Abstract
We evaluated the efficacy and safety of as-needed tadalafil in a diverse clinical population (with varying patient demographics, disease severity, and comorbid medical conditions) of Asian men with erectile dysfunction (ED). An integrated analysis of five double-blind, placebo-controlled trials (N = 1 046) was performed. Patients were randomly assigned to receive 10 mg tadalafil (N = 185), 20 mg tadalafil (N = 510), or placebo (N = 351). Efficacy assessments included the International Index of Erectile Function (IIEF), Sexual Encounter Profile (SEP) diary and Global Assessment Question (GAQ). Patients receiving 10 mg or 20 mg tadalafil showed significant improvement from baseline-to-end point on the Erectile Function domain of the International Index of Erectile Function (IIEF-EF) domain score in all clinical sub-populations analyzed, compared with patients receiving placebo (P < 0.001). The 10-mg and 20-mg tadalafil groups showed a mean success rate of 64.1% and 70.5% for sexual intercourse attempts (SEP3, successful intercourse), respectively, compared with 33.4% in the placebo group (P < 0.001), and 85.5% and 85.4% reported improved erections at end point GAQ, respectively, versus 43.5% in the placebo group (P < 0.001). Tadalafil was well tolerated across all groups studied. Headache and back pain were the most frequently reported adverse events. Overall, tadalafil was effective and well tolerated across a diverse clinical spectrum of Asian men with ED.
Keywords: Asia, comorbidity, erectile dysfunction, phosphodiesterase inhibitors, tadalafil
Introduction
The prevalence of erectile dysfunction (ED) is estimated to exceed 320 million men worldwide by the year 2025, with the largest projected increases in developing continents such as Africa, Asia, and South America 1. The diagnosis and treatment of ED has changed dramatically since safe and effective pharmacotherapies became available, and the increasing number of men seeking medical help for ED has resulted in a large and diverse treatment population.
Several risk factors for ED have been identified, including age, smoking, diabetes, depression, hyper-tension, and cardiovascular disease. Age is the variable that is most strongly associated with ED; the prevalence of complete ED triples from 5% at the age of 40 years to 15% at the age of 70 years 2. ED and cardiovascular disease share similar risk factors, and earlier studies have shown that ED may, in fact, be one of the first clinical manifestations of cardiovascular disease 3, 4. The severity of ischemic heart disease correlates with the severity of ED 5, and there is evidence of a higher prevalence and severity of ED in patients with hypertension and diabetes 2. In addition, patients with comorbid diabetes exhibit an impaired response to ED pharmacotherapy compared with those without diabetes 6, 7, 8.
Tadalafil is a phosphodiesterase type 5 (PDE5) inhibitor that has been shown to be safe and efficacious in the treatment of ED across a variety of clinical populations, including patients with the aforementioned risk factors 9, 10, 11. These studies, however, were performed in predominantly Caucasian sample populations, and disease prevalence, disease severity, and response to treatment may vary across ethnic groups 12. In this report, five double-blind placebo-controlled trials in Asian men with ED were integrated to determine the efficacy and safety of tadalafil in treating ED in clinical sub-populations of patients with risk factors for and comorbid conditions associated with ED.
Materials and methods
Study design
Five randomized, double-blind, placebo-controlled, parallel clinical trials with consistent designs were included in this integrated analysis (Table 1). The studies were conducted across nine Asian countries at 50 study sites between October 2000 and December 2003. The details of the study design have been published earlier 13, 14, 15, 16. In brief, the studies included a 4-week baseline period and a 12-week double-blind treatment period. Patients were randomly assigned to placebo (N = 351), 10 mg tadalafil (N = 185), or 20 mg tadalafil (N = 510). All five clinical trials included 20 mg tadalafil, and two of the studies included 10 mg tadalafil. The dosing strategy for the individual studies was based on regulatory requirements of the participating country(ies) and the anticipated approved dose at the time of study initiation. Patients were instructed to self-administer treatment as needed (up to once daily) before sexual intercourse, with no restrictions on food or alcohol intake. Dosage adjustments were not permitted in these studies.
Table 1. Summary of five Asian tadalafil clinical studies.
| Study |
Country(ies)/region(s) |
No. centers |
No. patients |
||
|---|---|---|---|---|---|
| Placebo | Tadalafil (10 mg) | Tadalafil (20 mg) | |||
| H6D-MC-LVCO | Taiwan (China) | 8 | 66 | 65 | 65 |
| H6D-MC-LVDW | Korea | 10 | 41 | – | 80 |
| H6D-MC-LVDX | India | 5 | 39 | – | 81 |
| H6D-MC-LVDY | Mainland China, Philippines, Singapore | 10 | 122 | 120 | 125 |
| H6D-MC-LVDZ | Hong Kong (China), Indonesia, Malaysia, Philippines, Singapore, Taiwan (China) | 17 | 83 | – | 159 |
All studies were conducted according to the Inter-national Conference on Harmonization (ICH) Good Clinical Practice Guidelines, founded on the Declaration of Helsinki. We obtained ethical committee approval and written informed consent from all patients before their entering into the study.
Study population
Men, at least 18 years of age, who had at least a 3-month history of ED, and anticipated having the same female sexual partner during the study period were eligible to participate. Patients were excluded from enrollment if they failed to achieve erection following radical prostatectomy or pelvic surgery, had clinically significant penile deformities or penile implants, had a recent history of stroke or spinal cord trauma, had cardiovascular disease (for example, unstable angina, recent myocardial infarction, recent myocardial revascularization, poorly controlled blood pressure), or had clinically significant renal or hepatic insufficiency. Additional exclusion criteria included current treatment with nitrates, cancer chemotherapy, or antiandrogens, and prior ineffective treatment with sildenafil.
