Abstract
Resveratrol is a dietary polyphenol espoused to have chemopreventive activity against a variety of human cancer types. We first reported that resveratrol significantly decreases the proliferation of both androgen-dependent and hormone-refractory prostate cancer cells. However, the effects of resveratrol in normal prostate epithelial and stromal cells, particularly with regard to its uptake, subcellular distribution and intracellular targets, have not been investigated. To advance the knowledge on accessibility and cellular disposition of resveratrol in prostate cells, [3H] resveratrol, fractionation of cell extracts into subcellular compartments, Western blot analysis, resveratrol affinity column chromatography and flow cytometry were used to study the uptake and intracellular distribution of resveratrol in normally cultured prostate stromal (PrSCs) and epithelial cells (PrECs). Pretreatment of both PrSCs and PrECs for 2 days with resveratrol modulated its uptake and selectively increased its distribution to the membrane and organelle compartments. Resveratrol affinity column chromatography studies showed differential expression of a previously identified resveratrol-targeting protein, quinone reductase 2 (QR2), in PrSCs and PrECs. Flow cytometric analysis comparing resveratrol-treated and untreated PrSCs showed a large decrease in G1-phase and a concomitant increase in S and G2/M-phases of the cell cycle. These results suggest that resveratrol suppresses PrSC proliferation by affecting cell cycle phase distribution, which may involve the participation by QR2.
Keywords: affinity column chromatography, prostate epithelial cells, prostate stromal cells, quinone reductase 2, resveratrol
Introduction
Resveratrol is a recently identified food-derived polyphenol claimed to protect against malignant and cardiovascular diseases, osteoporosis and nephrotoxic drugs 1, 2, 3, 4, 5, 6. The chemopreventive property of resveratrol is illustrated by suppression of cell proliferation and induction of apoptosis in numerous cancer cell types 7, 8, 9, 10. Animal studies provide support that resveratrol inhibits tumorigenesis at the stages of initiation, promotion and progression 11, 12. These results suggest that resveratrol might indeed confer chemoprotection even in humans.
Despite the relatively well-characterized responses elicited by resveratrol in transformed and malignant cells, its molecular actions and mechanisms in physiologically relevant settings and normal tissues remain largely unknown. It is to be noted that there is a paucity of data on the affects of resveratrol in normal cells, particularly regarding its uptake, cellular disposition and interaction with distinct targets. In earlier studies, we and others have shown that resveratrol displayed anti-prostate cancer (CaP) properties in androgen-dependent and hormone-refractory CaP cells 13, 14, 15, 16, 17, 18, 19. To advance the knowledge on the accessibility of resveratrol in prostate cells, we studied the kinetics of uptake and intracellular trafficking and distribution of this polyphenol using normally cultured prostate stromal (PrSCs) and epithelial cells (PrECs). We also investigated the cellular affects and targets of this polyphenol on PrSCs and PrECs. Our results suggest that pretreatment of both PrSCs and PrECs by resveratrol substantially modulated the cellular uptake of this polyphenol. Moreover, whereas resveratrol-targeting protein, quinone reductase 2 (QR2), was only detected at a low level in PrECs, it showed a robust expression in PrSCs, which, as we suggest, may contribute to the control of proliferation and cell cycle phase transition by resveratrol in PrSCs.
Materials and methods
Materials
Human PrSCs and PrECs were purchased from Lonza Walkersville Inc (Walkersville, MD, USA). Cells were cultured and passaged using Clonetics stromal cell growth medium (SCGM) and Clonetics prostate epithelial cell growth medium (PrEGM). Both growth media were provided as BulletKit containing the requisite cell type-specific growth factors, cytokines and supplements. Alternatively, cells were cultured using complete media supplemented with SingleQuots, as recommended by the manufacturer (Lonza Walkersville, MD, USA). Cells were maintained in CO2 incubator and media were changed every 3–4 days. All experiments used passages 2–5 PrSCs and PrECs. The Subcellular Proteome extraction kit was purchased from Calbiochem (San Diego, CA, USA). [3H] resveratrol (specific activity, 15 Ci mmol L−1) was obtained from Moravek Biochemicals (Brea, CA, USA). Epoxy-activated agarose resin and resveratrol were purchased from Sigma Chemical Co (St Louis, MO, USA). Resveratrol was dissolved in dimethyl sulfoxide as a 12.5-mmol L−1 stock and maintained in aliquots at −20°C. Primary antibodies for actin and secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Polyclonal anti-QR2 antibody was generated in rabbits by Cocalico Biologicals Inc (Reamstown, PA, USA). All other chemicals and solvents used were of analytical grade.
