p-NP can increase the rate of apoptosis in germinal cells 39, which could be considered as a parameter involved in the reduction of the number of germinal cells. In addition, induction of oxidative stress and reduction in the activity of scavenging enzymes, such as glutathione reductase and superoxide dismutase, by administration of 100 μg kg−1 of p-NP for 45 days in the testis of rats have been reported 9. ROS produced through oxidative stress attack the unsaturated fatty acids in the cell membranes of testis, which in turn causes disruption of the cell membrane and finally leads to cell damage. Therefore, p-NP toxicity, estrogenic property and induction of oxidative stress collectively can cause the above-mentioned abnormalities in the testis.
In our study, a significant reduction in the mean body and testis weight was found in the rats treated with p-NP at the end of treatment period. The same results have also been reported by other investigators 37, 40, 41. On the basis of the studies carried out so far, body and testis weight reduction due to the p-NP treatment is dose and time-dependent, and also relates to the stage of development in which the treatment starts 13, 14, 40, 42, 43. The reduction in body weight could be because of loss of appetite and anorexia in the treated rats, which results in the reduction of their food consumption 42, 44. In case of testis weight reduction, it should be mentioned that the seminiferous tubules occupy most of the testis volume, and also germinal cells are the most important compartments of testis tissue 38, so the testis weight reduction may be correlated with the abnormal changes in the above-mentioned parameters.
The co-administration of vitamin E with p-NP could compensate for the reduction of weight and volume of testis, volume of seminiferous tubules, and its diameter and thickness of basement membrane, as well as number of type A spermatogonia, spermatocyte, spermatid and Sertoli cells. It is well documented that the vitamin E, as a strong antioxidant, can prevent oxidative effects of chemicals, such as ethane dimethane sulfonate 19 and formaldehyde 20, and the DNA-breaking effect of γ-radiation 21. In addition, Chitra and Mathur 9 reported that vitamin E reversed the reducing effect of p-NP on scavenging enzymes. Also, co-administration of vitamin C along with bisphenol A could compensate for the effects of oxidative stress in epididymal epithelium of rats testis 10. Therefore, induced oxidative stress could be the most important reason for adverse effects of p-NP 9, 10 on the investigated parameters of the rats' testis in this study.
In conclusion, as the most important finding of this study, vitamin E could compensate for all the adverse effects of p-NP treatment on the testis structure. In addition, it was observed that the application of only vitamin E could significantly increase the seminiferous tubules diameter and basement membrane thickness, height of germinal epithelium as well as the number of type B spermatogonia and spermatid. Thus, we recommend consumption of this vitamin wherever the toxicity with p-NP is the matter of concern.
References
- Blake CA, Boockfor FR. Chronic administration of the environmental pollutant 4-tert-octylphenol to adult male rats interferes with the secretion of luteinizing hormone, follicle-stimulating hormone, prolactin, and testosterone. Biol Reprod. 1997;57:255–66. doi: 10.1095/biolreprod57.2.255. [DOI] [PubMed] [Google Scholar]
- Kinnberg K, Korsgaard B, Bjerregaard P, Jespersen A. Effects of nonylphenol and 17beta-estradiol on vitellogenin synthesis and testis morphology in male platyfish Xiphophorus maculatus. J Exp Biol. 2000;203:171–81. doi: 10.1242/jeb.203.2.171. [DOI] [PubMed] [Google Scholar]
- Murray TJ, Lea RG, Abramovich DR, Haites NE, Fowler PA. Endocrine disrupting chemicals: effects on human male reproductive health. Early Pregnancy. 2001;5:80–112. [PubMed] [Google Scholar]
- Han XD, Tu ZG, Gong Y, Shen SN, Wang XY, et al. The toxic effects of nonylphenol on the reproductive system of male rats. Reprod Toxicol. 2004;19:215–21. doi: 10.1016/j.reprotox.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Kyselova V, Peknicova J, Buckiova D, Boubelik M. Effects of p-nonylphenol and resveratrol on body and organ weight and in vivo fertility of outbred CD-1 mice. Reprod Biol Endocrinol. 2003;1:30. doi: 10.1186/1477-7827-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Suzuki A, Goto M, Lubahn DB, Handa H, et al. Tissue-specific estrogenic and non-estrogenic effects of a xenoestrogen, nonylphenol. J Mol Endocrinol. 2004;33:243–52. doi: 10.1677/jme.0.0330243. [DOI] [PubMed] [Google Scholar]