Efficacy and safety assessments
Measures used to evaluate the efficacy of tadalafil included the Erectile Function domain of the International Index of Erectile Function (IIEF-EF) 17, Sexual Encounter Profile (SEP) questions 2 ('Were you able to insert your penis into your partner's vagina?') and 3 ('Did your erection last long enough for you to have successful intercourse?'), and the Global Assessment Question (GAQ) ('Has the treatment you have been taking over the past study interval improved your erection?'). The IIEF was administered after a 4-week treatment-free run-in period (baseline) and at 4-week intervals during treatment (post-baseline). Patients responded to the questions in the SEP diary after each sexual attempt throughout the study and reviewed the diary with the investigator at each visit. The GAQ was assessed upon study completion or early termination.
Measures used to evaluate the safety of tadalafil included the assessment of adverse events, in addition to the monitoring of vital signs, electrocardiograms (ECGs), and clinical laboratory tests (blood chemistry, hematology, and urinalysis). Adverse events entered by the investigators were coded using the Medical Dictionary for Regulatory Activities (MedDRA) Version 5 Preferred Terms.
Clinical populations
All patient subgroups analyzed will be referred to as clinical sub-populations. The integrated analysis included clinical sub-populations based on known risk factors for ED, such as age, smoking, diabetes mellitus type I or type II, hypertension, and cardiovascular disease (defined based on terms reflective of cardiac, arterial, and/or venous disease, including those of microcirculation), and other characteristics including ED severity (defined by baseline IIEF-EF score) and prior sildenafil use.
During randomization, patients were stratified by baseline ED severity according to a modified definition of Cappelleri et al. 18 (IIEF-EF domain score < 11, severe; 11–16, moderate; 17–30, mild). In the analysis of efficacy by ED severity, patients classified as having no ED at baseline (IIEF-EF domain score ≥ 26) were excluded from analysis.
Statistical analysis
Analyses were conducted on an intent-to-treat basis. Efficacy analyses included all patients who had a baseline measurement and at least one post-baseline measurement. Safety analyses included all randomized patients.
The IIEF domain and SEP scores were analyzed using Analysis of Covariance (ANCOVA) models, including terms for baseline value of the efficacy variable, treatment group, protocol, and baseline-by-treatment group interaction (if P < 0.10). Pairwise comparisons of tadalafil doses versus placebo were based on least squares (LS) means adjusted using the method of Dunnett 19. Responses to the GAQ and percentages of patients returning to normal erectile function (IIEF-EF domain score ≥ 26) were analyzed using a logistic regression model, and included the same terms as the ANCOVA models except that the baseline IIEF-EF domain value was used. Patients with a baseline IIEF-EF domain score ≥ 26 were excluded from this return-to-normal erectile function analysis. The efficacy analyses of clinical sub-populations used similar statistical models. For the purpose of efficacy analyses by geographic region, Hong Kong (China), Indonesia, and Malaysia were combined as 'other' due to small individual country samples.
All analyses were performed using Statistical Analysis Software version 8.2 (SAS®, SAS Institute Inc., Cary, NC, USA).
Results
Demographic and baseline characteristics
A summary of baseline demographic characteristics and ED history is presented in Table 2. The mean patient age was 53 years (range: 22–82 years), and 17% (n = 175) of patients were over 65 years old. The majority of patients (88%) were of East or South-East Asian origin; 12% of patients were of West Asian origin, and almost all of these patients (120 of 127 patients) were enrolled in Study H6D-MC-LVDX (India). The most common ED etiology was organic in nature (49%), followed by mixed (33%) and psychogenic cases (17%), and 88% of patients had experienced persistent ED for at least 1 year. Approximately one quarter of patients enrolled had at least one of the three most commonly reported comorbidities—cardiovascular disease (28%), diabetes (26%), and hypertension (25%).
Table 2. Patient demographics and baseline characteristics.
| Characteristic |
Total |
Placebo |
10 mg Tadalafil |
20 mg Tadalafil |
P-value |
|---|---|---|---|---|---|
| (N = 1 046) | (N = 351) | (N = 185) | (N = 510) | ||
| Mean age, years (range) | 53 (22–82) | 53 (22–81) | 55 (29–82) | 52 (24–79) | 0.069 |
| Age > 65, n (%) | 175 (17) | 55 (16) | 49 (26) | 71 (14) | < 0.001 |
| Ethnicity, n (%) | < 0.001 | ||||
| Caucasian | 1 (0) | 0 (0) | 0 (0) | 1 (0) | |
| East/Southeast Asian | 917 (88) | 310 (88) | 184 (99) | 423 (83) | |
| West Asian | 127 (12) | 41 (12) | 1 (1) | 85 (17) | |
| Other | 1 (0) | 0 (0) | 0 (0) | 1 (0) | |
| Mean body mass index, kg m−2 (range) | 25.0 (13.8–50.4) | 25.0 (16.9–37.5) | 25.0 (17.2–50.4) | 25.1 (13.8–38.5) | 0.956 |
| Smokers, n (%) | 354 (34) | 114 (32) | 74 (40) | 166 (33) | 0.232 |
| Prior sildenafil use, n (%) | 483 (46) | 162 (46) | 61 (33) | 260 (51) | < 0.001 |
| Duration of ED ≥ 12 months, n (%) | 920 (88) | 321 (91) | 171 (92) | 428 (84) | < 0.001 |
| ED etiology, n (%)a | 0.063 | ||||
| Psychogenic | 180 (17) | 72 (21) | 23 (12) | 85 (17) | |
| Organic | 517 (49) | 169 (48) | 87 (47) | 261 (51) | |
| Mixed | 349 (33) | 110 (31) | 75 (41) | 164 (32) | |
| Erectile dysfunction severity, n (%)b,c | 0.686 | ||||
| Mild (IIEF: 17–30) | 408 (39) | 134 (38) | 67 (36) | 207 (41) | |
| Moderate (IIEF: 11–16) | 344 (33) | 115 (33) | 59 (32) | 170 (33) | |
| Severe (IIEF: 1–10) | 294 (28) | 102 (29) | 59 (32) | 133 (26) | |
| Medical history, n (%) | |||||
| Cardiovascular disease | 294 (28) | 91 (26) | 47 (25) | 156 (31) | 0.218 |
| Coronary artery disease | 31 (3) | 14 (4) | 4 (2) | 13 (3) | 0.368 |
| Depression | 8 (1) | 2 (1) | 2 (1) | 4 (1) | 0.810 |
| Diabetes mellitus | 273 (26) | 93 (26) | 22 (12) | 158 (31) | < 0.001 |
| Hypertension | 259 (25) | 80 (23) | 38 (21) | 141 (28) | 0.092 |
| Hyperlipidaemia | 83 (8) | 32 (9) | 12 (6) | 39 (8) | 0.533 |
Abbreviations: ED, erectile dysfunction; IIEF, International Index of Erectile Function; N, number of randomized patients per treatment group; n, number of patients.