Preparation and subcellular fractionation of whole cell extracts
Cells were collected by centrifugation and were lysed in ice-cold RIPA buffer (50 mmol L−1 Tris, pH 7.4, 150 mmol L−1 NaCl, 1 mmol L−1 EDTA [ethylenediaminetetraacetic acid], 1% Triton X-100, 1% deoxycholate and 0.1% SDS). Protease inhibitor cocktail (1%, Sigma) and 1 mmol L−1 dithiothreitol were added to the buffer immediately before use. The cell suspension mixture was incubated on ice for 20 min with intermittent mixing and then centrifuged at 14 000 × g. The clear supernatant was stored in aliquots at −70°C for further analysis. Fractionation of the supernatant from control and treated cells was performed using the Subcellular Proteome extraction kit, into four cellular compartments, namely, F1 (cytosol), F2 (membrane/organelle), F3 (nucleus) and F4 (cytoskeleton). Protein content of cell lysates and cellular fractions was determined by coomassie protein assay kit (Pierce, Rockford, IL, USA) with bovine serum albumin as standard.
Measurement of resveratrol uptake
Cells pretreated with 0, 10 or 50 μmol L−1 resveratrol for 2 days were incubated with 5 nmol L−1 [3H] resveratrol for 0, 5, 10 and 20 min in serum-free medium at 37°C, 95% humidity and 5% CO2. At the end of the indicated labeling period, cells were harvested, washed thrice with ice-cold phosphate-buffered saline to remove unincorporated resveratrol. Attached cells were lysed with 1 mL 0.1 mol L−1 NaOH. The amount of cell-associated radioactivity was determined by mixing the pooled cell lysates containing 6–16 μg soluble proteins with 4 mL of scintillation cocktail, Liquiscint (National Diagnostics, Atlanta, GA, USA), and were counted using a liquid scintillation counter (Beckman LS 6500, Jersey City, NJ, USA).
Preparation of resveratrol affinity column and analysis of control and resveratrol-treated PrSC and PrEC extracts by resveratrol affinity chromatography
Resveratrol was immobilized on epoxy-activated agarose as described 20, and used to analyze extracts prepared from control and 2-day, 25 μmol L−1 resveratrol-treated PrSCs and PrECs. Lysates were prepared and fractionated by sequential elution with low (0.35 mol L−1) and high (1.0 mol L−1) NaCl, followed by 1 mmol L−1 ATP and finally 1 mmol L−1 resveratrol, to displace resveratrol-targeting proteins, denoted as RTPs, binding with different affinity to the resveratrol-immobilized column as described 20. Specificity of protein binding to the affinity resin was assessed by competing extracts with 1 mmol L−1 resveratrol before fractionation. The eluted fractions were concentrated, separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) and visualized by silver staining.
Western blot analysis
Expression of QR2 in eluted fractions of untreated and resveratrol-treated cells was determined by Western blot analysis. Enhanced chemiluminescence was used to detect immunoreactive bands 13, 20.
Cell culture and proliferation assay
PrSCs and PrECs were maintained in complete culture media supplemented with SingleQuots, as described above. When cells reached > 70% confluence, they were subcultured by splitting into 1:4 cell ratio and maintained in T-25 flasks containing fresh culture media. After an overnight incubation, cells were treated with increasing doses of resveratrol, as specified in the text. Cell numbers were determined at the indicated times by trypan blue exclusion, as previously described 21, 22, 23.
Cell cycle analysis
Prostate stromal cells were treated with different concentrations of resveratrol (0, 10 and 50 μmol L−1) for 2, 4 and 6 days, and cell cycle phase distribution was assayed by flow cytometry as described 22, 24, 25. The cellular DNA content was obtained and the percentage of cells in the respective phases (G1, S and G2/M) of the cell cycle was quantified.
Results
[3H]resveratrol uptake in PrSCs and PrECs
To obtain information on whether resveratrol is readily accessible to prostate cells, time-course of uptake of resveratrol by PrSCs or PrECs was studied. Control and resveratrol-pretreated human PrSCs or PrECs (see Materials and methods) were labeled with [3H]resveratrol and the amount of radioactivity taken up by the cells was determined at different time points after labeling. Figure 1A showed [3H] resveratrol bound to stromal cell extracts, evident by 14 c.p.m. per μg soluble protein measured when labeling was immediately terminated by harvesting (within 1 min) and processing (within 2 min), suggesting that there was an initial, rapid association of [3H] resveratrol with cellular components. By following the amount of radioactivity in cell extracts after 5, 10 or 20 min of labeling, it can be seen that uptake of [3H]resveratrol increased progressively in both control and resveratrol-pretreated PrSCs (Figure 1A). Uptake of resveratrol may be further modulated by resveratrol, as in PrSCs pretreated with increasing doses (10 or 50 μmol L−1) of unlabeled resveratrol for 2 days, the uptake of [3H] resveratrol into treated cells increased in a dose-dependent manner, more substantially than that in untreated cells (Figure 1A), suggesting that pretreatment may affect the cell membrane properties in ways that stimulated the uptake of resveratrol.
Figure 1.