- Kimura N, Kimura T, Suzuki M, Totsukawa K. Effect of gestational exposure to nonylphenol on the development and fertility of mouse offspring. J Reprod Dev. 2006;52:789–95. doi: 10.1262/jrd.18007. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci. 2003;75:40–6. doi: 10.1093/toxsci/kfg150. [DOI] [PubMed] [Google Scholar]
- Chitra KC, Mathur PP. Vitamin E prevents nonylphenol-induced oxidative stress in testis of rats. Indian J Exp Biol. 2004;42:220–3. [PubMed] [Google Scholar]
- Chitra KC, Rao KR, Mathur PP. Effect of bisphenol A and co-administration of bisphenol A and vitamin C on epididymis of adult rats: a histological and biochemical study. Asian J Androl. 2003;5:203–8. [PubMed] [Google Scholar]
- Chapin RE, Delaney J, Wang Y, Lanning L, Davis B, et al. The effects of 4-nonylphenol in rats: a multigeneration reproduction study. Toxicol Sci. 1999;52:80–91. doi: 10.1093/toxsci/52.1.80. [DOI] [PubMed] [Google Scholar]
- Qiu YL, Wu DS, Zeng XG, Zhang H. Adverse effects of nonylphenol on the reproductive development of F1 male SD rats in sexual maturation period. Sichuan Da Xue Xue Bao Yi Xue Ban. 2005;36:382–5. [PubMed] [Google Scholar]
- de Jager C, Bornman MS, Oosthuizen JM. The effect of p-nonylphenol on the fertility potential of male rats after gestational, lactational and direct exposure. Andrologia. 1999;31:107–13. doi: 10.1046/j.1439-0272.1999.00246.x. [DOI] [PubMed] [Google Scholar]
- de Jager C, Bornman MS, van der Horst G. The effect of p-nonylphenol, an environmental toxicant with oestrogenic properties, on fertility potential in adult male rats. Andrologia. 1999;31:99–106. doi: 10.1046/j.1439-0272.1999.00245.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zeng X, Cheng W, Wu D. Adverse effects of nonylphenol on the reproductive function of adult male SD rats. Sichuan Da Xue Xue Bao Yi Xue Ban. 2003;34:295–7. [PubMed] [Google Scholar]
- Zhang H, Long DM, Zhan L, Wu DS. Effects of nonylphenol on testis tissue development and apoptosis of F1 generation male SD rats in weaning. Sichuan Da Xue Xue Bao Yi Xue Ban. 2006;37:421–3. [PubMed] [Google Scholar]
- Latchoumycandane C, Mathur PP. Effects of vitamin E on reactive oxygen species-mediated 2,3,7,8-tetrachlorodi-benzo-p-dioxin toxicity in rat testis. J Appl Toxicol. 2002;22:345–51. doi: 10.1002/jat.866. [DOI] [PubMed] [Google Scholar]
- Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:15–4. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahinturk V, Guclu C, Baycu C. Protective effects of vitamin E on ethane dimethane sulfonate-induced testicular toxicity in rats. Asian J Androl. 2007;9:117–24. doi: 10.1111/j.1745-7262.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- Zhou DX, Qiu SD, Zhang J, Tian H, Wang HX. The protective effect of vitamin E against oxidative damage caused by formaldehyde in the testes of adult rats. Asian J Androl. 2006;8:584–8. doi: 10.1111/j.1745-7262.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- Songthaveesin C, Saikhun J, Kitiyanant Y, Pavasuthipaisit K. Radio-protective effect of vitamin E on spermatogenesis in mice exposed to gamma-irradiation: a flow cytometric study. Asian J Androl. 2004;6:331–6. [PubMed] [Google Scholar]
- Azzi A, Stocker A. Vitamin E: non-antioxidant roles. Prog Lipid Res. 2000;39:231–55. doi: 10.1016/s0163-7827(00)00006-0. [DOI] [PubMed] [Google Scholar]
- Chen H, Liu J, Luo L, Baig MU, Kim JM, et al. Vitamin E, aging and Leydig cell steroidogenesis. Exp Gerontol. 2005;40:728–36. doi: 10.1016/j.exger.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Butkevich IP, Vershinina EA. [Nociceptive sensitivity to longterm exposure to an irritant in formaline test of males and females of rat in postnatal development] Zh Evol Biokhim Fiziol. 2005;41:76–81. [PubMed] [Google Scholar]
- Nagao T, Saito Y, Usumi K, Nakagomi M, Yoshimura S, et al. Disruption of the reproductive system and reproductive performance by administration of nonylphenol to newborn rats. Hum Exp Toxicol. 2000;19:284–96. doi: 10.1191/096032700678815909. [DOI] [PubMed] [Google Scholar]