The cause of erectile dysfunction was defined subjectively according to each investigator's clinical opinion.
Erectile dysfunction severity categories were based on a modified version of the definition by Cappelleri et al. 18.
The subsequent assessment of erectile function by the IIEF at baseline indicated that a small percentage of patients had an erectile function domain score in the normal range (26–30).
Differences (P < 0.001) were observed between the treatment groups for the following baseline characteristics: proportion of patients > 65 years old, ethnicity, prior sildenafil use, proportion of patients with ED duration > 1 year, and proportion of patients with comorbid diabetes. Study H6D-MC-LVDX (India) investigated only placebo and 20 mg tadalafil, and included almost all of the West Asian patients in this integrated analysis; therefore, the 10-mg tadalafil group contained a lower proportion of West Asian patients, a higher percentage of patients > 65 years old, and a lower percentage of patients with diabetes (patients in Study H6D-MC-LVDX had a mean age of 45 years, and 63% of patients had pre-existing comorbid diabetes).
Of the 1 046 patients enrolled in the five studies, 976 (93%) completed 12 weeks of treatment (placebo 92%, 10 mg tadalafil 92%, 20 mg tadalafil 95%), as shown in Figure 1.
Figure 1.
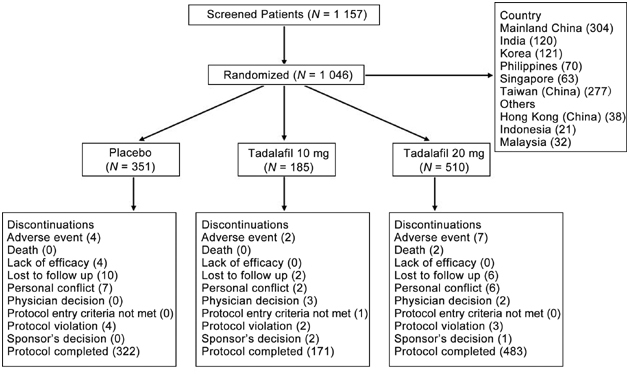
Patient disposition.
Overall efficacy results
Tadalafil-treated patients in both the 10-mg and the 20-mg groups showed greater improvement than placebo-treated patients (P < 0.001) on all efficacy measures (Table 3), including the IIEF-EF domain score, the mean per-patient 'yes' response to SEP2 (successful penetration) and SEP3 (successful intercourse), and the GAQ. Tadalafil at a dose of 20 mg showed numerically (although not statistically) greater improvement in efficacy measures than 10 mg tadalafil. The percentage of patients achieving normal erectile function (IIEF-EF domain score ≥ 26) at the end point was greatest for the 20-mg tadalafil group (45%, [n = 222]); P < 0.001 vs. placebo, P = 0.102 vs. 10 mg), followed by the 10-mg tadalafil group (36%, [n = 63]; P < 0.001 vs. placebo) and the placebo group (12%, [n = 40]).
Table 3. Summary of major efficacy variables at the study end pointa.
| |
Placebo (N = 351) |
10 mg Tadalafil (N = 185) |
20 mg Tadalafil (N = 510) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | End point | Change | n | Baseline | End point | Change | n | Baseline | End point | Change | |
| IIEF-EF domain (mean) | 347 | 14.6 | 16.7 | 2.0 | 181 | 13.9 | 21.9 | 7.2* | 502 | 14.9 | 23.0 | 8.3* |
| Mean per-patient 'yes' response to SEP questions (%) | ||||||||||||
| SEP2. Successful penetration | 348 | 51.3 | 55.3 | 4.0 | 181 | 43.6 | 77.3 | 26.0* | 502 | 54.2 | 82.9 | 31.5* |
| SEP3. Successful intercourse | 348 | 20.7 | 33.4 | 13.8 | 181 | 15.4 | 64.1 | 44.5* | 502 | 20.4 | 70.5 | 50.9* |
| GAQ question (%) | ||||||||||||
| Improved erections | 338 | 43.5 | 179 | 85.5* | 493 | 85.4* | ||||||
| IIEF-EF ≥ 26 at end point, n (%) | 336 | 40 (11.9) | 175 | 63 (36.0) | 489 | 222 (45.4)* | ||||||
| IEF domains (mean) | ||||||||||||
| Intercourse satisfaction | 347 | 6.2 | 8.0 | 1.7 | 181 | 5.9 | 10.2 | 3.9* | 502 | 6.6 | 10.5 | 4.2* |
| Overall satisfaction | 347 | 4.3 | 5.1 | 0.8 | 181 | 4.4 | 6.6 | 2.3* | 502 | 4.4 | 6.8 | 2.5* |
Abbreviations: GAQ, Global Assessment Question; IIEF, International Index of Erectile Function; N, number of patients randomized per treatment group; n, number of patients having both baseline and end point data; SEP, Sexual Encounter Profile.