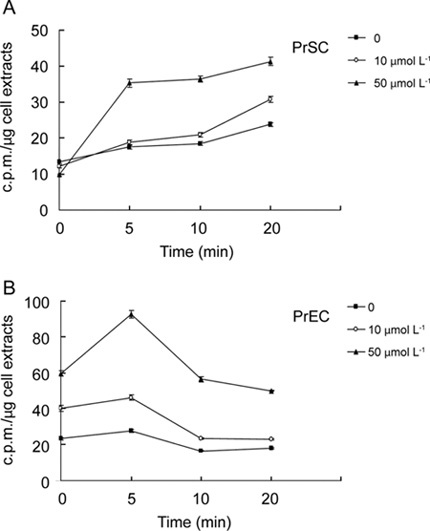
The uptake of [3H]resveratrol by prostate stromal (PrSCs) and epithelial cells (PrECs). (A) Time-course of resveratrol uptake by PrSCs. (B) Time-course of resveratrol uptake by PrECs. Cells were pretreated with 0, 10 or 50 μmol L−1 resveratrol for 2 days, washed extensively to remove resveratrol and then incubated with [3H]-labeled resveratrol for the indicated time durations. The uptake of resveratrol by both cells was measured as described in Materials and methods. Data points are expressed as mean ± SD from 3–6 determinations.
Time-course study on uptake of resveratrol in PrECs was also performed. Similar to what was observed in PrSCs, there was a substantial amount of labeled resveratrol bound to PrEC extracts, evident by 22 c.p.m. per μg soluble protein when cells were harvested and processed immediately (Figure 1B). In untreated PrECs, intracellular radioactivity was unchanged during the first 5 min and interestingly, gradually decreased thereafter perhaps because of efflux. However, dose-dependent increase in uptake of resveratrol was observed in resveratrol-treated PrECs compared with untreated cells (Figure 1B).
It is noteworthy that, in both PrSCs and PrECs pretreated with 50 μmol L−1 resveratrol, the initial uptake of radioactive resveratrol within the first 5 min was more rapid and robust than in control and 10 μmol L−1 resveratrol-treated cells. In PrSCs, this was followed by a steady maintenance of the level of labeled resveratrol over the next 20 min, whereas in PrECs, a decline of radioactive resveratrol to a new steady state was observed (Figure 1). These results suggest that, although different uptake kinetics was observed between PrSCs and PrECs (with optimal uptake occurring at 10 min for PrSCs and at 5 min for PrECs), treatment by resveratrol, particularly at a dose of 50 μmol L−1, consistently stimulated the uptake of resveratrol in both cell types.
Subcellular distribution of resveratrol in PrSCs and PrECs
Determination of the intracellular disposition of resveratrol is of interest as it might provide information on the identity and nature of cellular targets with which resveratrol binds specifically. To analyze the intracellular localization of resveratrol in PrSCs, cells were incubated with [3H] resveratrol for 10 min, harvested and fractionated into the compartments of cytosol, organelle/membrane, nucleus and cytoskeleton. The amount of radioactive resveratrol in each compartment was determined. In parallel experiments, we also tested whether the subcellular distribution of this polyphenol may be altered by earlier exposure of cells for 2 days to 25 μmol L−1, resveratrol, a concentration found to pronouncedly induce anti-CaP effect in previous studies 13. Figure 2A shows that (i) ∼40% radioactivity was recovered in the organelle/membrane fraction in both control and resveratrol-treated cells, (ii) ∼30% increase in radioactivity in the organelle/membrane was observed in resveratrol-treated cells and (iii) the subcellular distribution of resveratrol in untreated cells followed the pattern of membrane/organelle > nucleus > cytoskeleton = cytosol, with no substantial pattern change in the resveratrol-treated cells. In PrECs, subcellular distribution of resveratrol decreased as follows: membrane/organelle > cytosol = cytoskeleton > nucleus. Resveratrol treatment increased the radioactivity associated with the membrane/organelle fraction by ∼35% (Figure 2B). These results suggest that once resveratrol gains entry into cells, it is rapidly and preferentially delivered to the organelle/membrane fraction.
Figure 2.
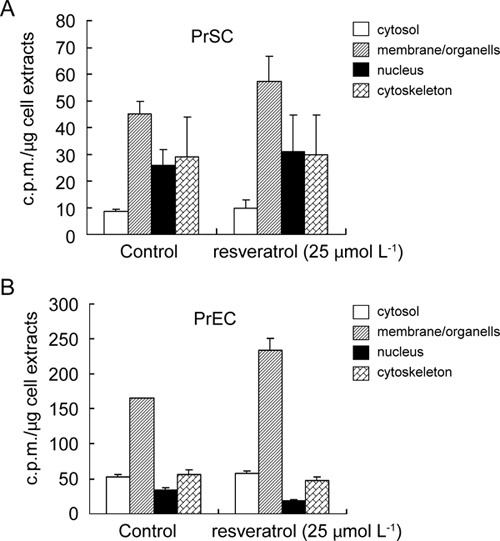
Subcellular distribution of resveratrol in PrSCs and PrECs. (A): Subcellular distribution of [3H] resveratrol in untreated and 25 μmol L−1 resveratrol-treated PrSC extracts was analyzed by fractionation into the cytosol, membrane/organelle, nucleus and cytoskeleton. (B): Subcellular distribution of [3H] resveratrol in untreated and 25 μmol L−1 resveratrol-treated PrEC extracts. Cells were pretreated with 0 or 25 μmol L−1 resveratrol for 2 days before incubation with [3H]-labelled resveratrol. The amount of radioactivity associated with each fraction was measured as described in Materials and methods. Data points are expressed as mean ± SD from 3–6 determinations.
