Abstract
To investigate the expression pattern of rat Eppin (epididymal protease inhibitor; official symbol Spinlw1), we detected mRNA transcripts and subsequent protein translation of Eppin in several sorts of tissues by RT-PCR and western blotting. Then immunohistochemistry was performed for more detailed observation. The testicular transcription level was monitored by real-time PCR throughout postnatal development. We found that rat Eppin was specifically expressed in the testis and epididymis. The testicular transcription was slight in neonatal (1-day) and infantile stages (5-, 7- and 10-day). It increased sharply thereafter, with maximum expression level (about 38-fold compared with that of 1-day old rat) detected in prepubertal stage (15-day). Then a slightly declined but stable level (about 20-fold compared with that of 1-day old rat) was kept in pubertal-early adult (30-day) and adult (60-day) stages of postnatal maturation. In the adult rat, EPPIN protein was mainly localized in the elongated spermatids and epididymal epithelial cells. Sperm in the epididymal duct were all covered with EPPIN and its level kept constant during incubation under conditions used to achieve capacitation. Its stage-specific expression in the testis suggests that EPPIN may be important during spermatogenesis especially for the spermatid elongation. The abundant production of epididymal EPPIN indicated indirectly that it might play a role in the function of the epididymis.
Keywords: expression pattern, postnatal development, rat Eppin, spermatogenesis and maturation
Introduction
Spermatogenesis is a very complicated process and is regulated by different factors, such as hormones 1, 2, specifically expressed proteases and their cognate inhibitors 3, 4. Many testis-expressed proteins are indispensable during spermatogenesis 5, 6. When released from testis, spermatozoa undergo a post-testicular maturation process in the epididymis to acquire both progressive motility and fertilization ability 7, 8. Rigorous microenvironments in the epididymal ducts are created by epididymal epithelium secreting or absorbing proteins 9, leading to changes or modifications of sperm surface molecules. Among these sperm surface molecules, some act as decapacitation factors 10, some act as inhibitors to repress proteases 11 and some mediate sperm–egg interaction 12, 13. Studies on these factors from testis and epididymis help us learn more about the fertilization process and identify new targets for male contraception.
Eppin (epididymal protease inhibitor), a newly identified gene, is specifically expressed in the testis and epididymis of human 14 and mouse 15. The human Eppin gene, located on chromosome 20, expresses three mRNAs encoding two isoforms of a cysteine-rich protein containing both Kunitz-type and WAP (whey acidic protein)-type four disulfide core protease inhibitor consensus sequences 14. Male monkeys immunized with recombinant human EPPIN developed high titers of EPPIN, and all the high-titer monkeys were reversibly infertile without hormone disruption 16. These results make us believe that EPPIN may be an excellent target for male contraception and, therefore, the underlying mechanisms are of great interest to many researchers. However, only the antibacterial activity of EPPIN has been reported up to now 17. Considering the difficulties of experimenting on monkeys or human, it is necessary to carry out the studies on rodents. A detailed description about gene structure and protein distribution of mouse Eppin has been published by Sivashanmugam et al. 15. However, we still know nothing about rat Eppin. Rat is a commonly used model in the field of reproductive science for its strong fertility and short reproduction cycle. Therefore, research on rat Eppin may provide us another important model to investigate Eppin's function and its contraception mechanism.
In the present study, experiments were designed to disclose the expression pattern of rat Eppin for the first time. We detected mRNA transcripts and subsequent protein translation of rat Eppin in several sorts of tissues by reverse transcription PCR (RT-PCR), sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting. Then immunohistochemistry was performed for more detailed observation. The Eppin transcription level was also monitored by real-time PCR throughout the postnatal development of rat testis.
Materials and methods
Materials and animals
All chemicals and reagents used in this study were of molecular biology grade. Eppin (I-12) is an affinity-purified goat polyclonal antibody raised against a peptide mapping near the N-terminus of EPPIN of human origin (Santa Cruz, CA, USA), and it is recommended for detection of EPPIN of mouse, rat and human origin by western blotting and immunofluorescence. The mouse anti-tubulin monoclonal antibody was purchased from Boster Biological Technology Ltd. (Wuhan, China). The EvaGreen 20 × in water was purchased from Biotium Inc (Hayward, CA, USA). All the other chemicals and reagents used in this study were purchased from Sigma (St. Louis, MO, USA) unless where specifically described.
Adult male and female (10-week-old) Sprague–Dawley (SD) rats were used. Animals were maintained with food and water in a temperature-controlled room. This project had the clearance from the Institute Animal Ethics Committee of Nanjing Medical University and experiments were conducted in accordance with the declaration of Helsinki and the guiding principles in the care and use of animals.