Least squares mean end point and change reported, with the exception of GAQ and return to normal erectile function (IIEF-EF ≥ 26) results.
P < 0.001 vs. placebo.
Efficacy in clinical populations
The IIEF-EF domain score and SEP3 efficacy measures were further analyzed by clinical sub-populations (Figure 2 and Figure 3, respectively). Baseline IIEF-EF domain scores were numerically lower for patients > 65 years (vs. ≤ 65 years old) and for patients with comorbid diabetes, hypertension, or cardiovascular disease (vs. without). However, baseline-to-end point improvement in IIEF-EF domain score was not limited by the presence of risk factors, with the 10-mg and 20-mg tadalafil groups showing greater (P < 0.001) mean improvement than the placebo group across all clinical sub-populations (Figure 2).
Figure 2.
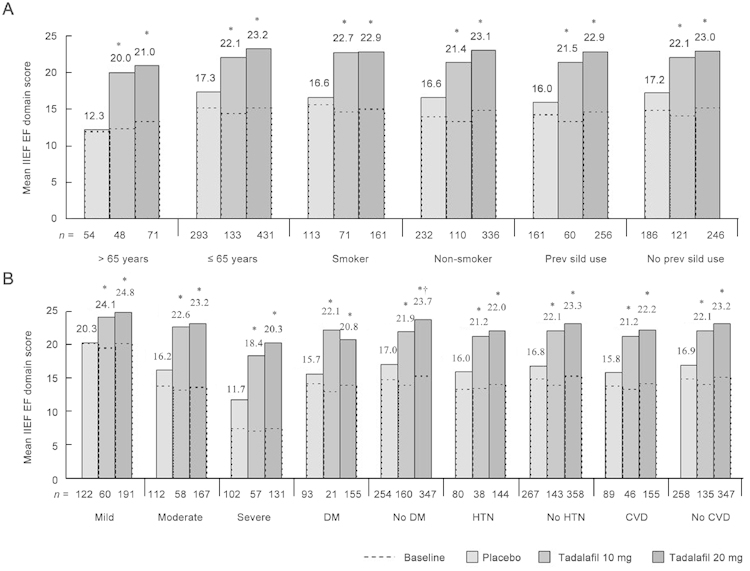
Baseline mean International Index of Erectile Function (IIEF) EF domain scores and least squares mean scores at the end point for placebo, 10-mg tadalafil, and 20-mg tadalafil groups. (A): Scores for clinical sub-populations based on patient demographics, (B): scores for clinical sub-populations based on ED severity and comorbid medical conditions. ED severity categories were determined based on a modified version of the definition described by Cappelleri et al. 18. ***: IIEF-EF domain score < 11, severe; 11–16, moderate; 17–30, mild. DM, diabetes mellitus; HTN, hypertension; CVD, cardiovascular disease. *P < 0.001, least squares mean improvement vs. placebo. †P < 0.05, least squares mean improvement of 20 mg vs. 10 mg tadalafil.
Figure 3.
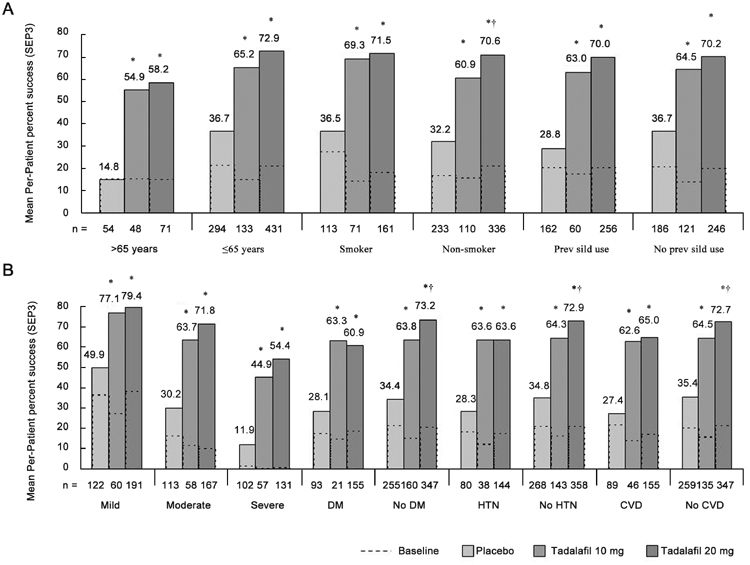
Baseline mean per-patient percentage of successful intercourse (Sexual Encounter Profile question 3 [SEP3]) and least squares mean percentage at the end point for the placebo, 10-mg tadalafil, and 20-mg tadalafil groups. (A): Scores for clinical sub-populations based on patient demographics, (B): scores for clinical sub-populations based on ED severity and comorbid medical conditions. ED severity categories were based on a modified version of the definition described by Cappelleri et al. 18: IIEF-EF domain score < 11, severe; 11–16, moderate; 17–30, mild. DM, diabetes mellitus; HTN, hypertension; CVD, cardiovascular disease. *P < 0.001, least squares mean improvement vs. placebo. †P < 0.05, least squares mean improvement of 20 mg vs. 10 mg tadalafil.