Fractionation of cytoplasmic extracts from control and resveratrol-treated PrSCs and PrECs by resveratrol affinity chromatography
To learn the identity of the intracellular proteins that interact with resveratrol, resveratrol affinity chromatography was used to detect, select and capture RTPs in PrSCs and PrECs, as well as to analyze the proteins whose level might change in response to the resveratrol treatment. Cell extracts from PrSCs, with and without 2-day treatment with 25 μmol L−1 resveratrol, were separately applied to resveratrol affinity columns. Fractions were eluted with 0.35 mol L−1 and 1 mol L−1 NaCl, followed by 1 mmol L−1ATP and finally 1 mmol L−1 resveratrol. The eluted fractions were concentrated and resolved by 10% SDS–PAGE, followed by silver staining and immunoblot analysis. A typical silver-stained elution profile from PrSC extracts is illustrated in Figure 3A, comparing the elution profile of extracts prepared from untreated and resveratrol-treated PrSCs on resveratrol affinity columns. In the profile shown, fractions from control cell extracts were identified as 1, whereas fractions from resveratrol-treated PrSCs without and with the first competing cell extract with resveratrol before the fractionation were identified as 2 and 3, respectively. The silver-stained profile representing the unbound material and the material eluted with 0.35 mol L−1 and 1.0 mol L−1 NaCl, 1 mmol L−1 ATP and 1 mmol L−1 resveratrol was shown in the upper panel of Figure 3A. Correspondingly, the same fractions were also analyzed for the presence of QR2, an already identified RTP, by western blot analysis using QR2-specific antibody (shown in lower panel of Figure 3A). To confirm the identity of QR2, purified recombinant protein was added as a marker (shown as QR2 lane). The results showed that (i) QR2 appeared in the unbound fraction when extracts were first preincubated with excess resveratrol before fractionation, showing that the retention of this protein on the affinity column was effectively competed by resveratrol, (ii) QR2 was found exclusively in the resveratrol-eluted fraction and (iii) PrSC treated with resveratrol showed increased QR2, as in the resveratrol-eluted fraction of the treated samples, increased presence of QR2 was evident relative to similarly fractionated untreated cell extract (Figure 3A). Similar experiments were also performed on PrECs. Extracts from untreated and resveratrol-treated PrECs were also fractionated on a resveratrol affinity column. Materials eluted with 0.35 mol L−1 and 1.0 mol L−1 NaCl, 1 mmol L−1 ATP and 1 mmol L−1 resveratrol, were separated by SDS–PAGE, visualized by silver staining, and QR2 present in various eluted fractions were monitored by western blot analysis (Figure 3B). No specific binding and/or expression of QR2 were detected by immunoblot analysis in either control or resveratrol-treated PrEC extracts (Figure 3B). These results suggested that QR2 is differentially expressed in PrSCs and PrECs. To validate this possibility, the expression of QR2 was investigated by western blot analysis using unfractionated PrSC and PrEC extracts. As expected, QR2 was below detection level in PrECs, whereas it was clearly expressed in PrSCs (Figure 3C), thus supporting and confirming the relative low and high abundance of QR2 found in PrEC and PrSC extracts fractionated on resveratrol affinity columns (Figures 3A and 3B). These results reinforced the notion that specific RTPs, for example, QR2 exist and are differentially expressed in PrSC and PrECs.
Figure 3.
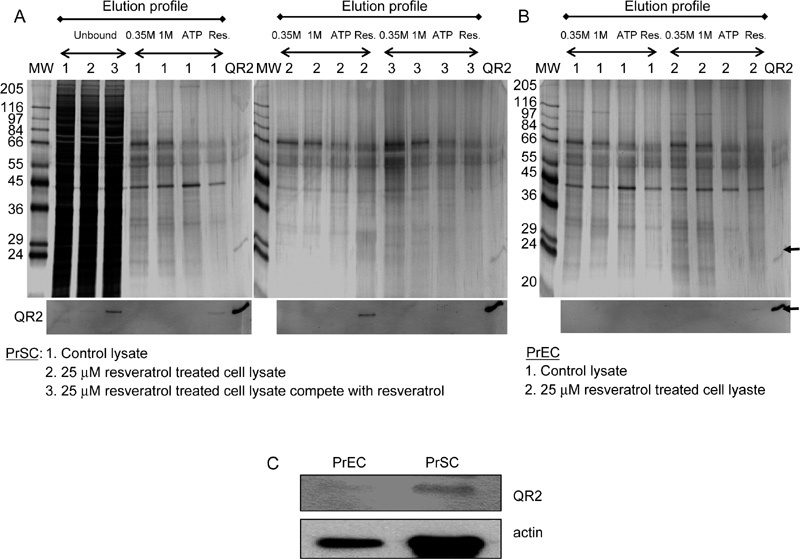
Analysis of PrSC and PrEC extracts on resveratrol-immobilized affinity columns. (A) Elution profile of QR2 in control and 25 μmol L−1 resveratrol-treated PrSC extracts, with and without competition by resveratrol, before fractionation on resveratrol affinity column. Extract obtained from untreated and resveratrol-treated PrSCs was fractionated on resveratrol affinity columns as detailed in Materials and methods. The fractions shown here represent unbound material and material eluted with 0.35 mol L−1 and 1.0 mol L−1 NaCl, 1 mmol L−1 ATP and 1 mmol L−1 resveratrol. Purified recombinant QR2 was added as a marker. Fractions were separated by SDS–PAGE and visualized by silver staining. QR2 in unbound and various eluted fractions were determined by western blot analysis. (B): Elution profile of QR2 in control and 25 μmol L−1 resveratrol-treated PrEC extracts on resveratrol affinity column. Extract from untreated and resveratrol-treated PrECs was fractionated on resveratrol affinity columns as detailed in Materials and methods. The fractions shown here represent material eluted with 0.35 mol L−1 and 1.0 mol L−1 NaCl, 1 mmol L−1 ATP and 1 mmol L−1 resveratrol. Purified recombinant QR2 was added as a marker. Fractions were separated by SDS–PAGE, and visualized by silver staining. QR2 in various eluted fractions were determined by western blot analysis. (C): Western blot analysis of QR2 and actin from whole cell extracts of control PrSCs and PrECs.
