Abstract
Regulatory T cells play important roles in cancer development and progression by limiting the generation of innate and adaptive anti-tumor immunity. We hypothesized that in addition to natural CD4+CD25+ Tregs and myeloid-derived suppressor cells, tumor antigen-specific regulatory T cells interfere with the detection of anti-tumor immunity following immunotherapy. Using samples from prostate cancer patients immunized with a DNA vaccine encoding prostatic acid phosphatase (PAP) and a trans-vivo delayed type hypersensitivity (tvDTH) assay, we found that the detection of PAP-specific effector responses following immunization was prevented by the activity of PAP-specific regulatory cells. These regulatory cells were CD8+CTLA-4+, and their suppression was relieved by blockade of CTLA-4, but not IL-10 or TGF-β. Moreover, antigen-specific CD8+ regulatory T cells were detected prior to immunization in the absence of PAP-specific effector responses. These PAP-specific CD8+CTLA-4+ suppressor T cells expressed IL-35, which was decreased following blockade of CTLA-4, and inhibition of either CTLA-4 or IL-35 reversed PAP-specific suppression of tvDTH response. PAP-specific CD8+CTLA-4+ T cells also suppressed T-cell proliferation in an IL-35-dependent, contact-independent fashion. Taken together, these findings suggest a novel population of CD8+CTLA-4+ IL-35-secreting tumor antigen-specific regulatory T cells arise spontaneously in some prostate cancer patients, persist during immunization, and can prevent the detection of antigen-specific effector responses by an IL-35-dependent mechanism.
Introduction
The ability of the immune system to respond to infectious pathogens, non-inherited antigens, and malignant tumor antigens is counterbalanced by an equally important system of regulatory immune responses which seek to limit the self-reactive potential of these effector responses. These responses can be mediated by a variety of cell types (including the recently identified myeloid-derived suppressor cells), but traditionally have been thought of as antigen non-specific CD4+ T cells. CD4+ regulatory T cells are broadly defined as either 'natural' (CD4+CD25+) (1) or 'induced' Tregs. Induced Tregs are generated as uncommitted CD4+ T cells, which gain distinct suppressive functions based on particular antigenic stimulation and are characterized by their secretion of various cytokines: IL-10-secreting type 1 T-regulatory (Tr1) cells (2), transforming growth factor β (TGF-β)-secreting Th3 cells (3), and the recently identified IL-35-secreting iTr35 population (4). These iTr35 cells are particularly important to regulatory T cell function, as IL-35 expression (composed of a heterodimer of the IL-12p35 and Ebi3 subunits) has been shown to be required for the maximal regulatory function of murine and human regulatory T cells, and can propagate infectious tolerance in part by converting conventional CD4+ T cells into iTr35 regulatory cells (4–7).
While most reports of regulatory T cell populations have largely focused on their antigen non-specific function, the immune system has also been shown to have the ability to mobilize antigen-specific regulatory T-cell responses, notably in several models of autoimmunity and transplantation (8–16). These antigen-specific populations require their cognate antigen to become activated, but once active can suppress effector responses in an antigen non-specific fashion (14–16). Emerging evidence has also shown a role for antigen-specific regulation in cancer, with the identification of CD4+ T cells specific for various tumor antigens that have suppressive function (17–24). Much of this research has been conducted in patients with melanoma, with the identification of CD4+ regulatory T cells that recognize antigens such as LAGE1 (17), ARTC1 (18), or other tumor antigens (19). As in other models of antigen-specific regulatory T cells, these responses required ligand-specific activation, but once activated could suppress proliferation in a non-specific fashion. In two seminal reports by Wang and colleagues, the blockade of either IL-10 or TGF-β did not abrogate the suppressive activity of antigen-specific regulatory T cells, suggesting other cytokines or cell-contact dependent mechanisms are responsible for mediating suppression (17, 18).
The association between regulatory immune responses and the development and progression of cancer has been well established in a number of malignancies, including prostate cancer (25–27). Numerous reports in both rodent models as well as patients have shown that prostate tumor-bearing individuals have increased frequencies of CD4+ Tregs compared to healthy individuals, both in the periphery (3, 27–30) as well as infiltrating the tumor (27, 30–33). Tregs have also been shown to be associated with prostate cancer disease progression, as patients with advanced disease were found to have higher percentages of peripheral Tregs than individuals with early stage disease (3, 29, 30). Immunosuppressive factors produced by Tregs, such as IL-10 and TGF-β, have been shown to contribute to prostate cancer development and progression (34, 35). Additionally, many current prostate cancer therapies have been shown to enhance Treg frequency, including radiation therapy (36), androgen deprivation (37, 38), and chemotherapy (39).
The use of anti-tumor immunotherapies for prostate cancer has seen several advances in recent years, with the FDA approval of sipuleucel-T for advanced prostate cancer (40) and another randomized clinical trial evaluating a viral-based vaccine (PROSTVAC) showing a significant survival benefit (41). However, as in other established cancer therapies, regulatory immune responses have also been shown to have a profound detrimental effect on the immune and clinical success of these immunotherapies. For example, baseline effector T-cell responses to a variety of prostate tumor-associated antigens have been shown to be suppressed by concurrent Treg responses, including effector responses to prostatic acid phosphatase (PAP) and prostate specific antigen (PSA), the antigens targeted by sipuleucel-T and PROSTVAC, respectively (42). Furthermore, while immunotherapies aim to augment anti-tumor effector responses, several reports have found that they can also enhance regulatory immune responses which can suppress concurrent effector responses (38, 43–47), including a report of a DNA vaccine in patients with either non-small cell lung, esophageal, or prostate cancer in which regulatory responses were found to suppress effector responses generated following immunization (48).
We recently reported the results of a phase I clinical trial evaluating a DNA vaccine encoding PAP in which patients with early recurrent prostate cancer received six biweekly immunizations (49). In this trial, 8/22 individuals developed PAP-specific effector IFNγ-secreting T-cells that persisted for several months after immunization, which correlated with a favorable change in PSA doubling time (a decrease in the rate of PSA rise), a possible prognostic marker in patients with early recurrent prostate cancer (50–54). However, three of these eight ‘responding’ patients did not have an immune response two weeks following the final immunization, and several other individuals were not found to have a detectable effector immune response up to a year following immunization. We hypothesized that the presence of PAP-specific regulatory cells might have prevented the detection of PAP-specific effector responses post-immunization. Consequently, in this report we used samples from this trial to investigate whether antigen-specific regulatory T cell responses existed, and if so to further characterize the phenotype and function of this population.
Materials and Methods
Patient Populations
Patient PBMC used for the studies were obtained from individuals previously treated on a clinical trial at the University of Wisconsin Carbone Cancer Center (49). This included 21 subjects with PSA-recurrent prostate cancer, without radiographic evidence of metastases, and not receiving concurrent androgen deprivation. All subjects gave written, IRB-approved consent for the use of residual blood products to be used for immunological research. PBMC had been collected by leukapheresis prior to, and two weeks following, vaccination with six biweekly intradermal injections of a DNA vaccine encoding PAP, and by blood draw at three month intervals for one year following immunization. All subjects also received an intramuscular tetanus booster immunization prior to receiving the DNA vaccinations which was used as a recall antigen. PBMC were cryopreserved in aliquots in liquid nitrogen until use.
Mice
CB-17 SCID mice were bred at the University of Wisconsin Gnotobiotic Laboratory facility. All animals were housed and treated in accordance with guidelines outlined by the University of Wisconsin and the National Institutes of Health, and under an IACUC-approved protocol.
Trans-vivo Delayed-Type Hypersensitivity
7.5–10 × 106 PBMC obtained from patients prior to and after immunization were co-injected into the footpads of 6-to-8-week old SCID mice with 1 µg of recombinant human PAP protein (Fitzgerald Industries, Acton, MA), or recombinant human prostate-specific antigen (PSA, Fitzgerald Industries) as a non-specific antigen control. The response to tetanus toxoid (TT/D; Aventis Pasteur, Bridgewater, NJ) recall antigen alone plus PBMC was used as a positive control, and PBMC plus PBS was used as a negative control. Antigen–driven swelling was determined as previously described (55). DTH reactivity after 24 hours was measured as the change in footpad thickness in multiples of 10−4 inches, measured using a dial thickness gauge (Mitutoyo, Japan), and net swelling is the antigen-specific swelling subtracted for the contribution obtained with PBMC plus PBS. To determine the effect of neutralizing antibodies, PBMC were mixed with PAP or PSA antigen and injected into the footpads of SCID mice with 25 µg of either control IgG or rabbit anti-human TGF-β (R&D Systems, Minneapolis, MN), goat anti-human IL-10 (R&D Systems), or 1 µg of mouse anti-human CTLA-4 monoclonal Ab (clone AS32, Ab Solutions, Mountain View, CA). The extent of bystander suppression was measured as inhibition of recall antigen response in the presence of PAP or PSA antigens and calculated as previously described (56). To reverse bystander suppression of DTH responses, PBMC were first mixed with PAP protein and 25 µg of TT/D, and then combined with either control IgG, anti-human CTLA-4 mAb, MHC class I (clone W6/32, Biolegend, San Diego, CA), MHC class II antibodies (clone L243, Biolegend), mouse anti-human IL-12p35 (R&D Systems, clone 27537), mouse anti-human IL-12p40 (R&D Systems, clone 24901), goat anti-human IL-23p19 (R&D Systems, AF1716), goat anti-mouse IL-27p28 (cross-reactive with human, R&D Systems, AF1834), or mouse anti-human Ebi3 (clone V1.4F5.25 (6)) and the mixture was then injected into the footpads of SCID mice. DTH reactivity was again measured after 24 hours as described above. Results are expressed as the change over the swelling induced by injection of PBMC + PBS alone. Absolute net tvDTH response of > 15 × 10−4 inches, and changes in net tvDTH > 10 × 10−4 inches to a particular antigen, were considered as consistent with the presence or gain of a DTH immune response (16, 55, 57). Given the nature of the testing, data shown are typically from single measurements, but with experiments repeated 2–3 times. We have previously demonstrated the reproducibility of this tvDTH assay (58). In separate experiments, PBMC were depleted of CD8+ T cells, or enriched/selected for CD8+ T cells, by magnetic bead selection (StemCell Technologies, Vancouver, BC), according to the manufacturer’s instructions. In other studies, CD8+ enriched/selected T cells were further depleted of subpopulations using PE-labeled antibodies specific for CTLA-4 (eBiosciences, San Diego, CA, clone 14D3) or control antigen followed by magnetic bead selection (StemCell Technologies). For these T-cell subset studies the purity of CD3+CD8+ cells and effective depletion of CD8+ T cells from PBMC was determined by flow cytometry, and was routinely found to be >94%. As a result of this separation, contaminating CD4+CTLA-4+ T cells amongst the added back CD8+ T cells would expected to be at most 0.06% of the total injected population.