Total RNA extraction from multiple tissues and RT-PCR analysis
Adult male and female SD rats (10-week-old) were killed by CO2 asphyxiation to obtain multiple tissues including heart, brain, kidney, lung, skeletal muscle, liver, spleen, testis, epididymis, ovaries and uterus. Total RNAs were extracted from the tissues using TRIzol reagent 18. Briefly, 50-mg tissue was homogenized in 1 mL TRIzol reagent followed by storage at room temperature for 5 min. Chloroform was added and the mixture was centrifuged (12 000 × g, 5 min), and the aqueous phase was collected. RNA was precipitated from the aqueous phase by addition of isopropanol. The recovered pellets were washed twice with 1 mL of 75% ethanol, air dried and solubilized in 20-μL diethylpyrocarbonate-treated water for concentration determination.
First-strand complementary DNAs (cDNAs) were synthesized using 2 μg of total RNA of each tissue sample. PCR analysis was performed to generate rat Eppin fragments (405 bp) using the synthesized cDNAs as templates. Primers were designed according to cDNA sequence obtained from PubMed (gene ID of rat Eppin: 685161; gene ID of rat Gapdh: 24383). The Eppin primers were: sense: 5′-CCAGGATGAAGTTTTCCAG-3′ antisense: 5′-CGTTCAGGTGGAATTGCTT-3′. The Gapdh primers were: sense: 5′-TCCCTCAAGATTGTCAGCAA-3′ antisense: 5′-AGATCCACAACGGATACATT-3′.
Sperm collection
The medium used for rat sperm culture and capacitation was BWW (the complete Biggers, Whitten and Whittingham [BWW] media), as described by Zhang et al. 19. The complete BWW minus calcium or bicarbonate (the incomplete BWW) was used for sperm culture without supporting capacitation. Adult male SD rats were killed by CO2 asphyxiation, and bilateral epididymides were surgically removed. Both epididymides were minced in two different media, one in the complete BWW and the other in the incomplete BWW media. Radial slits were made in each cauda epididymidis to let the rat sperm swim out. Then the epididymides were discarded. Sperm were counted with a hemocytometer and the concentration diluted to 2 × 106 cells per mL. The suspension was placed in a 1.5-mL microcentrifuge tube and incubated for 3 h at 37°C in 5% CO2. Sperm cultured in the complete or incomplete BWW were collected by centrifugation and total proteins were extracted as described below.
Cells and tissue lysate preparation
For total protein extraction, rat epididymis and testis samples were minced in extraction buffer (7 mol L−1 urea, 2 mol L−1 thiourea, 4% CHAPS, 65 mmol L−1 dithiothreitol and 1% cocktail) and sonicated. The mixture was kept on ice, shaken every 15 min for totally 1 h and then centrifuged at 12 000 × g for 30 min. Supernatants were collected for concentration determination before SDS-PAGE 19.
Western blot analysis
To detect EPPIN protein expression, western blotting was performed according to the procedure described by Zhang et al. 19. Briefly, protein samples in loading buffer were reduced by the addition of β-mercaptoethanol, and heated to 100°C for 5 min. SDS-PAGE was performed on 12.5% polyacrylamide gel. Proteins were transferred to polyvinylidene fluoride membrane at 200 mA for 1 h and blocked using 5% skimmed milk powder in Tris-buffered saline with 0.1% Tween-20. The blots were immunostained using Eppin (I-12) (1:1 000) as the primary antibody and horseradish peroxidase-conjugated anti-goat IgG (1:1 000; Beijing Zhong Shan Golden Bridge Biotechnology Co Ltd, China) as the secondary antibody. Reactive protein bands were visualized by enhanced chemiluminescence using the manufacturer's protocol (Cell Signaling Technology Inc, Danvers, MA, USA).
Immunohistochemical localization of EPPIN in rat testis and epididymis
Testis and epididymis of adult rat were collected, fixed in 4% paraformaldehyde and embedded in paraffin. Tissue sections (5-μm thick) were cut on a microtome and mounted on glass slides for EPPIN detection according to the protocol described by Khobarekar 20. Briefly, sections were dewaxed, rehydrated and immersed in a solution of methanol containing 0.5% (v/v) hydrogen peroxide for 20 min to quench endogenous peroxidase activity. Nonspecific binding was blocked by incubating sections with normal rabbit serum 2 h at room temperature. Slides were incubated overnight at 4°C in a humidified chamber with Eppin (I-12) diluted to 1:500 in phosphate-buffered saline (PBS). Sections were washed thrice in PBS and incubated for 1 h with horseradish peroxidase-conjugated anti-goat IgG (1:1 000). After washing thrice with PBS, the slides were incubated with 0.05% (w/v) DAB (3,3′-diaminobenzidine) in PBS containing 0.2% (v/v) H2O2. The nuclei were counterstained with hematoxylin solution and the section mounted for image analysis.