The SEP3 results (Figure 3) paralleled those of the IIEF-EF domain. Tadalafil treatment at doses of both 10 mg and 20 mg was associated with greater (P < 0.001) baseline-to-end point improvement in sexual intercourse for all clinical sub-populations. Most sub-populations had end point success rates between 60% and 80% patients over 65 years of age and patients with severe ED at baseline were exceptions, with mean success rates (for 20-mg tadalafil groups) of 58% and 54%, respectively. However, despite the lower end point values in these groups, patients experienced greater improvements than their younger and less severely affected counterparts; the SEP3 LS mean change difference was 43.4% for patients > 65 years vs. 36.3% for patients ≤ 65 years old and 42.5% for patients with severe ED vs. 29.5% for patients with mild ED (20-mg tadalafil groups).
In these IIEF-EF domain and SEP3 subgroup analyses, 20 mg tadalafil showed numerically greater improvement in all efficacy measures than 10 mg tadalafil, except in patients with diabetes. SEP3 improvement was statistically greater (P < 0.05) for 20 mg vs. 10 mg tadalafil in the following clinical sub-populations: non-smokers, patients without diabetes, patients without hypertension, and patients without cardiovascular disease. Also, tadalafil treatment was effective across efficacy measures, irrespective of geographic region, as shown in Figure 4.
Figure 4.
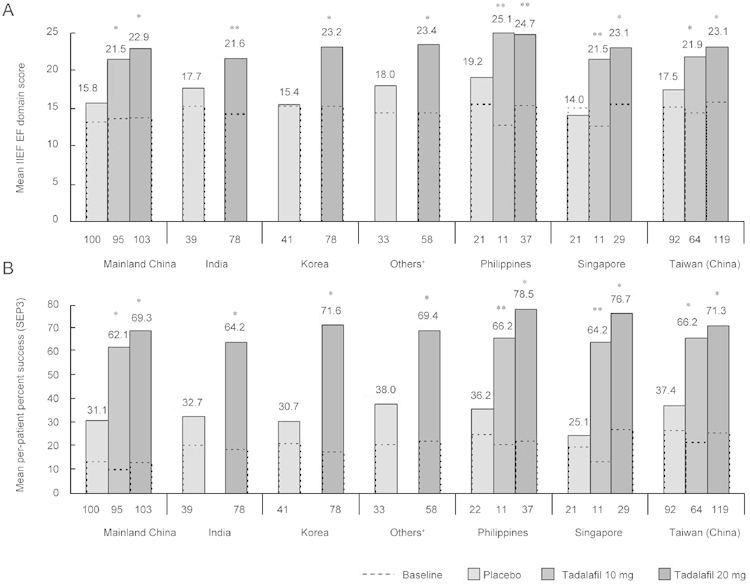
Efficacy analyses by geographic region. (A): Baseline mean International Index of Erectile Function (IIEF) EF domain scores and least squares mean scores at end point for placebo, 10-mg tadalafil, and 20-mg tadalafil groups. (B): Baseline mean per-patient percentage of successful intercourse (Sexual Encounter Profile question 3 [SEP3]) and least squares mean percentage at end point for placebo, 10-mg tadalafil, and 20-mg tadalafil groups. †Others = Hong Kong (China), Indonesia, Malaysia. *P < 0.001, **P < 0.05, least squares mean improvement vs. placebo.
Safety results
The incidence of treatment-emergent adverse events (TEAEs) is summarized in Table 4. The most common TEAEs reported by the combined tadalafil group were headache, back pain, and myalgia. The rate of discontinuation due to adverse events in the tadalafil group was 1.3% compared with 1.1% in the placebo group (Figure 1). Twelve patients (nine treated with 20 mg tadalafil and three treated with placebo) experienced serious adverse events (SAEs), including two deaths (both treated with 20 mg tadalafil). The causes of death were colon cancer and murder; neither was considered related to the study drug in the opinion of the investigator. One SAE (worsening of coronary artery disease) occurring in a patient on 20 mg tadalafil was considered possibly related to study drug by the investigator and this case is described elsewhere 16. No clinically meaningful changes in vital signs, ECG findings, or laboratory tests attributable to tadalafil were observed.
Table 4. Summary of treatment-emergent adverse events occurring in ≥ 2% of patients in the combined tadalafil treatment group.
| Adverse eventsa | Placebo | Tadalafil (10 mg) | Tadalafil (20 mg) | Tadalafil combined | ||||
|---|---|---|---|---|---|---|---|---|
| (N = 351) | (N = 185) | (N = 510) | (N = 695) | |||||
| n | % | n | % | n | % | n | % | |
| Headache | 10 | 2.8 | 9 | 4.9 | 56 | 11.0 | 65 | 9.4 |
| Back pain | 8 | 2.3 | 8 | 4.3 | 26 | 5.1 | 34 | 4.9 |
| Myalgia | 0 | 0.0 | 5 | 2.7 | 16 | 3.1 | 21 | 3.0 |
| Dizziness | 6 | 1.7 | 6 | 3.2 | 13 | 2.5 | 19 | 2.7 |
| Flushing | 4 | 1.1 | 5 | 2.7 | 14 | 2.7 | 19 | 2.7 |
| Dyspepsia | 1 | 0.3 | 3 | 1.6 | 12 | 2.4 | 15 | 2.2 |
| Gastritis | 2 | 0.6 | 0 | 0.0 | 15 | 2.9 | 15 | 2.2 |
| Nasal congestion | 3 | 0.9 | 4 | 2.2 | 11 | 2.2 | 15 | 2.2 |
| Cough | 10 | 2.8 | 6 | 3.2 | 8 | 1.6 | 14 | 2.0 |
Abbreviations: N, number of patients randomized; n, number of patients reporting adverse events.