IFNγ ELISPOT
ELISPOT was performed as previously described (53). In brief, wells of nitrocellulose 96-well microtiter (ELISPOT) plates were coated with an anti-IFNg capture monoclonal antibody (Endogen, Rockford, IL). Cryopreserved PBMC, obtained at various times prior to or after vaccination, were then thawed and cultured for 48 hours in the presence of media only (no antigen), 2 µg/ml PAP protein (Research Diagnostics Inc., Flanders, NJ), 250 ng/ml tetanus toxoid (Calbiochem, San Diego, CA), or 5 µg/ml phytohemaglutinin (PHA, positive mitogenic control, Fisher, Pittsburgh, PA). ELISPOT plates were then washed and probed for 1.5 hours with a biotinylated anti-IFNg antibody (Endogen), streptavidin-labeled alkaline phosphatase for one hour, and then developed with BCIP/NBT colorimetric substrate (BioRad, Hercules, CA) for 15–30 minutes. The number of spots per well was determined with an automated ELISPOT reader (Autoimmun Diagnostika GmbH, Strassberg, Germany) and normalized to 106 PBMC. The mean number of spots detected under media-only conditions at each time point was subtracted from the antigen-specific conditions to enumerate antigen-specific IFNγ spot-forming units (sfu) +/− standard deviation. Comparison of experimental wells with control, no-antigen, wells was performed using a two-tailed t test, with p ≤ 0.05 used to define a significant T-cell response.
Protein stimulations, cell sorting, RNA, cDNA, and quantitative real-time PCR
PBMC samples were stimulated for 72 hours with media alone (RPMI 1640 media supplemented with L-glutamine, penicillin, streptomycin, and 10% human AB serum), or either recombinant prostate specific antigen (PSA, 2µg/mL, Chemicon) or recombinant human PAP (2µg/mL, Chemicon). Furthermore, PSA- or PAP-stimulated cultures were also treated with either a blocking antibody specific for CTLA-4 (clone AS32), or a murine IgG control. Cultures were collected, stained with antibodies specific for CD3, CD8, and CTLA-4, and sorted by flow cytometry (FACSAria, BD Biosciences), collecting the following populations: CD3+CD8+CTLA-4+, CD3+CD8+CTLA-4−, and CD3+CD8− (CD3: clone OKT3, eBioscience; CD8: clone SK1, eBioscience; CTLA-4: clone 14-D3, eBioscience). RNA was then collected from sorted cells using the Qiagen mRNA kit, and cDNA was reverse-transcribed with the iScript cDNA Synthesis kit (BioRad). The cDNA samples were then subjected to 40 cycles of amplification with primers specific for IL-12A, IL-12B, IL-23A, IL-27, Ebi3, IL-10 (as previously described (6)), or the ribosomal protein P0 as a control gene (as previously described (59)) in a MyiQ™2 Two-Color Real-Time PCR Detection System (Biorad) and were quantified by the comparative cycling threshold method. Fold induction results were analyzed by the 2−ΔΔCt method (60) relative to P0 expression and the media-only treatment group, and statistical differences between CTLA-4 and IgG-treated samples was performed using a two-tailed t test, with p≤ 0.05 used to define a significant T-cell response.
In vitro PKH26 suppression assays
In vitro suppression assays were performed with modifications to a previously established protocol (4, 6). PBMC samples from patients with PAP-specific, CTLA-4 and IL-35-regulated CD8+ suppressive T cells were stimulated for 72 hours in the presence of PAP, PSA, or media alone. Following stimulation, cultures were sorted for CD8+CTLA-4+ and CD8+CTLA-4− T cells by flow cytometry (as above), and the sorted cells were labeled with 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE, Sigma-Aldrich). For direct suppression assays, sorted cells were co-cultured in 96-well round-bottom plates with unstimulated, autologous PBMC labeled with PKH26 (Sigma-Aldrich). Sorted cells were added back at titrated amounts: either their natural frequency as determined by flow cytometry, or ten-fold lower or higher. To set PKH26- gates as well as to calculate the percent suppression, a 'No Add Back' control was included using PKH26-labeled unstimulated PBMC alone. Co-cultures were stimulated with media alone or a mixture of anti-CD3/anti-CD28-coated beads (clones OKT3 and CD28.2, respectively; Biolegend, San Diego, CA) and 20U/mL recombinant human IL-2 (Fitzgerald Industries). Additionally, stimulated cultures were also treated with IL-35 blocking antibodies (a combination of antibodies directed against the IL12p35 subunit (clone 27537) and Ebi3 (clone V1.4F5.25)) or a murine IgG control. Following a seven day incubation, cells were harvested and labeled with fluorescent antibodies directed against CD3 (clone OKT3), CD4 (clone RPA-T4, BD Pharmingen), and CD8 (clone SK1). Lymphocytes were gated based on size, CFSE- staining (to exclude sorted cells added back to the experiment), either CD4+ or CD8+ expression, and PKH26 staining. Percent suppression was calculated using the following formula: 100−100*((ExpαCD3/28 − ExpMedia Alone) / (No Add BackαCD3/28 − No Add BackMedia Alone)). For transwell suppression assays, sorted cells were added to the top chamber of 96-well transwell plates (Millipore 96-well cell culture 0.4µm insert plate), and PKH26-labeled unstimulated, autologous PBMC were added to the bottom chamber (Millipore 96-well Feeder/Transport Tray). Either media alone or anti-CD3/CD28-coated latex beads along with 20U/mL IL-2 were added to the bottom chamber, and cultures were incubated seven days. Following this incubation, insert plates were removed and cells in the bottom chamber were collected and analyzed for proliferation as above.
Results
PAP-specific T effector immune responses following immunization are suppressed by PAP-specific T regulatory cells in a CTLA-4-dependent fashion
Patients with early recurrent prostate cancer were vaccinated six times biweekly with a DNA vaccine encoding PAP (49, 53). Eight of 22 patients experienced at least a two-fold increase in the PSA doubling time, and this was significantly associated with the development of long-term PAP-specific IFNγ-secreting immune responses (53). One of these patients (ID007) is highlighted in Figure 1A, showing the development of a durable PAP-specific IFNγ-secreting immune response. While this patient eventually developed a PAP-specific immune response many months after immunization, he had no detectable IFNγ-secreting immune response two weeks following the final immunization. A similar finding of delayed T effector responses (but no response two weeks following the final immunization) was also seen in two other patients who experienced a greater than two-fold increase in PSA doubling time (ID005 and ID014). While conceivable that an effector immune response might have taken months to develop, we reasoned that an alternative explanation might be that PAP-specific effector T-cell responses were augmented during vaccination, but that the detection of these effector responses was prevented by a concurrent regulatory immune response following immunization.
Figure 1. PAP antigen-specific T effector immune responses following immunization are suppressed by PAP-specific T regulatory cells in a CTLA-4-dependent fashion.
Panel A. IFNγ ELISPOT responses of patient ID007 prior to (Pre) or two weeks following (Post) six biweekly immunizations, and three-month intervals thereafter. PBMC were evaluated for IFNγ responses following stimulation with media alone (No Ag, grey), PAP (black), tetanus toxoid (TT/D, vertical hatches), or a PHA positive control (horizontal hatches). Shown are the mean and standard deviation of quadruplicate samples in spot-forming units (sfu). Panel B. Pre- (grey) or post-immunization (black) PBMC from three patients (ID005, ID007, and ID014) were injected into the footpads of SCID mice with the indicated antigens (TT/D, PAP, or PSA) and blocking antibodies (IgG control, anti-CTLA-4, anti-TGFβ, or anti-IL-10). DTH swelling responses (10−4 in.) were measured after 24 hours. Data shown are representative of at least two independent experiments. Differences in mean tvDTH between groups were analyzed by student’s t-test, with * indicating p<0.05. Panel C. Post-immunization samples from patient ID005 and ID014 were used in tvDTH assays measuring responses to TT/D alone or in combination with PAP or PSA, and in the presence or absence of a CTLA-4 blocking antibody. Data shown are representative of at least two independent experiments. Differences between mean DTH were compared using a student’s t-test, with * indicating p<0.05.