- Soto AM, Justicia H, Wray JW, Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ Health Perspect. 1991;92:167–73. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya UR, Mishra M, Patro J, Panda MK. Effect of vitamins C and E on spermatogenesis in mice exposed to cadmium. Reprod Toxicol. 2008;25:84–8. doi: 10.1016/j.reprotox.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Mandarim-de-Lacerda CA. Stereological tools in biomedical research. An Acad Bras Cienc. 2003;75:469–86. doi: 10.1590/s0001-37652003000400006. [DOI] [PubMed] [Google Scholar]
- Latendresse JR, Warbrittion AR, Jonassen H, Creasy DM. Fixation of testes and eyes using a modified Davidson's fluid: comparison with Bouin's fluid and conventional Davidson's fluid. Toxicol Pathol. 2002;30:524–33. doi: 10.1080/01926230290105721. [DOI] [PubMed] [Google Scholar]
- Howard C, Reed M. Bios Scientific Publishers, Oxford, Unnited Kindom; 1998. Unbiased Stereology: Three-Dimentional Measurment in Microscopy. [Google Scholar]
- Mouton PR.Length and surface area. In: Mouton PR, editor. Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists Baltimore and London: The Johns Hopkins University Press; 2002103–5. [Google Scholar]
- Dalgaard M, Pilegaard K, Ladefoged O. In utero exposure to diethylstilboestrol or 4-n-nonylphenol in rats: number of sertoli cells, diameter and length of seminiferous tubules estimated by stereological methods. Pharmacol Toxicol. 2002;90:59–65. doi: 10.1034/j.1600-0773.2002.900202.x. [DOI] [PubMed] [Google Scholar]
- Ferrando RE, Nyengaard JR, Hays SR, Fahy JV, Woodruff PG. Applying stereology to measure thickness of the basement membrane zone in bronchial biopsy specimens. J Allergy Clin Immunol. 2003;112:1243–5. doi: 10.1016/j.jaci.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Peng B, Zhang RD, Dai XS, Deng XZ, Wan Y, et al. Quantitative (stereological) study of the effects of vasectomy on spermatogenesis in rhesus monkeys (Macaca mulatta) Reproduction. 2002;124:847–56. doi: 10.1530/rep.0.1240847. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yang F, Cai YQ, Xu Y. Oxidative damage in unfertilized eggs of Chinese rare minnow (Gobiocypris rarus) exposed to nonylphenol. Environ Toxicol Chem. 2008;27:213–9. doi: 10.1897/07-074.1. [DOI] [PubMed] [Google Scholar]
- Boekelheide K, Fleming SL, Johnson KJ, Patel SR, Schoenfeld HA. Role of Sertoli cells in injury-associated testicular germ cell apoptosis. Proc Soc Exp Biol Med. 2000;225:105–15. doi: 10.1046/j.1525-1373.2000.22513.x. [DOI] [PubMed] [Google Scholar]
- Nagao T, Wada K, Marumo H, Yoshimura S, Ono H. Reproductive effects of nonylphenol in rats after gavage administration: a two-generation study. Reprod Toxicol. 2001;15:293–315. doi: 10.1016/s0890-6238(01)00123-x. [DOI] [PubMed] [Google Scholar]
- O'Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr Rev. 2001;22:289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- McClusky LM, de Jager C, Bornman MS. Stage-related increase in the proportion of apoptotic germ cells and altered frequencies of stages in the spermatogenic cycle following gestational, lactational, and direct exposure of male rats to p-nonylphenol. Toxicol Sci. 2007;95:249–56. doi: 10.1093/toxsci/kfl141. [DOI] [PubMed] [Google Scholar]
- Takagi H, Shibutani M, Masutomi N, Uneyama C, Takahashi N, et al. Lack of maternal dietary exposure effects of bisphenol A and nonylphenol during the critical period for brain sexual differentiation on the reproductive/endocrine systems in later life. Arch Toxicol. 2004;78:97–105. doi: 10.1007/s00204-003-0517-0. [DOI] [PubMed] [Google Scholar]
- Latendresse JR, Newbold RR, Weis CC, Delclos KB. Polycystic kidney disease induced in F (1) Sprague–Dawley rats fed para-nonylphenol in a soy-free, casein-containing diet. Toxicol Sci. 2001;62:140–7. doi: 10.1093/toxsci/62.1.140. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Flynn KM, Delclos KB, Newbold RR. Maternal and offspring toxicity but few sexually dimorphic behavioral alterations result from nonylphenol exposure. Neurotoxicol Teratol. 2000;22:583–91. doi: 10.1016/s0892-0362(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Cooper S, Latendresse JR, Doerge DR, Twaddle NC, Fu X, et al. Dietary modulation of p-nonylphenol-induced polycystic kidneys in male Sprague–Dawley rats. Toxicol Sci. 2006;91:631–42. doi: 10.1093/toxsci/kfj171. [DOI] [PubMed] [Google Scholar]
- Cunny HC, Mayes BA, Rosica KA, Trutter JA, Van Miller JP. Subchronic toxicity (90-day) study with para-nonylphenol in rats. Regul Toxicol Pharmacol. 1997;26:172–8. doi: 10.1006/rtph.1997.1154. [DOI] [PubMed] [Google Scholar]