Adverse events listed by MedDRA 5.0 coding term and ordered by decreasing frequency for the combined tadalafil treatment group.
Discussion
Earlier studies have shown the efficacy and safety of PDE5 inhibitors in Asian men 20, 21; however, this integrated analysis is the first to report on the efficacy and safety of tadalafil in Asian men across a broad spectrum of clinical sub-populations. This integrated analysis of 1 046 Asian men with ED showed that treatment with tadalafil at 10 mg or 20 mg improves erectile function irrespective of patient demographic characteristics (age, smoking status, prior sildenafil use) and comorbid medical conditions (diabetes, hypertension, cardiovascular disease).
The improvements in erectile function observed in this Asian population mirror those observed in earlier studies in Western populations 22, 23, 24. Patients who received 20 mg tadalafil in this analysis had, on average, an IIEF-EF domain score of 23.0 at the end point, corresponding to mild ED as defined by Cappelleri et al. 18. The mean success rate for intercourse attempts (SEP3) with 20 mg tadalafil was 70.5%, which is comparable to results reported in Japan (69.4%) 25, the US and Puerto Rico (67.6%) 24, Canada (61.5%) 22, and Western Europe (73.9%) 23.
The efficacy of tadalafil was evident irrespective of patient age, prior sildenafil use, smoking status, or ED severity. In this study, younger patients (≤ 65 years) had numerically better scores than older patients on each of the efficacy measures at baseline (all patients > 65 years old vs. ≤ 65 years old: IIEF-EF 12.7 vs. 15.1; SEP3 15.4 vs. 20.4), which is consistent with the Massachusetts Male Aging Study, which reported an increase in moderate and severe ED from age 40 to 70 2. The magnitude of improvement following tadalafil treatment (corrected for placebo response) was more pronounced for older patients, however, which may be expected with lower baseline scores.
Both tadalafil doses showed greater improvements in erectile function than placebo (P < 0.001) for each ED severity level (mild, moderate, or severe ED). In this analysis, patients with severe ED at baseline had a 54% success rate of sexual intercourse at end point following 20-mg tadalafil treatment, which is comparable to the 50% success rate observed in an integrated analysis of 11 double-blind, placebo-controlled studies of tadalafil across a diverse population of Caucasian, African descent, Hispanic, and Asian patients 11.
Marumo and co-workers 26 recently showed that patients with severe ED experienced greater improvement in erectile function with 20 mg than 10 mg tadalafil, whereas, improvement in the overall ED population was less pronounced. In this study, this was also the case, because the difference in SEP3 between 20 mg and 10 mg tadalafil widened as the severity of ED increased from mild to moderate to severe. Surprisingly, this was not the case for patients of more advanced age or with comorbidities, factors that are normally associated with more severe ED. In fact, the diabetic sub-population showed numerically greater efficacy improvements with 10 mg than with 20 mg tadalafil. However, the 10 mg tadalafil diabetes subgroup was the smallest clinical sub-population examined (n = 21 reporting), and the resulting standard error was relatively large. Unfortunately, inadequate power (particularly in the older and comorbid sub-populations) and imbalances between individual trials (some of which omitted 10 mg tadalafil) limit interpretation of the 20 mg versus 10 mg tadalafil comparisons in this analysis.
Comorbid diabetes, hypertension, coronary artery disease, and depression are known risk factors for ED, and are therefore observed at a higher prevalence in patients with ED than in the general population. In this report, approximately one quarter of the patients with ED had at least one of the three most common comorbid conditions: cardiovascular disease, diabetes, and hypertension. This frequency of diabetes is higher than that reported in Western ED populations 23, 24 and is largely due to the high frequency of diabetes in Indian patients with ED (63% in Study H6D-MC-LVDX of this analysis). As expected, baseline erectile function scores were numerically lower for patients with comorbid diabetes, hypertension, and cardiovascular disease; however, the magnitude of improvement with treatment was not impaired. For patients with diabetes, these findings are consistent with those from an earlier published placebo-controlled study, in which tadalafil improved all efficacy measures compared with placebo in patients with ED and either type I or type II diabetes 27.
Tadalafil was safe and well tolerated in the five clinical studies included in this report. The most frequently reported TEAEs associated with tadalafil included headache, back pain, and myalgia. These events are consistent with the known safety profile of PDE5 inhibition and have been reported earlier in studies with PDE5 inhibitors 9, 10, 21, 28, 29. The rates of adverse events with tadalafil treatment reported by this Asian population were relatively low compared with those in Western populations; for example, headache was reported by 11.0% of patients in the 20-mg tadalafil group in this study but by 17.0% and 17.9% of patients from Canada 22 and Western Europe 23, respectively. A recent Japanese tadalafil study reported similar rates of adverse events (headache 18.6% for 20 mg tadalafil) 25, suggesting that less frequent adverse event reporting is not uniform across Asia. Geographical variation in adverse event reporting in clinical trials has been described earlier, and may be related to cultural differences, such as attitudes towards health and authority 30.