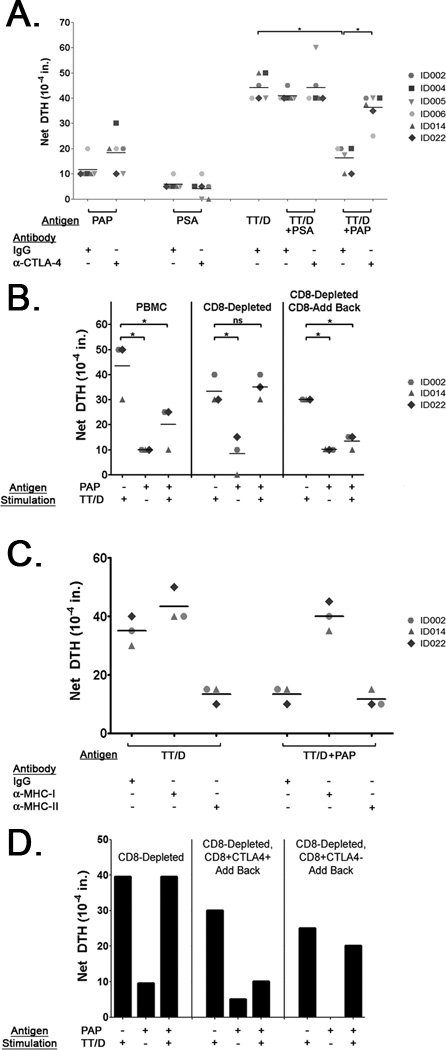
To distinguish between these two possibilities, we used the trans-vivo delayed-type hypersensitivity (tvDTH) assay, a method used extensively to identify antigen-specific regulatory T-cell responses in human organ transplant recipients (55). This technique involves injecting patient peripheral blood samples into the footpads of SCID mice along with the antigen of interest, and measuring footpad swelling 24 hours later as an indicator of an inflammatory immune response. As shown in Figure 1B, while these three patients had baseline recall responses to tetanus toxoid that remained following a booster immunization (given prior to the priming PAP DNA immunization), PAP-specific tvDTH immune responses were not detected either pre-immunization or two weeks following the final immunization. These findings agree with previously published results using standard T-cell proliferation and IFNγ ELISPOT assays (49, 53). However, when a blocking antibody specific for CTLA-4 was co-administered, PAP-specific immune responses were uncovered in all three patients two weeks following the final immunization (Figure 1B). Co-administration of blocking antibodies to the immunosuppressive cytokines IL-10 and TGF-β did not uncover a PAP-specific effector response (Figure 1B).
We reasoned that the uncovering of effector responses upon treatment with a CTLA-4 blocking antibody could be a result of targeting CTLA-4 expressed on effector cells following activation, or alternatively could be a result of blocking CTLA-4 expressed by regulatory cells (61). To distinguish between these possibilities, we evaluated the same patients for bystander suppression of a recall tetanus response, a technique we have routinely used to identify antigen-specific regulatory T cells in models of transplant tolerance (55). This method involves co-injecting patient peripheral blood samples with a recall antigen (either tetanus toxoid or inactivated Epstein-Barr virus) and an experimental antigen being evaluated for regulatory immune responses. As with other antigen-specific regulatory immune responses, the experimental antigen-specific regulatory cells require their cognate antigen for activation, but once activated can suppress in a non-specific fashion (thus suppressing the tetanus bystander immune response). While samples were unavailable to conduct this analysis with patient ID007, post-immunization samples from patients ID005 and ID014 were found to generate a strong immune response to the tetanus antigen. This response was unaffected by co-administration of a control protein (prostate-specific protein, PSA; Figure 1C). However, when PAP was injected along with the tetanus antigen, the bystander immune response to tetanus was significantly diminished. Furthermore, this PAP-specific bystander suppression could be alleviated with co-administration of a blocking antibody directed against CTLA-4. Blocking CTLA-4 did not have an effect on DTH in animals treated with tetanus and the control protein PSA, indicating that this blocking antibody was not targeting the intrinsic effector activity of tetanus-specific effector immune responses. These results suggest that PAP-specific regulatory cells were present along with PAP-specific effector immune responses following immunization, and that these regulatory cells mediate suppression via CTLA-4.
Immunization increases the frequency of CTLA-4-regulated antigen-specific effector responses
To determine if the development of PAP-specific effector responses regulated by CTLA-4 is a common phenomenon among patients immunized with a PAP DNA vaccine, we studied remaining pre- and post-immunization samples from 21 patients who received six biweekly immunizations with a DNA vaccine targeting PAP. As shown in Table I, PAP-specific effector responses regulated by CTLA-4 were uncommon prior to immunization, with only one patient (ID004) showing a CTLA-4-regulated PAP-specific effector response. However, following immunization, eight individuals (ID002, ID005, ID006, ID007, ID014, ID016, ID017, and ID022) were found to have PAP-specific effector responses that were regulated by CTLA-4. The frequency of patients with these CTLA-4-regulated effector responses was significantly higher following immunization than pre-treatment (p=0.021, Fisher’s exact test). Similar to the patients evaluated in Figure 1, PAP-specific effector responses were restored by blocking CTLA-4, but were unaffected by the co-administration of antibodies specific for IL-10 or TGFβ (Supplementary Figure 1).
Table 1.
| PAP | ||||||
|---|---|---|---|---|---|---|
| Pre-Immunization (Net DTH, 10−4 in.) | Post-Immunization (Net DTH, 10−4 in.) | |||||
| Subject ID | +IgG* | +α-CTLA-4* | CTLA-4-Regulated PAP Response |
+IgG* | +α-CTLA-4* | CTLA-4-Regulated PAP Response |
| 02 | 10 ± 0.0 | 15 ± 5.8 | 5 | 10 | 35 | 25† |
| 03 | 30 | 20 | −10 | 30 | 26.7 ± 5.8 | −3.3 |
| 04 | 12.5 ± 5 | 25 ± 10 | 12.5† | 15 ± 5.8 | 13.3 ± 2.9 | −1.7 |
| 05 | 11.25 ± 2.5 | 12.5 ± 5 | 1.25 | 8.3 ± 5.8 | 16.7 ± 5.8 | 8.4† |
| 06 | 20 ± 0 | 22.5 ± 5 | 2.5 | 18.3 ± 2.9 | 45 ± 8.7 | 36.7† |
| 07 | 5 | 10 | 5 | 10 | 50 | 40† |
| 08 | 25 | 25 | 0 | 25 | 20 | −5 |
| 09 | 30 | 40 | 10 | 35 | 35 | 0 |
| 10 | 26.7 ± 5.8 | 27.5 | 0.8 | 20 | 15 | −5 |
| 11 | 10 | 10 | 0 | 0 | 5 | 5 |
| 12 | 10 | 20 | 10 | 5 | 0 | −5 |
| 13 | 30 | 30 | 0 | 30 | 20 | −10 |
| 14 | 10 ± 0 | 16.7 ± 5.8 | 6.7 | 10 ± 0 | 26.7 ± 2.9 | 16.7† |
| 15 | 20 ± 0 | 17.5 | −2.5 | 27.5 | 10 | −17.5 |
| 16 | 10 ± 0 | 10 ± 0 | 0 | 5 ± 5 | 30 ± 0 | 25† |
| 17 | 10 | 10 | 0 | 5 | 20 | 15† |
| 18 | 20 | 20 ± 0 | 0 | 5 | 15 | 10 |
| 19 | 20 | 20 | 0 | 20 | 30 | 10 |
| 20 | 30 | 40 | 10 | 5 | 15 | 10 |
| 21 | 20 | 20 | 0 | 10 | 0 | −10 |
| 22 | 8 ± 4.5 | 10 ± 5 | 2 | 8.75 ± 2.5 | 26.7 ± 7.6 | 17.95† |
Indicates CTLA-4-regulated PAP response >15in−4 in at least one assay
Shown are the mean ± standard deviation values calculated from triplicate assays. When sample availability precluded triplicate experimental assays, shown is the average experimental values.
PAP-specific regulatory responses are present prior to immunization, and are CD8+ CTLA-4+ T cell-dependent
To determine if PAP-specific regulatory cells were present in prostate cancer patients prior to immunization, samples from patients obtained prior to immunization were evaluated for PAP-specific bystander suppression of tetanus immune responses. As shown in Figure 2A, pre-immunization samples from patients identified as having CTLA-4-regulated PAP-specific effector responses (see Table I) lacked effector responses to PAP or PSA. However, while these patients had strong immune responses to the tetanus antigen, co-treatment with PAP resulted in a significant suppression of the tetanus immune response in 6/6 individuals. This suppression was alleviated by the co-administration of a blocking antibody specific for CTLA-4 (Figure 2A), and was antigen-specific, as co-administration of PSA did not result in suppression of the tetanus immune response. Moreover, the suppression of responses to the tetanus antigen was found not to be due to the PAP protein itself, as samples from other patients without CTLA-4-regulated responses did not demonstrate this bystander suppression (data not shown).
Figure 2. PAP-specific regulatory cells exist prior to immunization, and are CD8+CTLA-4+.