Effects of resveratrol on growth and cell cycle distribution of cultured PrSCs and PrECs
To obtain information on the role that QR2 might have in proliferation and overall cellular responsiveness to resveratrol in PrSCs and PrECs, the growth modulatory effects of resveratrol were investigated. PrSCs and PrECs were treated with 0, 10 or 50 μmol L−1 resveratrol for 2 days, and cell numbers were determined by trypan blue exclusion using a hemocytometer. Resveratrol treatment resulted in dose-dependent growth inhibition in PrSCs, but had no effect on PrEC growth (Figure 4A). To test whether resveratrol-induced growth suppression in PrSCs might involve alterations in cell cycle control, flow cytometry studies were performed. Cells treated with 10 μmol L−1 resveratrol showed minimum affects on cell cycle phase distribution, whereas cells exposed to 50 μmol L−1 resveratrol for 2, 4 or 6 days showed a substantial change in cell cycle phase distribution. For example, 2-day resveratrol-treated cells showed a decrease in G1-phase cell population (92% in control versus 40% in treated cells) accompanied by a concomitant accumulation in the S- and G2/M-phase cell population (2% and 5% in control versus 22% and 32% in treated cells) (Figure 4B).
Figure 4.
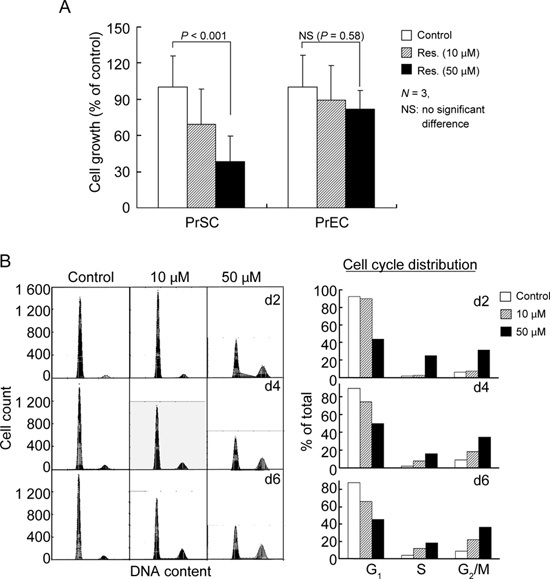
Effects of treatment by resveratrol on cell growth of cultured PrSCs and PrECs, and effects of resveratrol treatment on cell cycle distribution of cultured PrSCs. (A): Growth of PrSCs and PrECs in response to treatment with 0, 10 or 50 μmol L−1 of resveratrol for 2 days. Results represent means of triplicate experiments ± s.d. The significance of cell number decrease was determined by paired t-test. (B): Effects of resveratrol on the changes of cell cycle phase distribution in PrSCs. Cells were treated with 0, 10 or 50 μmol L−1 of resveratrol for 2, 4 and 6 days, and the effect on cell cycle distribution was analyzed by flow cytometry and presented as histograms (left panel). The percentage of cells in G1, S and G2/M-phases were calculated and presented as bar graph (right panel).