Sertoli cell isolation and culture
Primary culture of rat Sertoli cells were performed according to the previously described method 21. Briefly, testis were collected from adult rat, decapsulated and cut into small pieces in Hanks Balanced Salts Solution. The dispersed seminiferous tubules were then digested in 0.25% trypsin (Amresco, Solon, OH, USA) at 32°C and 0.1% collagenase (type I, Invitrogen, Grand Island, NY, USA) at 34°C for 30 min. Digested cell suspension was filtered through a 100-mesh filter and washed thrice in Dulbecco's modified Eagle's medium and Ham's F-12 nutrient mixture (DMEM–F12, Sigma–Aldrich, St. Louis, MO, USA) supplemented with 5% fetal bovine serum (FBS). Sertoli cells were seeded in 100-mm tissue culture dishes (Falcon, Becton Dickinson Labware, Lincoln Park, NJ, USA) at a density of 1.5 × 106 cells per mL in DMEM–F12 medium with 5% FBS and cultured at 34°C in a water-saturated atmosphere of 5% CO2 in 95% air. About 48 h later, cells were hypotonically treated with 20 mmol L−1 Tris (pH 7.4) for 1–2 min followed by two washes with sterile PBS. Sertoli cells were cultured until it was abundant enough for total RNA extraction. The RT-PCR was done as described above.
Real-time polymerase chain reaction
Total RNA from rat testis of each postnatal time point (1 day, 5 days, 7days, 15 days, 30 days and 60 days) were collected and cDNAs were synthesized in the same way described above. Primers were specially designed for the real-time PCR analysis. The Eppin primers were: sense: 5′-AAGAAATGTCTAAACCCCCAAC-3′ antisense: 5′-CCCTGGCAACCACCATAGA-3′. The Gapdh primers were: sense: 5′-TCCTACCCCCAATGTATCCG-3′ antisense: 5′-CCTTTAGTGGGCCCTCGG-3′.
PCR samples were prepared in a final volume of 20 μL containing TaKaRa Taq HS (5 U μL−1, TaKaRa Biotechnology Co. Ltd), 10 × PCR buffer (Mg2+ Plus), dNTP mixture (2.5 μmol L−1), forward primer (20 μmol L−1), reverse primer (20 μmol L−1), cDNA templates (50 ng) and 20 × EvaGreen (1 μL). The reaction was performed in the ABI7300 instrument using the following conditions: 50°C for 2 min, 95°C for 10 min, 95°C for 15 s and 63°C for 1 min. Melting curve analysis and agarose gel electrophoresis were used to monitor accumulation of the PCR products.
Data analysis
Computations were carried out with the Stata statistical package (Stata9.0, Texas, USA). All values were expressed as the mean ± s.e.m. Statistical significance between mean values was determined by one-way ANOVA (analysis of variance) followed by the Student–Neuman–Keuls test, and was accepted at the 0.05 level.
Results
Tissue distribution of rat Eppin mRNA
Male rat tissue cDNAs from liver, spleen, heart, brain, kidney, lung and skeleton muscle were all negative, whereas testis and epididymis showed a 405-bp band predicted for rat Eppin (Figure 1, EPPIN). The expression of Eppin mRNA was not detected in female rat tissues including liver, spleen, ovary, uterus, heart, brain, kidney, lung and skeleton muscle. Gapdh was used as the internal control in this experiment to ensure that the RNA extraction and RT-PCR were successful (Figure 1, GAPDH).
Figure 1.
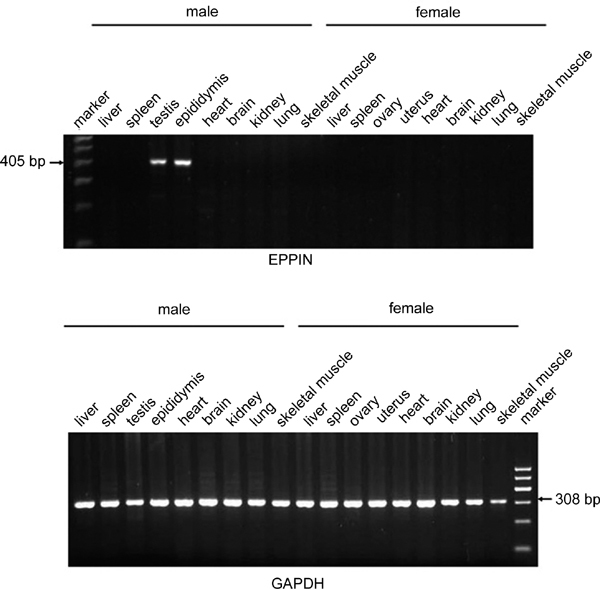
Reverse transcriptase PCR (RT-PCR) analysis of multiple tissue complementary DNAs (cDNAs) from both male and female adult rats. RNA was extracted from each tissue with TRIzol reagent. Then complementary DNA (cDNA) was synthesized from 2 μg of the extracted RNA. Finally, PCRs were performed to generate rat Eppin (epididymal protease inhibitor) (405 bp) and Gapdh (308 bp) fragments using the synthesized cDNA as templates. The rat Eppin transcripts were only expressed in the testis and epididymis.