Panel A. PBMC samples from multiple prostate cancer patients were tested for pre-existing PAP-specific regulatory responses by tvDTH. PBMC were stimulated with the described antigens and antibody treatments, and tvDTH reactivity was measured. Data shown are representative of at least two independent experiments. Differences in mean tvDTH among different antigen/antibody treatment groups (indicated by solid lines) were compared using a student’s t-test, with * indicating p≤ <0.05. Panel B. PAP-specific regulatory responses were evaluated in tvDTH studies using samples from patients identified in Panel A. These studies were conducted using whole PBMC (left section), PBMC samples that had been depleted of CD8+ T-cells (center section), or PBMC samples that had been depleted of CD8+ T-cells and were subsequently supplemented with autologous CD8+ T-cells (right section). Data shown are representative of at least two independent experiments. Statistical differences between group mean DTH values (indicated by solid lines) were analyzed using a student’s t-test, with * indicating p≤0.05. Panel C. PBMC from patients with CD8-dependent regulatory responses identified in Panel B were evaluated for MHC dependency in tvDTH bystander suppression assays using antibodies blocking MHC class I or MHC class II. Statistical differences between group mean DTH values (indicated by solid lines) were analyzed using a student’s t-test, with * indicating p≤0.05. Panel D. PBMC samples from patient ID022 were evaluated for tetanus bystander suppression by tvDTH. PBMC samples were depleted of CD8+ T-cells, and tested alone (left section) or supplemented with magnetic bead-sorted CD8+CTLA-4+ (center section) or CD8+CTLA-4− T-cells (right section) along with antigens described.
After identifying a PAP-specific regulatory cell population, we sought to identify the cell population mediating this suppression. Surprisingly, when CD8+ T cells were depleted from PBMC samples of patients with regulatory responses, we found that PAP-specific bystander suppression was lost and a tetanus-specific response could be detected (Figure 2B). Furthermore, when purified CD8+ T cells were added back to CD8-depleted PBMC, this PAP-specific bystander regulation was re-established. To evaluate whether these CD8+ T cells were being directly activated as a result of encountering PAP-derived epitopes bound to MHC class I, we conducted bystander regulation assays in the presence of blocking antibodies targeting MHC class I or II. As the tetanus recall response is mediated primarily by CD4+ T cells, blocking MHC class II (but not MHC class I) abrogated a tetanus effector response (Figure 2C). However, while co-treatment of tetanus and PAP resulted in the suppression of the tetanus immune response, blocking MHC class I relieved this suppression. When this CD8+ T cell was further interrogated based on surface expression of CTLA-4, the addition of CD8+CTLA-4+ cells to CD8-depleted PBMC restored the suppression of tetanus immune responses (Figure 2D), whereas the addition of CD8+CTLA-4− T cells did not, suggesting the presence of a PAP-specific population of CD8+CTLA-4+ suppressive T cells.
CTLA-4 blockade decreases the expression of IL-35 by antigen-specific CD8+CTLA-4+ T cells
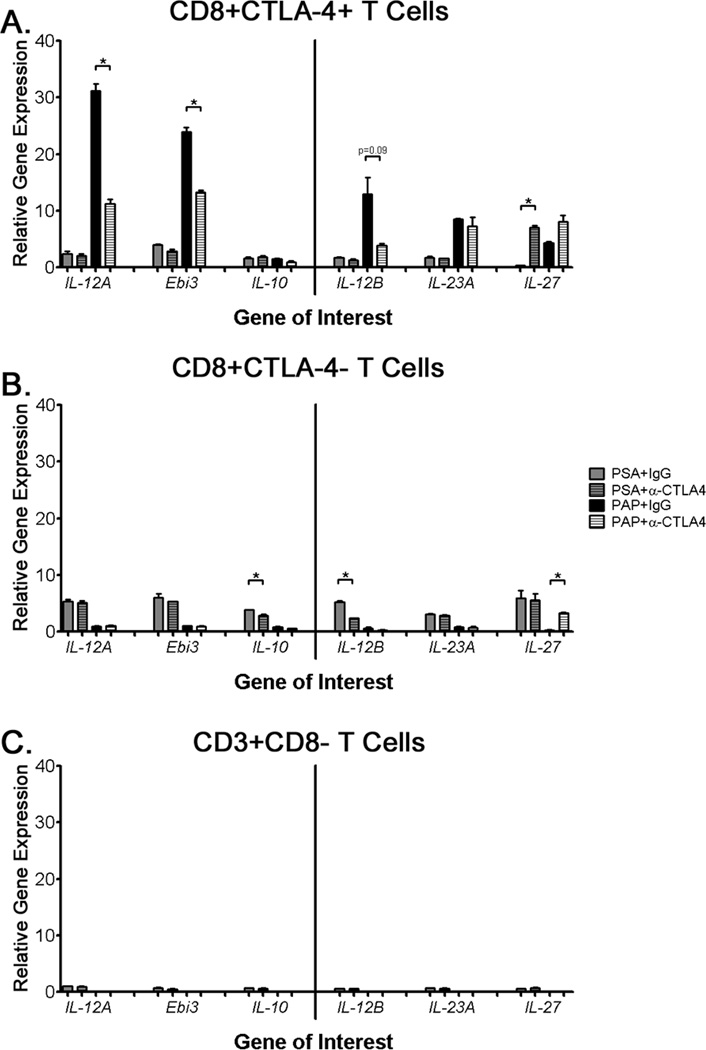
After finding that IL-10 and TGF-β did not play a role in mediating PAP-specific regulatory cell function (Figure 1C), we explored the potential contribution of IL-35. Pre-immunization PBMC samples from a patient with a CTLA-4-regulated immune response (ID002) were stimulated for 72 hours with PAP in the presence of either CTLA-4 blocking antibody or an IgG control. CD3+CD8+CTLA-4+ cells were then isolated by cell sorting (flow gating schema shown in Supplementary Figure 2A) and assessed for cytokine expression by quantitative real-time PCR. As shown in Figure 3A, stimulation with PAP resulted in a 20–30 fold induction in expression of IL-12A and Ebi3 subunits of IL-35 in CD8+CTLA-4+ T cells compared with PSA-treated cells. When regulatory responses were inhibited using an anti-CTLA-4 antibody, the expression of the IL-35 subunits was significantly decreased in a PAP-specific fashion. An antigen-specific, CTLA-4-regulated increase in IL-35 expression was not observed in other T cell populations (Figure 3B, 3C). Additionally, we did not detect any CTLA-4-regulated expression of other components of the IL-12 family (IL-12B, IL-23A, and IL-27 - Figure 3). Consistent with previous results, we did not detect significant expression, nor a CTLA-4-regulated decrease in expression, of IL-10 by quantitative real-time PCR (Figure 3A), ELISA, or multiplex cytokine analysis (data not shown). Similar results were also observed in each of the other two patients that had been found to have PAP-specific CD8+ suppressive immune responses (Supplementary Figure 3).
Figure 3. CTLA-4 blockade decreases expression of IL-35 by antigen-specific CD8+CTLA-4+ T cells.
Samples from a patient with CTLA-4-regulated PAP-specific immune responses (ID002) were stimulated for 72 hours with PAP, PSA, or media alone in the presence of an anti-CTLA-4 antibody or an IgG control (PSA+IgG: grey, PSA+anti-CTLA-4: hatched gey, PAP+IgG: black, PAP+anti-CTLA-4: white hatched). Cells were then sorted via flow cytometry for the following populations: CD3+CD8+CTLA-4+ (panel A), CD3+CD8+CTLA-4− (panel B), or CD3+CD8− (panel C). Relative mRNA expression of IL-12A, Ebi3, IL-10, IL-12B, IL-23A, and IL-27 was determined by quantitative RT-PCR, and were normalized against the ribosomal protein P0 as an internal control and the media-alone cell-sorted sample. Differences between control IgG and anti-CTLA-4 treatment group means were calculated using a student’s t-test, with * indicating p≤0.05. Data shown is representative of three independent experiments.
CD8+CTLA-4+ T cells suppress T-cell proliferation in an IL-35-dependent, contact-independent fashion
To directly assess the suppressive function of PAP-specific CD8+CTLA-4+ T cells, PBMC were stimulated with PAP or the control antigen PSA and sorted for CD8+CTLA-4+ and CD8+CTLA-4− T cells (shown in Supplementary Figure 3B). These sorted cells were then assessed for their ability to suppress the proliferation of autologous T cells in the presence or absence of antibodies blocking IL-35. As shown in Figure 4A, PAP-stimulated CD8+CTLA-4+ T cells had a marked, titratable suppressive effect on the proliferation of autologous CD4+ and CD8+ T cells, an effect which was abrogated by the addition of antibodies blocking both subunits of IL-35. This suppression was not seen when CD8+CTLA-4− T cells were added, nor cells sorted from cultures stimulated with either PSA or media alone (graphically represented in Figure 4B). Similar results were obtained from other patients with PAP-specific CD8+ suppressive immune responses (Supplementary Figure 4A).
Figure 4. CD8+CTLA-4+ T cells suppress T-cell proliferation in an IL-35-dependent fashion.