Discussion
Resveratrol has shown potent antitumor activities against a variety of human cancer types, including prostate cancer. Previous studies have also reported that resveratrol treatment showed no effect on viability and growth in normal PrECs even at a dose of 50 μmol L−1 26. However, whether resveratrol affects normal PrSC proliferation and gene expression have not been reported, nor was the uptake of resveratrol in PrECs previously investigated. Using PrSCs and PrECs, we provide evidence on four new observations regarding the affects of resveratrol in normal prostate cells: (i) earlier exposure to resveratrol stimulates its uptake in both PrSCs and PrECs (Figure 1), (ii) resveratrol pretreatment increases its cellular trafficking into the membrane/organelle compartment in PrECs and PrSCs (Figure 2), (iii) using a biospecific affinity approach, we have identified QR2 as a resveratrol-targeting protein in PrSCs (Figure 3) and (iv) resveratrol suppresses cell proliferation and modulates cell cycle progression in PrSCs, but has no noticeable effect on proliferation of human PrECs. Inhibition of PrSC proliferation was not accompanied by induction of cell death, as assayed by the trypan blue exclusion assay and also by the absence of sub-G1 DNA in flow cytometric analysis (data not shown). Similarly, PrECs treated with up to 50 μmol L−1 resveratrol did not show evidence of apoptosis (data not shown). As the increased proliferation of stromal cells is thought to drive the enlargement of the prostate gland as men age, a condition clinically referred to benign prostatic hyperplasia 27, 28, 29, 30, it is conceivable that the growth suppressive effects of resveratrol in PrSCs may prove to be beneficial to patients diagnosed with such a condition.
The results of this study show that resveratrol pretreatment changes the time and extent of its uptake in both PrSCs and PrECs, suggesting that resveratrol may function as an auto-regulator, promoting and enhancing its own transport and uptake into responsive cells. These may have important potential implications, as they directly relate to the unsettled issue of bioavailability of resveratrol as well as provide support to recent reports showing that resveratrol sensitizes cancer cells to other chemotherapeutic agents, which act by binding to cell surface death receptors, for example, TRAIL 31. Data showing that resveratrol increases its own uptake, as illustrated by our experiments using normal prostate cells, also raise the tantalizing possibility that resveratrol might perturb membrane structure and function in ways that facilitate the targeting of death receptors and, hence, improve responsiveness to chemotherapeutic agents without compromising the likelihood for developing chemoresistance 31, 32, 33. It is noteworthy to point out that the present studies also showed considerable binding of [3H]resveratrol to cell extracts of PrSCs and PrECs, even when cells are immediately harvested after labeling with resveratrol, and that there was 1.6-fold labeling in PrECs compared with PrSCs (Figure 1). A possible explanation is that there is an initial burst of binding of resveratrol to available membrane targets, more so in PrECs than in PrSCs. The nature of such targets remains to be elucidated. One might surmise that integrin αVβ3 is a reasonable candidate, as integrin αVβ3 has been found to contain a receptor site for resveratrol 34; moreover, differential, altered expression pattern of integrin αVβ3 might exist in PrSCs and PrECs. Experiments exploring and testing these hypotheses are underway in our laboratory.
The resveratrol subcellular distribution analysis revealed that ∼40% of the grape polyphenol was preferentially localized to the organelle/membrane fraction in both PrSCs and PrECs (Figure 2), providing support to the thesis that the major and primary intracellular targets of resveratrol reside in the organelle/membrane fraction. Although resveratrol treatment increased the subcellular distribution of this polyphenol to the organelle/membrane fraction more robustly in PrECs than in PrSCs, the target affected is unlikely to be QR2, which is barely detected in PrECs, nor are they ones involved in control of proliferation and cell cycle transition, as growth of PrECs are noticeably unresponsive to treatment by resveratrol. Thus, the identity and nature of cellular targets differentially affected by resveratrol, particularly the ones localized to the organelle/membrane fractions, between PrSCs and PrECs must await further studies in the future.
Another significant finding in this study is the identification of resveratrol-targeting protein, QR2, in PrSCs. Previously, we have found that QR2 shows prostate cancer stage-dependent differences in the level of its expression 20. The observation that QR2 was present in PrSCs and only barely detectable in PrECs (Figure 3B) shows that differential expression of QR2 also exists between different normal prostate cell types. The relationship between QR2 and cellular response to resveratrol was investigated by comparing the anti-proliferative effects of resveratrol in PrSCs and PrECs, clearly showing that only the growth of PrSCs were affected by treatment with resveratrol, concomitant with increased expression of QR2 (Figures 3 and 4). These results and the observed differential expression of QR2 between PrSCs and PrECs are consistent with the notion that QR2 has a role in the control of mitogenesis by resveratrol in PrSCs. Moreover, because the prostate stroma seems to be involved in the carcinogenic evolution and progression of prostate epithelial tumor cells, it might be suggested that resveratrol, by targeting QR2, functions as a chemopreventive agent against prostate cancer by its integrative action against prostate cancer cells 13, 14 as well as through its growth and gene regulatory control of normal PrSCs. It is noteworthy that QR2 has recently been found to act as a low-affinity melatonin receptor, MT3 35, 36; this raises the possibility that QR2 might contribute to the prostate cancer chemopreventive activities of melatonin 37, 38, 39. We hypothesize that QR2, by binding to melatonin, facilitates and promotes the cytosolic sequestration of androgen receptor, thereby inhibiting cell proliferation and prostate-specific antigen expression. Further studies comparing the uptake of resveratrol, its intracellular distribution, and binding and interaction with target proteins, including QR2, in normal and cancerous prostate cells treated with melatonin are warranted and are actively pursued in our laboratory.