Rat EPPIN protein expression in mRNA-positive tissue
Western blotting analysis was performed to see whether EPPIN protein was produced in Eppin mRNA-positive tissues. Tissue lysates from testis, caput, corpus and cauda epididymis all showed strong reactivity with a band at about 26 kDa (Figure 2). The specificity of the Eppin (I-12) probe was confirmed by western blots of recombinant human EPPIN purified from bacteria. Bacterial recombinant human EPPIN reacted to Eppin (I-12) with bands at 17 kDa and 34 kDa predicted to be the monomer and dimmer, respectively. No staining was seen when the primary antibody was absorbed with recombinant human EPPIN (data not shown).
Figure 2.
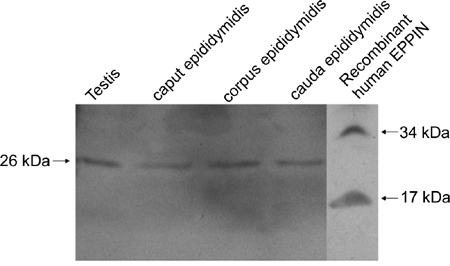
Western blotting analysis of rat EPPIN (epididymal protease inhibitor) in mRNA-positive tissue. Tissue proteins (100 μg) from testis, caput, corpus and cauda epididymis were electrophoretically separated, electrotransferred, immunostained and detected by chemiluminescence. Samples from testis, caput, corpus and cauda epididymis all showed a strong band at about 26 kDa. The specificity of the Eppin (I-12) probe was confirmed by western blots of recombinant human EPPIN purified from bacteria. The 17 and 34 kDa bands were predicted to be the monomer and dimmer, respectively.
Localization of EPPIN protein in the testis and epididymis of adult rat
We localized the EPPIN protein in rat testis and epididymis by immunohistochemistry using Eppin (I-12) probe. In the testis, intense staining was mainly localized in the elongated spermatids (Figure 3A and B). In the cauda epididymis, protein was present throughout the cytoplasm of epithelial cells and in the lumen (Figure 3D and E). Massive EPPIN was attached to the sperm surface and no positive staining was seen in the interstitial tissue. Signals for EPPIN protein were further confirmed by recombinant protein neutralization. No staining was detected if the primary antibody was pre-incubated with excess recombinant human EPPIN (Figure 3C and F).
Figure 3.
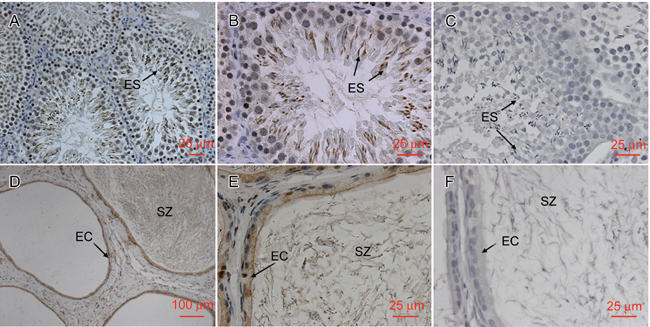
Localization of EPPIN (epididymal protease inhibitor) protein in rat testis and epididymis. Testis and epididymis from adult rat were fixed in 4% paraformaldehyde, paraffin embedded and sectioned for immunohistochemistry analysis. Sections stained with Eppin (I-12) probe. In the testis, (A and B) the labels were primarily in the elongated spermatids (ES). In the cauda epididymis (D and E), the labels were primarily in the epithelial cells (EC) and spermatozoa (SZ). No signal was seen in the testis (C) and cauda epididymis (F) when the primary antibody was neutralized with recombinant human EPPIN. (D): scale bar = 100 μm; (A, B, C, E and F): scale bars = 25 μm.
Detection of Eppin transcripts in primarily cultured Sertoli cells
As the testis section showed strong staining in elongated spermatids rather than Sertoli cells, which were previously reported to be the main source of EPPIN in human and mouse testis, experiments were designed to detect the Eppin transcripts in Sertoli cells in vitro. As shown in Figure 4, a 308-bp band predicted for rat Gapdh was seen in the PCR product using Sertoli cell cDNA as templates. However, it didn't produce any band when primers specific for Eppin's coding sequence were added. The testis cDNA prepared as described in Section 2.2 and deionized water were used as positive and negative control, respectively.
Figure 4.