PBMC from a patient with CTLA-4- and IL-35-regulated PAP-specific bystander suppressive immune response (ID014) were stimulated for three days with PAP, PSA, or media alone. Cells were sorted by flow cytometry for CD8+CTLA-4+ or CD8+CTLA-4− T cells, labeled with CFSE, and added-back to autologous PKH26-labeled PBMC at either their natural frequency, or a fold higher or lower. Co-cultures were stimulated for seven days with either media alone, anti-CD3/CD28-coated beads along with IgG, or anti-CD3/CD28-coated beads along with anti-IL-35 blocking antibodies. Following this incubation, the proliferation of CFSE- CD4+ and CD8+ T cells was measured by PKH26 dilution, and percent suppression was calculated compared to the proliferation of unstimulated PBMC without any cells added back. Panel A shows the proliferation of CD4+ (left panels) and CD8+ (right panels) to which were added increasing frequencies of PAP-stimulated CD8+CTLA-4+ or CD8+CTLA-4− sorted T cells (indicated next to y-axis). Co-cultures were also treated with either a control IgG (top panels) or IL-35 blocking antibodies (lower panels – indicated to far left of x-axis). Panel B, graphical representation of suppression assays conducted using sorted CD8+CTLA-4+ (black) or CD8+CTLA-4− (grey) T cells that were stimulated with either PAP (bottom row), PSA (middle row), or media alone (top row). Co-cultures were treated with anti-CD3/CD28-coated beads along with IgG (solid lines), or IL-35 blocking antibodies (dashed lines). Following this incubation, the proliferation of CFSE- CD4+ (left panels) and CD8+ (right panels) T cells was measured by PKH26 dilution. Data shown are representative of at least two independent experiments from the same patient.
To evaluate the contact-dependence of this regulation, similar in vitro suppression assays were performed utilizing a transwell system (6, 7). In this system, PAP-stimulated CD8+CTLA-4+ T cells physically separated from the responding autologous T-cell population were found to mediate similar levels of suppression compared to direct suppression assays, and this suppression could be alleviated upon co-treatment with antibodies blocking both subunits of IL-35 (Figure 5). T cells lacking CTLA-4+ expression lacked this suppressive activity, as did cells isolated from unstimulated PBMC or PSA-stimulated cultures. Similar results were obtained from another patient with PAP-specific CD8+CTLA-4+ suppressive immune responses (Supplementary Figure 4B), showing that PAP-specific CD8+CTLA-4+ suppressive immune responses can mediate suppression in an IL-35-dependent, contact-independent fashion.
Figure 5. CD8+CTLA-4+ T cells suppress T-cell proliferation in a contact-independent fashion.
PBMC from a patient with CTLA-4− and IL-35-regulated PAP-specific bystander suppressive immune response (ID002) were stimulated for three days with PAP (bottom row), PSA (middle row), or media alone (top row). Cells were sorted by flow cytometry for CD8+CTLA-4+ (black) or CD8+CTLA-4− (grey) T cells, and added-back to the top chamber of a 96 transwell plate at either their natural frequency, or a fold higher or lower (indicated along the x-axis). Autologous PKH26-labeled PBMC were added to the bottom chamber of the transwell plates, and stimulated for seven days with either media alone, anti-CD3/CD28-coated beads along with IgG (solid lines), or anti-CD3/CD28-coated beads along with anti-IL-35 blocking antibodies (dashed lines). Following this incubation, cells were collected from the bottom chamber and measured for the proliferation of naive CD4+ (left panels) and CD8+ (right panels) T cells by PKH26 dilution, and percent suppression was calculated compared to the proliferation of naïve PBMC without any cells added back. Data shown are representative of at least two independent experiments.
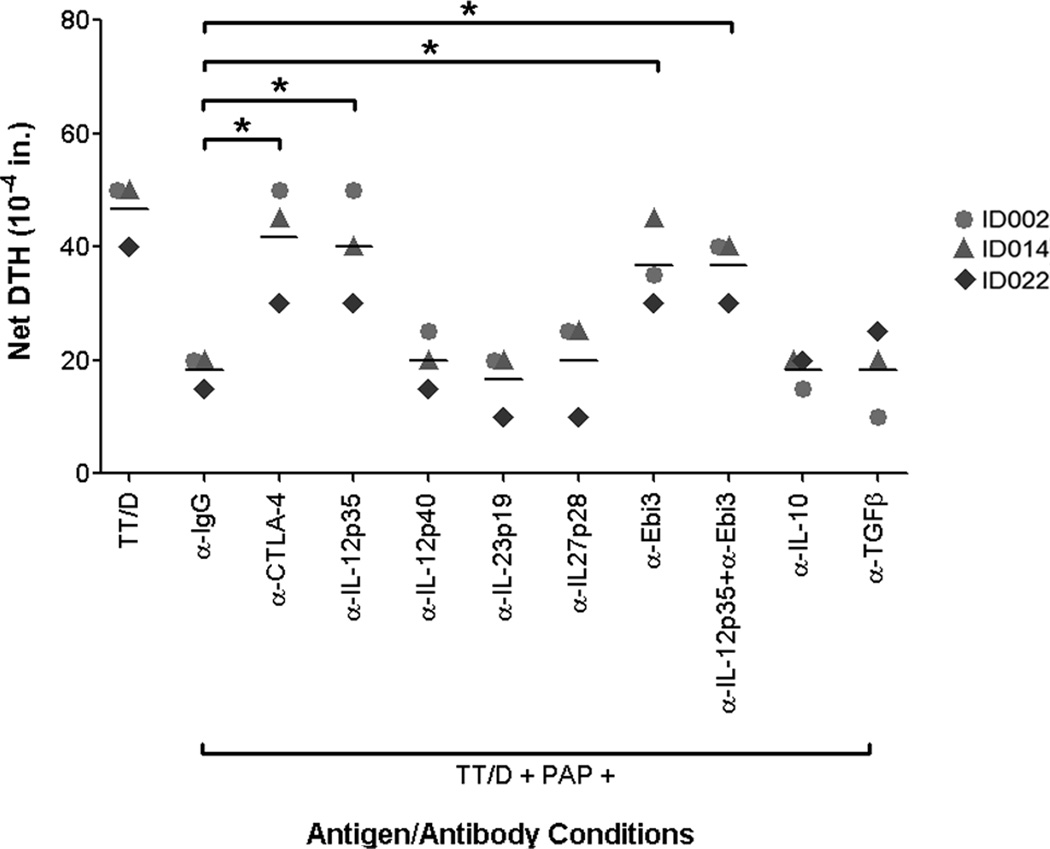
PAP-specific regulatory responses mediate tvDTH suppression via IL-35
After identifying PAP-specific IL-35 expression in CD8+CTLA-4+ T cells, and finding that this expression is decreased by inhibiting CTLA-4-mediated regulatory responses, we studied the role of IL-35 in mediating PAP-specific suppressive responses in the tvDTH in vivo setting. Samples from three patients with pre-existing PAP-specific CD8+CTLA-4+ regulatory responses were evaluated for bystander suppression in the presence of various blocking antibodies. Specific neutralization of IL-35 (by blocking IL12p35 or Ebi3, alone or in combination) significantly relieved the PAP-specific bystander suppression of tetanus immune response in 3/3 patients tested (Figure 6). Treatment with blocking antibodies targeting the other members of the IL-12 family (IL-12p40, IL-23p19, or IL-27p28), as well as blocking the immunosuppressive cytokines IL-10 and TGFβ, did not relieve this PAP-specific bystander suppression, further illustrating that PAP-specific regulatory cells mediate suppression via CTLA-4 and IL-35.
Figure 6. PAP-specific CD8+ regulatory responses mediate tvDTH suppression via IL-35.
PBMC from patients with CTLA-4-regulated PAP-specific bystander suppressive immune responses were stimulated with TT/D alone or TT/D and PAP in the presence of either an IgG control or blocking antibodies directed against CTLA-4, IL-12p35, IL-12p40, IL-23p19, IL-27p28, Ebi3, a combination of IL-12p35 and Ebi-3 (both IL-35 subunits), IL-10, or TGFβ. Data shown are representative of at least two independent experiments. Differences between treatment group mean DTH values (indicated by solid lines) were compared using a student’s t-test, with * indicating p<0.05.
Discussion
While cancer vaccines have shown promise in eliciting anti-tumor immune responses, albeit with modest clinical benefits, the effector responses these vaccines aim to augment are often countered by concurrent regulatory responses. While reports evaluating the effects of regulatory responses on vaccine efficacy have focused on CD4+CD25+ regulatory T cell responses and MDSC, here we show that CD8+ antigen-specific suppressive responses also exist in individuals with prostate cancer, and these regulatory responses can have a profound effect limiting the detection of antigen-specific effector T cell responses. Using samples from a recently completed clinical trial evaluating a DNA vaccine targeting PAP, we identified individuals who had antigen-specific effector immune responses following immunization that were suppressed by concurrent regulatory responses. CTLA-4-regulated PAP-specific effector responses were found to be common following immunization, but such effectors were rare prior to immunization. However, PAP-specific regulatory T cells, revealed by bystander suppression of a recall antigen response, were identified prior to immunization in at least 33% (7/21) of prostate cancer patients. PAP-specific regulatory T cells were found to mediate suppression via IL-35 in a contact-independent fashion, and were not found to rely on more extensively-characterized suppressive cytokines such as IL-10 or TGF-β (62). The data indicate that tumor antigen-specific immunosuppressive responses mediated by CD8+CTLA-4+ IL-35-expressing T cells arise spontaneously in some patients, and that these responses affect the detection (and possibly the function) of effector immune responses resulting from immunization.
Our results suggest that antigen-specific CD8+ cells might be a significant obstacle in efforts to augment effective anti-tumor immune responses to prostate tumor-associated antigens. This is of particular importance with regard to PAP, as it is the target of the FDA-approved sipuleucel-T immunotherapy for patients with advanced prostate cancer (40). Tumor antigen-specific CD8+ T cell responses to tumor-associated antigens were reported by Andersen and colleagues, who identified peripheral CD8+ regulatory T cells in patients with melanoma, renal or breast cancer that were specific for heme oxygenase-1 (HO-1), a protein involved in the termination of inflammatory responses (63). They found that HO-1-specific CD8+ Tregs had enhanced suppressive function compared to CD4+ Tregs, and that this suppression was contact-independent. However, they did not detect secretion of either IL-10 or TGF-β by these CD8+ regulatory cells, leaving open a role for other immunosuppressive cytokines such as IL-35.