Acknowledgments
This research was supported in part by the NCI Clinical Nutrition Research Unit Grant CA 29502 and by the NCI Grant 1RO3CA109932 to TCH. Some of the initial uptake studies used funds from United States Army Prostate Cancer Award W81XWH-04-1-0059 to Dr Joseph M Wu who also provided editorial assistance.
References
- Dong Z. Molecular mechanism of the chemopreventive effect of resveratrol. Mutat Res. 2003. pp. 523–524.pp. 145–50. [DOI] [PubMed]
- Savouret JF, Quesne M. Resveratrol and cancer: a review. Biomed Pharmacother. 2002;56:84–7. doi: 10.1016/s0753-3322(01)00158-5. [DOI] [PubMed] [Google Scholar]
- Wu JM, Wang ZR, Hsieh TC, Bruder JL, Zou JG, et al. Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (Review) Int J Mol Med. 2001;8:3–17. doi: 10.3892/ijmm.8.1.3. [DOI] [PubMed] [Google Scholar]
- Singh SU, Casper RF, Fritz PC, Sukhu B, Ganss B, et al. Inhibition of dioxin effects on bone formation in vitro by a newly described aryl hydrocarbon receptor antagonist, resveratrol. J Endocrinol. 2000;167:183–95. doi: 10.1677/joe.0.1670183. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Das DK, Tosaki A, Bagchi M, Kothari SC. Benefits of resveratrol in women's health. Drugs Exp Clin Res. 2001;27:233–48. [PubMed] [Google Scholar]
- Morales AI, Buitrago JM, Santiago JM, Fernandez-Tagarro M, Lopez-Novoa JM, et al. Protective effect of trans-resveratrol on gentamicin-induced nephrotoxicity. Antioxid Redox Signal. 2002;4:893–8. doi: 10.1089/152308602762197434. [DOI] [PubMed] [Google Scholar]
- Bhat KP, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Ann NY Acad Sci. 2002;957:210–29. doi: 10.1111/j.1749-6632.2002.tb02918.x. [DOI] [PubMed] [Google Scholar]
- Gusman J, Malonne H, Atassi G. A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis. 2001;22:1111–7. doi: 10.1093/carcin/22.8.1111. [DOI] [PubMed] [Google Scholar]
- Tsan MF, White JE, Maheshwari JG, Chikkappa G. Anti-leukemia effect of resveratrol. Leuk Lymphoma. 2002;43:983–7. doi: 10.1080/10428190290021669. [DOI] [PubMed] [Google Scholar]
- Mouria M, Gukovskaya AS, Jung Y, Buechler P, Hines OJ, et al. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome C release and apoptosis. Int J Cancer. 2002;98:761–9. doi: 10.1002/ijc.10202. [DOI] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Schneider Y, Duranton B, Gosse F, Schleiffer R, Seiler N, et al. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr Cancer. 2001;39:102–7. doi: 10.1207/S15327914nc391_14. [DOI] [PubMed] [Google Scholar]
- Hsieh TC, Wu JM. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Exp Cell Res. 1999;249:109–15. doi: 10.1006/excr.1999.4471. [DOI] [PubMed] [Google Scholar]
- Hsieh TC, Wu JM. Grape-derived chemopreventive agent resveratrol decreases prostate-specific antigen (PSA) expression in LNCaP cells by an androgen receptor (AR)-independent mechanism. Anticancer Res. 2000;20:225–8. [PubMed] [Google Scholar]
- Narayanan BA, Narayanan NK, Re GG, Nixon DW. Differential expression of genes induced by resveratrol in LNCaP cells: P53-mediated molecular targets. Int J Cancer. 2003;104:204–12. doi: 10.1002/ijc.10932. [DOI] [PubMed] [Google Scholar]
- Jones SB, DePrimo SE, Whitfield ML, Brooks JD. Resveratrol-induced gene expression profiles in human prostate cancer cells. Cancer Epidemiol Biomarkers Prev. 2005;14:596–604. doi: 10.1158/1055-9965.EPI-04-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NK, Narayanan BA, Nixon DW. Resveratrol-induced cell growth inhibition and apoptosis is associated with modulation of phosphoglycerate mutase B in human prostate cancer cells: two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis and mass spectrometry evaluation. Cancer Detect Prev. 2004;28:443–52. doi: 10.1016/j.cdp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Gao S, Liu GZ, Wang Z. Modulation of androgen receptor-dependent transcription by resveratrol and genistein in prostate cancer cells. Prostate. 2004;59:214–25. doi: 10.1002/pros.10375. [DOI] [PubMed] [Google Scholar]
- Lin HY, Shih A, Davis FB, Tang HY, Martino LJ, et al. Resveratrol induced serine phosphorylation of p53 causes apoptosis in a mutant p53 prostate cancer cell line. J Urol. 2002;168:748–55. [PubMed] [Google Scholar]