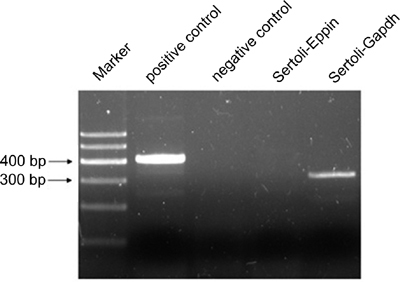
Detection of Eppin (epididymal protease inhibitor) mRNA in primarily cultured Sertoli cells. Sertoli cells were isolated from adult testis and primarily cultured. The mRNA was extracted for Reverse transcriptase PCR (RT-PCR) analysis. A 308-bp band predicted for rat Gapdh was seen in the PCR product using Sertoli cell complementary DNA (cDNA) as templates. However, it did not produce any band when primers specific for Eppin's coding sequence were added. The testis cDNA prepared as described in Section 2.2 and deionized water were used as positive and negative control, respectively.
Detection of EPPIN levels on sperm before and after incubation under conditions used to achieve capacitation
Proteins were extracted from spermatozoa cultured in the complete or incomplete BWW and analyzed by western blotting using β-tubulin as internal control. Intense bands at about 26 kDa and 22 kDa were observed in both lanes, which indicated that EPPIN protein did not scale off heavily from the spermatozoa during incubation under conditions used to achieve capacitation. (Figure 5).
Figure 5.
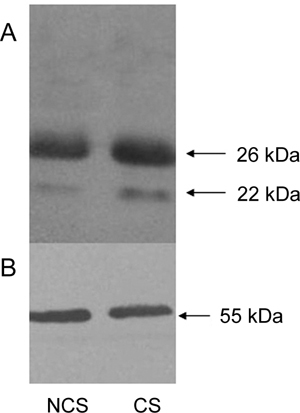
Rat EPPIN (epididymal protease inhibitor) did not scale off heavily from sperm during incubation under conditions used to achieve capacitation. Western blots were performed to detect EPPIN levels on spermatozoa. Protein samples on the polyvinylidene fluoride membrane were first detected with Eppin (I-12) probe (A), then washed, reblocked and detected with mouse anti-tubulin monoclonal antibody (B). Molecular weights of the bands were indicated on the right. Data represent one typical experiment of several experiments. Lane NCS: protein from sperm before capacitation. Lane CS: protein from sperm after incubation under conditions used to achieve capacitation.
Postnatal developmental expression pattern of Eppin transcripts in rat testis
We investigated the postnatal developmental expression pattern of Eppin transcripts in rat testis and the specific age points were selected on the basis of previous reference 22. RT-PCR analysis resulted in a specific band at expected size for rat Eppin. As shown in Figure 6, Eppin transcripts were expressed from the neonatal period to adulthood. The transcriptions were slight in neonatal (1-day-old) and infantile stages (5-, 7- and 10-day-old). It increased sharply thereafter, with maximum expression level (about 38-fold compared with that of 1-day-old rat) detected in prepubertal stage (15-day old). Then a slightly declined but stable level (about 20-fold compared with that of 1-day-old rat) was kept in pubertal–early adult (30-day old) and adult (60-day-old) stages of postnatal maturation.
Figure 6.
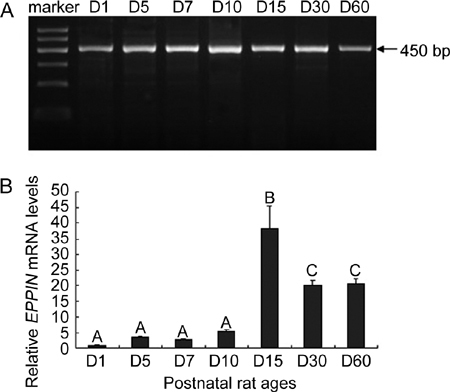
Postnatal developmental expression pattern of Eppin (epididymal protease inhibitor) transcripts in rat testis. Representative reverse transcriptase PCR (RT-PCR) assay of Eppin in testicular samples from 1-(D1), 5-(D5), 7-(D7), 10-(D10), 15-(D15), 30-(D30) and 60-day-old (D60) rats are presented (A). The molecular weight was indicated on the right. Semiquantitative data on the expression levels of the targets in rat testis along postnatal development are shown (B). We collected testis samples from several animals (at least three rats) at each age point and mixed them together as one sample. Each mixed sample was detected thrice simultaneously by real-time PCR. Relative levels were obtained in each sample by normalization to Gapdh. For presentation of data, expression level in the 1-day-old sample was taken as one unit and the others were normalized accordingly, thus allowing semiquantitative comparison. Values are the mean ± s.e.m. of the three readings at each age point. Groups with different superscript letters are statistically different (P < 0.05, ANOVA [analysis of variance] followed by Student–Neuman–Keuls test).