While we show that CD8+CTLA-4+ T cells specific for PAP have suppressive activity in vivo and in vitro, the specific target and mechanism of action of this suppression remains unclear. Our ability to isolate these CD8+CTLA-4+ regulatory T cells two weeks following the final immunization suggests that CTLA-4 expression may be sustained in this population (as opposed to the transient expression of CTLA-4 by effector T cells). As such, the expression of CTLA-4+ itself could potentially have a suppressive effect by preventing effector T cells from having access to B7 ligands on antigen presenting cells. However, IL-35 appears to be the central mediator of contact-independent tolerance in this model, which could occur via a number of mechanisms. It could be that IL-35 produced by this regulatory population is acting directly on effector T cells, including the PAP-specific effector cells identified following immunization and thus preventing their detection following immunization. IL-35 could also be acting to propagate these regulatory responses through the generation of additional induced regulatory T cells, as has previously been identified (4, 5). Alternatively, these CD8+CTLA-4+ regulatory T cells could be acting on a population of antigen-presenting cells, dampening their ability of activate effector responses. Identifying the precise target of suppression of this regulatory population will lead to a better understanding of how these regulatory cells may effect the generation of effector responses following immunization.
While we focus on the characterization of PAP-specific regulatory CD8+ T cells, our results certainly do not exclude the possibility that PAP-specific CD4+ regulatory cells exist. A recent report has found that PAP-specific immune responses in prostate cancer patients are enhanced by depletion of CD4+CD25+ Tregs (42), and immunization of rodents with a vaccine targeting PAP was found to elicit IL-10-secreting immune responses (64). Indeed, preliminary data from our studies indicate that CD4+ T cells may also contribute to PAP antigen-specific regulation, as one of the patients we identified as having PAP-specific CD8+ regulatory responses also had CD4+CD25hi T-cells that could suppress T-cell proliferation in vitro (Olson et al., unpublished observation). The impact of PAP antigen-specific regulatory immune responses (both CD8+ and potentially CD4+ T cells) on the generation of PAP effector responses will be evaluated in future studies.
While ours is the first report to our knowledge to identify IL-35-secreting antigen-specific regulatory T cells in patients with prostate cancer, CD8+ regulatory T cells have previously been identified in both murine models of prostate cancer as well as patients with prostate tumors. Kiniwa and colleagues identified human prostate tumor infiltrating CD8+ T cells that could suppress the non-specific activation of CD4+ T cells (65). An intriguing finding from this study was that they report a contact-independent mechanism of suppression that was not mediated by either IL-10 or TGF-β (65). Additionally, another report from Shafer-Weaver and colleagues identified murine prostate tumor-infiltrating CD8+ T cells that were induced to become regulatory cells once at the site of tumor, though it remains unclear whether this conversion was mediated by tumor-derived factors, or possibly through tumor-infiltrating regulatory cells (66). This study also found that CD8+ regulatory T cells mediate suppression in a contact-independent fashion, and while they identified a role for TGF-β in mediating this suppression, they found that blocking TGFβ did not completely alleviate contact-independent suppression (66). Given recent findings suggesting the crucial importance of IL-35 in propagating CD4+ Treg-mediated suppression (4–6), our findings suggest that IL-35 may also play an important role in mediating suppression by CD8+ regulatory T cells.
While CD8+ suppressive T cells have been detected in multiple model systems the origin of these CD8+ suppressor cells remains unclear. Possible origins include (i) a distinct thymic lineage like 'natural' CD4+CD25+ Tregs, (ii) an uncommitted population of naive CD8+ T cells, or (iii) conversion from traditional effector CD8+ T cells. We previously identified populations of CD8+CTLA-4+ suppressive T cells that mediate maternal/offspring tolerance to minor histocompatibility antigens, HA-1 and HY-1. In this model, a distinct population of tetramerdim CD8+CTLA-4+ suppressive T cells was identified, whereas antigen-specific CD8+ effector T cells were found to be tetramerbright (16, 67, 68). This may suggest that distinct lineages of CD8+ T cells arise whose functions are based on T-cell receptor avidity, which could be addressed in future studies using previously identified HLA-A2-restricted T-cell epitopes specific for PAP (69). While this model of distinct regulatory and effector antigen-specific T cell populations would account for the presence and function of antigen-specific regulatory cells, it does not account for the influence of the environment on T-cell function (6, 34, 66, 70). Research into T-cell plasticity has shown that T cells can change their functional phenotype depending on the conditions within the microenvironment, perhaps by regulatory CD4+ and/or CD8+ T cells or by the tumor itself, including a report of CD8+ T cells being induced into a regulatory phenotype in a murine model of prostate cancer (66). Additionally, iTr35 cells have also been shown to be induced by the tumor microenvironment in murine models of both melanoma and colorectal adenocarcinoma (4). This concept of ‘local tolerance’ (tolerance being induced within the microenvironment, rather than dichotomous central and peripheral tolerance), and a potential role for IL-35 in contributing to this induction of local tolerance, warrants further investigation, as does the prevalence of CD8+CTLA-4+ IL-35-secreting regulatory T cells within prostate tumors.
It remains unclear whether the presence of antigen-specific regulatory responses is beneficial or not in the context of immunization. A priori, the presence of antigen-specific regulation might predict for individuals unlikely to respond to an antigen-specific vaccine. On the other hand, we were able to detect this type of response in individuals who subsequently had evidence of PAP-specific IFNγ-secreting T cells. Moreover, of the eight individuals in whom CTLA-4-regulated PAP responses were detected after immunization, five subsequently experienced at least a 200% increase in PSA doubling time (53). Consequently, it is conceivable that the presence of antigen-specific regulation belies the existence of prior effector or memory antigen-specific cells whose function becomes actively suppressed, suggesting that the presence of antigen-specific regulation might indicate individuals with existing effector cells that might be more readily augmented with immunization. As such, we believe that the detection of antigen-specific regulatory responses should be included in the baseline evaluation of patients receiving antigen-specific vaccines, and potentially at times thereafter. Furthermore, while we focused on PAP antigen-specific regulatory immune responses and did not detect regulatory responses for another prostate tumor antigen (PSA), we certainly do not exclude the possibility that antigen-specific regulatory immune responses exist for other prostate cancer tumor-associated antigens that are targets for immunotherapeutic vaccines. The association of these findings with immunological response, specifically whether the presence of antigen-specific CD8+CTLA-4+ IL-35-secreting regulatory cells affects the generation and kinetics of T-cell effector responses, and more importantly clinical benefit, will be evaluated in sufficiently-powered future studies. Such associations might ultimately be used to determine whether a particular individual is likely, or not, to respond to a particular antigen-specific vaccine. This is of particular relevance for the PAP tumor antigen, as this is the antigen target of the recently FDA-approved sipuleucel-T immunotherapy for patients with advanced prostate cancer (40).
Given the potentially detrimental impact regulatory responses can have on anti-tumor vaccines, there has been great interest in combining vaccination with strategies aimed at depleting regulatory responses in many different malignancies, including prostate cancer (46, 71–74). A recent report of a Phase I trial by Fong and colleagues in which prostate cancer patients received granulocyte macrophage colony-stimulating factor (GM-CSF) and/or a CTLA-4-blocking antibody found that a combination of both treatments acted synergistically to increase expansion of activated CD8+ T cells (74). Several groups have reported the detection of antigen-specific T cells following CTLA-4 blockade, even years after antigen-specific vaccination, suggesting that antigen-specific T-cell regulation might be a mechanism that could exist for long periods of time but be directly targeted (75). Furthermore, the central role of IL-35 in mediating suppression suggests that antibodies directed against IL-35 may also have efficacy targeting the function of regulatory cells and may be a suitable target for future clinical evaluation. By identifying individuals with these pre-existing regulatory responses, it may be possible to prioritize patients who may respond ideally to these combinatorial therapies.
Supplementary Material
Acknowledgements
The authors would like to thank Kate Vignali, Karen Forbes, Dr. Creg Workman, Dr. William Singleterry, Dr. Dario Campana, and Dr. Joshua Lang for technical assistance and helpful discussions, and James Davies, Edward Dunphy, Dr. Thomas Frye, and Dr. Laura Johnson for PBMC preparation. We would also like to thank Dr. Paula Kavathas for critical reading of the manuscript.
Funding: This work was supported for BMO, JTB, and DGM by NIH (R01 CA142608), by the US Army Medical Research and Materiel Command Prostate Cancer Research Program (W81XWH-05-1-0404 and W81XWH-11-1-0196), and by the University of Wisconsin Carbone Cancer Center. WJB is supported by NIH (R01-AI066219-05), and WJB and EJG are supported by the EU-sponsored One Study. DAAV is supported by National Institutes of Health (R01 AI091977), NCI Comprehensive Cancer Center Support CORE grant (CA21765) and the American Lebanese Syrian Associated Charities (ALSAC).
Abbreviations
- Ebi3
Epstein Barr induced factor 3
- iTr
Induced T regulatory cell
- PAP
Prostatic acid phosphatase
- PSA
Prostate specific antigen
- TT/D
Tetanus/diphtheria toxoid
- tvDTH
trans-vivo delayed type hypersensitivity
Footnotes
Author Contributions: B.M.O., E.J.G., J.T.B., D.G.M., and W.J.B. designed the research, interpreted the data, and assisted with the manuscript. B.M.O., E.J.G, and J.T.B. performed the experiments, and B.M.O. wrote the manuscript. D.A.A.V. provided essential reagents and key discussions in designing the research, and assisted with the paper.