The pharmacokinetic profile of tadalafil is comparable in Asian and Caucasian healthy male subjects 31, 32. However, the efficacy and safety of PDE5 inhibitors may be influenced by comorbidities 6, concomitant medication 33 (including, potentially, Chinese traditional medicine), and cultural factors, including sexual behavior and dosing compliance (that is, administering the dose at an appropriate interval prior to intercourse) 9, 34. Earlier research has suggested that tadalafil dosing and sexual intercourse patterns vary geographically and culturally 35, 36. Examination of the efficacy of tadalafil according to time after dosing in this Asian population would be a valuable future investigation.
In conclusion, this integrated analysis showed that tadalafil treatment is as efficacious and safe in Asian men with ED and comorbid conditions or risk factors (such as diabetes, hypertension, cardiovascular disease, and/or older age) as in the Caucasian population. Both 10-mg and 20-mg doses of tadalafil were well tolerated and effective in improving erectile function across a wide clinical spectrum of ED patients in Asia.
Acknowledgments
This study was supported by Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, Indiana, USA. Authors Wei Shen and Vladimir Kopernicky are employees of Eli Lilly and Company. The authors acknowledge the contributions of Gabrielle Gallagher and Haoyue Zeigler in writing and statistical support, respectively. Dr. Gallagher and Ms. Zeigler are employees of Eli Lilly and Company.
References
- Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50–6. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- Billups KL. Erectile dysfunction as an early sign of cardiovascular disease. Int J Impot Res. 2005;17 Suppl 1:S19–24. doi: 10.1038/sj.ijir.3901425. [DOI] [PubMed] [Google Scholar]
- Maas R, Schwedhelm E, Albsmeier J, Boger RH. The pathophysiology of erectile dysfunction related to endothelial dysfunction and mediators of vascular function. Vasc Med. 2002;7:213–25. doi: 10.1191/1358863x02vm429ra. [DOI] [PubMed] [Google Scholar]
- Greenstein A, Chen J, Miller H, Matzkin H, Villa Y, et al. Does severity of ischemic coronary disease correlate with erectile function. Int J Impot Res. 1997;9:123–6. doi: 10.1038/sj.ijir.3900282. [DOI] [PubMed] [Google Scholar]
- Fonseca V, Seftel A, Denne J, Fredlund P. Impact of diabetes mellitus on the severity of erectile dysfunction and response to treatment: analysis of data from tadalafil clinical trials. Diabetologia. 2004;47:1914–23. doi: 10.1007/s00125-004-1549-6. [DOI] [PubMed] [Google Scholar]
- Price DE, Gingell JC, Gepi-Attee S, Wareham K, Yates P, et al. Sildenafil: study of a novel oral treatment for erectile dysfunction in diabetic men. Diabet Med. 1998;15:821–5. doi: 10.1002/(SICI)1096-9136(199810)15:10<821::AID-DIA697>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Ziegler D, Merfort F, van Ahlen H, Yassin A, Reblin T, et al. Efficacy and safety of flexible-dose vardenafil in men with type 1 diabetes and erectile dysfunction. J Sex Med. 2006;3:883–91. doi: 10.1111/j.1743-6109.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- Brock GB, McMahon CG, Chen KK, Costigan T, Shen W, et al. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analyses. J Urol. 2002;168:1332–6. doi: 10.1016/S0022-5347(05)64442-4. [DOI] [PubMed] [Google Scholar]
- Carson CC, Rajfer J, Eardley I, Carrier S, Denne JS, et al. The efficacy and safety of tadalafil: an update. BJU Int. 2004;93:1276–81. doi: 10.1111/j.1464-410X.2004.04819.x. [DOI] [PubMed] [Google Scholar]
- Lewis RW, Sadovsky R, Eardley I, O'Leary M, Seftel A, et al. The efficacy of tadalafil in clinical populations. J Sex Med. 2005;2:517–31. doi: 10.1111/j.1743-6109.2005.00068.x. [DOI] [PubMed] [Google Scholar]
- Morgentaler A, Barada J, Niederberger C, Donatucci C, Garcia CS, et al. Efficacy and safety of tadalafil across ethnic groups and various risk factors in men with erectile dysfunction: use of a novel noninferiority study design. J Sex Med. 2006;3:492–503. doi: 10.1111/j.1743-6109.2006.00209.x. [DOI] [PubMed] [Google Scholar]
- Chen K-K, Jiann BP, Lin JS, Lee S-S, Huang S-T, et al. Efficacy and safety of on-demand oral tadalafil in the treatment of men with erectile dysfunction in Taiwan: a randomized, double-blind, parallel, placebo-controlled clinical study. J Sex Med. 2004;1:201–8. doi: 10.1111/j.1743-6109.2004.04029.x. [DOI] [PubMed] [Google Scholar]
- Choi HK, Ahn TY, Kim JJ, Kim SC, Paick JS, et al. A double-blind, randomised- placebo, controlled, parallel group, multicentre, flexible-dose escalation study to assess the efficacy and safety of sildenafil administered as required to male outpatients with erectile dysfunction in Korea. Int J Impot Res. 2003;15:80–6. doi: 10.1038/sj.ijir.3900944. [DOI] [PubMed] [Google Scholar]
- Guo YL, Zhu JC, Pan TM, Ding Q, Wang YX, et al. Efficacy and safety of on-demand tadalafil for the treatment of erectile dysfunction in South-East Asian men. Int J Urol. 2006;13:721–7. doi: 10.1111/j.1442-2042.2006.01393.x. [DOI] [PubMed] [Google Scholar]