Discussion
Identical to the results obtained from mouse 15 and human 14, rat Eppin transcripts were expressed specifically in the testis and epididymis as shown in Figure 1. Bands predicted for rat Eppin were obtained in testis preparations at all developmental stages (Figure 6). Interestingly, however, the relative levels of transcription varied throughout the developmental process and the expression profile (Figure 6) strongly resembled that of mouse Eppin as previously reported 22. It reached the highest level in 15-day-old rats, which was considered to be the prepubertal stage of postnatal maturation 23. Given that the intratubular androgen receptor (AR) in the testis peaked on days 14–17 24, the increased AR availability seemed likely to enhance the expression of Eppin transcripts in the testis. The prepubertal period is characterized by a rapid growth of the testis, the transformation of the seminiferous cords into tubules and the initiation of spermatogenesis 4. Thus it is reasonable to think that the maximal level of transcription in prepubertal stage may have important roles in these developmental events.
To observe the protein expression, western blotting analysis was performed. Total proteins from testis, caput, corpus and cauda epididymis showed a strong band at about 26 kDa, which was different from its deduced molecular weight at 17 kDa. This could be due to glycosylation or β-mercaptoethanol-stable disulfide bonding that can produce anomalous molecular weights or aggregates. The 26-kDa isoform also existed in human and mouse (data not shown). Tissue sections were immunostained for more detailed observation. Results in Figure 3 strongly suggested the germ cell-specific expression of EPPIN in testis. However, no signal can be detected in the Sertoli cells, which were previously documented to be the main source of mouse EPPIN. A failure to find Eppin transcripts in the Sertoli cell in vitro (Figure 4) further confirmed the difference of EPPIN's origin between rat and mouse. Strong staining was observed in the elongated spermatids. The stage-specific production of EPPIN may reflect its strict translational regulation in germ cell. However, the elongated spermatid-only expression of EPPIN seemed contradictory to the RNA profile (Figure 6), as 15 days after birth is well before the first appearance of round spermatids. This is possibly because the protein peak tends to lag that of transcripts. That is to say, the Eppin transcripts may exist abundantly in an earlier subset of germ cell, such as primary spermatocyte, which has been reported to proliferate remarkably on postnatal day 15. Studies are now being carried out in our laboratory to investigate what type of germ cell is the main source of Eppin mRNA in testis.
Two predicted domains in EPPIN sequence, the Kunitz-type and WAP-type domain, have endowed EPPIN with potential protease inhibitory activity, which has been partially confirmed using recombinant human EPPIN 25. It has been proposed that proteases of testicular origin would act like scissors and help germ cells in migrating along Sertoli cell membranes during spermatogenesis 4. Therefore, EPPIN may be among a wide range of inhibitors that would restrict the activity of those testicular proteases in a finely tuned regulatory fashion to preserve homeostasis, although its spectrum of action remains unclear.
In addition to the testis, epididymis also expressed EPPIN protein. As shown on the cross-sections, EPPIN protein existed in the epithelial cells and lumen of epididymal duct. Sperm with normal appearance released from testis are infertile and need to experience an epididymis maturation process to acquire forward motility and the capacity to fertilize ova 7, 8, 26. The epididymis function of maturing sperm is highly dependent on the microenvironments created by its epithelial cells secreting and absorbing protein molecules 9. Those molecules can form protein complexes and adhere to the sperm surface to regulate sperm function 27, 28. Sperm association has been reported for many human epididymal proteins, such as ARP, HE2, HE4 and HE5/CD52 29, 30. EPPIN is such a kind of epididymis-secreted protein and has been generally thought to be a member of an enormous protein network. On the human sperm surface, an EPPIN protein complex containing lactotransferrin, clusterin and semenogelin may provide microbicidal properties that protect spermatozoa as well as regulate the sperm's transition to a motile, capacitated state 31. The modification of sperm surface proteins is also important for epididymal sperm maturation and this proteolysis event needs to be well controlled. Thus several protease inhibitors expressed in certain epididymal regions are expected to be responsible for this control. EPPIN, a serine protease inhibitor with Kunitz and WAP domains present in the epididymis, may be important to repress some of those proteases 32. Disruptions of these functions might partially contribute to the reversible contraception in EPPIN-immunized monkeys.
Mammalian sperm must experience a capacitation process in the female reproductive tract before they acquire the ability to fertilize oocytes 33. This process is believed to be initiated by the release of surface-associated decapacitation factors, a function already corroborated in a group of sperm surface proteins 34, 10. In the present study, we found that rat EPPIN did not scale off heavily from sperm during incubation under conditions used to achieve capacitation (Figure 6). This may suggest that EPPIN was not such a kind of decapacitation factor.
In conclusion, rat EPPIN is specifically expressed in the testis and epididymis, which provides indirect evidence for its important role in spermatogenesis and maturation. Studies on rat EPPIN may offer us a convenient animal model to investigate EPPIN's function and male contraception.