Competing interests: D.G.M., W.J.B., and D.A.A.V. have submitted patents that are pending and are entitled to a share in net income generated from licensing of these patent rights for commercial development.
References
- 1.Fehervari Z, Sakaguchi S. Development and function of CD25+CD4+ regulatory T cells. Curr Opin Immunol. 2004;16:203–208. doi: 10.1016/j.coi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 3.Akin Y, Koksoy S, Yucel S, Erdogru T, Baykara M. Increased peripheral CD4+CD25high Treg in prostate cancer patients is correlated with PSA. Saudi medical journal. 2011;32:1003–1008. [PubMed] [Google Scholar]
- 4.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. IL-35-mediated induction of a potent regulatory T cell population. Nature immunology. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi V, Collison LW, Guy CS, Workman CJ, Vignali DA. Cutting edge: Human regulatory T cells require IL-35 to mediate suppression and infectious tolerance. J Immunol. 2011;186:6661–6666. doi: 10.4049/jimmunol.1100315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol. 2009;182:6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 9.Rees MA, Rosenberg AS, Munitz TI, Singer A. In vivo induction of antigen-specific transplantation tolerance to Qa1a by exposure to alloantigen in the absence of T-cell help. Proc Natl Acad Sci U S A. 1990;87:2765–2769. doi: 10.1073/pnas.87.7.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 11.Zhang ZX, Yang L, Young KJ, DuTemple B, Zhang L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat Med. 2000;6:782–789. doi: 10.1038/77513. [DOI] [PubMed] [Google Scholar]
- 12.Olivares-Villagomez D, Wang Y, Lafaille JJ. Regulatory CD4(+) T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J Exp Med. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen TL, Sullivan NL, Ebel M, Teague RM, DiPaolo RJ. Antigen-specific TGF-beta-induced regulatory T cells secrete chemokines, regulate T cell trafficking, and suppress ongoing autoimmunity. J Immunol. 2011;187:1745–1753. doi: 10.4049/jimmunol.1004112. [DOI] [PubMed] [Google Scholar]
- 14.Chai JG, Bartok I, Chandler P, Vendetti S, Antoniou A, Dyson J, Lechler R. Anergic T cells act as suppressor cells in vitro and in vivo. European journal of immunology. 1999;29:686–692. doi: 10.1002/(SICI)1521-4141(199902)29:02<686::AID-IMMU686>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 15.von Herrath MG, Harrison LC. Antigen-induced regulatory T cells in autoimmunity. Nature reviews. 2003;3:223–232. doi: 10.1038/nri1029. [DOI] [PubMed] [Google Scholar]
- 16.Cai J, Lee J, Jankowska-Gan E, Derks R, Pool J, Mutis T, Goulmy E, Burlingham WJ. Minor H antigen HA-1-specific regulator and effector CD8+ T cells, and HA-1 microchimerism, in allograft tolerance. J Exp Med. 2004;199:1017–1023. doi: 10.1084/jem.20031012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 18.Wang HY, Peng G, Guo Z, Shevach EM, Wang RF. Recognition of a new ARTC1 peptide ligand uniquely expressed in tumor cells by antigen-specific CD4+ regulatory T cells. J Immunol. 2005;174:2661–2670. doi: 10.4049/jimmunol.174.5.2661. [DOI] [PubMed] [Google Scholar]
- 19.Vence L, Palucka AK, Fay JW, Ito T, Liu YJ, Banchereau J, Ueno H. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2007;104:20884–20889. doi: 10.1073/pnas.0710557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehe C, Ghebeh H, Al-Sulaiman A, Al Qudaihi G, Al-Hussein K, Almohareb F, Chaudhri N, Alsharif F, Al-Zahrani H, Tbakhi A, Aljurf M, Dermime S. The Wilms' tumor antigen is a novel target for human CD4+ regulatory T cells: implications for immunotherapy. Cancer Res. 2008;68:6350–6359. doi: 10.1158/0008-5472.CAN-08-0050. [DOI] [PubMed] [Google Scholar]
- 21.Bonertz A, Weitz J, Pietsch DH, Rahbari NN, Schlude C, Ge Y, Juenger S, Vlodavsky I, Khazaie K, Jaeger D, Reissfelder C, Antolovic D, Aigner M, Koch M, Beckhove P. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest. 2009;119:3311–3321. doi: 10.1172/JCI39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Burg SH, Piersma SJ, de Jong A, van der Hulst JM, Kwappenberg KM, van den Hende M, Welters MJ, Van Rood JJ, Fleuren GJ, Melief CJ, Kenter GG, Offringa R. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc Natl Acad Sci U S A. 2007;104:12087–12092. doi: 10.1073/pnas.0704672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welters MJ, Kenter GG, Piersma SJ, Vloon AP, Lowik MJ, Berends-van der Meer DM, Drijfhout JW, Valentijn AR, Wafelman AR, Oostendorp J, Fleuren GJ, Offringa R, Melief CJ, van der Burg SH. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res. 2008;14:178–187. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 24.Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Advances in cancer research. 2010;107:57–117. doi: 10.1016/S0065-230X(10)07003-X. [DOI] [PubMed] [Google Scholar]
- 25.Knutson KL, Disis ML, Salazar LG. CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol Immunother. 2007;56:271–285. doi: 10.1007/s00262-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Betts GJ, Clarke SL, Richards HE, Godkin AJ, Gallimore AM. Regulating the immune response to tumours. Advanced drug delivery reviews. 2006;58:948–961. doi: 10.1016/j.addr.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, Pisa P. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 28.Tien AH, Xu L, Helgason CD. Altered immunity accompanies disease progression in a mouse model of prostate dysplasia. Cancer Res. 2005;65:2947–2955. doi: 10.1158/0008-5472.CAN-04-3271. [DOI] [PubMed] [Google Scholar]
- 29.Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, Schlom J, Tsang KY. Enhanced functionality of CD4+CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res. 2008;14:1032–1040. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 30.Gray A, de la Luz Garcia-Hernandez M, van West M, Kanodia S, Hubby B, Kast WM. Prostate cancer immunotherapy yields superior long-term survival in TRAMP mice when administered at an early stage of carcinogenesis prior to the establishment of tumor-associated immunosuppression at later stages. Vaccine. 2009;27(Suppl 6):G52–G59. doi: 10.1016/j.vaccine.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degl'Innocenti E, Grioni M, Capuano G, Jachetti E, Freschi M, Bertilaccio MT, Hess-Michelini R, Doglioni C, Bellone M. Peripheral T-cell tolerance associated with prostate cancer is independent from CD4+CD25+ regulatory T cells. Cancer Res. 2008;68:292–300. doi: 10.1158/0008-5472.CAN-07-2429. [DOI] [PubMed] [Google Scholar]
- 32.Comperat E, Egevad L, Camparo P, Roupret M, Vaessen C, Valdman A, Jonmarker S, Capron F, Cussenot O, Charlotte F. Clinical significance of intratumoral CD8+ regulatory T cells in prostate carcinoma. Analytical and quantitative cytology and histology / the International Academy of Cytology [and] American Society of Cytology. 2010;32:39–44. [PubMed] [Google Scholar]
- 33.Valdman A, Jaraj SJ, Comperat E, Charlotte F, Roupret M, Pisa P, Egevad L. Distribution of Foxp3−, CD4− and CD8− positive lymphocytic cells in benign and malignant prostate tissue. Apmis. 2010;118:360–365. doi: 10.1111/j.1600-0463.2010.02604.x. [DOI] [PubMed] [Google Scholar]
- 34.Barrack ER. TGF beta in prostate cancer: a growth inhibitor that can enhance tumorigenicity. Prostate. 1997;31:61–70. doi: 10.1002/(sici)1097-0045(19970401)31:1<61::aid-pros10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 35.Stearns ME, Garcia FU, Fudge K, Rhim J, Wang M. Role of interleukin 10 and transforming growth factor beta1 in the angiogenesis and metastasis of human prostate primary tumor lines from orthotopic implants in severe combined immunodeficiency mice. Clin Cancer Res. 1999;5:711–720. [PubMed] [Google Scholar]
- 36.Kachikwu EL, Iwamoto KS, Liao YP, DeMarco JJ, Agazaryan N, Economou JS, McBride WH, Schaue D. Radiation enhances regulatory T cell representation. International journal of radiation oncology, biology, physics. 2011;81:1128–1135. doi: 10.1016/j.ijrobp.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorrentino C, Musiani P, Pompa P, Cipollone G, Di Carlo E. Androgen deprivation boosts prostatic infiltration of cytotoxic and regulatory T lymphocytes and has no effect on disease-free survival in prostate cancer patients. Clin Cancer Res. 2011;17:1571–1581. doi: 10.1158/1078-0432.CCR-10-2804. [DOI] [PubMed] [Google Scholar]
- 38.Tang S, Moore ML, Grayson JM, Dubey P. Increased CD8+ T cell function following castration and immunization is countered by parallel expansion of regulatory T cells. Cancer Res. 2012;72:1975–1985. doi: 10.1158/0008-5472.CAN-11-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England journal of medicine. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 41.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadaschik B, Su Y, Huter E, Ge Y, Hohenfellner M, Beckhove P. Antigen Specific T-Cell Responses Against Tumor Antigens are Controlled by Regulatory T Cells in Patients With Prostate Cancer. J Urol. 2012;187:1458–1465. doi: 10.1016/j.juro.2011.11.083. [DOI] [PubMed] [Google Scholar]