- Wang Z, Hsieh TC, Zhang Z, Ma Y, Wu JM. Identification and purification of resveratrol targeting proteins using immobilized resveratrol affinity chromatography. Biochem Biophys Res Commun. 2004;323:743–9. doi: 10.1016/j.bbrc.2004.08.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietrantonio AM, Hsieh TC, Wu JM. Specific processing of poly(ADP-ribose) polymerase, accompanied by activation of caspase-3 and elevation/reduction of ceramide/hydrogen peroxide levels, during induction of apoptosis in host HL-60 cells infected by the human granulocytic ehrlichiosis (HGE) agent. IUBMB Life. 2000;49:49–55. doi: 10.1080/713803590. [DOI] [PubMed] [Google Scholar]
- Hsieh TC, Kunicki J, Darzynkiewicz Z, Wu JM. Effects of extracts of Coriolus versicolor (I'm-Yunity) on cell-cycle progression and expression of interleukins-1 beta,-6, and -8 in promyelocytic HL-60 leukemic cells and mitogenically stimulated and nonstimulated human lymphocytes. J Altern Complement Med. 2002;8:591–602. doi: 10.1089/107555302320825101. [DOI] [PubMed] [Google Scholar]
- Hsieh TC, Wu P, Park S, Wu JM. Induction of cell cycle changes and modulation of apoptogenic/anti-apoptotic and extracellular signaling regulatory protein expression by water extracts of I'm-Yunity (PSP) BMC Complement Altern Med. 2006;6:30. doi: 10.1186/1472-6882-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietrantonio AM, Hsieh TC, Olson SC, Wu JM. Regulation of G1/S transition and induction of apoptosis in HL-60 leukemia cells by fenretinide (4HPR) Int J Cancer. 1998;78:53–61. doi: 10.1002/(sici)1097-0215(19980925)78:1<53::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Bedner E, Smolewski P. Flow cytometry in analysis of cell cycle and apoptosis. Semin Hematol. 2001;38:179–93. doi: 10.1016/s0037-1963(01)90051-4. [DOI] [PubMed] [Google Scholar]
- Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3′-kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther. 2006;5:1335–41. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- Egevad L, Carbin BE, Castellanos E, Hellstrom M, Valdman A. Atypical stromal hyperplasia of the prostate. Scand J Urol Nephrol. 2008;42:484–7. doi: 10.1080/00365590802086230. [DOI] [PubMed] [Google Scholar]
- Wang W, Li Y, Hong A, Wang J, Lin B, et al. NDRG3 is an androgen regulated and prostate enriched gene that promotes in vitro and in vivo prostate cancer cell growth. Int J Cancer. 2009;124:521–30. doi: 10.1002/ijc.23961. [DOI] [PubMed] [Google Scholar]
- Schauer IG, Ressler SJ, Rowley DR. Keratinocyte-derived chemokine induces prostate epithelial hyperplasia and reactive stroma in a novel transgenic mouse model. Prostate. 2009;69:373–84. doi: 10.1002/pros.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu YN, Xia SJ. Stroma-epithelium crosstalk in prostate cancer. Asian J Androl. 2009;11:28–35. doi: 10.1038/aja.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill C, Walsh SE, Morrissey C, Fitzpatrick JM, Watson RW. Resveratrol sensitizes androgen independent prostate cancer cells to death-receptor mediated apoptosis through multiple mechanisms. Prostate. 2007;67:1641–53. doi: 10.1002/pros.20653. [DOI] [PubMed] [Google Scholar]
- Merino D, Lalaoui N, Morizot A, Schneider P, Solary E, et al. Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol Cell Biol. 2006;26:7046–55. doi: 10.1128/MCB.00520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yue P, Khuri FR, Sun SY. Decoy receptor 2 (DcR2) is a p53 target gene and regulates chemosensitivity. Cancer Res. 2005;65:9169–75. doi: 10.1158/0008-5472.CAN-05-0939. [DOI] [PubMed] [Google Scholar]
- Lin HY, Lansing L, Merillon JM, Davis FB, Tang HY, et al. Integrin alphaVbeta3 contains a receptor site for resveratrol. FASEB J. 2006;20:1742–4. doi: 10.1096/fj.06-5743fje. [DOI] [PubMed] [Google Scholar]
- Nosjean O, Ferro M, Coge F, Beauverger P, Henlin JM, et al. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem. 2000;275:31311–7. doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- Vella F, Ferry G, Delagrange P, Boutin JA. NRH:quinone reductase 2: an enzyme of surprises and mysteries. Biochem Pharmacol. 2005;71:1–12. doi: 10.1016/j.bcp.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Jung B, Ahmad N. Melatonin in cancer management: progress and promise. Cancer Res. 2006;66:9789–93. doi: 10.1158/0008-5472.CAN-06-1776. [DOI] [PubMed] [Google Scholar]
- Connor TP. Melatonin as an adjuvant to therapeutic prostate cancer vaccines. J Pineal Res. 2008;45:224. doi: 10.1111/j.1600-079X.2008.00563.x. [DOI] [PubMed] [Google Scholar]
- Shiu SY. Towards rational and evidence-based use of melatonin in prostate cancer prevention and treatment. J Pineal Res. 2007;43:1–9. doi: 10.1111/j.1600-079X.2007.00451.x. [DOI] [PubMed] [Google Scholar]