Acknowledgments
This study was supported by grants from the National Eleventh-Five Science and Technology Support Program of China (2006BAI03B12); the National Basic Research Program of China (973 Program) (2009CB941703) and PCSIRT (IRT0631). The authors thank Michael O'Rand, PhD, for results analysis and English-language editing.
References
- De Ronde W, Meuleman EJ. Hormonal contraception in men. Ned Tijdschr Geneeskd. 2007;151:2558–61. [PubMed] [Google Scholar]
- Sikka SC, Wang R. Endocrine disruptors and estrogenic effects on male reproductive axis. Asian J Androl. 2008;10:134–45. doi: 10.1111/j.1745-7262.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- Said TM, Paasch U, Glander HJ, Agarwal A. Role of caspases in male infertility. Hum Reprod Update. 2004;10:39–51. doi: 10.1093/humupd/dmh003. [DOI] [PubMed] [Google Scholar]
- Le Magueresse-Battistoni B. Serine proteases and serine protease inhibitors in testicular physiology: the plasminogen activation system. Reproduction. 2007;134:721–9. doi: 10.1530/REP-07-0114. [DOI] [PubMed] [Google Scholar]
- Thakur SC, Kumar V, Ghosh I, Bharadwaj A, Datta K. Appearance of hyaluronan binding protein 1 proprotein in pachytene spermatocytes and round spermatids correlates with spermatogenesis. J Androl. 2006;27:604–10. doi: 10.2164/jandrol.05142. [DOI] [PubMed] [Google Scholar]
- Odet F, Duan C, Willis WD, Goulding EH, Kung A, et al. Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol Reprod. 2008;79:26–34. doi: 10.1095/biolreprod.108.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford JM. Effects of duct ligation on the fertilizing ability of spermatozoa from different regions of the rabbit epididymis. J Exp Zool. 1967;166:271–82. doi: 10.1002/jez.1401660210. [DOI] [PubMed] [Google Scholar]
- Orgebin-Crist MC. Sperm maturation in rabbit epididymis. Nature. 1967;216:816–8. doi: 10.1038/216816a0. [DOI] [PubMed] [Google Scholar]
- Dacheux JL, Gatti JL, Dacheux F. Contribution of epididymal secretory proteins for spermatozoa maturation. Microsc Res Tech. 2003;61:7–17. doi: 10.1002/jemt.10312. [DOI] [PubMed] [Google Scholar]
- Nixon B, MacIntyre DA, Mitchell LA, Gibbs GM, O'Bryan M, et al. The identification of mouse sperm-surface-associated proteins and characterization of their ability to act as decapacitation factors. Biol Reprod. 2006;74:275–87. doi: 10.1095/biolreprod.105.044644. [DOI] [PubMed] [Google Scholar]
- Jalkanen J, Kotimäki M, Huhtaniemi I, Poutanen M. Novel epididymal protease inhibitors with Kazal or WAP family domain. Biochem Biophys Res Commun. 2006;269:15957–60. doi: 10.1016/j.bbrc.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Cohen DJ, Ellerman DA, Busso D, Morgenfeld MM, Piazza AD, et al. Evidence that human epididymal protein ARP plays a role in gamete fusion through complementary sites on the surface of the human egg. Biol Reprod. 2001;65:1000–5. doi: 10.1095/biolreprod65.4.1000. [DOI] [PubMed] [Google Scholar]
- Busso D, Cohen DJ, Maldera JA, Dematteis A, Cuasnicu PS. A novel function for CRISP1 in rodent fertilization: involvement in sperm-zona pellucida interaction. Biol Reprod. 2007;77:848–54. doi: 10.1095/biolreprod.107.061788. [DOI] [PubMed] [Google Scholar]
- Richardson RT, Sivashanmugam P, Hall SH, Hamil KG, Moore PA, et al. Cloning and sequencing of human Eppin: a novel family of protease inhibitors expressed in the epididymis and testis. Gene. 2001;270:93–102. doi: 10.1016/s0378-1119(01)00462-0. [DOI] [PubMed] [Google Scholar]
- Sivashanmugam P, Hall SH, Hamil KG, French FS, O'Rand MG, et al. Characterization of mouse Eppin and a gene cluster of similar protease inhibitors on mouse chromosome 2. Gene. 2003;312:125–34. doi: 10.1016/s0378-1119(03)00608-5. [DOI] [PubMed] [Google Scholar]
- O'rand MG, Widgren EE, Sivashanmugam P, Richardson RT, Hall SH, et al. Reversible immunocontraception in male monkeys immunized with Eppin. Science. 2004;306:1189–90. doi: 10.1126/science.1099743. [DOI] [PubMed] [Google Scholar]