- 43.Hus I, Schmitt M, Tabarkiewicz J, Radej S, Wojas K, Bojarska-Junak A, Schmitt A, Giannopoulos K, Dmoszynska A, Rolinski J. Vaccination of B-CLL patients with autologous dendritic cells can change the frequency of leukemia antigen-specific CD8+ T cells as well as CD4+CD25+FoxP3+ regulatory T cells toward an antileukemia response. Leukemia. 2008;22:1007–1017. doi: 10.1038/leu.2008.29. [DOI] [PubMed] [Google Scholar]
- 44.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–636. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Francois V, Ottaviani S, Renkvist N, Stockis J, Schuler G, Thielemans K, Colau D, Marchand M, Boon T, Lucas S, van der Bruggen P. The CD4(+) T-cell response of melanoma patients to a MAGE-A3 peptide vaccine involves potential regulatory T cells. Cancer Res. 2009;69:4335–4345. doi: 10.1158/0008-5472.CAN-08-3726. [DOI] [PubMed] [Google Scholar]
- 46.Kavanagh B, O'Brien S, Lee D, Hou Y, Weinberg V, Rini B, Allison JP, Small EJ, Fong L. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112:1175–1183. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicholaou T, Ebert LM, Davis ID, McArthur GA, Jackson H, Dimopoulos N, Tan B, Maraskovsky E, Miloradovic L, Hopkins W, Pan L, Venhaus R, Hoffman EW, Chen W, Cebon J. Regulatory T-cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma. Clin Cancer Res. 2009;15:2166–2173. doi: 10.1158/1078-0432.CCR-08-2484. [DOI] [PubMed] [Google Scholar]
- 48.Gnjatic S, Altorki NK, Tang DN, Tu SM, Kundra V, Ritter G, Old LJ, Logothetis CJ, Sharma P. NY-ESO-1 DNA vaccine induces T-cell responses that are suppressed by regulatory T cells. Clin Cancer Res. 2009;15:2130–2139. doi: 10.1158/1078-0432.CCR-08-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNeel DG, Dunphy EJ, Davies JG, Frye TP, Johnson LE, Staab MJ, Horvath DL, Straus J, Alberti D, Marnocha R, Liu G, Eickhoff JC, Wilding G. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol. 2009;27:4047–4054. doi: 10.1200/JCO.2008.19.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, Partin AW. Death in patients with recurrent prostate cancer after radical prostatectomy: prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J Clin Oncol. 2007;25:1765–1771. doi: 10.1200/JCO.2006.08.0572. [DOI] [PubMed] [Google Scholar]
- 51.Slovin SF, Wilton AS, Heller G, Scher HI. Time to detectable metastatic disease in patients with rising prostate-specific antigen values following surgery or radiation therapy. Clin Cancer Res. 2005;11:8669–8673. doi: 10.1158/1078-0432.CCR-05-1668. [DOI] [PubMed] [Google Scholar]
- 52.Lee AK, Levy LB, Cheung R, Kuban D. Prostate-specific antigen doubling time predicts clinical outcome and survival in prostate cancer patients treated with combined radiation and hormone therapy. International journal of radiation oncology, biology, physics. 2005;63:456–462. doi: 10.1016/j.ijrobp.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 53.Becker JT, Olson BM, Johnson LE, Davies JG, Dunphy EJ, McNeel DG. DNA vaccine encoding prostatic acid phosphatase (PAP) elicits long-term T-cell responses in patients with recurrent prostate cancer. J Immunother. 2010;33:639–647. doi: 10.1097/CJI.0b013e3181dda23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keizman D, Huang P, Antonarakis ES, Sinibaldi V, Carducci MA, Denmeade S, Kim JJ, Walczak J, Eisenberger MA. The change of PSA doubling time and its association with disease progression in patients with biochemically relapsed prostate cancer treated with intermittent androgen deprivation. Prostate. 2011;71:1608–1615. doi: 10.1002/pros.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, Chin T, Kusaka S, Geissler F, Pelletier RP, Orosz CG. Human allograft acceptance is associated with immune regulation. J Clin Invest. 2000;106:145–155. doi: 10.1172/JCI9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Derks RA, Jankowska-Gan E, Xu Q, Burlingham WJ. Dendritic cell type determines the mechanism of bystander suppression by adaptive T regulatory cells specific for the minor antigen HA-1. J Immunol. 2007;179:3443–3451. doi: 10.4049/jimmunol.179.6.3443. [DOI] [PubMed] [Google Scholar]
- 57.Xu Q, Lee J, Jankowska-Gan E, Schultz J, Roenneburg DA, Haynes LD, Kusaka S, Sollinger HW, Knechtle SJ, VanBuskirk AM, Torrealba JR, Burlingham WJ. Human CD4+CD25low adaptive T regulatory cells suppress delayed-type hypersensitivity during transplant tolerance. J Immunol. 2007;178:3983–3995. doi: 10.4049/jimmunol.178.6.3983. [DOI] [PubMed] [Google Scholar]
- 58.Jankowska-Gan E, Rhein T, Haynes LD, Geissler F, Mulder A, Kalayoglu M, Sollinger H, Burlingham WJ. Human liver allograft acceptance and the "tolerance assay". II. Donor HLA-A, -B but not DR antigens are able to trigger regulation of DTH. Human immunology. 2002;63:862–870. doi: 10.1016/s0198-8859(02)00450-0. [DOI] [PubMed] [Google Scholar]
- 59.Daud II, Scott ME. Validation of reference genes in cervical cell samples from human papillomavirus-infected and -uninfected women for quantitative reverse transcription-PCR assays. Clin Vaccine Immunol. 2008;15:1369–1373. doi: 10.1128/CVI.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mathieu MG, Knights AJ, Pawelec G, Riley CL, Wernet D, Lemonnier FA, Straten PT, Mueller L, Rees RC, McArdle SE. HAGE, a cancer/testis antigen with potential for melanoma immunotherapy: identification of several MHC class I/II HAGE-derived immunogenic peptides. Cancer Immunol Immunother. 2007;56:1885–1895. doi: 10.1007/s00262-007-0331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jarnicki AG, Lysaght J, Todryk S, Mills KH. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 63.Andersen MH, Sorensen RB, Brimnes MK, Svane IM, Becker JC, thor Straten P. Identification of heme oxygenase-1-specific regulatory CD8+ T cells in cancer patients. J Clin Invest. 2009;119:2245–2256. doi: 10.1172/JCI38739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson LE, Frye TP, Arnot AR, Marquette C, Couture LA, Gendron-Fitzpatrick A, McNeel DG. Safety and immunological efficacy of a prostate cancer plasmid DNA vaccine encoding prostatic acid phosphatase (PAP) Vaccine. 2006;24:293–303. doi: 10.1016/j.vaccine.2005.07.074. [DOI] [PubMed] [Google Scholar]
- 65.Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, Thompson TC, Old LJ, Wang RF. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13:6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 66.Shafer-Weaver KA, Anderson MJ, Stagliano K, Malyguine A, Greenberg NM, Hurwitz AA. Cutting Edge: Tumor-specific CD8+ T cells infiltrating prostatic tumors are induced to become suppressor cells. J Immunol. 2009;183:4848–4852. doi: 10.4049/jimmunol.0900848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burlingham WJ, Goulmy E. Human CD8+ T-regulatory cells with low-avidity T-cell receptor specific for minor histocompatibility antigens. Human immunology. 2008;69:728–731. doi: 10.1016/j.humimm.2008.08.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Halteren AG, Jankowska-Gan E, Joosten A, Blokland E, Pool J, Brand A, Burlingham WJ, Goulmy E. Naturally acquired tolerance and sensitization to minor histocompatibility antigens in healthy family members. Blood. 2009;114:2263–2272. doi: 10.1182/blood-2009-01-200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olson BM, Frye TP, Johnson LE, Fong L, Knutson KL, Disis ML, McNeel DG. HLA-A2-restricted T-cell epitopes specific for prostatic acid phosphatase. Cancer Immunol Immunother. 2010;59:943–953. doi: 10.1007/s00262-010-0820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Addey C, White M, Dou L, Coe D, Dyson J, Chai JG. Functional plasticity of antigen-specific regulatory T cells in context of tumor. J Immunol. 2011;186:4557–4564. doi: 10.4049/jimmunol.1003797. [DOI] [PubMed] [Google Scholar]
- 71.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 72.Curran MA, Allison JP. Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res. 2009;69:7747–7755. doi: 10.1158/0008-5472.CAN-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gregor PD, Wolchok JD, Ferrone CR, Buchinshky H, Guevara-Patino JA, Perales MA, Mortazavi F, Bacich D, Heston W, Latouche JB, Sadelain M, Allison JP, Scher HI, Houghton AN. CTLA-4 blockade in combination with xenogeneic DNA vaccines enhances T-cell responses, tumor immunity and autoimmunity to self antigens in animal and cellular model systems. Vaccine. 2004;22:1700–1708. doi: 10.1016/j.vaccine.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 74.Fong L, Kwek SS, O'Brien S, Kavanagh B, McNeel DG, Weinberg V, Lin AM, Rosenberg J, Ryan CJ, Rini BI, Small EJ. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, Ku GY, Jungbluth AA, Segal NH, Rasalan TS, Manukian G, Xu Y, Roman RA, Terzulli SL, Heywood M, Pogoriler E, Ritter G, Old LJ, Allison JP, Wolchok JD. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.