- Yip WC, Chiang HS, Mendoza JB, Tan HM, Li MK, et al. Efficacy and safety of on demand tadalafil in the treatment of East and Southeast Asian men with erectile dysfunction: a randomized double-blind, parallel, placebo-controlled clinical study. Asian J Androl. 2006;8:685–92. doi: 10.1111/j.1745-7262.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Siegel RL, Osterloh IH, Rosen RC. Relationship between patient self-assessment of erectile function and the erectile function domain of the international index of erectile function. Urology. 2000;56:477–81. doi: 10.1016/s0090-4295(00)00697-x. [DOI] [PubMed] [Google Scholar]
- Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–121. [Google Scholar]
- Lim PH, Li MK, Ng FC, Chia SJ, Consigliere D, et al. Clinical efficacy and safety of sildenafil citrate (Viagra) in a multi-racial population in Singapore: a retrospective study of 1520 patients. Int J Urol. 2002;9:308–15. doi: 10.1046/j.1442-2042.2002.00425.x. [DOI] [PubMed] [Google Scholar]
- Tan HM, Moh CL, Mendoza JB, Gana T, Albano GJ, et al. Asian sildenafil efficacy and safety study (ASSESS-1): a double-blind, placebo-controlled, flexible-dose study of oral sildenafil in Malaysian, Singaporean, and Filipino men with erectile dysfunctionThe Assess-1 Study Group. Urology 200056635–40. [DOI] [PubMed] [Google Scholar]
- Carrier S, Brock GB, Pommerville PJ, Shin J, Anglin G, et al. Efficacy and safety of oral tadalafil in the treatment of men in Canada with erectile dysfunction: a randomized, double-blind, parallel, placebo-controlled clinical trial. J Sex Med. 2005;2:685–98. doi: 10.1111/j.1743-6109.2005.00097.x. [DOI] [PubMed] [Google Scholar]
- Eardley I, Gentile V, Austoni E, Hackett G, Lembo D, et al. Efficacy and safety of tadalafil in a Western European population of men with erectile dysfunction. BJU Int. 2004;94:871–7. doi: 10.1111/j.1464-410X.2004.05049.x. [DOI] [PubMed] [Google Scholar]
- Seftel AD, Wilson SK, Knapp PM, Shin J, Wang WC, et al. The efficacy and safety of tadalafil in United States and Puerto Rican men with erectile dysfunction. J Urol. 2004;172:652–7. doi: 10.1097/01.ju.0000132857.39680.ce. [DOI] [PubMed] [Google Scholar]
- Nagao K, Kimoto Y, Marumo K, Tsujimura A, Vail GM, et al. Efficacy and safety of tadalafil 5, 10, and 20 mg in Japanese men with erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled study. Urology. 2006;68:845–51. doi: 10.1016/j.urology.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Marumo K, Imaoka T, Fujimoto K, Watts S, Stothard D, et al. A comparison of the efficacy and tolerability of tadalafil 10 mg and 20 mg in Japanese patients with severe erectile dysfunction. J Sex Med. 2007;4:745–52. doi: 10.1111/j.1743-6109.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- De Tejada IS, Emmick J, Anglin G, Fredlund P, Pullman W. The effect of as-needed IC351 treatment of erectile dysfunction in men with diabetes. Diabetologia. 2001;44 Suppl 1:A295. [Google Scholar]
- Carson CC, Burnett AL, Levine LA, Nehra A. The efficacy of sildenafil citrate (Viagra) in clinical populations: an update. Urology. 2002;60 Suppl 2:12–27. doi: 10.1016/s0090-4295(02)01687-4. [DOI] [PubMed] [Google Scholar]
- Montorsi F, Verheyden B, Meuleman E, Junemann KP, Moncada I, et al. Long-term safety and tolerability of tadalafil in the treatment of erectile dysfunction. Eur Urol. 2004;45:339–44. doi: 10.1016/j.eururo.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Joelson S, Joelson IB, Wallander MA. Geographical variation in adverse event reporting rates in clinical trials. Pharmacoepidemiol Drug Saf. 1997;6 Suppl 3:S31–5. doi: 10.1002/(sici)1099-1557(199710)6:3+<s31::aid-pds288>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- Forgue ST, Patterson BE, Bedding AW, Payne CD, Phillips DL, et al. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2006;61:280–8. doi: 10.1111/j.1365-2125.2005.02553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Uenaka K, Imaoka T. Pharmacological, pharmacokinetic, and clinical profile of tadalafil (Cialis) Nippon Yakurigaku Zasshi. 2008;131:469–77. doi: 10.1254/fpj.131.469. [DOI] [PubMed] [Google Scholar]
- Corona G, Razzoli E, Forti G, Maggi M. The use of phosphodiesterase 5 inhibitors with concomitant medications. J Endocrinol Invest. 2008;31:799–808. doi: 10.1007/BF03349261. [DOI] [PubMed] [Google Scholar]
- Hatzichristou D, Moysidis K, Apostolidis A, Bekos A, Tzortzis V, et al. Sildenafil failures may be due to inadequate patient instructions and follow-up: a study on 100 non-responders. Eur Urol. 2005;47:518–22. doi: 10.1016/j.eururo.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ahn TY, Lee SW, Kim SW, Yang DY, Park NC, et al. Treatment preferences in men with erectile dysfunction: an open label study in Korean men switching from sildenafil citrate to tadalafil. Asian J Androl. 2007;9:760–70. doi: 10.1111/j.1745-7262.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- Yaman O, Chlosta P, Kovalev V, Shenfeld O, Pacik D, et al. Switching from sildenafil (viagra) to tadalafil (cialis) in men with erectile dysfunction in Central/Eastern Europe and Eastern Mediterranean regions: assessment of sexual attempt behavior and psychological and interpersonal relationship scales. J Sex Med. 2005;2 Suppl 1:65. [Google Scholar]