- Yenugu S, Richardson RT, Sivashanmugam P, Wang Z, O'rand MG, et al. Antimicrobial activity of human EPPIN, an androgen-regulated, sperm-bound protein with a whey acidic protein motif1. Biol Reprod. 2004;71:1484–90. doi: 10.1095/biolreprod.104.031567. [DOI] [PubMed] [Google Scholar]
- García EM, Vázquez JM, Parrilla I, Ortega MD, Calvete JJ, et al. Localization and expression of spermadhesin PSP-I/PSP-II subunits in the reproductive organs of the boar. Int J Androl. 2008;31:408–417. doi: 10.1111/j.1365-2605.2007.00784.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu J, Huo R, Mao Y, Lu Y, et al. ERp57 is a potential biomarker for human fertilization capability. Mol Hum Reprod. 2007;13:633–9. doi: 10.1093/molehr/gam049. [DOI] [PubMed] [Google Scholar]
- Khobarekar BG, Vernekar VJ, Prabagaran E, Raghavan VP, Bandivdekar AH. Studies on the expression of 80-kDa human sperm antigen in rat testis and epididymis. J Histochem Cytochem. 2007;55:753–62. doi: 10.1369/jhc.6A7132.2007. [DOI] [PubMed] [Google Scholar]
- Qian J, Bian Q, Cui L, Chen J, Song L, Wang X. Octylphenol induces apoptosis in cultured rat Sertoli cells. Toxicol Lett. 2006;166:178–86. doi: 10.1016/j.toxlet.2006.06.646. [DOI] [PubMed] [Google Scholar]
- Denolet E, De Gendt K, Allemeersch J, Engelen K, Marchal K, et al. The effect of a sertoli cell-selective knockout of the androgen receptor on testicular gene expression in prepubertal mice. Mol Endocrinol. 2006;20:321–34. doi: 10.1210/me.2005-0113. [DOI] [PubMed] [Google Scholar]
- Barreiro ML, Suominen JS, Gaytán F, Pinilla L, Chopin LK, et al. Developmental, stage-specific, and hormonally regulated expression of growth hormone secretagogue receptor messenger RNA in rat testis. Biol Reprod. 2003;68:1631–40. doi: 10.1095/biolreprod.102.008862. [DOI] [PubMed] [Google Scholar]
- Weber MA, Groos S, Aumüller G, Konrad L. Post-natal development of the rat testis: steroid hormone receptor distribution and extracellular matrix deposition. Andrologia. 2002;34:41–54. doi: 10.1046/j.1439-0272.2002.00465.x. [DOI] [PubMed] [Google Scholar]
- McCrudden MT, Dafforn TR, Houston DF, Turkington PT, Timson DJ. Functional domains of the human epididymal protease inhibitor, eppin. FEBS J. 2008;275:1742–50. doi: 10.1111/j.1742-4658.2008.06333.x. [DOI] [PubMed] [Google Scholar]
- Turner TT. De Graaf's thread: the human epididymis. J Androl. 2008;29:237–50. doi: 10.2164/jandrol.107.004119. [DOI] [PubMed] [Google Scholar]
- Schröter S, Osterhoff C, McArdle W, Ivell R. The glycocalyx of the sperm surface. Hum Reprod Update. 1999;5:302–13. doi: 10.1093/humupd/5.4.302. [DOI] [PubMed] [Google Scholar]
- Mishra S, Somanath PR, Huang Z, Vijayaraghavan S. Binding and inactivation of the germ cell-specific protein phosphatase PP1g2 by sds22 during epididymal sperm maturation. Biol Reprod. 2003;69:1572–9. doi: 10.1095/biolreprod.103.018739. [DOI] [PubMed] [Google Scholar]
- Kirchhoff C. Molecular characterization of epididymal proteins. Rev Reprod. 1998;3:86–95. doi: 10.1530/ror.0.0030086. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Frenette G, Girouard J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J Androl. 2007;9:483–91. doi: 10.1111/j.1745-7262.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Widgren EE, Richardson RT, O'rand MG. Characterization of an EPPIN protein complex from human semen and spermatozoa. Biol Reprod. 2007;77:476–84. doi: 10.1095/biolreprod.107.060194. [DOI] [PubMed] [Google Scholar]
- Sipilä P, Jalkanen J, Huhtaniemi IT, Poutanen M. Novel epididymal proteins as targets for the development of post-testicular male contraception. Reproduction. 2009;137:379–89. doi: 10.1530/REP-08-0132. [DOI] [PubMed] [Google Scholar]
- De Jonge C. Biological basis for human capacitation. Hum Reprod Update. 2005;11:205–14. doi: 10.1093/humupd/dmi010. [DOI] [PubMed] [Google Scholar]
- Oliphant G, Reynolds AB, Thomas TS. Sperm surface components involved in the control of the acrosome reaction. Am J Anat. 1985;174:269–83. doi: 10.1002/aja.1001740308. [DOI] [PubMed] [Google Scholar]


